Abstract
Derangements in cerebrovascular structure and function can impair cognitive performance throughout ageing and in cardiometabolic disease states, thus increasing dementia risk. Modifiable lifestyle factors that cause a decline in cardiometabolic health, such as physical inactivity, exacerbate these changes beyond those that are associated with normal ageing. The purpose of this review was to examine cerebrovascular, cognitive and neuroanatomical adaptations to ageing and the potential benefits of exercise training on these outcomes in adults 50 years or older. We systematically searched for cross-sectional or intervention studies that included exercise (aerobic, resistance or multimodal) and its effect on cerebrovascular function, cognition and neuroanatomical adaptations in this age demographic. The included studies were tabulated and described narratively. Aerobic exercise training was the predominant focus of the studies identified; there were limited studies exploring the effects of resistance exercise training and multimodal training on cerebrovascular function and cognition. Collectively, the evidence indicated that exercise can improve cerebrovascular function, cognition and neuroplasticity through areas of the brain associated with executive function and memory in adults 50 years or older, irrespective of their health status. However, more research is required to ascertain the mechanisms of action.
Keywords: Dementia, exercise training, cerebrovascular function, cognition, ageing
Introduction
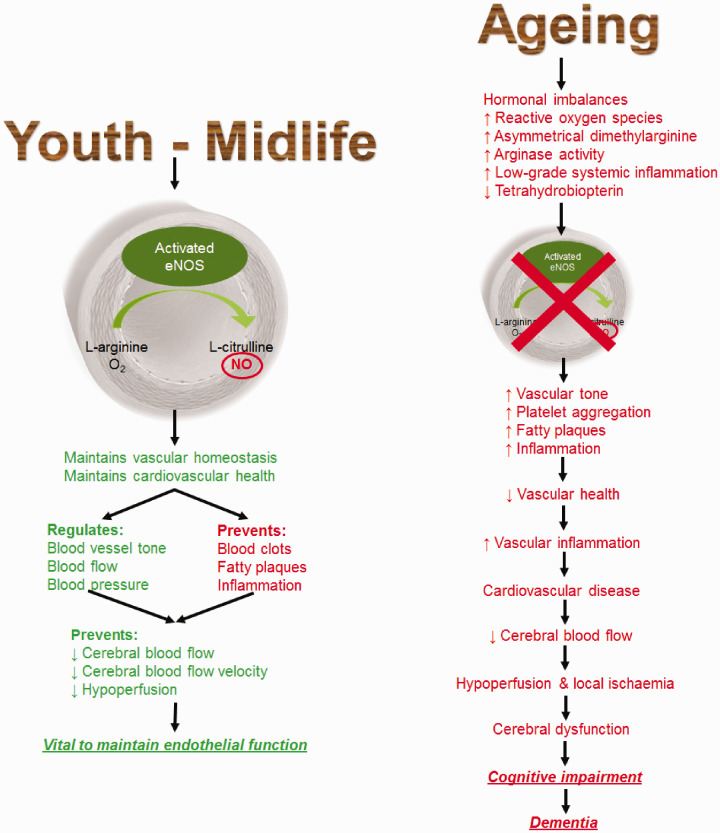
The current worldwide incidence of dementia is more than 50 million people and is expected to treble in the next 30 years.1,2 Dementia is associated with adverse changes in cerebrovascular structure and function, which contribute to a decline in cognition.3–5 The greatest risk factor for developing dementia is advanced age.1 However, there are various modifiable risks factors that lead to impaired vascular function and contribute to dementia. These include behavioural risk factors (limited educational engagement, physical inactivity and excessive alcohol consumption), metabolic risk factors (obesity, hypertension, dyslipidaemia, hyperglycaemia and homocysteinaemia) and cardiovascular diseases (coronary heart disease, heart failure, arrhythmia, stroke, diabetes and renal disease).6–16 Of these, low-level educational engagement, chronic renal disease, diabetes mellitus, hypertension and physical inactivity account for the largest degree of dementia burden globally, particularly in developed countries such as Australia.2,17 These modifiable risk factors are associated with and characterised by endothelial dysfunction,18 which can impair cerebrovascular function. This, in turn, may promote the development of cerebral dysfunction, cerebral pathology, impaired cognition (in addition to the age-related decline) and eventually a neurodegenerative disease state such as dementia (Figure 1).18
Figure 1.
Ageing is associated with hormonal imbalances and increased low-grade systemic inflammation. It is also associated with the increased production of reactive oxygen species (ROS), which may be due to diminished nuclear regulation factor 2 and superoxide dismutase expression and increased expression of nicotinamide adenine dinucleotide phosphate oxidase complexes, resulting in increased mitochondrial superoxide production.120 Uncoupled endothelial nitric oxide (NO) synthase (eNOS) increases superoxide production by catalysing nicotinamide adenine dinucleotide phosphate, instead of synthesising NO. Increased arginase activity reduces l-arginine supply, thus promoting the uncoupling of eNOS.69,70 This may also be associated with diminished tetrahydrobiopterin availability and increased asymmetrical dimethylarginine concentrations, which subsequently acts as a competitive inhibitor of eNOS, thus reducing NO biosynthesis.71 These promote and lead to endothelial dysfunction, which subsequently manifests as local ischemia and micro-haemorrhages in the microvasculature, leading to reductions in capillary density and BBB function (i.e. reduced cerebrovascular function).121,122 It may also be a result of increased conduit artery stiffness, which then diffuses to the cerebral circulation and increases pulsatility of the cerebral microvasculature, reducing CBF and promoting ischaemic-induced leukoaraiosis.123 Nevertheless, increased inflammation and oxidative stress, hypoperfusion and decreased BBB integrity are potentiated by these events.3,121,124–128 This subsequently promotes increased microglial activity, amyloid-β production and decreased amyloid-β clearance, which may act as a trigger for enhanced S100B and glial fibrillary acidic protein secretion to form astrocytes, thus promoting the inflammatory cycle and the continued accumulation of neurotoxic products.3,121,124–128 Further, the increase in amyloid-β accumulation in the brain can further compromise cerebrovascular function that manifests into neurodegeneration and further structural and functional changes within the brain.129
There is increasing evidence that exercise training may help to maintain optimal cerebrovascular function and thereby prevent or slow the development of cognitive impairment.19–47 Currently, there are limited randomised controlled studies examining the effects of exercise training on cerebrovascular function and its association with cognitive function in middle-aged and older adults, as both should be interpreted together and not as separate functions. The purpose of this review is to examine the benefits of exercise training on cerebrovascular and cognitive function in ageing. Firstly, we will review the impact of ageing on cerebrovascular function, cognition and structural changes within the brain. Secondly, the effects of aerobic exercise training (AT) and resistance exercise training (RT), alone and in combination, on structural adaptations, cerebral blood flow (CBF) and cognition in middle-aged and older adults will be discussed. Thirdly, we will determine if there are any published randomised control trials examining the association between CBF and cognition function in this cohort. Finally, we will discuss why cerebrovascular structure and function and cognition may change following exercise training.
Methods
This review implemented the procedures and following the guidelines outlined in the Peer Review of Electronic Search Strategies: 2015 Guideline Statement. Appropriate literature was searched for systematically using seven databases (PubMed, CINAHL, Cochrane, Science Direct, Web of Science, Scopus and MEDLINE) to explicitly find, select, evaluate and interpret relevant research, as well as Google Scholar as a method to ensure completeness.48 The inclusion criteria were that original articles must be written in English, peer-reviewed, describe a cross-sectional study or an intervention trial in middle-aged or older adults (≥50 years old) and include the use of exercise (aerobic, resistance or multimodal). In this review, we defined AT as training performed over months and years, which comprises repeated bouts of exercise that primarily utilise energy produced via aerobic respiration, such as running, cycling and swimming. RT is defined as repeated bouts of muscle contraction against an applied force or resistance, which primarily aims to improve muscular strength, endurance, size and definition. Multimodal or combined exercise training (CT) is defined as the combination of at least two different forms of exercise training, such as AT and RT. The search terms used were: exercise (including physical activity or exercise training), ageing, blood–brain barrier (BBB), CBF, CBF velocity (CBFv), cerebral perfusion, cerebral volume (whole brain and specific regions such as the hippocampus), neuroplasticity, cognition, transcranial Doppler (TCD) ultrasonography, magnetic resonance imaging (MRI) and arterial spin labelling (ASL).
The search was completed by April 2019 and included all studies conducted up until this period. An updated search was performed prior to submission of the article in February 2020, with limited results being returned other than those who have published method papers with results still pending. Additionally, a search was performed on the International Clinical Trials Registry Platform. It was found that there were over 34 registered trials in this field of research, which were about to commence, are ongoing or due to be completed by the end of 2020.
After removing duplicates, those identified were screened initially by title then abstract and, if deemed suitable, were read in full and included in the review. Further, the reference lists of suitable articles were screened to ensure completeness. The studies identified for inclusion in the review have had their primary and secondary results tabulated and their primary results described narratively. Knowledge gaps and future directions of research were identified based upon the findings of these studies.
Age-related cognitive decline and structural changes
Whilst crystallised intelligence (i.e. acquired knowledge) remains relatively unchanged, our fluid intelligence (ability to respond to novel situations), such as processing speed, attention, memory, language, executive functions and visuospatial ability, and visual construction proficiency decreases with ageing.49 The changes in these cognitive domains in normal ageing are summarised in Table 1, which indicates that the majority of these abilities decline at some stage throughout the lifespan. The exceptions to this include certain aspects of memory (implicit memory and memory retention), language (vocabulary and visual confrontation naming), visuospatial ability and similarity association and proverb description and reasoning of familiar material (executive function domain).49–57
Table 1.
