Abstract

The primary task of a battery is to store energy and to power electronic devices. This has hardly changed over the years despite all the progress made in improving their electrochemical performance. In comparison to batteries, electronic devices are continuously equipped with new functions, and they also change their physical appearance, becoming flexible, rollable, stretchable, or maybe transparent or even transient or degradable. Mechanical flexibility makes them attractive for wearable electronics or for electronic paper; transparency is desired for transparent screens or smart windows, and degradability or transient properties have the potential to reduce electronic waste. For fully integrated and self-sufficient systems, these devices have to be powered by batteries with similar physical characteristics. To make the currently used rigid and heavy batteries flexible, transparent, and degradable, the whole battery architecture including active materials, current collectors, electrolyte/separator, and packaging has to be redesigned. This requires a fundamental paradigm change in battery research, moving away from exclusively addressing the electrochemical aspects toward an interdisciplinary approach involving chemists, materials scientists, and engineers. This Outlook provides an overview of the different activities in the field of flexible, transient, and transparent batteries with a focus on the challenges that have to be faced toward the development of such multifunctional energy storage devices.
Short abstract
Multifunctional batteries offering new properties like flexibility, transparency, or degradability open up possibilities in wearable, optoelectronic, implantable, or ingestible electronic devices.
1. Introduction
Progress in portable electronic devices has been so rapid that the performance and range of features of a device just 10 years old seem awfully old-fashioned. What has not changed so much is their physical appearance. Even modern devices remain mostly solid, rigid, and fragile. However, this has begun to change, and there are prototypes and new models on the market that incorporate flexibility/bendability,1−3 transparency,4−6 or degradability/transience into the design.2,7−10 Clearly, such innovations also affect the battery as the main energy source. For fully integrated and self-sufficient systems, the battery as part of the device must have the same physical properties.
The rapid evolution of multipurpose (transparent, flexible, and degradable) electronic devices has the potential to revolutionize our life. Biodegradable medical implants, which serve as platforms with sensing and stimulation functions to support biological processes such as wound healing, tissue regeneration, and brain activity,11 are just one example of how technological innovations in electronics fit together with practical application scenarios that will profoundly influence our future lifestyles.
In contrast to flexible, transparent, and transient electronics,7,9,10 corresponding breakthroughs in batteries are still limited, which is certainly not due to the lack of scientific and technological interest, but due to the high complexity of the battery structure. The implementation of new physical, mechanical, and chemical properties into a multifunctional battery requires that all its components (active materials, current collectors, electrolyte/separator, and packaging) have to be flexible, transparent, and/or degradable, while maintaining the electrochemical function. From a materials science point of view, this is a great challenge because the materials currently used in lithium-ion batteries (LIBs) do not offer any of these properties. In fact, typical batteries are rigid bodies enclosed in a metal container. Even in the case of the much lighter pouch cells, the battery is protected in a rigid plastic case. Such a rigorous design is necessary to meet all the requirements for safe and long-lasting operation. Accordingly, the fabrication and assembly of a multifunctional battery require a broad expertise in materials and their processing.
In this Outlook, we want to highlight some of the latest developments in the field of flexible/stretchable, transparent, and degradable batteries. Looking beyond electrochemical storage capability, our focus is on the challenges coming with equipping all different battery components with the additional properties. The structure follows that of the battery discussing each component from active materials, current collectors, electrolytes/separators, and packaging.
2. Flexible and Stretchable Batteries
The interest in flexible electronics, such as wearable devices, on-skin sensors, flexible displays, or environmental sensors, has triggered immense research activities in the field of energy storage systems that can be bent, folded, crumpled, and stretched while maintaining their electrochemical properties.12 However, the high standards of today’s battery technology and user expectations have set the bar very high for good electrochemical performance, affordable prices, and high safety.13,14
There are two general approaches to introduce flexibility in batteries: intrinsically stiff materials are replaced by soft and bendable compounds, or stiff materials are processed into structures that are flexible.15−17 In both cases, for stable performance the contacts between the battery constituents have to be guaranteed under repeated deformation. Furthermore, leakage and evaporation of electrolyte have to be prevented by appropriate packaging. While these aspects are mostly given in rigid batteries with solid cases, they can represent a serious safety issue in pouch cells and soft batteries.
In the following section, we present and discuss the most relevant approaches for flexible battery components and introduce some concepts for a stretchable battery design.
Active Materials and Electrode Structure
Intrinsic electrochemical stability and interfacial adhesion define the robustness of a flexible battery. Using established chemistries, the remaining challenge is reduced to strengthening the adhesive forces between the battery components. Conductive additives and binders help with sandwiching the electrode layer between the self-standing, relatively strong components of the current collector and separator. Therefore, research efforts focus on combining the performance of existing chemical systems like lithium-ion, lithium sulfur (Li/S), or zinc-ion (Zn) batteries with structures that exhibit new mechanical properties. Similar to traditional batteries, the electrode materials are usually present in the form of powders that have to be processed in such a way that a strong adhesion to the current collector is achievable. Certainly, the requirements to avoid any delamination are much stricter for a flexible or stretchable than for a rigid battery.18,19
LIBs clearly represent the state-of-the-art in portable energy storage due to their high energy and power density as well as their long-term stability.20 However, the current standard electrode architectures in LIBs do not allow physical bending without considerable performance loss.21 Through careful structural engineering, flexible LIBs have been realized. Oh et al. utilized carbon-nanotube-decorated α-iron oxide particles and lithium iron phosphate as the anode and cathode, respectively.22 Fibrous mats of these materials (Figure 1a) as electrodes as well as a gel polymer electrolyte allowed for stress-minimized bending of the electrochemical pouch cell. Their study focused on the synthesis of the electrochemically active materials and collecting the data in the bent state. For applications in flexible devices, however, one-time bending is far from practical relevance. Bendability over 5000 cycles has been introduced into well-known LIB chemistry system based on lithium cobalt oxide (LiCoO2) and graphite through patterning, resulting in a high contact area at the interfaces, which promotes adhesion and helps the stress distribution during bending.18
Figure 1.
Various architectures reported to be highly stress accommodating and advantageous for flexible and stretchable electrochemical energy storage: (a) Fiber mats. Used with permission from ref (22). Copyright 2019 Royal Society of Chemistry. (b) Fabrics. Used with permission from ref (28). Copyright 2018 Springer Nature. (c–e) Stress accommodating interfaces and interlayers through: (c) Sliding contacts. Used with permission from ref (42). Copyright 2018 John Wiley and Sons. (d) Patterning processes. Used with permission from ref (18). Copyright 2018 John Wiley and Sons. (e) Hydrogel interlayer. Used with permission from ref (68). Copyright 2019 John Wiley and Sons.
Zn-ion batteries represent another promising technology with relatively high capacity. Active materials for this type of battery are low-cost, abundant, safe, ecofriendly, and sustainable. Like LIBs, zinc-based flexible batteries consist mostly of well-established material combinations.23,24 Zamarayeva et al. reported a Zn metal anode along with a manganese oxide (MnO2) cathode, where performance retention after bending was facilitated through highly flexible binders such as poly(vinyl alcohol) (PVA) and poly(acrylic acid) (PAA).25
A third technology intensively studied for flexible energy storage is Li/S batteries. Although the long-term stability has not yet been satisfactorily solved, the high theoretical capacity makes this system attractive.26,27 The anode has to facilitate a uniform lithium distribution, while the cathode has to be composed of a conducting fine-pore network to accommodate the insulating sulfur. An example was presented by Chang et al. employing metal-coated carbon fibers and a N-doped carbon to collect the sulfur (Figure 1b).28
Other battery chemistries based on metallic lithium26,29 or silicon30,31 are still in their infancy. They struggle with quick capacity fading, even in the classical rigid design, and are therefore not yet ready for implementation in flexible devices.
Current Collector and Conductive Additive
Although copper and aluminum32 exhibit certain flexibility as thin foils, the main challenge utilizing them in a flexible battery is the poor adhesion of the electrode materials to the metal surfaces.33,34 This problem can be reduced by maximizing the contact area between the two materials, for example, by using components with large surface areas or interlocked structures.18 Researchers have also tested metal nanowires35,36 and electrochemically deposited metal films.37 However, the most promising alternatives are different types of carbon-based structures due to their low weight, high mechanical stability, and tunable adhesive forces.38−41 Li et al. utilized carbon nanotubes and graphite as the electron collector and conductive additive (Figure 1c).42
To ensure physical contact of the components, traditional cell manufacturing relies on binders. Their adhesive forces add to the integrity of the layer as well as to the interlayer stability. Besides the common polyvinylidene fluoride (PVDF), another approach makes use of hydrogels as binders, with which Wu et al. achieved high stability in a silicon nanoparticle anode.43 Directed deposition guided through surface architecture can further enhance the interconnection between the current collector and the active material layer.40,44
Electrolyte and Separator
When selecting the electrolyte, its stable voltage window and its state of matter at the working temperature decide whether a separator or a matrix is necessary, and this also determines the permeability requirements of the packaging. Whereas the combination of liquid electrolytes with most common battery separators, gel, and polymer electrolytes are inherently flexible,45,46 most solid-state electrolytes are fragile and inflexible and require a mixed matrix approach to achieve flexibility.47,48
In balancing volatility and flexibility, gel polymer electrolytes based on PVDF, PAA, polyionic liquids, or poly(ethylene oxide) (PEO) seem to be particularly promising.24,49−54 These materials are mechanically flexible, safe, and easier to pack compared to liquid electrolytes. Mechanical stability is particularly important, when metal anodes (like lithium or zinc) are involved, and dendrite formation is a major concern for their functionality.53,55 The main challenge of polymer electrolytes is their low ionic conductivity at room temperature. Gel electrolytes are reported, among others, for Zn56−58 and Li/S batteries,27,59−61 and they have demonstrated good compressibility,56 which makes them good interlayer materials for stress accommodation.62
Packaging
Most of the active components require robust packaging that is impermeable to water vapor and oxygen, which excludes the use of polymers as the sole packing material.63 Traditional packaging, mainly metal cases, provides stability, impermeability, and electric terminals. For flexible batteries, the standard materials are multilayered laminates64,65 composed of a thin metal layer, mostly aluminum, ensuring low water, solvent, and oxygen permeability with an exterior protective and an interior heat-sealable polymer film.65−67 These materials meet the safety requirements in LIBs only at the price of additional stiffness. For less-sensitive battery chemistries like aqueous Zn-ion batteries, flexible but sufficiently safe packaging is provided by polymers like polyethylene naphthalate (PEN)/polyvinyl chloride (PVC).25
Performance Comparison in Full Cells
For a flexible full cell battery, all components must provide both the appropriate electrochemical performance and the intrinsic mechanical stability upon bending. Therefore, the fabrication of a flexible full cell is not the mere assembly of the components, but the combination of the different layers with robust interfaces, so that they do not lose contact under mechanical stress. Cha et al. proposed a flexible battery based on a patterning process for interlocking the different layers (Figure 1d).18 Hydrogel interlayers, which accommodate occurring stresses and/or soft packaging, which avoid additional stresses, are common strategies as well (Figure 1e).68
Generally, choosing a robust battery chemistry is as important as a stress accommodating structure for a flexible battery.55,69,70 To date, this fact seems to lead to a trade-off between capacity and bending capability (Figure 2). Unfortunately, high-capacity materials like those used in Li/S batteries are already exposed to high internal stress due to their high lithium uptake and large volume change. This effect is amplified, e.g., in the sulfur cathode, which possesses weaker interactions resulting in capacity loss upon bending.71
Figure 2.