Summary of the cognitive domain changes throughout the lifespan.
| References | Cognitive domain | Stable, increases or decreases |
|---|---|---|
| Harada et al.,49 Kochunov et al.52 | Processing speed | Decreases (from third decade of life) |
| Harada et al.,49 Salthouse et al.55 | Attention (Auditory, selective and divided attention) | Decreases (later life) |
| Harada et al.,49 Haaland et al.51, Piolino et al.,53 Rönnlund et al.,54 Salthouse et al.,55 Singh-Manoux et al.,56 Zelinski and Burnight57 | Memory • Explicit memory (episodic and semantic memory) • Implicit memory (procedural) • Memory acquisition • Memory retention • Memory retrieval |
Decreases (episodic – throughout life; semantic – later
life) Stable Decreases (throughout life) Stable Decreases (later life) |
| Harada et al.,49 Singh-Manoux et al.,56 Zelinski and Burnight57 | Language
(overall) • Vocabulary • Visual confrontation naming • Verbal fluency |
Increases Increases (increases throughout life) Increases (until seventh decade)/decreases (from seventh decade of life) Decreases (throughout life) |
| Harada et al.,49 | Visuospatial ability Visual construction proficiency |
Stable Decreases (throughout life) |
| Harada et al.,49 De Luca and Leventer50 Singh-Manoux et al.,56 | Executive function • Concept formation, abstraction and mental flexibility • Response inhibition • Inductive reasoning • Reasoning (unfamiliar material) • Similarity association, proverb description and reasoning (familiar material) • Executive function (associated with a speeded motor component) |
Decreases (throughout life, rapid from seventh decade of
life) Decreases (throughout life) Decreases (from fourth to fifth decade of life) Decreases (throughout life) Stable/increases Decreases (throughout life) |
Our understanding of the anatomical and functional changes that occur within the brain during ageing is not well defined. These changes may vary between individuals and populations and are undoubtedly related to modifiable (environmental) and unmodifiable (genetic) changes. They may be affected by a variety of disease states that promote cerebrovascular dysfunction, oxidative stress and inflammation.58–61 Further, actual cognitive changes may lag behind neuroanatomical changes by decades and, therefore, may not correlate.60 Nonetheless, there appears to be consensus that the brain’s grey matter declines with age in conjunction with expansion of the cerebral ventricles,62 thus contributing to age-related cortical thinning. Further, the prefrontal, medial temporal and parietal cortices are some of the most vulnerable regions to senescent changes.49,58,60 This is likely a consequence of neuronal death and reduced plasticity due to decreases in synaptic density.49 Additionally, white matter also decreases with age, but there is limited information about structural changes compared with functional connectivity.49,59 Functional connectivity is reduced within the default mode network, which is comprised of the precuneus and the post cingulate, medial prefrontal and lateral parietal cortices and has been correlated to reduced attention, memory and executive function.58,59 In post-mortem examinations, these anatomical changes are associated with the deterioration of the BBB, thus suggesting that these changes are associated with impaired cerebrovascular function.62 In support of this, reduced CBF and metabolism are associated with reduced functional connectivity and anatomical changes in healthy individuals and are exacerbated in those with Alzheimer’s disease.63 Since poor cardiometabolic status intensifies endothelial dysfunction, cerebrovascular dysfunction and cognitive decline, interventions that improve cardiometabolic health, such as regular exercise, may be beneficial in preventing or slowing the progress of the chain of events leading to dementia.5,64
Ageing and cerebrovascular function
As we age, the endothelium’s ability to produce adequate concentrations of nitric oxide (NO) to maintain optimal vascular health and cerebrovascular function decreases.5 Even in healthy individuals, CBF is estimated to be continually reduced by 0.38–0.45% annually from midlife onwards until age 80 years, after which the trajectory is unknown.19
The mechanisms that lead to age-related endothelial dysfunction and diminished NO concentrations are complex and are not fully understood. What is clear is that the senescent phenotype favours decreased vasodilator tone, platelet aggregation, vascular smooth muscle cell proliferation and inflammation, which is probably associated with elevated oxidative stress caused by an increase in reactive oxygen species (ROS) production.65 In animal models, ROS production has been demonstrated to be a key factor leading to endothelial dysfunction, cerebrovascular dysfunction and cognitive impairment.66–68 Further, there is increased uncoupling of endothelial nitric oxide synthase (eNOS) in ageing, resulting from a diminished l-arginine supply,69,70 and increased concentrations of asymmetrical dimethylarginine, which acts as a competitive inhibitor of eNOS, thus reducing NO biosynthesis.71
Endothelial dysfunction may also be induced by hormonal imbalances and increased angiotensin activity. Both of these are associated with the senescent phenotype and promote vascular inflammation and ROS production. They may also promote increased endothelial expression of endothelin-1, which induces vasoconstriction while suppressing the effects of NO.70,72,73 Additionally, adiposity from mid-to-late life reduces systemic adiponectin concentrations, which may have a role in maintaining cerebrovascular function and reducing vascular inflammation via upregulating eNOS activity.74,75 In any case, NO bioavailability becomes diminished as it reacts with superoxide, resulting in peroxynitrite formation and continuation of the inflammatory cycle leading to endothelial cell dysfunction and the promotion of atherosclerosis and cardiovascular diseases.5 Ultimately, this impairs or causes a decline in the effectiveness of CBF regulatory mechanisms. The resulting decline in CBF leads to cerebral hypoperfusion, cerebral dysfunction and the development of cognitive impairment, as well as the potential development of leukoaraiosis (i.e. white matter lesions resulting from small blood vessel damage frequently observed as white matter hyperintensities on MRI) and increased amyloid-β production that is characteristic of dementia.3,5
Why exercise training might improve cerebrovascular and cognitive function – evidence from animal studies
Acutely, CBF increases concurrently with cardiac output and oxygen uptake (O2) during incremental exercise, probably due to vasodilatation caused by shear stress and the increased demand for the endothelial-derived NO to maintain this vasodilatory state. However, increased neuronal metabolic demand in regions of the brain that control motor function and the autonomic nervous system activities also increases during exercise.76 Increased neuronal metabolism results in increased carbon dioxide and metabolite production, triggering vasodilatation of the cerebral microvasculature and increased CBF and local perfusion, which permits for adequate removal of these waste products via the venous network.76 The venous network also dilates in response to increased blood flow through the arterial network during exercise. It would be presumed that this chronic adaptation would be partly due to increased efficiency of this process.
Exercise training promotes angiogenesis within areas of the brain that were previously ischaemic, via the upregulation of eNOS and endothelial progenitor cell production in mice and Sprague–Dawley rats.77,78 The reduction in endothelial expressed low-density lipoprotein (LDL) receptor-related protein 1 that occurs during ageing has been linked to decreased cerebral perfusion in animals, and exercise training has been demonstrated to reverse this process, thereby improving cerebrovascular function.75,79,80 These findings suggest that exercise training may improve endothelial function, which would possibly increase cerebrovascular function, which is related to cognition.
In support of this, it was reported that in middle-aged female mice, AT improved cerebrovascular function, peripheral endothelial function as well as favourable neuroanatomical changes, such as decreased hippocampal astrocyte hypertrophy.81 This was in contrast to the sedentary group who had increased hippocampal astrocyte hypertrophy, reduced vascular and cerebrovascular function and myelin dysregulation.81 Additionally aged Wistar rats that underwent swimming training for 1 h per weekday for eight weeks demonstrated significantly increased CBF and brain capillary vascularity, in addition to increased brain microvessel vascular endothelial growth factor (VEGF) and eNOS concentrations and reduced plasma malondialdehyde concentration, compared to aged sedentary rats and rats immersed in water for leisure.82 However, these were still below to be lower than young sedentary rats, thus supporting the notion that CBF decreases with age and that exercise training can improve these outcomes possibly by upregulating VEGF and eNOS and reducing oxidative stress.
In aged animals, there are a lack of studies that directly test the effects of exercise on CBF and cognition. One study using a mouse model of Alzheimer’s disease reported improvements in both CBF and cognition using a pharmaceutical intervention, while another demonstrated that exercise improved neurogenesis and cognition in aged mice.83,84 This suggests that exercise, which can improve both CBF and cognition in animals, may result in improved outcomes associated with the ageing brain. In support of this was another study that reported middle-aged to older female cynomolgus monkeys that underwent AT had improved cognition (determined by the Wisconsin General Testing Apparatus) and vascular volume within the cerebral cortex compared to aged-matched sedentary controls.85 However, these findings were found to be abolished following a three-month period of physical inactivity, which also suggest exercise is a vital component in maintaining neurovascular function throughout the ageing process.
In summary, these novel animal studies highlight that exercise training improves cerebrovascular function and cognition by improving vascular health, specifically endothelial function. These few novel studies provide insight as to why exercise may improve cerebrovascular and cognitive function in humans, as they improve parameters that are associated with ageing, such as decreased endothelial function leading to reduced cerebrovascular function, BBB integrity and ROS formation.
Results of studies
The effects of exercise training on cerebrovascular function and structure
The studies that have been included in the results have measured and reported cerebrovascular function slightly different from each other or have at least focused on one aspect of cerebrovascular function. Hence, it is important to address terms used throughout the text here. Cerebrovascular responsiveness (CVR) is the ability of the vasculature to respond to cognitive or physiological stimuli.86,87 Specifically, the response of smaller healthy vessels to a stimulus is to dilate, which results in an increase in CBF.86,87 The physiological mechanism that ensures that CBF is maintained and kept constant during changes in systemic blood pressure is referred to as cerebrovascular autoregulation (i.e. cerebrovascular conductance).5,26 This is stimulated by chemical and mechanical stimuli, which induce a myriad of molecular pathways, including those described above, which result in modulating the vascular resistance applied to the cerebral vasculature and, therefore, the CBF.5 Further, cerebrovascular function can be described as declining if cerebral pulsatility (i.e. cerebral arterial stiffness) increases.20 This will be observed in conjunction with a decline in CBFV.20 These functions can be determined by medical imaging systems (e.g. MRI and ASL) and TCD ultrasonography.
Aerobic exercise training (Table 2)
Table 3.
Summary of research that has examined the effects of resistance exercise training on cerebrovascular function, cognition and neural structural adaptations.
| References | Study design | Participant description | Group allocation | Primary method used to evaluate CBF/cognition | Effect of exercise on primary outcome | Effect of exercise on other outcomes |
|---|---|---|---|---|---|---|
| Cerebrovascular function | ||||||
| Studies conducted in apparently healthy individuals | ||||||
| Xu et al.89 | Cross-sectional | Adults (57–76 years
old) Sedentary Participation in one or more strength-training sessions/week Participation in aerobic exercise training |
Total participants (n =59)
Resistance trained (n = 31) |
MRI | ↑ cerebrovascular perfusion (strength-trained females) | No association between ↑ cerebral perfusion with either aerobic or flexibility training |
| Cognition | ||||||
| Studies conducted in apparently healthy individuals or self-reported memory complaints | ||||||
| Busse et al.93 | Randomised control trial Nine months resistance exercise 2 × 60 min/week Varied intensity |
Adults (62–86 years old) Sedentary Subjective memory complaints |
Exercise (n = 14) Control (n = 17) |
Cognitive battery | ↑ memory performance | ↑ muscle strength |
| Cassilhas et al.27 | Randomised control trial 24 weeks resistance exercise 3 × 60 min/week Moderate or high intensity |
Healthy males (65–75 years old) Sedentary |
Moderate intensity (exercise; n = 19) High intensity (exercise; n = 20) Stretching without overload (control; n = 23) |
Cognitive battery | ↑ cognition, memory and executive function | ↓ POMS score (↑ performance) ↑ IGF1 ↑ muscle strength |
| Studies conducted in those with mild cognitive impairment | ||||||
| Fiatarone Singh et al.31 | Randomised control trial 26 weeks resistance exercise 2–3 × 60–100 min/week Moderate–high intensity |
Adults (>55 years old) MCI |
Exercise + sham cognitive training (n = 22)
Cognitive training + sham exercise (n = 24) Exercise + cognitive training (n = 27) Sham exercise + sham cognitive training (control; n = 27) |
ADAS-cog Cognitive battery |
↑ ADAS-cog performance post intervention ↑ ADAS-cog performance (participants with normal scores doubled from baseline, i.e. 24–48% one-year post-intervention) |
↑ executive function ↑ executive function one year post-intervention ↑ visual memory ↑ speed/attention (all groups) |
| Neural structural adaptations | ||||||
| Studies conducted in those with mild cognitive impairment | ||||||
| Bolandzadeh et al.24, Liu-Ambrose et al.,35 Liu-Ambrose et al.36 |
Randomised control trial One-year resistance exercise 1 × 60 min/week Or 2 × 60 min/week High intensity |
Females (65–75 years old) Community dwelling Not participated in resistance training in the last six months |
1 × 60 min/week (exercise; n = 46) 2 × 60 min/week (exercise; n = 47) Balance and tone (control; n = 42) |
MRI Cognitive battery |
↑ Stroop performance (↑10.9-12.6%) ↑ executive function ↑ left middle temporal gyrus and left anterior insula function – improved functional plasticity of response inhibition (2 sessions/week) ↑ flanker task performance (2 session/week) ↓ whole-brain volume ↓ trail making task time (all groups) |
↓ white matter lesions (2 sessions/week) ↑ gait speed ↑ muscle power (2 session/week) ↑ muscle strength |
| Nagamatsu et al.39 ten Brinke et al.45 |
Randomised control trial 26 weeks resistance exercise Or 26 weeks aerobic exercise 2 × 60 min/week Moderate–high intensity |
Females (70–80 years old) Mild cognitive impairment |
Aerobic exercise (n = 30)
Resistance exercise (n = 28) Balance and tone (control; n = 28) |
MRI Stroop test RAVLT Associated memory tasks |
↑ Stroop test performance ↑ memory performance and conflict resolution performance (resistance exercise) ↑ right lingual and occipital-fusiform gyri and right frontal pole functional plasticity (resistance exercise) ↑ hippocampal volume by 4% (aerobic exercise) |
↑ cardiovascular capacity ↑ physical function (aerobic exercise) |
MRI: magnetic resonance imaging; ADAS-Cog: Alzheimer disease assessment scale–cognitive subscale; POMS: profile of mood states; RAVLT: Rey’s auditory verbal learning test; IGF1: insulin-like growth factor-1; MCI: mild cognitive impairment.
Ainslie et al.19 measured CBFV in the middle cerebral artery (MCA) in apparently healthy aerobic exercise trained and sedentary males. Their estimation of the annual decrease in CBFV was 0.45%. However, CBFV remained 17% higher in aerobic exercise-trained individuals compared to their age-matched sedentary counterparts, suggesting a difference in cerebrovascular function of 10 years between the active and sedentary individuals. A major limitation to this study was that CVR was not evaluated. Brown et al.26 assessed whether higher aerobic fitness was associated with superior cognitive in a group of healthy aerobic exercise trained and sedentary older women. Trained females had a higher O2max compared with the sedentary group and increased cerebrovascular conductance. Cognition was negatively correlated with age and positively correlated with maximal oxygen uptake (O2max). These cross-sectional studies provide an indication that aerobically trained individuals may have superior cerebrovascular function and that aerobic fitness, cerebrovascular function and cognition are interrelated.
Table 2.
Summary of research that has examined the effects of aerobic exercise training on cerebrovascular function, cognition and neural structural adaptations.
| References | Study design | Participant description | Group allocation | Primary method used to evaluate CBF/cognition | Effect of exercise on primary outcome | Effect of exercise on other outcomes |
|---|---|---|---|---|---|---|
| Cerebrovascular function | ||||||
| Studies conducted in apparently healthy individuals | ||||||
| Ainslie et al.19 | Cross-sectional | Healthy males (17–79 years old)
Endurance-trained/sedentary |
Trained (n = 154) Untrained (n = 153) |
TCD | ↑ MCA CBFV (9.1 cm·s−1·year−1 of life) | ↑ O2 max |
| Akazawa et al.20 | Non-randomised control trial 12 weeks aerobic exercise 4-6 × 30-45 min/week Moderate intensity |
Healthy adults (52–66 years old) Sedentary |
Exercise (n = 10) | TCD | ↓ cerebral pulsatility index | ↓ arterial stiffness ↑ O2 max ↓CHO |
| Brown et al.26 | Cross-sectional | Females (50–90 years old) Endurance trained/sedentary |
Trained (n = 28) Untrained (n = 13) |
TCD | ↑ cerebrovascular conductance | ↓ resting mean arterial pressure ↑ O2 max |
| Chapman et al.28 | Randomised control trial 12 weeks aerobic exercise 3 × 60 min/week Moderate intensity |
Healthy adults (57–75 years old) Sedentary |
Exercise (n = 18) Control (n = 19) |
MRI | ↑ ACC blood flow ↑ hippocampal blood flow |
↑ memory performance ↑ O2max ↑ ability to perform exercise |
| Maass et al.37 | Randomised control trial 12 weeks aerobic exercise 3 × 30 min/week Moderate intensity |
Healthy adults (60–77 years old) | Exercise (n = 21) Stretching (control; n = 19) |
MRI | ↑ O2VAT correlated with ↑ hippocampal perfusion & head volume, which correlated with ↑ recognition memory & early recall | ↑ hippocampal perfusion in younger participants ↓ hippocampal perfusion in older participants |
| Vicente-Campos et al.46 | Randomised control trial 28 weeks aerobic exercise 3–4 × 50 min/week Moderate intensity |
Health adults (60–75 years old) Sedentary |
Exercise (n = 22) Control (n = 21) |
TCD | ↑ vasomotor reactivity ↑ MCA CBFV |
↑ waking velocity & cardiorespiratory capacity ↑ HDL ↓ CHO, LDL & TG ↓ BP |
| Studies conducted in those diagnosed with a cardiovascular disease | ||||||
| Anazodo et al.21 | Non-randomised control trial 24-week aerobic exercise-based cardiac rehabilitation program |
Adults Coronary artery disease |
Exercise (n = 17) | ASL | ↑ ACC blood flow | ↓ resting CBF and CVR to hypercapnia at baseline |
| Ivey et al.33 | Randomised control trial 24 weeks aerobic exercise-based rehabilitation program 3 × 40 min/week Moderate intensity |
Adults (>60 years old) Remote stroke (>6 months) Mild-to-moderate gait defects |
Exercise (n = 19) Control (n = 19) |
TCD | ↑ bilateral cerebrovascular vasomotor reactivity | ↑ O2max ↑ walking speed |
| Cognition | ||||||
| Studies conducted in those who are overweight but apparently healthy | ||||||
| Anderson-Hanley et al.22 | Randomised control trial 12 weeks aerobic exercise 5 × 45 min/week Moderate intensity |
Adults (≥55 years old) Overweight |
Cycle (control; n = 41) Cybercycle (experimental; n = 38) |
Cognitive battery | ↑ score on cognitive battery | ↑ BDNF ↓ BMI, fat mass and blood glucose ↑ lean mass and insulin |
| Studies conducted in those with mild cognitive impairment | ||||||
| Baker et al.23 | Randomised control trial 24 weeks aerobic exercise 4 × 45–60 min/week High intensity |
Adults (55–85 years old) Mild cognitive impairment Sedentary |
Exercise (n = 19) Stretching (control; n = 10) |
Cognitive battery | ↑ cognitive scores in multiple tests (female) ↑ cognitive score in 1 test (male) |
↓ CHO & LDL ↑ glucose utilisation (female) ↓ insulin, cortisol and BDNF (female) ↑ IGF1, cortisol and HOMA-IR (male) |
| Studies conducted in those diagnosed with Alzheimer’s disease | ||||||
| Hoffmann et al.32 | Randomised control trial 16 weeks aerobic exercise 3 × 60 min/week Moderate–high intensity |
Adults (50–90 years old) Mild Alzheimer’s disease |
Exercise (n = 102) Control (n = 88) |
SDMT (cognitive testing) | No change between the two arms of the study ↑ SDMT from baseline (experimental arm) |
↑ Neuropsychiatric inventory from baseline (experimental arm only) |
| Sobol et al.43 | Cross-sectional | Adults (50–90 years old) Mild Alzheimer’s disease |
Undefined (n = 185) | Cognitive battery | ↑ cognition correlated with ↑ 30 s chair sit-to-stand performance | ↑ dual-task performance correlated with ↑ performance in the cognitive battery |
| Neural structural adaptations | ||||||
| Studies conducted in apparently healthy individuals | ||||||
| Erickson et al.30 | Randomised control trial One-year aerobic exercise 3 × 40 min/week Moderate intensity |
Healthy adults (55–80 years old) Sedentary |
Exercise (n = 60) Stretching (control; n = 60) |
MRI | ↑ hippocampal volume | ↑ spatial memory ↑ BDNF ↑ O2max |
| Studies conducted in those with mild cognitive impairment | ||||||
| Chirles et al.29 | Non-randomised control trial 12 weeks aerobic exercise 4 × 30 min/week Low–moderate intensity |
Adults (60–88 years old) Mild cognitive impairment |
MCI (exercise; n = 16) Non-MCI (exercise; n = 19) |
MRI | ↑ right parietal lobe connectivity (MCI group) ↓ right parietal lobe connectivity (healthy group) ↑ left post central gyrus connectivity (both) |
↑ neural connectivity from baseline (MCI group) No change in neural connectivity from baseline (healthy group) |
| Smith et al.42 | Non-randomised control trial 12 weeks aerobic exercise 4 × 30 min/week Low–moderate intensity |
Adults (60–88 years old) Mild cognitive impairment Low levels of physical activity |
MCI (exercise; n = 17) Non-MCI (exercise; n = 18) |
MRI Semantic memory task |
↑ semantic memory ↓ neural activation post exercise |
↑ O2peak |
TCD: transcranial Doppler ultrasound; ASL: arterial spin labelling; MRI: magnetic resonance imaging; CBF: cerebral blood flow; CBFV: cerebral blood flow velocity; MCA: middle cerebral artery; ACC: anterior cingulate cortex; CVR: cerebrovascular responsiveness. SDMT: symbol digit modalities test; CHO: total cholesterol; LDL: low-density lipoprotein; TG: triglycerides; BMI: body mass index; IGF1: insulin-like growth factor-1; HOMA-IR: homeostatic model assessment of insulin resistance; BDNF: brain-derived neurotrophic factor; MCI: mild cognitive impairment.