Performance comparison of bending tests with flexible cablelike (outlined) and two-dimensional (filled) electrochemical cells. The graph shows their initial capacity vs number of bending cycles as well as their capacity retention after the specified bending cycles (indicated by symbol size). Data are colored according to battery chemistries with LIBs (green) and Li/S (orange) and Zn (blue) batteries.
In general, the comparison of performance values has some limitations and should be considered together with information on device structure, materials use, and experimental details. Whereas cycling performance parameters are already on the way to be standardized,72,73 no guidelines for performance testing under bending are available. Cycling data broadly range from performance reports after one flection up to thousands of bending cycles.18,25,28,55,71,149−161 Furthermore, the results of mechanical testing depend not only on the bending radius but also on the angle, speed, and tension stemming from packing and bending stresses of the various layers.18 The development of evaluation standards seems to be urgently needed to better classify and compare future progress in research on flexible batteries.
Current Challenges
Due to the elaborate manufacturing process and the associated high costs, as well as the fact that the technology is not yet ready for the market, most flexible batteries are still at the stage of company R&D42 and in the startup phase. Companies like Blue Spark Technologies or Molex LLC produce zinc polymer batteries for personal electronics. Big players in the field like Samsung SDI, Apple Inc., or Panasonic Corporation released or patented flexible batteries but are, however, not yet using them in their devices. Foldable phones currently on the market still contain bulky batteries and are, therefore, only bendable along defined axes, and they still come with technical and durability issues.74 It is expected that future cells with improved power efficiency and longer battery life will increase the use and demand for flexible devices in the coming years.
By balancing material properties and introducing hierarchical architectures, flexible full cell assemblies could reach the performance and stability needed for practical device integration. A robust flexible battery technology with stable electrochemistry would open up fascinating possibilities for including other functionalities. First attempts have already been made and many more areas will benefit from research on flexible batteries: functional energy storage textiles,75,76 pressure sensing hybrid batteries,56,77 self-charging batteries,78−81 electrochromic microbatteries as screen components,82−84 transparent cells for integration into smart windows,84 or in solar cells,85 as well as batteries with transient functionalities.86 For all these new functionalities, flexibility can enhance their applicability and bring them one step closer to interesting customer-ready products.
3. Degradable Batteries
Transient or degradable electronics is an emerging type of technology wherein devices are built to operate for a well-defined period and, when no longer needed, can degrade at controlled rates.87 Such electronic platforms can offer a multitude of applications including implantable biomedical devices that resorb in the body after realizing their diagnostic functions, ecofriendly devices that reduce the problems associated with growing electronic-waste, and data-secure hardware systems which self-destruct to prevent unauthorized access to sensitive information.7,10 To realize self-sufficient degradable electronics, the development of transient batteries as an autonomous on-board power source is important. To date, there are very few examples of transient batteries in the literature. The main challenges are the deficit of suitable soluble materials, fabrication schemes, and battery designs that must fulfill completely different requirements than for traditional batteries. To ensure rapid progress in this field, it is necessary to expand the materials toolbox beyond classical battery materials and to optimize the battery structure with respect to transiency. Inspiration can be drawn from research activities in the field of bioelectronics, nature-inspired/derived biomaterials, and green electronics.
In the following section, a critical discussion of the materials used as components in transient batteries and their full cell assembly will be presented.
Active Materials and Electrode Structure
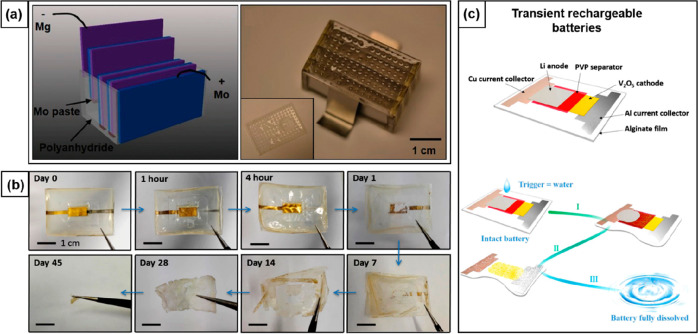
Transient primary batteries have been mainly developed based on the use of Mg metal (Figure 3a),88−92 or sodium ion chemistry.93,94 Mg as an anode material offers excellent electrochemical properties and good biocompatibility. However, its rapid corrosion in aqueous environments presents a serious challenge. To overcome this issue, several strategies such as alloying of Mg with biocompatible metals (Al, Zn, Zr),90,91,95−97 surface coating with biomaterials (β-tricalcium phosphate (β-TCP)),97,98 and design optimization (use of electroplated Mg)99 have been proposed. Mg and its alloys are coupled with benign cathode materials (e.g., Fe, Mo, W, molybdenum oxide, polypyrrole, Au) to develop fully degradable batteries. Jia et al. utilized a bioresorbable Mg alloy anode and Au nanoparticles as the cathode to demonstrate a biodegradable Mg–air battery, whose enzymatic degradation was observed in buffered protease solution at 37 °C (Figure 3b).90 Degradable primary sodium-ion batteries were developed using aqueous electrolytes and naturally occurring or edible electrode materials such as melanin, activated carbon, and manganese oxide. These electrode materials can reversibly bind with the sodium ions that are present inside the human body, thus offering in vitro applications.93,94
Figure 3.
Transient behavior in batteries. (a) Schematic illustration and optical image of a biodegradable primary Mg–Mo battery in a stacked configuration with four cells in series. Used with permission from ref (88). Copyright 2014 WILEY-VCH. (b) Optical images demonstrating the biodegradation profile of a Mg–air battery in buffered protease solution at 37 °C. Used with permission from ref (90). Copyright 2017 American Chemical Society. (c) Schematic illustrations of a degradable rechargeable LIB and its triggered dissolution process: upon contact with water, the encapsulation and separator of the battery dissolved first (I), followed by the reaction of Li metal with water to produce LiOH (II). The generated base reacted with V2O5 and Al metal leading to the complete dissolution of the battery (III). Used with permission from ref (101). Copyright 2015 American Chemical Society.
For secondary/rechargeable systems, the most representative type comprises LIBs (Figure 3c).100,101 Fu et al. demonstrated the first rechargeable transient LIB based on dissoluble electrodes including vanadium oxide (V2O5) as the cathode and lithium (Li) metal as the anode.101 In this work, V2O5 was selected as the cathode due to its comparatively high theoretical capacity and its ability to dissolve in alkali solution, formed by the reaction of Li metal with water. Subsequent research efforts focused on improving the electrochemical performance while still achieving fast transience behavior. Nevertheless, their application in biomedical and ecofriendly devices is still severely limited due to the generation of an alkaline environment that can have adverse biological and ecological effects. As an alternative, one could target greener energy-storage materials derived from abundant resources like redox-active biopolymers, which can be tuned via nano-/microstructuring to undergo programmed degradation.
Current Collector and Conductive Additive
Biodegradable metals such as Mg, Mg-based alloys, Fe, Mo, and W serve as current collectors for transient primary batteries. Most of these metals are known to play significant physiological functions and demonstrate appropriate degradation rates in vivo.102,103 They are used either as self-supporting foils compatible with most processes, temperatures, and solvents104 or as highly flexible polymer-supported thin films. The difference in their dissolution behavior arises from surface morphology, grain structure, and presence of pit holes that control the kinetics of dissolution.105 Secondary transient batteries have been demonstrated with thin layers of relatively inert metals such as Cu, Al, and Ni deposited onto degradable polymeric substrates as current collectors. As a promising substitute to metallic conductors, researchers have also reported the use of carbon-based current collectors (e.g., carbon black) that are blended with degradable polymers to achieve transiency.100,106
Electrolyte and Separator
The electrolytes used in transient batteries so far can be classified into nonaqueous electrolytes, aqueous electrolytes, and polymer electrolytes. The nonaqueous electrolytes were used in transient secondary LIBs in combination with a separator made of water-soluble polymers such as PVA, PEO, and polyvinylpyrrolidone (PVP).100,101,107−109 The organic electrolytes provide a considerable advantage in terms of their large voltage window, which enables high power density, but their toxicity is a major concern. Environmentally benign aqueous electrolytes were mostly used to activate transient primary batteries designed for implantable medical devices.110 For example, using physiological fluid (PBS) as the electrolyte, polycaprolactone (PCL)-coated biodegradable Mg–Fe batteries delivered an average power of 30 μW for 100 h, sufficient to power a commercial pacemaker for up to 4 days.89 Volatilization of solvent leading to changing electrical properties and a small potential window of aqueous electrolytes hinder practical applications.110 As an alternative, polymer electrolytes have been proposed that can also function as a separator and as glue to improve mechanical integrity.96 Reported examples tested for transient batteries are the solid polymer electrolyte composed of sodium chloride and PCL111 and gel electrolytes based on a biocompatible ionic liquid (choline nitrate) embedded in silk90 (Figure 4a,b). Unfortunately, the performance of the gel electrolyte was found to decline at high discharge rates due to its low ion migration rate.96
Figure 4.
(a) Schematic illustration of an encapsulated thin-film Mg–air battery, (b) fabrication procedure and a digital image of the gel electrolyte (choline nitrate (ionic liquid) embedded in silk fibroin) used in the battery. (c) Open circuit voltage changes of the encapsulated battery without and with an additional crystallized silk protection layer in phosphate buffer solution (PBS) and in air. Used with permission from ref (90). Copyright 2017 American Chemical Society. (d) Schematic illustration of a biodegradable Mg-MoO3 battery, (e) powering an LED in PBS for over 16 h. (f) Optical images at various stages of battery degradation in PBS. Used with permission from ref (11). Copyright 2018 WILEY-VCH.