One of the first exercise training studies undertaken evaluated the effects of AT in healthy, sedentary and overweight older adults.46 CBFv in the MCA in response to breath-holding was higher in the trained group compared to the control group. Additionally, AT decreased blood pressure and improved individual lipid profiles, in addition to increasing exercise performance. This suggested that AT improved cerebral responsiveness by modulating cardiovascular markers, thus contributing to improved cardiovascular health and, consequently, improved endothelial function. This was supported by a recent interventional study that investigated the effects of AT assessing the response of cerebral pulsatility to an acute bout of exercise before and after exercise training in older sedentary adults.20 The trained group had improvements in CBFV and a decreased PI, as well as lower total cholesterol concentrations and increased peak oxygen uptake (O2peak) post-intervention. However, based on the primary findings of the study, it was indicated that arterial stiffness had declined post-intervention during the acute bout of exercise and that chronic exercise training may be necessary for sustaining the improvements in systemic and cerebrovascular functions.
The benefits of regular AT are also evident in patients with established endothelial dysfunction. Anazodo et al.21 measured changes in both resting CBF and CVR to hypercapnia in patients with coronary artery disease and healthy patients of the same age, following an AT-based program. At baseline, patients were reported to have lower CBF, reduced CVR to hypercapnia, and some atrophied brain regions compared to their healthy counterparts. Following the exercise intervention, CBF was increased in the anterior cingulate cortex (ACC) in the coronary artery disease patients. In fact, the increase in CBF to this region was equal to the shortfall measured at baseline, i.e. the difference between the exercise intervention group and the control group at baseline.
A similar improvement in cerebral perfusion was also observed in overweight adults, who suffered from a stroke and were at risk of developing mild cognitive impairment (MCI).33 AT improved bilateral CVR to hypercapnia which were accompanied by a 19% improvement in O2peak, compared with a 4% decline in the control group. Hence, AT can improve CVR in these stroke survivors and potentially protect against further neurological insults. However, it was noted that those using statins (58%) had higher CVR at baseline and lower training-induced elevation of CVR, indicating that statin use alone has already improved CVR with less scope for further improvement. Nonetheless, this study shows that AT can improve cerebrovascular function even in individuals with established cerebrovascular dysfunction.
A larger study which assigned older sedentary adults to a one-year walking program30 showed increased hippocampal volume, systemic brain-derived neurotrophic factor (BDNF) concentrations, spatial memory and O2 max. The authors of the study also ran correlation analyses between hippocampal volume and O2 max, as well as correlations between BDNF and hippocampal volume. It was reported that increases in exercise capacity and systemic BNDF concentrations were associated with increased hippocampal volume which was, in turn, positively associated with improved spatial memory. Hence, it was concluded that AT increased BDNF which reversed both senescent-related hippocampal atrophy and decline in spatial memory.
The changes elicited in regions of the brain that are responsible for cognition following AT have not been clear. A study that evaluated the effects of AT on hippocampal vascularity in healthy older adults, indicated varied results.37 O2 at ventilatory threshold increased by 10% after training, which correlated with the increases in hippocampal CBF perfusion and brain volume as measured by functional MRI (fMRI). Hippocampal volume and perfusion were also correlated positively to changes in recognition memory and early recall. This suggests that hippocampal vascularity decreases with age and that the effect of exercise training may attenuate this decline. A later study aimed to determine if functional connectivity of the default mode network (described above) could be improved in non-MCI and MCI older adults who took part in AT.29 It was noted that right parietal lobe connectivity increased in the MCI group, but decreased in the non-MCI group, while left post central gyrus connectivity increased in all of the participants. It was also noted that there was increased neural connectivity in 10 regions of the brain that spanned from all major lobes of the cerebrum as well the insular lobe and the cerebellum in the MCI group. However, these changes after training were not evident in the non-MCI group, suggesting that exercise training may impart protective effects on cognition in elderly adults with MCI by increasing the plasticity in the posterior cingulate cortex and the precuneus and thereby improving neural recruitment. While mechanisms mediating these changes occurred were not proposed, it could be hypothesised that enhanced cerebrovascular function increased the availability of neural growth factors such as BDNF in these areas of the brain, resulting in synaptogenesis.88 However, further testing is needed to support this hypothesis.
Chapman et al.28 assigned cognitively healthy, sedentary adults to an AT program. Following training, CBF was increased at rest in the ACC, which is responsible for executive functioning and autonomic cardiovascular control. The trained group also demonstrated improved immediate and delayed memory performance, which was associated with a general increase in hippocampal perfusion. The authors suggested that exercise training could assist in diminishing the biological and cognitive consequences of the senescent phenotype in sedentary older adults by increasing neuroplasticity in both the ACC and hippocampus. However, measurements of physical volume of these brain areas were not undertaken. Hence, this study indicates that improvements in cerebrovascular function, particularly within the ACC and hippocampus, following AT are associated with improved cognitive capacity in sedentary older adults and that these changes may diminish the progression of the senescent phenotype described above.
In summary, there is strong evidence that AT improves cerebrovascular function. The studies presented indicate that improved vascular function and health and exercise capacity may be associated with these improvements as well as increased systemic BDNF concentrations.
Resistance exercise training (Table 3)
Xu et al.89 investigated the impact of flexibility, RT and AT on cerebrovascular perfusion in older adults. It was reported that females who participated in RT had greater cerebrovascular perfusion than those who did not and that this finding remained significant after adjusting for health, educational status and the other types of exercise training performed. It was also reported that there was no association between cerebral perfusion and AT or flexibility training. While interesting, these results were based on self-reported subjective data obtained from a small sample size and did not include the intensity and duration of exercise and should be examined with caution. Additionally, the authors of this study did not offer an explanation or propose a mechanism whereby RT could improve cerebrovascular perfusion. The adaptations to RT and cardiovascular function are not well understood compared to AT. To highlight this, a recent cross-sectional study of nearly 400,000 United States residents aimed to determine whether meeting the current physical activity guidelines for moderate-to-vigorous intensity aerobic physical activity, RT or both were associated with chronic health conditions.90 It was reported that meeting the guidelines for both forms of physical activity resulted in less risk of developing any cardiovascular disease, including stroke, and that meeting the strength component of the guidelines alone resulted in less risk of cardiovascular disease development than moderate-to-vigorous intensity aerobic physical activity. Hence, it may be plausible that strength training can reduce the incidence of systemic vascular disease and may also reduce cerebrovascular dysfunction, through an unknown and unexplored mechanism.
Other than the longitudinal data presented above, there have been no studies that examine the effect of RT on cerebrovascular function.
The effects of exercise training on cognitive function
Aerobic exercise training (Table 2)
A population-based study examined the relationship between aerobic exercise-trained individuals and executive function at the start of adulthood and the risk of developing cognitive impairment in later life.41 Over one million young Swedish males who were subject to mandatory conscription examinations between 1968 and 2005 were evaluated to ascertain the risk of developing cognitive impairment. Aerobic fitness and cognitive performance at 18 years of age was associated with an increased risk of developing cognitive impairment in later life. Specifically, those with poor aerobic fitness had a >7-fold increased risk of developing early-onset dementia and early-onset MCI, while those with poor cognitive performance had a >8-fold increased risk compared with those who did not. Another cross-sectional study, which utilised the baseline data from their AT study involving older participants with mild Alzheimer’s disease, reported that a greater performance in the 30 s chair sit-to-stand test correlated with increased cognition.43 Sobol et al.43 concluded that there was a strong association between superior cognitive performance, physical function and the ability to carry-out dual tasks, thus suggesting that interventions that improve physical function and the ability to multi-task may impart benefits on improving cognitive outcomes and prevent further deterioration in patients with mild Alzheimer’s disease. Taken together, these studies suggest that physical fitness is strongly associated with cognitive performance and that the former has a positive effect on the latter.19,26,41,43
Smith et al.42 determined if AT could improve semantic memory activation during fMRI in physically inactive older adults with MCI in comparison to appropriately matched cognitively-intact individuals. O2 peak and performance in list-learning tasks increased (indicating improved semantic memory performance), and there was a decrease in activation intensity (i.e. improved neural operating efficiency) post exercise. This study did not evaluate any cerebrovascular functions, but did suggest that the improved neural efficiency may be due to improved cerebral perfusion, even with low–moderate intensity AT.
Baker et al.23 evaluated the impact of AT on cognitive function and biomarkers of Alzheimer’s disease in older adults with amnestic MCI. After training, female participants had improved cognition, cortisol and BDNF concentrations compared to the stretching (control) group. Aerobically trained men, however, had increased insulin-like growth factor-1 (IGF1) and only performed favourably in one of the cognitive battery tests. Hence, this study concluded that AT can improve cognition and that these effects may be greater in women who are at greater risk of developing cognitive impairment than men, possibly due to an altered hypothalamic–pituitary–adrenal axis response. While this may in part be true, these findings and the increased risk of developing MCI in women may also be due to loss of the vasoprotective effects of oestrogen post-menopause.91 Taken together, these studies20,23,46 suggest that AT improves cognitive function and indicate that improved lipid profiles are important for maintaining vascular health and, therefore, cerebrovascular function. Further, it suggests that there may be a sex difference in responses to exercise training, which should be considered in future studies.
In another intervention study, overweight older adults participated in three-month traditional cycling or cyber-cycling (a cycle ergometer with an attached virtual reality component) training demonstrated increased cognitive performance and systemic BDNF concentrations compared to their baseline results.22 Cyber-cycling participants had greater increases than those who underwent traditional cycling, resulting in a medium effect size improvement of cognitive performance compared to the control group. This suggested that virtual reality coupled with AT increased neuroplasticity more than standard AT and that cyber-cycling imparts a 23% relative reduction in the risk of MCI development. This study did not have a sedentary control group but relied on improvements from baseline measurements and did not provide details of potential mechanism/s by which a virtual reality component could elicit improved executive functioning, other than suggesting that increased BDNF concentrations may have elicited these results. Hence, this may provide a future direction for researchers to consider when prescribing exercise coupled with a virtual reality program.
A later and larger study evaluated the effects of AT in sedentary older adults with mild Alzheimer’s disease.32 There were no differences between the control and training groups in cognitive performance. However, participants with over 80% compliance to the exercise program and trained at 70% of their maximal heart rate or greater exhibited improvements in cognitive performance from baseline compared to the control group, thus suggesting a dose–response relationship between AT and cognitive performance. Finally, it was reported that neuropsychiatric scores were improved from baseline in the exercise group compared to the control group, whose scores declined over the course of the program. Hence, it was concluded that AT could impede or slow the progression of neuropsychiatric symptoms and that increased adherence to AT is imperative to improve cognition in those who are already cognitively impaired.
In summary, there is evidence that AT can improve cognitive performance. Again, this appears to be related to improved exercise capacity. However, the mechanism leading to these changes is not clear, but may involve increased systemic BDNF, vascular health and hormonal changes. Further studies are required to determine the mechanism of action leading to improved cognitive performance.
Resistance exercise training (Table 3)
Cassilhas et al.27 conducted one of the first studies investigating the effects of RT on cognition in healthy, sedentary, older males. A limitation to the exercise protocol used in this study was that no progressive overload, periodisation or adjustments added or made that adjusted for intensity and conditioning to this program. The program may have been completed in this manner for simplicity, since the control group performed the same routine once per week without any overload applied. In any case, both exercise groups demonstrated improved cognition in comparison to the control group. Lean mass, systemic IGF1 concentrations and muscle strength were increased in both of the exercise groups when compared to the control group, with these changes being more prominent in the high-intensity exercise group. Additionally, IGF1 concentrations decreased in the control group suggesting IGF1 may be mediating the change in cognition. These results suggest that RT could improve cognitive function possibly by increased growth factors, which may act as mediators of neurogenic pathways within the brain.27,92 However, no correlational results were generated from this study.
This finding was supported by a later study that investigated the effects of RT on cognitive performance and muscle strength in sedentary older adults with subjective memory complaints.93 It was found that the exercise group, particularly the women, demonstrated improved memory performance within the cognitive battery administered and increased muscle strength compared to the control group. This finding indicates that RT may improve memory deficits in elderly participants with memory complaints.
Finally, Fiatarone Singh et al.31 evaluated 26 weeks of either progressive RT, cognitive training or a combination of both on global cognitive function in older adults with MCI. The cognitive training incorporated the COGPACK program, which is a neuro-rehabilitation program incorporating adaptive computerised exercises of memory, executive function, attention and processing speed.98 Upon study completion, those in the RT group demonstrated improvements to a greater extent than the other groups in the Alzheimer Disease Assessment Scale–Cognitive Subscale (ADAS-Cog), which assesses cognitive decline. In this group, the proportion of participants attaining normal ADAS-Cog scores increased from only 24% at baseline to 48% following one year of training. They also performed 74% higher in executive function testing than the other groups in a 12-month follow-up post-intervention, indicating that the improvements in executive function were sustained for at least one year. Additionally, the RT group demonstrated increased visual memory performance compared to the other groups immediately post-intervention. These findings indicate that progressive RT improves global cognitive and executive functions and that cognitive training prevents any further decreases in overall memory and cognition. Interestingly, combined training did not demonstrate an enhanced benefit compared to RT alone, possibly because it was too challenging for participants to successfully engage for a full 100 min, separating RT and cognitive training may have assisted this. Why cognition was maintained a year after RT cessation was unclear, and biomarkers such as IGF1 and BDNF were not measured.
The cognitive improvements observed by Busse et al.93 were more evident in females than males and Xu et al.89 reported that sex differences could account for differences in improvements of cerebral perfusion and cognition induced by RT. Two larger trials examined this finding by investigating the efficacy of chronic exercise training, particularly RT, on executive functions and cognition in elderly females with MCI.24,35,36,39,45 The EXCEL study randomised participants to either AT or RT or to a balance and tone (control) group.39,45 Compared to the control and AT groups, participants in the RT group increased performances in the Stroop test, memory tasks and conflict resolution, as well as enhancing functional plasticity in the right lingual and occipital–fusiform gyri and right frontal pole, assessed by fMRI. The AT group reported a 4% increase in hippocampal volume compared with the other groups but did not show improvement in memory performance to the recall tasks, as one would expect an increase in hippocampal volume to correlate with improved memory function. These findings suggest that RT improves cognitive performance and regional brain plasticity to a greater extent than AT in elderly females at risk of further cognitive decline.
The other large-scale study aimed to determine if performing once or twice weekly RT sessions for one year could improve executive function and cognition in older females compared to balance and muscular toning exercises.24,35,36 Both RT groups improved by 10.9–12.6% in the Stroop test compared with a control group who reported a 0.5% decrease and demonstrated improved executive function compared to the control group. However, only the participants performing two RT sessions per week had increased functional plasticity in the left middle temporal gyrus and left anterior insula, as well as superior performance in the flanker task and muscle power measurements. Additionally, MRI revealed a decrease in whole-brain volume in both groups compared with the control group. The authors of the study cautiously suggest that this may have been due to an increase in amyloid-β removal in conjunction with other neural proteins, as this had been a finding in previous studies that specifically measured and noted reductions in cerebral volume following treatments with pharmaceuticals.94,95 This study did not measure any neurodegenerative or inflammatory biomarkers and could only speculate that this may be the case. In support of this, however, the twice per week RT group experienced a decrease in white matter lesions, which one-third of the participants were reported to possess. These findings warrant further research into the effect of exercise on cerebral volume. In any case, it was concluded that two RT sessions performed twice a week may improve cognition, executive function, selective attention and conflict resolution in older females and that this dosage of exercise could potentially convey favourable functional plasticity changes in a manner similar to AT.
Taken together, these studies indicate that RT improves cognitive performance. However, none of these studies offer an insight as to why RT appears to exert a greater effect in women than men. Future studies might benefit from measuring different hormones, such as androgens and oestrogens. It has been suggested that phytoestrogens, such as resveratrol, increase central oestrogen receptor activity, while promoting upregulation of eNOS and, therefore, NO production, thus leading to improvements in cerebrovascular function.91 Further, these studies did not investigate possible effects of changes in body composition, as it is well known that a result of RT is increased lean mass. Any increase in tissue, whether positive or negative, demands increased capillarisation and recruitment of the existing vasculature, which is largely mediated through VEGF, a protein that promotes angiogenesis and potentiates the effects of NO. Specifically, central VEGF concentrations and increased cerebral capillarisation have been demonstrated in animal studies to be upregulated centrally via the centrally derived lactate receptor hydroxycarboxylic acid receptor 1 (HCAR1) following increased plasma lactate concentrations and shear stress.96,97
The effects of multimodal exercise training on cerebrovascular function, structure and/or cognition (Table 4)
Table 4.
Summary of research that has examined the effects of multimodal exercise training on cognition and neural structural adaptations.
| References | Study design | Participant description | Group allocation | Primary method used to evaluate CBF/cognition | Effect of exercise on primary outcome | Effect of exercise on other outcomes |
|---|---|---|---|---|---|---|
| Cerebrovascular function | ||||||
| Studies conducted in those diagnosed with a cardiovascular disease | ||||||
| Moore et al.38 | Randomised control trial 19-week multimodal exercise 3 × 45–60 min/week Varied intensity |
Adults (>50 years old) >6 months post stroke |
Exercise (n = 20) Stretching (control; n = 20) |
MRI ACE-R |
↑ middle temporal lobe tissue CBF No change in grey matter tissue volume ↑ ACE score (6 points) |
No change in HOMA-IR ↑ O2peak ↑ walking speed ↑ balance ↑ physical function ↑ HDL ↓ diastolic blood pressure |
| Cognition | ||||||
| Studies conducted in those diagnosed with a dementia | ||||||
| Bossers et al. 25 | Randomised control trial Nine-week multimodal exercise 4 × 30 min/week Varied intensity Or Nine-week aerobic exercise 4 × 30 min/week Moderate–high intensity |
Adults (80–90 years old) Diagnosis of dementia |
Multimodal exercise (n = 33)
Aerobic exercise (n = 34) Social (control; n = 34) |
Cognitive battery | ↑ global cognition, visual & verbal memories, executive
function (multimodal exercise) ↑ executive function (aerobic exercise) |
↑ walking endurance, muscle strength & balance
(multimodal exercise) ↑ walking endurance (aerobic exercise) |
| Vreugdenhil et al.47 | Randomised control trial 16 weeks multimodal exercise 10 resistance + 30 min walking Intensity and weekly session were undefined |
Adults (mean 77 years old) Alzheimer’s disease Lower levels of physical activity |
Exercise (n = 20) Control (n = 20) |
MMSE ADAS-Cog |
↑ MMSE scores (2.6 points) ↑ ADAS-Cog exam performance (7.1 points) |
↑ lower body strength ↓ waist-to-hip ratio ↑ mobility (2.9s faster on timed up & go test) ↑ Instrumental activities of daily living scores (1.6 points) |
| Studies conducted in apparently healthy individuals or self-reported memory complaints | ||||||
| Lautenschlager et al.34 | Randomised control trial 24 weeks multimodal exercise 3 × 50 min/week Moderate intensity |
Healthy adults (>50 years old) Self-reported memory problems (excluding dementia) |
Exercise (n = 85) Control (n = 85) |
ADAS-Cog | ↑ 0.26 points post-intervention ↑ 0.73 points 18 months post-intervention |
↑ physical activity 18 months post intervention ↑ word list delayed recall test ↑ clinical dementia rating |
| Neural structural adaptations | ||||||
| Studies conducted in apparently healthy individuals | ||||||
| Nishiguchi et al.40 | Randomised control trial 12 weeks multimodal exercise 1 × 90 min/week + pedometer-based walking activity Varied intensity |
Healthy adults (>60 years old) | Exercise (n = 24) Control (n = 24) |
MRI Cognitive battery |
↑ memory and executive function ↓ activation in visual short-term memory centres (e.g. bilateral prefrontal cortex) |
↑ average daily steps (54%) ↑ 100% adherence to program |
| Studies conducted in those with mild cognitive impairment | ||||||
| Suzuki et al.44 | Randomised control trial 24 weeks multimodal exercise 2 × 90 min/week Varied intensity |
Adults (55–95 years old) MCI |
Exercise (n = 45) Education (control; n = 45) |
MRI ADAS-Cog Cognitive battery |
↓ whole brain cortical atrophy ↑ cognitive battery and ADAS-Cog exam scores |
↓ baseline CHO and ↑ BDNF associated with improvements in cognitive function pre-exercise |
ADAS-Cog: Alzheimer disease assessment scale–cognitive subscale; MMSE: mini-mental state examination; MRI: magnetic resonance imaging; ACE: Addenbrooke’s cognitive examination; CHO: total cholesterol; BDNF: brain-derived neurotrophic factor; MCI: mild cognitive impairment.
One of the first intervention studies examined the effects of CT (of the participants’ choice) on cognition in older participants with self-reported memory problems.34 The rate of cognitive decline (evaluated using ADAS-Cog exam) improved by 0.26 points post-intervention, while the control group had declined by 1.04 points, both of which were considered as clinically significant. Eighteen months post-intervention, the intervention group had improved by a further 0.73 points, whilst the control group improved 0.04 points from the previous ADAS-Cog examination. The intervention group also reported a modest improvement in a word list delayed recall test and clinical dementia rating. These findings were supported by a later study that investigated the effects of CT on cognition in physically inactive older Alzheimer’s disease patients.47 It was reported that exercise training improved mini-mental state examination (MMSE) scores by 2.6 points and ADAS-Cog by 7.1 points. Taken together, these studies suggest that CT improves both cognitive and physical function in elderly individuals with either self-reported memory issues or Alzheimer’s disease. However, the latter study did not define intensity, dose or progression, thus it is unknown if there was a dose–response relationship in relation to the study outcomes.