Packaging
The concept of transience relies on packaging strategies balancing device operation lifetime and dissolution rate. Effective encapsulation is required to ensure stable operation for the desired period before degradation starts. To date, biodegradable polymers such as sodium alginate, silk, polyanhydrides, PVA, and PCL were applied as encapsulation layers for transient batteries.88−90,101,107 Their properties such as crystallinity, thickness, and composition were tuned to obtain a more predictable or programmed battery lifetime.112 For instance, an additional crystallized silk film on top of an encapsulated primary Mg battery extended its stable operation in PBS solution from 64 to 109 min (Figure 4c).90 Nonetheless, the high water permeation rates of biodegradable polymers limit the development of batteries with longer lifetimes.113 One viable solution can be the coating of biodegradable packaging materials with thin films of metal oxides and/or nitrides (SiO2, Si3N4), or with hydrophobic compounds (beeswax, paraffin). The low water permeability of these materials can facilitate longer battery lifetimes together with programmed degradation.114
Performance Comparison in Full Cells
To improve the practical application of transient batteries, continuous efforts should be invested in fabricating degradable full cell batteries. Rogers and co-workers first reported a biodegradable, polyanhydride-encapsulated primary Mg–Mo battery that exhibited a stable voltage of 0.4–0.7 V for 24 h and demonstrated transience in PBS solution. The battery, however, suffered from low power density and comparably short lifetime.88 Huang et al. developed a high-performance primary Mg-MoO3 battery with extended lifetime, delivering a stable voltage of 0.6 V for 250 h. The battery powered a standard LED for 16 h in PBS solution and was shown to be fully degradable both in vivo and in vitro (Figure 4d–f).11 Fu et al. fabricated the first transient secondary LIB that operated in organic electrolytes yet rapidly dissolved in an aqueous environment due to triggered cascade reactions.101 The battery provided a high working voltage of 2.8 V but could only be charged and discharged for four cycles. These examples show that, by careful selection of different battery components and fabrication schemes, innovative prototypes of transient batteries can be achieved. Clearly there is a trade-off between a battery’s electrochemical performance and transient behavior, and therefore, it is necessary to find an optimum balance.
Current Challenges
The field of transient batteries is still in its infancy, but it is expected that in the future transient batteries would serve as advanced power supplies for green, disposable, and transient electronics. To boost the development, new electrode materials and electrolytes must be investigated, and their degradation behavior should be studied in great depth. New modes of trigger such as light, heat, temperature, pH, etc. can be utilized to precisely control the onset of the degradation process. At the end of life, the resulting degradation products can ideally be recaptured and recycled to enhance economic and environmental viability of transient electronics. Further efforts should be directed toward developing strategies for seamless integration of such batteries in functional transient systems to achieve completely self-powered devices.115,116
4. Transparent Batteries
Although it still seems a bit like science fiction, research on transparent electronics has increased significantly. The most fascinating property of such devices is that their transparency makes it possible to superimpose a virtual electronic image on a real background. Displays become invisible, which opens up the possibility of integrating them into car windshields, eyeglasses, or windows to display information without obstructing the view. In smartphones or tablets, transparency enables unique designs. If the energy source is to be built-in, it must be transparent not to impair the optical appearance.
Up to now, the literature on transparent batteries is relatively scarce, although a few instructive examples of transparent energy storage devices have been reported.117,118 Similar to flexible batteries, which have to overcome the rigidity of typical battery components, and to degradable batteries, which have to replace nondegradable components with transient materials, transparent batteries face the challenge that neither traditional electrodes nor usual separators are transparent.
In this section, we focus on presenting some recent progress about different components and their combination into transparent batteries.
Active Materials and Electrode Structure
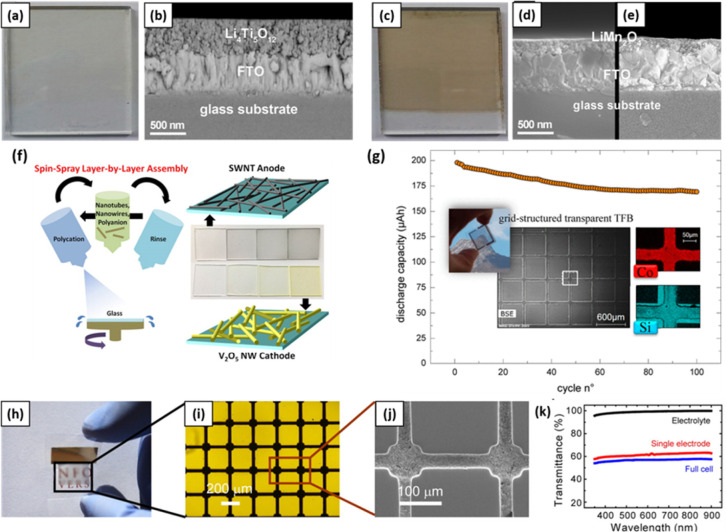
There are three major approaches to reach transparency for active materials. Firstly, wide-band-gap transparent conducting oxides that show high transmission in the visible range can be applied as active materials.119,120 Al-doped ZnO thin films, deposited by a midfrequency sputtering technique, as the anode for LIBs offered a high transmittance of 84% and a high specific capacity of 301 mA h g–1.121 The electrochemical tests, however, were performed on nontransparent stainless-steel substrates. The second strategy is to reduce the dimensions of active materials and decrease the electrode thickness down to a scale below their optical absorption length.122 In Figure 5a–e, Roeder et al. applied a sol–gel dip-coating technique to prepare a transparent full cell battery consisting of a 600 nm thick Li4Ti5O12 anode and a 150 nm thick LiMn2O4 cathode.123 Li4Ti5O12 presented a visible light transmittance of 30–75%, transitioning from dark-blue to colorless depending on the charge/discharge state. The LiMn2O4 electrode showed a green color in the uncharged state and turned to orange when being charged. The cycling behavior of the full cell was not reported. A transparent LIB composed of single-walled carbon nanotubes and V2O5 nanowires as the anode and cathode, respectively, was produced by layer-by-layer assembly (Figure 5f). The transmittance of these thin film electrodes was adjusted by the number of sprayed layers. The 255–300 nm thick anode showed a transmittance of 87%, while the cathode presented 93% transmittance at a thickness of 150–300 nm.124 Another approach toward transparent electrodes involves the preparation of gridlike structures with feature dimensions below the visual acuity of human eyes.125 The grid-structure approach was used for fabricating thin-film electrodes in a transparent, all-solid inorganic LIB. LiCoO2/lithium phosphorus oxynitride (LiPON)/Si structures were fabricated on glass substrates using photolithography and etching processes to achieve a transmittance of 60% with 65.3% of open area (Figure 5g).126 Yang et al. used a microfluidics-assisted method to process Li4Ti5O12 and LiMn2O4 into grid-structured electrodes for a transparent LIB. A transmittance of 62% in the visible and near-infrared range for the electrode was obtained (Figure 5h–k). The single electrode had 65% areal vacancy and exhibited a transmittance of 62%.125 Since the transparency of the electrodes decreases with increasing thickness and covered area, but the stored energy increases linearly with the mass loading of the active material, a trade-off between electrode loading and transparency has to be accepted when working with gridlike structures.
Figure 5.
(a) Photograph and (b) cross-section of a colorless Li4Ti5O12 thin-film electrode. (c) Photograph and (d, e) cross-section of a brownish LiMn2O4 thin-film electrode. Used with permission from ref (123). Copyright 2016 Elsevier B.V. (f) Transparent SWNT anode and V2O5 nanowire cathode. Used with permission from ref (124). Copyright 2015 American Chemical Society. (g) Thin-film battery with a grid-structured design of LiCoO2/LiPON/Si on glass substrates. Used with permission from ref (126). Copyright 2019 American Chemical Society. (h) Photograph of a transparent and flexible battery electrode, (i) magnified optical image, and (j) scanning electron microscopy image and UV–vis spectrum of the gel electrolyte, a single electrode, and the full battery. Used with permission from ref (125). Copyright 2011 National Academy of Sciences of the United States of America.
Current Collector and Conductive Additive
Current collectors for transparent batteries are expected to meet the following requirements: transparent, lightweight and thin, and electrochemically stable in the electrolyte and over the operating voltage window of the electrodes. In addition, to achieve high rate capability and energy density, their resistance should be as low as possible to provide enough conductivity, when high currents are applied. Transparent conductive films are well established and integral components of electronic devices such as touch screens, displays, and solar cells.127−129 For instance, commercially available indium-/fluorine-doped tin oxides were used as current collectors in transparent batteries.123,130−133 Alternative materials include metallic nanowires (24.5 Ω sq–1 sheet resistance, 71% transmittance),35 carbon nanotubes (57 Ω sq–1, 90%),134 graphene (350 Ω sq–1, 90%),135 and conductive polymers like poly(3,4-ethylenedioxythio-phene):poly(styrenesulfonate) (PEDOT:PSS, 260 Ω sq–1, 95%).136 Examples of opaque batteries include lithium/sulfur batteries fabricated on a graphene current collector, which were cycled between 1 and 3 V,137 and Cu nanowire arrays in combination with Fe3O4 as the LIB electrode working between 0.02 and 2.5 V.138 To further improve the transparency in these examples, the film thickness of the current collectors has to be decreased.
Electrolyte and Separator
In traditional batteries, the liquid electrolyte is completely transparent, while the separators (e.g., glass fiber, polypropylene/polyethylene) are opaque. Thus, transparent solid electrolytes have been considered as the most promising for transparent batteries. For practical use, transparent solid electrolytes, including inorganic and polymer electrolytes, should possess high transparency, good thermal/chemical stability, a wide electrochemical voltage window, low electronic conductivity, and high ionic conductivity (higher than 10–4 S cm–1). As an inorganic and transparent solid electrolyte, Al2O3-doped Li7La3Zr2O12 delivered a bulk conductivity of 9.9 × 10–4 S cm–1 at 25 °C, but the transmittance was only 30%.139 Polymer electrolytes mainly employ monomers with suitable donor atoms like O and N to coordinate cations, and to form polymer salt complexes.140 Polymer electrolytes with low crystallinity for high transparency141 and low viscosity for high ionic conductivity142 are preferred, such as PVDF and its copolymers with hexafluoropropylene (PVDF-HFP), PEO, polyacrylonitrile (PAN), and poly(methyl methacrylate) (PMMA). For instance, a gel electrolyte composed of a PVDF-HFP membrane infiltrated with 1 M LiClO4 in ethylene carbonate/diethyl carbonate (EC/DEC) achieved an ionic conductivity of 2 × 10–3 S cm–1 with a transmittance of about 99%.125
Packaging
There are no systematic studies of the various packaging methods for transparent full cell configurations. For all-solid-state batteries, usually tight adhesion between the electrode and electrolyte layers is achieved, which makes packaging obsolete.126,143 If the electrodes are fabricated on rigid substrates like glass, the batteries can be sealed simply by applying methyl ethyl ketone131 or double-coated adhesive polyester tape132 to the edges of the electrodes. To obtain better protection in air, a transparent battery was encapsulated into a closed thermoplastic PVC bag.125 Future work should focus on exploring transparent polymer materials with high transparency, low water vapor permeability, and thermal sealability, such as PEN, polyethylene terephthalate (PET), polycarbonate (PC), and polyphenylene sulfide (PPS).144
Performance Comparison in Full Cells
A fully transparent battery requires all the integrated components to be transparent. However, in most of the transparent battery reports, only the performance of individual components is reported. Here, the main challenge lies in ensuring long cyclability along with high transparency. Yang et al. fabricated a transparent and flexible battery with a transmittance of 57% using LiMn2O4 and Li4Ti5O12 as the cathode and anode materials. However, at a current density of 100 μA cm–2, it could only cycle for 15 times with a remaining capacity of 80 mA h g–1.125 Pat et al. reported a transparent all-solid-state battery by stacking silver paste/Li4Ti5O12 anode/Li3PO4 electrolyte/LiFePO4 cathode/ITO/glass. A capacity of 600 μA h g–1 was achieved with a high transparency of about 80%, but no data on cyclability were reported.143 Apart from the lack of long-term performance, energy densities of transparent batteries are insufficient for applications in smart windows and displays. To address this issue, an interesting strategy could be to explore high-theoretical-capacity battery systems, like the Li/S battery (1675 mA h g–1), Li–air battery (3840 mA h g–1), Zn–air battery (820 mA h g–1), and Al–air battery (2980 mA h g–1).
Current Challenges
Transparent batteries are realized through layer-by-layer assembly, microfluidics-assisted methods, sol–gel dip coating, and magnetron sputtering. In order to make transparent batteries even more attractive for emerging electronics, they should additionally be equipped with flexibility and electrochromic properties. In spite of all the progress, significant challenges still exist. Specifically, full cell configuration, cycle life, and their integration into devices [transparent smartphones/tablets (e.g., a commercially available smartphone needs an energy density of 246 W h kg–1),145 smart windows, or displays] are still missing. The capacity of the batteries has to be increased by exploring high-capacity systems as active materials for anodes and cathodes. To achieve high mass loadings of active materials in transparent electrodes, new electrode architectures have to be designed, and to maximize the voltage operation window, the electrode–electrolyte combination has to be carefully selected. Overall, to meet the requirements for practical use, energy density and lifespan of the batteries have to be improved without sacrificing the transparency.
5. Conclusions and Outlook
Driven by the rapidly changing market for consumer electronics, the topics of battery research have expanded significantly. In addition to the continuous improvement of electrochemical performance, which of course remains an important branch of research, the search for new electrochemical systems beyond lithium-ion and new battery designs has become the focus of interest. Batteries, which in addition to energy storage also offer new properties such as transparency, flexibility/stretchability, or degradability, open up fascinating possibilities for innovations in wearable, optoelectronic, implantable, or ingestible electronic devices.
Robotics,146 unmanned aerial vehicles (UAVs or drones),147 and prosthetic devices148 are some other popular areas that can greatly benefit from the advancements in multifunctional batteries. Power sources are usually the biggest constraints on the potential capabilities of these devices. Developing batteries with multiple functionalities could offer new degrees of freedom in designing these devices, which is not possible with the traditional bulky and rigid batteries. In fact, these batteries could seamlessly integrate onto the soft, elastic, and curvilinear surface of such electronics, thus becoming a structural element of the device rather than just a power source.
Regardless of whether the battery is flexible, transparent, or degradable, these properties are completely opposite to those of a conventional battery, and therefore, the entire battery design, including all components, their processing, and their arrangement and assembly, has to be rethought from scratch. Although there have been impressive advances in this area, they are mainly at the level of individual components, and examples of full cells that combine these additional features with acceptable electrochemical performance are very rare. Considering that the electrochemical performance of traditional batteries has been optimized over several decades, it is obvious that concerted and dedicated research efforts will be required to produce multifunctional batteries with the same level of energy storage capabilities. For a long time to come, there will probably still be a fine line between balancing the compromise between electrochemical performance and desired additional properties. Nevertheless, the prospect of having a truly multifunctional battery is fascinating and worth any research effort.
Acknowledgments
The authors acknowledge ETH Zurich for financial support (ETH Research Grants ETH-13 16-1 and ETH-50 19-2).
Author Contributions
† L.A.W., N.M., and T.L. contributed equally.
The authors declare no competing financial interest.
References
- Honda W.; Harada S.; Arie T.; Akita S.; Takei K. Wearable, Human-Interactive, Health-Monitoring, Wireless Devices Fabricated by Macroscale Printing Techniques. Adv. Funct. Mater. 2014, 24 (22), 3299–3304. 10.1002/adfm.201303874. [DOI] [Google Scholar]
- Najafabadi A. H.; Tamayol A.; Annabi N.; Ochoa M.; Mostafalu P.; Akbari M.; Nikkhah M.; Rahimi R.; Dokmeci M. R.; Sonkusale S.; Ziaie B.; Khademhosseini A. Biodegradable Nanofibrous Polymeric Substrates for Generating Elastic and Flexible Electronics. Adv. Mater. 2014, 26 (33), 5823–5830. 10.1002/adma.201401537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K.; Honda W.; Harada S.; Arie T.; Akita S. Toward Flexible and Wearable Human-Interactive Health-Monitoring Devices. Adv. Healthcare Mater. 2015, 4 (4), 487–500. 10.1002/adhm.201400546. [DOI] [PubMed] [Google Scholar]
- Wager J. F. Transparent electronics. Science 2003, 300 (5623), 1245–1246. 10.1126/science.1085276. [DOI] [PubMed] [Google Scholar]
- Xu X.; Zhou J.; Jiang L.; Lubineau G.; Ng T.; Ooi B. S.; Liao H.-Y.; Shen C.; Chen L.; Zhu J. Y. Highly transparent, low-haze, hybrid cellulose nanopaper as electrodes for flexible electronics. Nanoscale 2016, 8 (24), 12294–12306. 10.1039/C6NR02245F. [DOI] [PubMed] [Google Scholar]