Bossers et al.25 compared the effects of either CT or AT on cognitive and motor function in institutionalised elderly adults with dementia. Post-intervention, the CT group demonstrated increased global cognition, visual and verbal memories and executive function compared with the control group, while the AT group only demonstrated increases in executive function compared to the control group. Eighteen weeks post-intervention, there was a decline in cognitive function towards baseline values. Hence, it was concluded that a combined exercise program is more effective than AT alone in reducing cognitive and motor function decline in patients with dementia.
Suzuki et al.44 assigned older adults with MCI to a CT program. Memory function and whole brain cortical atrophy all improved after training compared to controls. Further, lower total cholesterol and higher BDNF concentrations were associated with increased cognitive function and memory in patients with MCI at baseline than those who had higher total cholesterol and lower BDNF concentrations. However, it was not determined how having higher or lower baseline concentrations of these biomarkers impacted the results throughout the course of the study, or if participants with more favourable concentrations of these biomarkers responded better to the exercise intervention compared with those who had a less favourable profile. Interestingly, these biomarkers were not measured upon completion of the study, as this could have provided a mechanism or possibly explained the improved response to exercise. This study provides further evidence that exercise training improves cognitive function and was supported by Nishiguchi et al.40 who evaluated the effects of CT on cognition and brain activation efficiency healthy older adults. The exercise group had improvements in memory and executive function when compared to the control group, but they had less activation in regions of the brain, such as the prefrontal cortex, that were associated with short-term and visual memory. Hence, it was concluded that the combined exercise increased the activation efficiency of the brain during cognitive tasks and that this was associated with improved memory and executive function.
Moore et al.38 examined a CT program in older participants who had previously suffered from a stroke six months prior or longer. Medial temporal lobe tissue CBF increased in the exercise group without incidence of grey matter atrophy compared to the control group, who reported a decrease in grey matter atrophy. Further, exercise training improved cognition by 6 points. Training increased O2peak, diastolic blood pressure, HDL and physical and cognitive function. Hence, it was suggested that CT could lead to improved short-term metabolic, cognitive and functional capacity of the brain. These results suggest a similar mechanism to AT, as markers of cardiovascular health had improved in the exercise group and provides further evidence that exercise training improves cognitive function.
In summary, there is evidence that CT improves both cerebrovascular function and cognitive performance, particularly in those that have been diagnosed with stroke, MCI or dementia. The mechanism of action is, again, not clear, but likely involves improved vascular function and health leading to increased CBF, increased exercise capacity and structural adaptations within the brain that lead to improved brain activation efficiency.
Why does cerebrovascular and cognitive function change following exercise training?
It is evident from this review that exercise training improves cerebrovascular structure and function and cognition. It is also evident that the mechanisms that lead to these improvements in humans are poorly defined. The link, or the associations, between the two do not appear clear. Very few studies have performed correlation analyses on the results generated from their studies or undertaken perturbation studies to understand the mechanisms involved. Erickson et al.30 analysed associations between hippocampal volume andO2 max and BDNF and hippocampal volume. They concluded that AT increased BDNF, which, in turn reversed both senescent-related hippocampal atrophy and decline in spatial memory. Aside from this, associations can only be made based upon the observations generated from the studies described. It is quite evident that as O2 max and/or physical function increases, so too does cerebrovascular function and cognition. However, this does not identify a direct mechanism by which exercise improves these parameters. Rather, it provides an insight into the potential mechanisms that could lead to change. O2 max, which represents cardiorespiratory fitness, is governed by the Fick equation (cardiac output multiplied by the arterial–venous oxygen difference). For O2 max to increase there would be one or several physiological adaptations following exercise training, which are defined elsewhere.98–100
Mechanistic evidence observed in animal studies
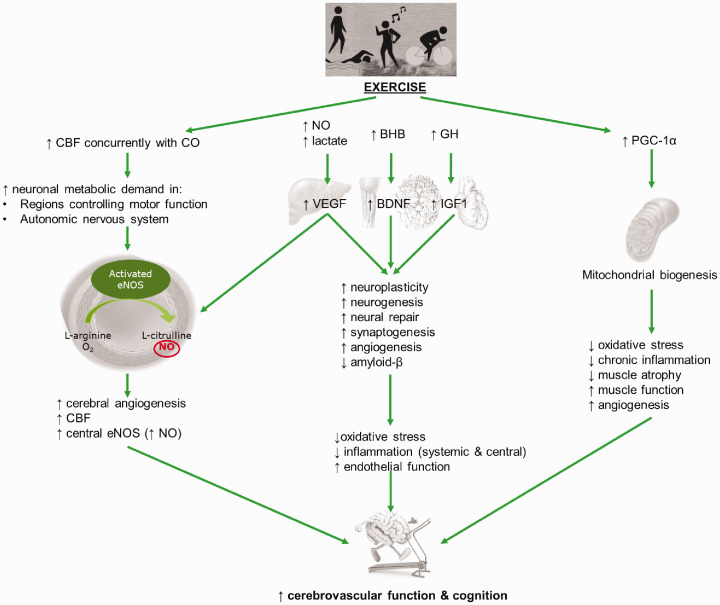
What occurs centrally after exercise training is poorly defined in humans. Centrally in mice there is an increase in oxidative enzyme synthesis, suggesting that exercise may improve central oxidative capacity.82,98–104 Therefore, it may be plausible that some of the adaptations that are described in skeletal muscle may also occur centrally, thus leading to improvements in cerebrovascular and cognitive functions. Specifically, chronic exercise in animal enhances endothelial function, upregulates eNOS expression and activity thereby increasing NO production and reduces ROS production. These changes reduce vascular inflammation and inhibit endothelial dysfunction. Additionally, exercise training enhances mitochondrial biogenesis via upregulating transcription factors, such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α).105 Specifically, PGC-1α upregulates oestrogen-related receptor alpha-α, which subsequently upregulates lactate dehydrogenase (LDH) B and indirectly inhibits LDH A, thus leading to increased lactate oxidation during exercise.106 This process increases the oxidative capacity of skeletal muscle and improves an individual’s exercise capacity, as well as contributing to reducing ROS production, chronic inflammation, angiogenesis and the loss of muscle atrophy and function that is associated with the senescent phenotype.105,106 However, further studies are required in order to demonstrate this.
The clinical studies described indicate that specific growth factors may have a role in conferring improvements in cerebrovascular and cognitive functions in at risk populations. Acute exercise results in increased arterial shear stress, enhanced synthesis of growth hormone and NO, as well as increased β-hydroxybutyrate and lactate production, all of which either stimulate increased IGF1 synthesis, promote increased BDNF expression and synthesis and/or HCAR1-induced VEGF upregulation, respectively.96,97,107–111 BDNF and IGF1 are expressed centrally and systemically and when produced systemically cross the BBB and augment the response of their centrally derived counterparts.97,112 These factors promote increased neuroplasticity, neurogenesis, neural repair, synaptogenesis and angiogenesis, in addition to possibly promoting the removal of amyloid-β.96,97,105,107,108,110–115 BDNF and IGF1 may also enhance glutamate synthesis centrally, via upregulating synapsin-1, thus contributing to improved cognition.114 Additionally, VEGF promotes angiogenesis and potentiates the effects of NO via phosphatidylinositol-4,5-bisphosphate hydrolysis, which subsequently activates the calmodulin and protein kinase B and C pathways, thus resulting in eNOS upregulation.82,96,110
In older animal models, ageing increases ROS production and chronic low-grade inflammation, resulting in arterial stiffening.82,101 Exercise training decreases inflammation and ROS production, modulates arterial structure and increases NO bioavailability, thus reducing arterial stiffness and improving endothelial function (reviewed in detail in Ref.116). This has also been suggested to occur in older physically inactive adults.101 Hence, it would be expected that exercise-induced improvements in endothelial function, which leads to increased CBF, would also improve the ability of these neurotrophic factors to reach the smaller cerebral vessels and potentiate growth, maintenance and efficiency of the areas of the brain that are responsible for cognitive and executive functioning, such as the hippocampus. While this hypothesis seems plausible and may assist in providing an explanation as to why exercise studies note changes in brain volume, plasticity and neural efficiency, future studies are required in order to validate this.
Summary
There is evidence that AT can improve CBF, cognition and neuroplasticity through areas of the brain associated with executive function and memory in older adults but there is still much to explore regarding the effect of RT and how both of these work in concert. This is largely due to the limited studies performed on the effects of RT on cerebrovascular function and cognition. Improvements in cognitive performance and structural adaptations have been reported with RT which, in some studies, was indicated to be superior to AT. There is some evidence that CT may be superior to AT or RT alone. While this seems logical given the systemic health benefits that CT imparts compared with a single exercise modality, more studies are needed to determine whether benefits also occur centrally. It is also evident that improved cerebrovascular function may act to prevent or slow the progress of cognitive impairment and ultimately dementia. There is currently limited literature that has researched the effects of exercise training on cognition in conjunction with cerebrovascular function, general health and well-being. There are also limited human studies that have aimed to determine the mechanisms by which exercise improves these parameters. While it appears that neurotrophic factors increase in an older population in parallel with exercise, no studies have directly attempted to measure cerebrovascular, inflammatory and metabolic markers that may act to increase their production and reduce ROS production by the potential pathways that we have summarised in Figure 2, which have been based on the current evidence and studies conducted in animal models. In any case, we can conclude that exercise training can improve CBF, cognition and neuroplasticity through areas of the brain associated with executive function and memory in older adults and this message should be promoted to the general public.
Figure 2.
A summary of the potential mechanisms that may be elicited by exercise in improving cerebrovascular function and cognition. CBF: cerebral blood flow; eNOS: endothelial nitric oxide synthase, NO: nitric oxide; BHB: beta-hydroxybutyrate; GH: growth hormone; VEGF: vascular endothelial growth factor; BDNF: brain-derived neurotrophic factor; IGF1: insulin-like growth factor-1; PGC-1a: peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
Future directions
At present, there are several significant gaps in our understanding of the benefits of exercise training on cerebrovascular and cognitive functions in ageing.
Few of the studies cited indicate a dose–response relationship between exercise training, in particular AT, on cerebrovascular and cognitive function in ageing. This is an important step because we do not know whether more is better or if something is better than nothing given that older adults are not meeting the current physical activity guidelines. Further, we do not know whether meeting these guidelines, especially by those who are physically inactive or sedentary, will benefit cerebrovascular and cognitive function in ageing. Randomised clinical trials that vary the frequency and/or duration of exercise would be beneficial in determining if some exercise is better than none.