- Ju S.; Facchetti A.; Xuan Y.; Liu J.; Ishikawa F.; Ye P.; Zhou C.; Marks T. J.; Janes D. B. Fabrication of fully transparent nanowire transistors for transparent and flexible electronics. Nat. Nanotechnol. 2007, 2 (6), 378–384. 10.1038/nnano.2007.151. [DOI] [PubMed] [Google Scholar]
- Irimia-Vladu M. “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 2014, 43 (2), 588–610. 10.1039/C3CS60235D. [DOI] [PubMed] [Google Scholar]
- Hwang S.-W.; Kim D.-H.; Tao H.; Kim T.-i.; Kim S.; Yu K. J.; Panilaitis B.; Jeong J.-W.; Song J.-K.; Omenetto F. G.; Rogers J. A. Materials and Fabrication Processes for Transient and Bioresorbable High-Performance Electronics. Adv. Funct. Mater. 2013, 23 (33), 4087–4093. 10.1002/adfm.201300127. [DOI] [Google Scholar]
- Hwang S.-W.; Park G.; Cheng H.; Song J.-K.; Kang S.-K.; Yin L.; Kim J.-H.; Omenetto F. G.; Huang Y.; Lee K.-M.; Rogers J. A. 25th Anniversary Article: Materials for High-Performance Biodegradable Semiconductor Devices. Adv. Mater. 2014, 26 (13), 1992–2000. 10.1002/adma.201304821. [DOI] [PubMed] [Google Scholar]
- Fu K. K.; Wang Z.; Dai J.; Carter M.; Hu L. Transient Electronics: Materials and Devices. Chem. Mater. 2016, 28 (11), 3527–3539. 10.1021/acs.chemmater.5b04931. [DOI] [Google Scholar]
- Huang X. Y.; Wang D.; Yuan Z. Y.; Xie W. S.; Wu Y. X.; Li R. F.; Zhao Y.; Luo D.; Cen L.; Chen B. B.; Wu H.; Xu H. X.; Sheng X.; Zhang M. L.; Zhao L. Y.; Yin L. A Fully Biodegradable Battery for Self-Powered Transient Implants. Small 2018, 14 (28), 1800994. 10.1002/smll.201800994. [DOI] [PubMed] [Google Scholar]
- Mackanic D. G.; Chang T. H.; Huang Z. J.; Cui Y.; Bao Z. N. Stretchable electrochemical energy storage devices. Chem. Soc. Rev. 2020, 49 (13), 4466–4495. 10.1039/D0CS00035C. [DOI] [PubMed] [Google Scholar]
- Hu L.; Wu H.; La Mantia F.; Yang Y.; Cui Y. Thin, Flexible Secondary Li-Ion Paper Batteries. ACS Nano 2010, 4 (10), 5843–5848. 10.1021/nn1018158. [DOI] [PubMed] [Google Scholar]
- Chen D.; Lou Z.; Jiang K.; Shen G. Device Configurations and Future Prospects of Flexible/Stretchable Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28 (51), 1805596. 10.1002/adfm.201805596. [DOI] [Google Scholar]
- Qian G.; Liao X.; Zhu Y.; Pan F.; Chen X.; Yang Y. Designing Flexible Lithium-Ion Batteries by Structural Engineering. ACS Energy Lett. 2019, 4 (3), 690–701. 10.1021/acsenergylett.8b02496. [DOI] [Google Scholar]
- Ryu J.; Song W.-J.; Lee S.; Choi S.; Park S. A Game Changer: Functional Nano/Micromaterials for Smart Rechargeable Batteries. Adv. Funct. Mater. 2020, 30 (2), 1902499. 10.1002/adfm.201902499. [DOI] [Google Scholar]
- Pomerantseva E.; Bonaccorso F.; Feng X.; Cui Y.; Gogotsi Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366 (646), eaan8285. 10.1126/science.aan8285. [DOI] [PubMed] [Google Scholar]
- Cha H.; Lee Y.; Kim J.; Park M.; Cho J. Flexible 3D Interlocking Lithium-Ion Batteries. Adv. Energy Mater. 2018, 8 (30), 1801917. 10.1002/aenm.201801917. [DOI] [Google Scholar]
- Wang S.; Chen Z.; Yang B.; Chen H.; Ruckenstein E. Mechanical deformation: A feasible route for reconfiguration of inner interfaces to modulate the high performance of three-dimensional porous carbon material anodes in stretchable lithium-ion batteries. J. Colloid Interface Sci. 2019, 555, 431–437. 10.1016/j.jcis.2019.07.101. [DOI] [PubMed] [Google Scholar]
- Goodenough J. B.; Park K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135 (4), 1167–1176. 10.1021/ja3091438. [DOI] [PubMed] [Google Scholar]
- de Biasi L.; Schwarz B.; Brezesinski T.; Hartmann P.; Janek J.; Ehrenberg H. Chemical, Structural, and Electronic Aspects of Formation and Degradation Behavior on Different Length Scales of Ni-Rich NCM and Li-Rich HE-NCM Cathode Materials in Li-Ion Batteries. Adv. Mater. 2019, 31 (26), 1900985. 10.1002/adma.201900985. [DOI] [PubMed] [Google Scholar]
- Oh S. H.; Kwon O. H.; Kang Y. C.; Kim J.-K.; Cho J. S. Highly integrated and interconnected CNT hybrid nanofibers decorated with α-iron oxide as freestanding anodes for flexible lithium polymer batteries. J. Mater. Chem. A 2019, 7 (20), 12480–12488. 10.1039/C9TA01374A. [DOI] [Google Scholar]
- Mo F.; Liang G.; Meng Q.; Liu Z.; Li H.; Fan J.; Zhi C. A flexible rechargeable aqueous zinc manganese-dioxide battery working at – 20 °C. Energy Environ. Sci. 2019, 12 (2), 706–715. 10.1039/C8EE02892C. [DOI] [Google Scholar]
- Li H.; Liu Z.; Liang G.; Huang Y.; Huang Y.; Zhu M.; Pei Z.; Xue Q.; Tang Z.; Wang Y.; Li B.; Zhi C. Waterproof and Tailorable Elastic Rechargeable Yarn Zinc Ion Batteries by a Cross-Linked Polyacrylamide Electrolyte. ACS Nano 2018, 12 (4), 3140–3148. 10.1021/acsnano.7b09003. [DOI] [PubMed] [Google Scholar]
- Zamarayeva A. M.; Jegraj A.; Toor A.; Pister V. I.; Chang C.; Chou A.; Evans J. W.; Arias A. C. Electrode Composite for Flexible Zinc–Manganese Dioxide Batteries through In Situ Polymerization of Polymer Hydrogel. Energy Technol. 2020, 8, 1901165. 10.1002/ente.201901165. [DOI] [Google Scholar]
- Kim J.-H.; Lee Y.-H.; Cho S.-J.; Gwon J.-G.; Cho H.-J.; Jang M.; Lee S.-Y.; Lee S.-Y. Nanomat Li–S batteries based on all-fibrous cathode/separator assemblies and reinforced Li metal anodes: towards ultrahigh energy density and flexibility. Energy Environ. Sci. 2019, 12 (1), 177–186. 10.1039/C8EE01879K. [DOI] [Google Scholar]
- Chen M.; Xu W.; Jamil S.; Jiang S.; Huang C.; Wang X.; Wang Y.; Shu H.; Xiang K.; Zeng P. Multifunctional Heterostructures for Polysulfide Suppression in High-Performance Lithium-Sulfur Cathode. Small 2018, 14 (49), 1803134. 10.1002/smll.201803134. [DOI] [PubMed] [Google Scholar]
- Chang J.; Shang J.; Sun Y.; Ono L. K.; Wang D.; Ma Z.; Huang Q.; Chen D.; Liu G.; Cui Y.; Qi Y.; Zheng Z. Flexible and stable high-energy lithium-sulfur full batteries with only 100% oversized lithium. Nat. Commun. 2018, 9 (1), 4480. 10.1038/s41467-018-06879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; Lu R.; Yuan H.; Amin K.; Mao L.; Yan W.; Wei Z. Nanowire Array-Coated Flexible Substrate to Accommodate Lithium Plating for Stable Lithium-Metal Anodes and Flexible Lithium–Organic Batteries. ACS Appl. Mater. Interfaces 2019, 11 (23), 20873–20880. 10.1021/acsami.9b05056. [DOI] [PubMed] [Google Scholar]
- Lin H.; Weng W.; Ren J.; Qiu L.; Zhang Z.; Chen P.; Chen X.; Deng J.; Wang Y.; Peng H. Twisted Aligned Carbon Nanotube/Silicon Composite Fiber Anode for Flexible Wire-Shaped Lithium-Ion Battery. Adv. Mater. 2014, 26 (8), 1217–1222. 10.1002/adma.201304319. [DOI] [PubMed] [Google Scholar]
- Cai X.; Liu W.; Zhao Z.; Li S.; Yang S.; Zhang S.; Gao Q.; Yu X.; Wang H.; Fang Y. Simultaneous Encapsulation of Nano-Si in Redox Assembled rGO Film as Binder-Free Anode for Flexible/Bendable Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11 (4), 3897–3908. 10.1021/acsami.8b18134. [DOI] [PubMed] [Google Scholar]
- Myung S. T.; Hitoshi Y.; Sun Y. K. Electrochemical behavior and passivation of current collectors in lithium-ion batteries. J. Mater. Chem. 2011, 21 (27), 9891–9911. 10.1039/c0jm04353b. [DOI] [Google Scholar]
- Son B.; Ryou M.-H.; Choi J.; Lee T.; Yu H. K.; Kim J. H.; Lee Y. M. Measurement and Analysis of Adhesion Property of Lithium-Ion Battery Electrodes with SAICAS. ACS Appl. Mater. Interfaces 2014, 6 (1), 526–531. 10.1021/am404580f. [DOI] [PubMed] [Google Scholar]
- Rahani E. K.; Shenoy V. B. Role of Plastic Deformation of Binder on Stress Evolution during Charging and Discharging in Lithium-Ion Battery Negative Electrodes. J. Electrochem. Soc. 2013, 160 (8), A1153–A1162. 10.1149/2.046308jes. [DOI] [Google Scholar]
- Deshmukh R.; Calvo M.; Schreck M.; Tervoort E.; Sologubenko A. S.; Niederberger M. Synthesis, Spray Deposition, and Hot-Press Transfer of Copper Nanowires for Flexible Transparent Electrodes. ACS Appl. Mater. Interfaces 2018, 10 (24), 20748–20754. 10.1021/acsami.8b04007. [DOI] [PubMed] [Google Scholar]
- Liu R.; Liu Y.; Chen J.; Kang Q.; Wang L.; Zhou W.; Huang Z.; Lin X.; Li Y.; Li P.; Feng X.; Wu G.; Ma Y.; Huang W. Flexible wire-shaped lithium-sulfur batteries with fibrous cathodes assembled via capillary action. Nano Energy 2017, 33, 325–333. 10.1016/j.nanoen.2016.12.049. [DOI] [Google Scholar]
- Zhu Y.; Yang M.; Huang Q.; Wang D.; Yu R.; Wang J.; Zheng Z.; Wang D. V2O5 Textile Cathodes with High Capacity and Stability for Flexible Lithium-Ion Batteries. Adv. Mater. 2020, 32 (7), 1906205. 10.1002/adma.201906205. [DOI] [PubMed] [Google Scholar]
- Dalton A. B.; Collins S.; Muñoz E.; Razal J. M.; Ebron V. H.; Ferraris J. P.; Coleman J. N.; Kim B. G.; Baughman R. H. Super-tough carbon-nanotube fibres. Nature 2003, 423 (6941), 703. 10.1038/423703a. [DOI] [PubMed] [Google Scholar]
- Dörfler S.; Strubel P.; Jaumann T.; Troschke E.; Hippauf F.; Kensy C.; Schökel A.; Althues H.; Giebeler L.; Oswald S.; Kaskel S. On the mechanistic role of nitrogen-doped carbon cathodes in lithium-sulfur batteries with low electrolyte weight portion. Nano Energy 2018, 54, 116–128. 10.1016/j.nanoen.2018.09.065. [DOI] [Google Scholar]