This raises the question as to whether it may be easier for the general public to participate in exercise of shorter duration, higher intensity and more frequently, especially if there are physiological benefits associated with this. High-intensity interval training has significantly grown in popularity and has numerous benefits that are either comparable to or exceed those elicited by more traditional exercise programs, such as steady-state moderate-intensity AT. These may be due to reduced perceived exertion and excess post-exercise O2, and people suffering from chronic disease may be more compliant with this form of exercise.117–119 Therefore, randomised control trials that focus on more vigorous exercise training performed at shorter durations would help to ascertain whether this method could produce favourable results.
Finally, it is currently unknown if lifelong exercise training of any kind is required to maintain cerebrovascular function and prevent the development of dementia or if participating in exercise later in life can mirror the benefits of lifelong exercise and potentially decrease the likelihood of dementia development. As indicated above, there is limited research that has longitudinally assessed data providing any an insight into the trajectory of cerebrovascular function and cognition with exercise training throughout the lifespan, as well as how the type, duration, frequency and intensity of exercise training may impact these parameters. Future studies that incorporate these aspects will undoubtedly provide novel information and assist in determining the mode, intensity and duration of exercise required to elicit benefits with regard to cerebrovascular function and cognition.
Acknowledgements
The authors would like to acknowledge the University of Southern Queensland for assistance in funding the production of this manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Nichols E, Szoeke CEI, Vollset SE, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lanc Neurol 2019; 18: 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Towards a Dementia Plan: A WHO Guide. Geneva: World Health Organization, 2018. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/.
- 3.Bangen KJ, Nation DA, Clark LR, et al. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front Aging Neurosci 2014; 6: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corriveau RA, Bosetti F, Emr M, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol 2016; 36: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toth P, Tarantini S, Csiszar A, et al. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 2017; 312: H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 2018; 362: k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalmijn S, van Boxtel MPJ, Verschuren MWM, et al. Cigarette smoking and alcohol consumption in relation to cognitive performance in Middle age. Am J Epidemiol 2002; 156: 936–944. [DOI] [PubMed] [Google Scholar]
- 8.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009; 302: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anstey KJ, Kingston A, Kiely KM, et al. The influence of smoking, sedentary lifestyle and obesity on cognitive impairment-free life expectancy. Int J Epidemiol 2014; 43: 1874–1883. [DOI] [PubMed] [Google Scholar]
- 10.Bunch TJ, Weiss JP, Crandall BG, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm 2010; 7: 433–437. [DOI] [PubMed] [Google Scholar]
- 11.Kokmen E, Whisnant JP, Fallon WM, et al. Dementia after ischemic stroke. Neurology 1996; 46: 154–110. [DOI] [PubMed] [Google Scholar]
- 12.Kuller LH, Lopez OL, Jagust WJ, et al. Determinants of vascular dementia in the cardiovascular health cognition study. Neurology 2005; 64: 1548–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prins ND, den Heijer T, Hofman A, Rotterdam Scan Study et al. Homocysteine and cognitive function in the elderly. Neurology 2002; 59: 1375–1310. [DOI] [PubMed] [Google Scholar]
- 14.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the cardiovascular health cognition study. J Am Soc Nephrol 2004; 15: 1904–1910. [DOI] [PubMed] [Google Scholar]
- 15.Solomon A, Kivipelto M, Wolozin B, et al. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord 2009; 28: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia. Neurology 2010; 75: 1195–1110. [DOI] [PubMed] [Google Scholar]
- 17.AIHW. Australia’s health 2018. Canberra: Australian Institute of Health and Welfare, 2018. [Google Scholar]
- 18.Hadi HAR, Carr CS, Al Suwaidi J.Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 2005; 1: 183–198. [PMC free article] [PubMed] [Google Scholar]
- 19.Ainslie PN, Cotter JD, George KP, et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 2008; 586: 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akazawa N, Tanahashi K, Kosaki K, et al. Aerobic exercise training enhances cerebrovascular pulsatility response to acute aerobic exercise in older adults. Physiol Rep 2018; 6: e13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anazodo UC, Shoemaker JK, Suskin N, et al. Impaired cerebrovascular function in coronary artery disease patients and recovery following cardiac rehabilitation. Front Aging Neurosci 2016; 7: 224. DOI: 10.3389/fnagi.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson-Hanley C, Arciero PJ, Brickman AM, et al. Exergaming and older adult cognition: a cluster randomized clinical trial. Am J Prev Med 2012; 42: 109–119. [DOI] [PubMed] [Google Scholar]
- 23.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 2010; 67: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolandzadeh N, Tam R, Handy TC, et al. Resistance training and white matter lesion progression in older women: exploratory analysis of a 12-month randomized controlled trial. J Am Geriatr Soc 2015; 63: 2052–2060. [DOI] [PubMed] [Google Scholar]
- 25.Bossers WJR, van der Woude LHV, Boersma F, et al. A 9-week aerobic and strength training program improves cognitive and motor function in patients with dementia: a randomized, controlled trial. Am J Geriatr Psychiatry 2015; 23: 1106–1116. [DOI] [PubMed] [Google Scholar]
- 26.Brown AD, McMorris CA, Longman RS, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging 2010; 31: 2047–2057. [DOI] [PubMed] [Google Scholar]
- 27.Cassilhas RC, Viana VAR, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc 2007; 39: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 28.Chapman S, Aslan S, Spence J, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci 2013; 5: 75. DOI: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chirles TJ, Reiter K, Weiss LR, et al. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J Alzheimers Dis 2017; 57: 845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011; 108: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiatarone Singh MA, Gates N, Saigal N, et al. The study of mental and resistance training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J Am Med Direct Assoc 2014; 15: 873–880. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann K, Sobol NA, Frederiksen KS, et al. Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: a randomized controlled trial. J Alzheimers Dis 2016; 50: 443–453. [DOI] [PubMed] [Google Scholar]
- 33.Ivey FM, Ryan AS, Hafer-Macko CE, et al. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke 2011; 42: 1994–2000. [DOI] [PubMed] [Google Scholar]
- 34.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008; 300: 1027–1037. [DOI] [PubMed] [Google Scholar]
- 35.Liu-Ambrose T, Nagamatsu LS, Graf P, et al. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med 2010; 170: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu-Ambrose T, Nagamatsu LS, Voss MW, et al. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging 2012; 33: 1690–1698. [DOI] [PubMed] [Google Scholar]
- 37.Maass A, Düzel S, Goerke M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry 2015; 20: 585–593, https://www.nature.com/articles/mp2014114#supplementary-information (accessed 19 March 2019). [DOI] [PubMed] [Google Scholar]
- 38.Moore SA, Hallsworth K, Jakovljevic DG, et al. Effects of community exercise therapy on metabolic, brain, physical, and cognitive function following stroke: a randomized controlled pilot trial. Neurorehabil Neural Repair 2015; 29: 623–635. [DOI] [PubMed] [Google Scholar]
- 39.Nagamatsu LS, Handy TC, Hsu CL, et al. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med 2012; 172: 666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishiguchi S, Yamada M, Tanigawa T, et al. A 12-week physical and cognitive exercise program can improve cognitive function and neural efficiency in community-dwelling older adults: a randomized controlled trial. J Am Geriatr Soc 2015; 63: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 41.Nyberg J, Åberg MAI, Schiöler L, et al. Cardiovascular and cognitive fitness at age 18 and risk of early-onset dementia. Brain 2014; 137: 1514–1523. [DOI] [PubMed] [Google Scholar]
- 42.Smith JC, Nielson KA, Antuono P, et al. Semantic memory functional MRI and cognitive function after exercise intervention in mild cognitive impairment. J Alzheimers Dis 2013; 37: 197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobol NA, Hoffmann K, Vogel A, et al. Associations between physical function, dual-task performance and cognition in patients with mild Alzheimer’s disease. Aging Ment Health 2016; 20: 1139–1146. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki T, Shimada H, Makizako H, et al. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS ONE 2013; 8: e61483. DOI: 10.1371/journal.pone.0061483. [DOI] [PMC free article] [PubMed]
- 45.ten Brinke LF, Bolandzadeh N, Nagamatsu LS, et al. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br J Sports Med 2015; 49: 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicente-Campos D, Mora J, Castro-Piñero J, et al. Impact of a physical activity program on cerebral vasoreactivity in sedentary elderly people. J Sports Med Phys Fitness 2012; 52: 537–544. [PubMed] [Google Scholar]
- 47.Vreugdenhil A, Cannell J, Davies A, et al. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomized controlled trial. Scand J Caring Sci 2012; 26: 12–19. [DOI] [PubMed] [Google Scholar]
- 48.McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016; 75: 40–46. [DOI] [PubMed] [Google Scholar]
- 49.Harada CN, Natelson Love MC, Triebel KL.Normal cognitive aging. Clin Geriatr Med 2013; 29: 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Luca CR, Leventer RJ.Developmental trajectories of executive functions across the lifespan. Executive functions and the frontal lobes. Hove, UK: Psychology Press, 2010, pp. 57–90. [Google Scholar]
- 51.Haaland KY, Price L, Larue A.What does the WMS–III tell us about memory changes with normal aging?. J Int Neuropsychol Soc 2003; 9: 89–96. [DOI] [PubMed] [Google Scholar]
- 52.Kochunov P, Williamson DE, Lancaster J, et al. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging 2012; 33: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piolino P, Desgranges B, Benali K, et al. Episodic and semantic remote autobiographical memory in ageing. Memory 2002; 10: 239–257. [DOI] [PubMed] [Google Scholar]
- 54.Rönnlund M, Nyberg L, Bäckman L, et al. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol Aging 2005; 20: 3–18. [DOI] [PubMed] [Google Scholar]
- 55.Salthouse TA, Toth JP, Hancock HE, et al. Controlled and automatic forms of memory and attention: process purity and the uniqueness of age-related influences. J Gerontol B Psychol Sci Soc Sci 1997; 52: P216–P228. [DOI] [PubMed] [Google Scholar]
- 56.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012; 344: d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zelinski EM, Burnight KP.Sixteen-year longitudinal and time lag changes in memory and cognition in older adults. Psychol Aging 1997; 12: 503–513. [DOI] [PubMed] [Google Scholar]
- 58.Cabeza R, Albert M, Belleville S, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci 2018; 19: 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Damoiseaux JS.Effects of aging on functional and structural brain connectivity. NeuroImage 2017; 160: 32–40. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy KM, Raz N.Normal aging of the brain. In: Toga AW. (ed) Brain mapping. Waltham: Academic Press, 2015, pp. 603–617. [Google Scholar]