- Pan H.; Chen J.; Cao R.; Murugesan V.; Rajput N. N.; Han K. S.; Persson K.; Estevez L.; Engelhard M. H.; Zhang J.-G.; Mueller K. T.; Cui Y.; Shao Y.; Liu J. Non-encapsulation approach for high-performance Li–S batteries through controlled nucleation and growth. Nature Energy 2017, 2 (10), 813–820. 10.1038/s41560-017-0005-z. [DOI] [Google Scholar]
- Jiang M.; Wang R.; Wang K.; Gao S.; Han J.; Yan J.; Cheng S.; Jiang K. Hierarchical porous Fe/N doped carbon nanofibers as host materials for high sulfur loading Li–S batteries. Nanoscale 2019, 11 (32), 15156–15165. 10.1039/C9NR04408F. [DOI] [PubMed] [Google Scholar]
- Li S.; Zhao Y.; Liu Z.; Yang L.; Zhang J.; Wang M.; Che R. Flexible Graphene-Wrapped Carbon Nanotube/Graphene@MnO2 3D Multilevel Porous Film for High-Performance Lithium-Ion Batteries. Small 2018, 14 (32), 1801007. 10.1002/smll.201801007. [DOI] [PubMed] [Google Scholar]
- Wu H.; Yu G.; Pan L.; Liu N.; McDowell M. T.; Bao Z.; Cui Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1943. 10.1038/ncomms2941. [DOI] [PubMed] [Google Scholar]
- Zou P.; Wang Y.; Chiang S.-W.; Wang X.; Kang F.; Yang C. Directing lateral growth of lithium dendrites in micro-compartmented anode arrays for safe lithium metal batteries. Nat. Commun. 2018, 9 (1), 464. 10.1038/s41467-018-02888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P.; Zhang Z. Battery Separators. Chem. Rev. 2004, 104 (10), 4419–4462. 10.1021/cr020738u. [DOI] [PubMed] [Google Scholar]
- Ghazi Z. A.; He X.; Khattak A. M.; Khan N. A.; Liang B.; Iqbal A.; Wang J.; Sin H.; Li L.; Tang Z. MoS2/Celgard Separator as Efficient Polysulfide Barrier for Long-Life Lithium–Sulfur Batteries. Adv. Mater. 2017, 29 (21), 1606817. 10.1002/adma.201606817. [DOI] [PubMed] [Google Scholar]
- Knauth P. Inorganic solid Li ion conductors: An overview. Solid State Ionics 2009, 180 (14), 911–916. 10.1016/j.ssi.2009.03.022. [DOI] [Google Scholar]
- Duan H.; Fan M.; Chen W.-P.; Li J.-Y.; Wang P.-F.; Wang W.-P.; Shi J.-L.; Yin Y.-X.; Wan L.-J.; Guo Y.-G. Extended Electrochemical Window of Solid Electrolytes via Heterogeneous Multilayered Structure for High-Voltage Lithium Metal Batteries. Adv. Mater. 2019, 31 (12), 1807789. 10.1002/adma.201807789. [DOI] [PubMed] [Google Scholar]
- He X.; Yan B.; Zhang X.; Liu Z.; Bresser D.; Wang J.; Wang R.; Cao X.; Su Y.; Jia H.; Grey C. P.; Frielinghaus H.; Truhlar D. G.; Winter M.; Li J.; Paillard E. Fluorine-free water-in-ionomer electrolytes for sustainable lithium-ion batteries. Nat. Commun. 2018, 9 (1), 5320. 10.1038/s41467-018-07331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik J. H.; Kim S.; Hong D. G.; Lee J. C. Gel Polymer Electrolytes Based on Polymerizable Lithium Salt and Poly(ethylene glycol) for Lithium Battery Applications. ACS Appl. Mater. Interfaces 2019, 11 (33), 29718–29724. 10.1021/acsami.9b05139. [DOI] [PubMed] [Google Scholar]
- Song J. Y.; Wang Y. Y.; Wan C. C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77 (2), 183–197. 10.1016/S0378-7753(98)00193-1. [DOI] [Google Scholar]
- Mackanic D. G.; Yan X.; Zhang Q.; Matsuhisa N.; Yu Z.; Jiang Y.; Manika T.; Lopez J.; Yan H.; Liu K.; Chen X.; Cui Y.; Bao Z. Decoupling of mechanical properties and ionic conductivity in supramolecular lithium ion conductors. Nat. Commun. 2019, 10 (1), 5384. 10.1038/s41467-019-13362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Rao Z.; Cheng Z.; Yuan L.; Li Z.; Huang Y. Ultrathin, Flexible Polymer Electrolyte for Cost-Effective Fabrication of All-Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2019, 9 (46), 1902767. 10.1002/aenm.201902767. [DOI] [Google Scholar]
- Wan J.; Xie J.; Kong X.; Liu Z.; Liu K.; Shi F.; Pei A.; Chen H.; Chen W.; Chen J.; Zhang X.; Zong L.; Wang J.; Chen L.-Q.; Qin J.; Cui Y. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 2019, 14 (7), 705–711. 10.1038/s41565-019-0465-3. [DOI] [PubMed] [Google Scholar]
- Zeng Y.; Zhang X.; Qin R.; Liu X.; Fang P.; Zheng D.; Tong Y.; Lu X. Dendrite-Free Zinc Deposition Induced by Multifunctional CNT Frameworks for Stable Flexible Zn-Ion Batteries. Adv. Mater. 2019, 31 (36), 1903675. 10.1002/adma.201903675. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Mo F.; Ma L.; Yang Q.; Liang G.; Liu Z.; Li H.; Li N.; Zhang H.; Zhi C. Highly Compressible Cross-Linked Polyacrylamide Hydrogel-Enabled Compressible Zn–MnO2 Battery and a Flexible Battery–Sensor System. ACS Appl. Mater. Interfaces 2018, 10 (51), 44527–44534. 10.1021/acsami.8b17607. [DOI] [PubMed] [Google Scholar]
- Wang D.; Li H.; Liu Z.; Tang Z.; Liang G.; Mo F.; Yang Q.; Ma L.; Zhi C. A Nanofibrillated Cellulose/Polyacrylamide Electrolyte-Based Flexible and Sewable High-Performance Zn–MnO2 Battery with Superior Shear Resistance. Small 2018, 14 (51), 1803978. 10.1002/smll.201803978. [DOI] [PubMed] [Google Scholar]
- Li M.; Meng J.; Li Q.; Huang M.; Liu X.; Owusu K. A.; Liu Z.; Mai L. Finely Crafted 3D Electrodes for Dendrite-Free and High-Performance Flexible Fiber-Shaped Zn–Co Batteries. Adv. Funct. Mater. 2018, 28 (32), 1802016. 10.1002/adfm.201802016. [DOI] [Google Scholar]
- Yao Y.; Wang H.; Yang H.; Zeng S.; Xu R.; Liu F.; Shi P.; Feng Y.; Wang K.; Yang W.; Wu X.; Luo W.; Yu Y. A Dual-Functional Conductive Framework Embedded with TiN-VN Heterostructures for Highly Efficient Polysulfide and Lithium Regulation toward Stable Li–S Full Batteries. Adv. Mater. 2020, 32 (6), 1905658. 10.1002/adma.201905658. [DOI] [PubMed] [Google Scholar]
- Li F.; Kaiser M. R.; Ma J.; Guo Z.; Liu H.; Wang J. Free-standing sulfur-polypyrrole cathode in conjunction with polypyrrole-coated separator for flexible Li-S batteries. Energy Storage Mater. 2018, 13, 312–322. 10.1016/j.ensm.2018.02.007. [DOI] [Google Scholar]
- Dong Q.; Shen R.; Li C.; Gan R.; Ma X.; Wang J.; Li J.; Wei Z. Construction of Soft Base Tongs on Separator to Grasp Polysulfides from Shuttling in Lithium–Sulfur Batteries. Small 2018, 14 (52), 1804277. 10.1002/smll.201804277. [DOI] [PubMed] [Google Scholar]
- Chen T.; Xue Y. H.; Roy A. K.; Dai L. M. Transparent and Stretchable High-Performance Supercapacitors Based on Wrinkled Graphene Electrodes. ACS Nano 2014, 8 (1), 1039–1046. 10.1021/nn405939w. [DOI] [PubMed] [Google Scholar]
- Salot R.; Martin S.; Oukassi S.; Bedjaoui M.; Ubrig J. Microbattery technology overview and associated multilayer encapsulation process. Appl. Surf. Sci. 2009, 256 (3), S54–S57. 10.1016/j.apsusc.2009.09.086. [DOI] [Google Scholar]
- Hu Y. H.; Sun X. L. Flexible rechargeable lithium ion batteries: advances and challenges in materials and process technologies. J. Mater. Chem. A 2014, 2 (28), 10712–10738. 10.1039/C4TA00716F. [DOI] [Google Scholar]
- Jansen A. N.; Amine K.; Newman A. E.; Vissers D. R.; Henriksen G. L. Low-cost, flexible battery packaging materials. JOM 2002, 54 (3), 29–54. 10.1007/BF02822616. [DOI] [Google Scholar]
- Kang H.-S.; Park E.; Hwang J.-Y.; Kim H.; Aurbach D.; Rosenman A.; Sun Y.-K. A Scaled-Up Lithium (Ion)-Sulfur Battery: Newly Faced Problems and Solutions. Adv. Mater. Technol. 2016, 1 (6), 1600052. 10.1002/admt.201600052. [DOI] [Google Scholar]
- Svens P.; Kjell M. H.; Tengstedt C.; Flodberg G.; Lindbergh G. Li-Ion Pouch Cells for Vehicle Applications-Studies of Water Transmission and Packing Materials. Energies 2013, 6 (1), 400–410. 10.3390/en6010400. [DOI] [Google Scholar]
- Chen X.; Huang H.; Pan L.; Liu T.; Niederberger M. Fully Integrated Design of a Stretchable Solid-State Lithium-Ion Full Battery. Adv. Mater. 2019, 31 (43), 1904648. 10.1002/adma.201904648. [DOI] [PubMed] [Google Scholar]
- Park S.-H.; Tian R.; Coelho J.; Nicolosi V.; Coleman J. N. Quantifying the Trade-Off between Absolute Capacity and Rate Performance in Battery Electrodes. Adv. Energy Mater. 2019, 9 (33), 1901359. 10.1002/aenm.201901359. [DOI] [Google Scholar]
- Tian R.; Park S.-H.; King P. J.; Cunningham G.; Coelho J.; Nicolosi V.; Coleman J. N. Quantifying the factors limiting rate performance in battery electrodes. Nat. Commun. 2019, 10 (1), 1933. 10.1038/s41467-019-09792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z.; Zhang Y.; Song N.; Li X. Towards flexible lithium-sulfur battery from natural cotton textile. Electrochim. Acta 2017, 246, 507–516. 10.1016/j.electacta.2017.06.069. [DOI] [Google Scholar]
- Talaie E.; Bonnick P.; Sun X.; Pang Q.; Liang X.; Nazar L. F. Methods and Protocols for Electrochemical Energy Storage Materials Research. Chem. Mater. 2017, 29 (1), 90–105. 10.1021/acs.chemmater.6b02726. [DOI] [Google Scholar]
- Li H. Practical Evaluation of Li-Ion Batteries. Joule 2019, 3 (4), 911–914. 10.1016/j.joule.2019.03.028. [DOI] [Google Scholar]