- 61.Reuter-Lorenz PA, Park DC.Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci 2010; 65: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole JH.Neuroimaging studies illustrate the commonalities between ageing and brain diseases. BioEssays 2018; 40: 1700221. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Wolk DA, Reddin JS, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology 2011; 77: 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bischof GN, Park DC.Obesity and aging: consequences for cognition, brain structure, and brain function. Psychosom Med 2015; 77: 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rossman MJ, LaRocca TJ, Martens CR, et al. Healthy lifestyle-based approaches for successful vascular aging. J Appl Physiol 2018; 125: 1888–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamel E, Nicolakakis N, Aboulkassim T, et al. Oxidative stress and cerebrovascular dysfunction in mouse models of Alzheimer’s disease. Exp Physiol 2008; 93: 116–120. [DOI] [PubMed] [Google Scholar]
- 67.Rutkai I, Merdzo I, Wunnava SV, et al. Cerebrovascular function and mitochondrial bioenergetics after ischemia-reperfusion in male rats. J Cerebr Blood Flow Metabol 2019; 39: 1056–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 2018; 17: e12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berkowitz DE, White R, Li D, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003; 108: 2000–2006. [DOI] [PubMed] [Google Scholar]
- 70.Deer RR, Stallone JN.Effects of estrogen on cerebrovascular function: age-dependent shifts from beneficial to detrimental in small cerebral arteries of the rat. Am J Physiol Heart Circulat Physiol 2016; 310: H1285–H1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pikula A, BöGer RH, Beiser AS, et al. Association of plasma ADMA levels with MRI markers of vascular brain injury: Framingham offspring study. Stroke 2009; 40: 2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Cavanagh EMV, Inserra F, Ferder L.Angiotensin II blockade: how its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. Am J Physiol Heart Circ Physiol 2015; 309: H15–H44. [DOI] [PubMed] [Google Scholar]
- 73.Flavahan S, Chang F, Flavahan NA.Local renin-angiotensin system mediates endothelial dilator dysfunction in aging arteries. Am J Physiol Heart Circ Physiol 2016; 311: H849–H854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishii M, Iadecola C.Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochim Biophys Acta 2016; 1862: 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trigiani LJ, Hamel E.An endothelial link between the benefits of physical exercise in dementia. J Cereb Blood Flow Metab 2017; 37: 2649–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Joris P, Mensink R, Adam T, et al. Cerebral blood flow measurements in adults: a review on the effects of dietary factors and exercise. Nutrients 2018; 10: 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gertz K, Priller J, Kronenberg G, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res 2006; 99: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 78.Zhang P, Yu H, Zhou N, et al. Early exercise improves cerebral blood flow through increased angiogenesis in experimental stroke rat model. J Neuroeng Rehabil 2013; 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herring A, Yasin H, Ambrée O, et al. Environmental enrichment counteracts Alzheimer’s neurovascular dysfunction in TgCRND8 mice. Brain Pathol 2008; 18: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanekiyo T, Bu G.The low-density lipoprotein receptor-related protein 1 and amyloid-β clearance in Alzheimer’s disease. Front Aging Neurosci 2014; 6: 93. DOI: 10.3389/fnagi.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Latimer CS, Searcy JL, Bridges MT, et al. Reversal of glial and neurovascular markers of unhealthy brain aging by exercise in Middle-aged female mice. Plos One 2011; 6: e26812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Viboolvorakul S, Patumraj S.Exercise training could improve age-related changes in cerebral blood flow and capillary vascularity through the upregulation of VEGF and eNOS. BioMed Res Int 2014; 2014: 1–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bracko O, Njiru BN, Swallow M, et al. Increasing cerebral blood flow improves cognition into late stages in Alzheimer’s disease mice. J Cerebr Blood Flow Metabol 2020; 40: 1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Praag H, Shubert T, Zhao C, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 2005; 25: 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rhyu IJ, Bytheway JA, Kohler SJ, et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience 2010; 167: 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serrador JM, Picot PA, Rutt BK, et al. MRI measures of Middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 2000; 31: 1672–1678. [DOI] [PubMed] [Google Scholar]
- 87.Willie CK, Colino FL, Bailey DM, et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 2011; 196: 221–237. [DOI] [PubMed] [Google Scholar]
- 88.Yamada K, Nabeshima T.Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci 2003; 91: 267–270. [DOI] [PubMed] [Google Scholar]
- 89.Xu X, Jerskey BA, Cote DM, et al. Cerebrovascular perfusion among older adults is moderated by strength training and gender. Neurosci Lett 2014; 560: 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bennie JA, De Cocker K, Teychenne MJ, et al. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 U.S. adults. Int J Behav Nutr Phys Act 2019; 16: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Evans H, Howe P, Wong R.Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 2017; 9: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trejo JL, Carro E, Torres-Alemán I.Circulating insulin-like growth factor 1 mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 2001; 21: 1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Busse AL, Filho WJ, Magaldi RM, et al. Effects of resistance training exercise on cognitive performance in elderly individuals with memory impairment: results of a controlled trial. Einstein (São Paulo) 2010; 8: 40–407.26761751 [Google Scholar]
- 94.Fox NC, Black RS, Gilman S, for the AN1792(QS-21)-201 Study Team et al. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 2005; 64: 1563–1572. [DOI] [PubMed] [Google Scholar]
- 95.Sparks DL, Lemieux SK, Haut MW, et al. Hippocampal volume change in the Alzheimer disease cholesterol-lowering treatment trial. Cleve Clin J Med 2008; 75 Suppl 2: S87–93. [DOI] [PubMed] [Google Scholar]
- 96.Devika NT, Jaffar Ali BM.Analysing calcium dependent and independent regulation of eNOS in endothelium triggered by extracellular signalling events. Mol Biosyst 2013; 9: 2653–2664. [DOI] [PubMed] [Google Scholar]
- 97.Morland C, Andersson KA, Haugen ØP, et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun 2017; 8: 15557. https://www.nature.com/articles/ncomms15557#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilmore JH, Costill DL, Gleim GW.Physiology of sport and exercise. Med Sci Sports Exerc 1995; 27: 792. [Google Scholar]
- 99.Levine BD.VO2max: what do we know, and what do we still need to know?. J Physiol (Lond) 2008; 586: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lundby C, Montero D, Joyner M.Biology of VO2max: looking under the physiology lamp. Acta Physiol (Oxf) 2017; 220: 218–228. [DOI] [PubMed] [Google Scholar]
- 101.Wilson MG, Ellison GM, Cable NT.Basic science behind the cardiovascular benefits of exercise. Heart 2015; 101: 758–765. [DOI] [PubMed] [Google Scholar]
- 102.Bassett DR, Howley ET.Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 2000; 32: 70–70. [DOI] [PubMed] [Google Scholar]
- 103.Navarro A, Gomez C, López-Cepero JM, et al. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol 2004; 286: R505–R511. [DOI] [PubMed] [Google Scholar]
- 104.Radak Z, Taylor AW, Ohno H, et al. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev 2001; 7: 90–107. [PubMed] [Google Scholar]
- 105.Brook MS, Wilkinson DJ, Phillips BE, et al. Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise. Acta Physiol (Oxf) 2016; 216: 15–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Summermatter S, Santos G, Pérez-Schindler J, et al. Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci Usa 2013; 110: 8738–8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016; 5: 10–7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marston KJ, Newton MJ, Brown BM, et al. Intense resistance exercise increases peripheral brain-derived neurotrophic factor. J Sci Med Sport 2017; 20: 899–903. [DOI] [PubMed] [Google Scholar]
- 109.Frystyk J.Exercise and the growth hormone-insulin-like growth factor axis. Med Sci Sports Exerc 2010; 42: 58–66. [DOI] [PubMed] [Google Scholar]
- 110.Kimura H, Esumi H.Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol 2003; 50: 49–60. [PubMed] [Google Scholar]
- 111.Delezie J, Handschin C.Endocrine crosstalk between skeletal muscle and the brain. Front Neurol 2018; 9: 698. DOI: 10.3389/fneur.2018.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Phillips C, Baktir MA, Srivatsan M, et al. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front Cell Neurosci 2014; 8. DOI: 10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paillard T, Rolland Y, de Souto Barreto P.Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: a narrative review. J Clin Neurol 2015; 11: 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ding Q, Vaynman S, Akhavan M, et al. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006; 140: 823–833. [DOI] [PubMed] [Google Scholar]
- 115.Vaynman S, Ying Z, Gomez-Pinilla F.Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 2004; 20: 2580–2590. [DOI] [PubMed] [Google Scholar]
- 116.Santos-Parker JR, LaRocca TJ, Seals DR.Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ 2014; 38: 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Campbell WW, Kraus WE, Powell KE, 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE* et al. High-intensity interval training for cardiometabolic disease prevention. Med Sci Sports Exerc 2019; 51: 1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ross LM, Porter RR, Durstine JL.High-intensity interval training (HIIT) for patients with chronic diseases. J Sport Health Sci 2016; 5: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Batacan RB, Duncan MJ, Dalbo VJ, et al. Effects of high-intensity interval training on cardiometabolic health: a systematic review and Meta-analysis of intervention studies. Br J Sports Med 2017; 51: 494–503. [DOI] [PubMed] [Google Scholar]
- 120.Chang F, Flavahan S, Flavahan NA.Superoxide inhibition restores endothelium-dependent dilatation in aging arteries by enhancing impaired adherens junctions. Am J Physiol Heart Circ Physiol 2018; 314: H805–H811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raz N, Yang Y, Dahle CL, et al. Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim Biophys Acta 2012; 1822: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown WR, Moody DM, Thore CR, et al. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci 2007; 257: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Webb AJS, Simoni M, Mazzucco S, et al. Increased cerebral arterial pulsatility in patients with leukoaraiosis. Stroke 2012; 43: 2631–2636. [DOI] [PubMed] [Google Scholar]
- 124.Asai H, Ikezu S, Tsunoda S, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 2015; 18: 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di Marco LY, Venneri A, Farkas E, et al. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol Dis 2015; 82: 593–606. [DOI] [PubMed] [Google Scholar]
- 126.Schipke CG, Menne F, Teipel SJ, DELCODE Study Group et al. Levels of the astrocyte-derived proteins gfap and s100b in the cerebrospinal fluid of healthy individuals and Alzheimer’s disease patients at different disease stages. Alzheimer’s Dement 2018; 14: P1458–P1459. [Google Scholar]
- 127.Tzeng Y-C, Ainslie PN.Blood pressure regulation IX: cerebral autoregulation under blood pressure challenges. Eur J Appl Physiol 2014; 114: 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zlokovic BV.Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 2011; 12: 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hawkes CA, Sullivan PM, Hands S, et al. Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 allele. Plos One 2012; 7: e41636. [DOI] [PMC free article] [PubMed] [Google Scholar]