- Zeng L.; Chen S.; Liu M.; Cheng H.-M.; Qiu L. Integrated Paper-Based Flexible Li-Ion Batteries Made by a Rod Coating Method. ACS Appl. Mater. Interfaces 2019, 11 (50), 46776–46782. 10.1021/acsami.9b15866. [DOI] [PubMed] [Google Scholar]
- Liu S.; Wang Z.; Yu C.; Wu H. B.; Wang G.; Dong Q.; Qiu J.; Eychmüller A.; David Lou X. W. A Flexible TiO2(B)-Based Battery Electrode with Superior Power Rate and Ultralong Cycle Life. Adv. Mater. 2013, 25 (25), 3462–3467. 10.1002/adma.201300953. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Yang Q.; Wang D.; Liang G.; Zhu Y.; Mo F.; Huang Z.; Li X.; Ma L.; Tang T.; Lu Z.; Zhi C. A Flexible Solid-State Aqueous Zinc Hybrid Battery with Flat and High-Voltage Discharge Plateau. Adv. Energy Mater. 2019, 9 (46), 1902473. 10.1002/aenm.201902473. [DOI] [Google Scholar]
- Wang J.; Yang G.; Chen J.; Liu Y.; Wang Y.; Lao C.-Y.; Xi K.; Yang D.; Harris C. J.; Yan W.; Ding S.; Kumar R. V. Flexible and High-Loading Lithium–Sulfur Batteries Enabled by Integrated Three-In-One Fibrous Membranes. Adv. Energy Mater. 2019, 9 (38), 1902001. 10.1002/aenm.201902001. [DOI] [Google Scholar]
- Liu Y.; Yao M.; Zhang L.; Niu Z. Large-scale fabrication of reduced graphene oxide-sulfur composite films for flexible lithium-sulfur batteries. J. Energy Chem. 2019, 38, 199–206. 10.1016/j.jechem.2019.03.034. [DOI] [Google Scholar]
- Sun Q.; Fang X.; Weng W.; Deng J.; Chen P.; Ren J.; Guan G.; Wang M.; Peng H. An Aligned and Laminated Nanostructured Carbon Hybrid Cathode for High-Performance Lithium–Sulfur Batteries. Angew. Chem., Int. Ed. 2015, 54 (36), 10539–10544. 10.1002/anie.201504514. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Amin K.; Cheng M.; Yuan H.; Mao L.; Yan W.; Wei Z. A carbon foam-supported high sulfur loading composite as a self-supported cathode for flexible lithium–sulfur batteries. Nanoscale 2018, 10 (46), 21790–21797. 10.1039/C8NR07964A. [DOI] [PubMed] [Google Scholar]
- Li P.; Jin Z.; Xiao D. Three-dimensional nanotube-array anode enables a flexible Ni/Zn fibrous battery to ultrafast charge and discharge in seconds. Energy Storage Mater. 2018, 12, 232–240. 10.1016/j.ensm.2017.11.017. [DOI] [Google Scholar]
- Wang K.; Zhang X.; Han J.; Zhang X.; Sun X.; Li C.; Liu W.; Li Q.; Ma Y. High-Performance Cable-Type Flexible Rechargeable Zn Battery Based on MnO2@CNT Fiber Microelectrode. ACS Appl. Mater. Interfaces 2018, 10 (29), 24573–24582. 10.1021/acsami.8b07756. [DOI] [PubMed] [Google Scholar]
- He B.; Zhou Z.; Man P.; Zhang Q.; Li C.; Xie L.; Wang X.; Li Q.; Yao Y. V2O5 nanosheets supported on 3D N-doped carbon nanowall arrays as an advanced cathode for high energy and high power fiber-shaped zinc-ion batteries. J. Mater. Chem. A 2019, 7 (21), 12979–12986. 10.1039/C9TA01164A. [DOI] [Google Scholar]
- Ma L.; Chen S.; Long C.; Li X.; Zhao Y.; Liu Z.; Huang Z.; Dong B.; Zapien J. A.; Zhi C. Achieving High-Voltage and High-Capacity Aqueous Rechargeable Zinc Ion Battery by Incorporating Two-Species Redox Reaction. Adv. Energy Mater. 2019, 9 (45), 1902446. 10.1002/aenm.201902446. [DOI] [Google Scholar]
- Zhang Q.; Li C.; Li Q.; Pan Z.; Sun J.; Zhou Z.; He B.; Man P.; Xie L.; Kang L.; Wang X.; Yang J.; Zhang T.; Shum P. P.; Li Q.; Yao Y.; Wei L. Flexible and High-Voltage Coaxial-Fiber Aqueous Rechargeable Zinc-Ion Battery. Nano Lett. 2019, 19 (6), 4035–4042. 10.1021/acs.nanolett.9b01403. [DOI] [PubMed] [Google Scholar]
- Song C.; Li Y.; Li H.; He T.; Guan Q.; Yang J.; Li X.; Cheng J.; Wang B. A novel flexible fibershaped dual-ion battery with high energy density based on omnidirectional porous Al wire anode. Nano Energy 2019, 60, 285–293. 10.1016/j.nanoen.2019.03.062. [DOI] [Google Scholar]
- Chen B. X.Foldable Phones Are Here. Do We Really Want Them? New York Times, February 11, 2020. https://www.nytimes.com/2020/02/11/technology/personaltech/foldable-phones-samsung.html (accessed 2020-08-18). [Google Scholar]
- Di J.; Zhang X.; Yong Z.; Zhang Y.; Li D.; Li R.; Li Q. Carbon-Nanotube Fibers for Wearable Devices and Smart Textiles. Adv. Mater. 2016, 28 (47), 10529–10538. 10.1002/adma.201601186. [DOI] [PubMed] [Google Scholar]
- Zhai S.; Karahan H. E.; Wei L.; Qian Q.; Harris A. T.; Minett A. I.; Ramakrishna S.; Ng A. K.; Chen Y. Textile energy storage: Structural design concepts, material selection and future perspectives. Energy Storage Mater. 2016, 3, 123–139. 10.1016/j.ensm.2016.02.003. [DOI] [Google Scholar]
- He B.; Zhang Q.; Li L.; Sun J.; Man P.; Zhou Z.; Li Q.; Guo J.; Xie L.; Li C.; Wang X.; Zhao J.; Zhang T.; Yao Y. High-performance flexible all-solid-state aqueous rechargeable Zn–MnO2 microbatteries integrated with wearable pressure sensors. J. Mater. Chem. A 2018, 6 (30), 14594–14601. 10.1039/C8TA05862H. [DOI] [Google Scholar]
- Trindade Soares P. H.; Nossol E. Self-Recharging Reduced Graphene Oxide-Prussian Blue Electrodes for Transparent Batteries. ACS Appl. Nano Mater. 2019, 2 (4), 2241–2249. 10.1021/acsanm.8b02122. [DOI] [Google Scholar]
- Song W.; Wang C.; Gan B.; Liu M.; Zhu J.; Nan X.; Chen N.; Sun C.; Chen J. High performance lithium-sulfur batteries for storing pulsed energy generated by triboelectric nanogenerators. Sci. Rep. 2017, 7 (1), 425. 10.1038/s41598-017-00545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X.; Tang W.; Song Y.; Chen H.; Zhang H.; Wang Z. L. Power management and effective energy storage of pulsed output from triboelectric nanogenerator. Nano Energy 2019, 61, 517–532. 10.1016/j.nanoen.2019.04.096. [DOI] [Google Scholar]
- Paolella A.; Faure C.; Bertoni G.; Marras S.; Guerfi A.; Darwiche A.; Hovington P.; Commarieu B.; Wang Z.; Prato M.; Colombo M.; Monaco S.; Zhu W.; Feng Z.; Vijh A.; George C.; Demopoulos G. P.; Armand M.; Zaghib K. Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries. Nat. Commun. 2017, 8, 14643. 10.1038/ncomms14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.; Oh Y.; Yoon I. S.; Kim S. H.; Ju B.-K.; Hong J.-M. Flash-induced nanowelding of silver nanowire networks for transparent stretchable electrochromic devices. Sci. Rep. 2018, 8 (1), 2763. 10.1038/s41598-018-20368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Wang Z.; Li H.; Xiong Y.; Liu Z.; Tang Z.; Huang Y.; Rogach A. L.; Zhi C. Light-permeable, photoluminescent microbatteries embedded in the color filter of a screen. Energy Environ. Sci. 2018, 11 (9), 2414–2422. 10.1039/C8EE00590G. [DOI] [Google Scholar]
- Tong Z.; Liu S.; Li X.; Mai L.; Zhao J.; Li Y. Achieving rapid Li-ion insertion kinetics in TiO2 mesoporous nanotube arrays for bifunctional high-rate energy storage smart windows. Nanoscale 2018, 10 (7), 3254–3261. 10.1039/C7NR07703C. [DOI] [PubMed] [Google Scholar]
- Sun H.; Zhang Y.; Zhang J.; Sun X.; Peng H. Energy harvesting and storage in 1D devices. Nat. Rev. Mater. 2017, 2 (6), 17023. 10.1038/natrevmats.2017.23. [DOI] [Google Scholar]
- Delaporte N.; Lajoie G.; Collin-Martin S.; Zaghib K. Toward Low-Cost All-Organic and Biodegradable Li-Ion Batteries. Sci. Rep. 2020, 10 (1), 3812. 10.1038/s41598-020-60633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. W.; Tao H.; Kim D. H.; Cheng H. Y.; Song J. K.; Rill E.; Brenckle M. A.; Panilaitis B.; Won S. M.; Kim Y. S.; Song Y. M.; Yu K. J.; Ameen A.; Li R.; Su Y. W.; Yang M. M.; Kaplan D. L.; Zakin M. R.; Slepian M. J.; Huang Y. G.; Omenetto F. G.; Rogers J. A. A Physically Transient Form of Silicon Electronics. Science 2012, 337 (6102), 1640–1644. 10.1126/science.1226325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L.; Huang X.; Xu H.; Zhang Y.; Lam J.; Cheng J.; Rogers J. A. Materials, Designs, and Operational Characteristics for Fully Biodegradable Primary Batteries. Adv. Mater. 2014, 26 (23), 3879–3884. 10.1002/adma.201306304. [DOI] [PubMed] [Google Scholar]
- Tsang M.; Armutlulu A.; Martinez A. W.; Bidstrup Allen S. A.; Allen M. G. Biodegradable magnesium/iron batteries with polycaprolactone encapsulation: A microfabricated power source for transient implantable devices. Microsystems Nanoeng. 2015, 1, 15024. 10.1038/micronano.2015.24. [DOI] [Google Scholar]
- Jia X. T.; Wang C. Y.; Ranganathan V.; Napier B.; Yu C. C.; Chao Y. F.; Forsyth M.; Omenetto F. G.; MacFarlane D. R.; Wallace G. G. A Biodegradable Thin-Film Magnesium Primary Battery Using Silk Fibroin-Ionic Liquid Polymer Electrolyte. ACS Energy Lett. 2017, 2 (4), 831–836. 10.1021/acsenergylett.7b00012. [DOI] [Google Scholar]
- Jia X.; Wang C.; Zhao C.; Ge Y.; Wallace G. G. Toward Biodegradable Mg-Air Bioelectric Batteries Composed of Silk Fibroin-Polypyrrole Film. Adv. Funct. Mater. 2016, 26 (9), 1454–1462. 10.1002/adfm.201503498. [DOI] [Google Scholar]
- Edupuganti V.; Solanki R. Fabrication, characterization, and modeling of a biodegradable battery for transient electronics. J. Power Sources 2016, 336, 447–454. 10.1016/j.jpowsour.2016.11.004. [DOI] [Google Scholar]
- Kim Y. J.; Wu W.; Chun S.-E.; Whitacre J. F.; Bettinger C. J. Biologically derived melanin electrodes in aqueous sodium-ion energy storage devices. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (52), 20912–20917. 10.1073/pnas.1314345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. J.; Chun S.-E.; Whitacre J.; Bettinger C. J. Self-deployable current sources fabricated from edible materials. J. Mater. Chem. B 2013, 1 (31), 3781–3788. 10.1039/c3tb20183j. [DOI] [PubMed] [Google Scholar]
- Khan M. M.; Rahman Z. U.; Deen K. M.; Shabib I.; Haider W. Sputtered Mg100-xZnx (0 ≤ × ≤ 100) systems as anode materials for a biodegradable battery aimed for transient bioelectronics. Electrochim. Acta 2020, 329, 135129. 10.1016/j.electacta.2019.135129. [DOI] [Google Scholar]
- Jia X. T.; Yang Y.; Wang C. Y.; Zhao C.; Vijayaraghavan R.; MacFarlane D. R.; Forsyth M.; Wallace G. G. Biocompatible Ionic Liquid-Biopolymer Electrolyte-Enabled Thin and Compact Magnesium-Air Batteries. ACS Appl. Mater. Interfaces 2014, 6 (23), 21110–21117. 10.1021/am505985z. [DOI] [PubMed] [Google Scholar]
- Xia J. T.; Yuan Z. H.; Cai F. S. Toward a Biocompatible and Degradable Battery Using a Mg-Zn-Zr Alloy with β-Tricalcium Phosphate Nanocoating as Anode. J. Mater. Eng. Perform. 2018, 27 (8), 4005–4009. 10.1007/s11665-018-3512-6. [DOI] [Google Scholar]
- Tsang M.; Armutlulu A.; Martinez A.; Herrault F.; Allen S. A. B.; Allen M. G. A MEMS-enabled biodegradable battery for powering transient implantable devices. 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS) 2014, 358–361. 10.1109/MEMSYS.2014.6765650. [DOI] [Google Scholar]
- Tsang M.; Armutlulu A.; Herrault F.; Shafer R. H.; Allen S. A. B.; Allen M. G. Development of Electroplated Magnesium Microstructures for Biodegradable Devices and Energy Sources. J. Microelectromech. Syst. 2014, 23 (6), 1281–1289. 10.1109/JMEMS.2014.2360201. [DOI] [Google Scholar]
- Chen Y. F.; Jamshidi R.; White K.; Cinar S.; Gallegos E.; Hashemi N.; Montazami R. Physical-Chemical Hybrid Transiency: A Fully Transient Li-Ion Battery Based on Insoluble Active Materials. J. Polym. Sci., Part B: Polym. Phys. 2016, 54 (20), 2021–2027. 10.1002/polb.24113. [DOI] [Google Scholar]
- Fu K.; Liu Z.; Yao Y.; Wang Z.; Zhao B.; Luo W.; Dai J.; Lacey S. D.; Zhou L.; Shen F.; Kim M.; Swafford L.; Sengupta L.; Hu L. Transient Rechargeable Batteries Triggered by Cascade Reactions. Nano Lett. 2015, 15 (7), 4664–4671. 10.1021/acs.nanolett.5b01451. [DOI] [PubMed] [Google Scholar]
- Zheng Y. F.; Gu X. N.; Witte F. Biodegradable metals. Mater. Sci. Eng., R 2014, 77, 1–34. 10.1016/j.mser.2014.01.001. [DOI] [Google Scholar]
- Yin L.; Cheng H.; Mao S.; Haasch R.; Liu Y.; Xie X.; Hwang S.-W.; Jain H.; Kang S.-K.; Su Y.; Li R.; Huang Y.; Rogers J. A. Dissolvable Metals for Transient Electronics. Adv. Funct. Mater. 2014, 24 (5), 645–658. 10.1002/adfm.201301847. [DOI] [Google Scholar]
- Li R. F.; Wang L.; Yin L. Materials and Devices for Biodegradable and Soft Biomedical Electronics. Materials 2018, 11, 2108. 10.3390/ma11112108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.-K.; Hwang S.-W.; Yu S.; Seo J.-H.; Corbin E. A.; Shin J.; Wie D. S.; Bashir R.; Ma Z.; Rogers J. A. Biodegradable Thin Metal Foils and Spin-On Glass Materials for Transient Electronics. Adv. Funct. Mater. 2015, 25 (12), 1789–1797. 10.1002/adfm.201403469. [DOI] [Google Scholar]
- Feig V. R.; Tran H.; Bao Z. N. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4 (3), 337–348. 10.1021/acscentsci.7b00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. K.; Wang Z.; Yan C.; Liu Z.; Yao Y.; Dai J.; Hitz E.; Wang Y.; Luo W.; Chen Y.; Kim M.; Hu L. All-component transient lithium-ion batteries. Adv. Energy Mater. 2016, 6 (10), 1502496. 10.1002/aenm.201502496. [DOI] [Google Scholar]
- Liu Z.; Fu K.; Wang Z. Y.; Zhu Y. J.; Wan J. Y.; Yao Y. G.; Dai J. Q.; Kim M.; Swafford L.; Wang C. S.; Hu L. B. Cut-and-stack nanofiber paper toward fast transient energy storage. Inorg. Chem. Front. 2016, 3 (5), 681–688. 10.1039/C5QI00288E. [DOI] [Google Scholar]
- Wang Z. Y.; Fu K.; Liu Z.; Yao Y. G.; Dai J. Q.; Wang Y. B.; Liu B. Y.; Hu L. B. Design of High Capacity Dissoluble Electrodes for All Transient Batteries. Adv. Funct. Mater. 2017, 27 (11), 1605724. 10.1002/adfm.201605724. [DOI] [Google Scholar]
- Stauss S.; Honma I. Biocompatible Batteries-Materials and Chemistry, Fabrication, Applications, and Future Prospects. Bull. Chem. Soc. Jpn. 2018, 91 (3), 492–505. 10.1246/bcsj.20170325. [DOI] [Google Scholar]
- She D. D.; Tsang M.; Allen M. Biodegradable batteries with immobilized electrolyte for transient MEMS. Biomed. Microdevices 2019, 21 (1), 17. 10.1007/s10544-019-0377-x. [DOI] [PubMed] [Google Scholar]
- Acar H.; Cinar S.; Thunga M.; Kessler M. R.; Hashemi N.; Montazami R. Study of Physically Transient Insulating Materials as a Potential Platform for Transient Electronics and Bioelectronics. Adv. Funct. Mater. 2014, 24 (26), 4135–4143. 10.1002/adfm.201304186. [DOI] [Google Scholar]
- Li R. F.; Wang L.; Kong D. Y.; Yin L. Recent progress on biodegradable materials and transient electronics. Bioact. Mater. 2018, 3 (3), 322–333. 10.1016/j.bioactmat.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.-K.; Hwang S.-W.; Cheng H.; Yu S.; Kim B. H.; Kim J.-H.; Huang Y.; Rogers J. A. Dissolution Behaviors and Applications of Silicon Oxides and Nitrides in Transient Electronics. Adv. Funct. Mater. 2014, 24 (28), 4427–4434. 10.1002/adfm.201304293. [DOI] [Google Scholar]
- Douglas A.; Muralidharan N.; Carter R.; Share K.; Pint C. L. Ultrafast triggered transient energy storage by atomic layer deposition into porous silicon for integrated transient electronics. Nanoscale 2016, 8 (14), 7384–7390. 10.1039/C5NR09095D. [DOI] [PubMed] [Google Scholar]
- Kutbee A. T.; Bahabry R. R.; Alamoudi K. O.; Ghoneim M. T.; Cordero M. D.; Almuslem A. S.; Gumus A.; Diallo E. M.; Nassar J. M.; Hussain A. M.; Khashab N. M.; Hussain M. M. Flexible and biocompatible high-performance solid-state micro-battery for implantable orthodontic system. npj Flex. Electron. 2017, 1, 7. 10.1038/s41528-017-0008-7. [DOI] [Google Scholar]
- Li J.; Jiang Q.; Yuan N.; Tang J. A review on flexible and transparent energy storage system. Materials 2018, 11 (11), 2280. 10.3390/ma11112280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Yan R.; Huang H.; Pan L.; Cao X.; deMello A.; Niederberger M. A Micromolding Method for Transparent and Flexible Thin-Film Supercapacitors and Hybrid Supercapacitors. Adv. Funct. Mater. 2020, 30 (46), 2004410. 10.1002/adfm.202004410. [DOI] [Google Scholar]
- Granqvist C. G.; Hultaker A. Transparent and conducting ITO films: new developments and applications. Thin Solid Films 2002, 411 (1), 1–5. 10.1016/S0040-6090(02)00163-3. [DOI] [Google Scholar]
- Choi C. K.; Kihm K. D.; English A. E. Optoelectric biosensor using indium-tin-oxide electrodes. Opt. Lett. 2007, 32 (11), 1405–1407. 10.1364/OL.32.001405. [DOI] [PubMed] [Google Scholar]
- Shi Q.; Lin S. S.; Wei C. B.; Li H.; Guo C. Q.; Su Y. F.; Fang H.; Dai M. J. Electrochemical and optoelectric behavior of Al-doped ZnO films as transparent anode for Li-ion batteries. Mater. Today Commun. 2019, 19, 471–475. 10.1016/j.mtcomm.2019.05.011. [DOI] [Google Scholar]
- Ozen S.; Senay V.; Pat S.; Korkmaz S. Optical, morphological properties and surface energy of the transparent Li4Ti5O12 (LTO) thin film as anode material for secondary type batteries. J. Phys. D: Appl. Phys. 2016, 49 (10), 105303. 10.1088/0022-3727/49/10/105303. [DOI] [Google Scholar]
- Roeder M.; Beleke A. B.; Guntow U.; Buensow J.; Guerfi A.; Posset U.; Lorrmann H.; Zaghib K.; Sextl G. Li4Ti5O12 and LiMn2O4 thin-film electrodes on transparent conducting oxides for all-solid-state and electrochromic applications. J. Power Sources 2016, 301, 35–40. 10.1016/j.jpowsour.2015.09.063. [DOI] [Google Scholar]
- Gittleson F. S.; Hwang D.; Rya W.-H.; Hashmi S. M.; Hwang J.; Goh T.; Taylor A. D. Ultrathin Nanotube/Nanowire Electrodes by Spin Spray Layer-by-Layer Assembly: A Concept for Transparent Energy Storage. ACS Nano 2015, 9 (10), 10005–10017. 10.1021/acsnano.5b03578. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Jeong S.; Hu L. B.; Wu H.; Lee S. W.; Cui Y. Transparent lithium-ion batteries. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (32), 13013–13018. 10.1073/pnas.1102873108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oukassi S.; Baggetto L.; Dubarry C.; Le Van-Jodin L.; Poncet S.; Salot R. Transparent Thin Film Solid-State Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11 (1), 683–690. 10.1021/acsami.8b16364. [DOI] [PubMed] [Google Scholar]
- Hecht D. S.; Hu L. B.; Irvin G. Emerging Transparent Electrodes Based on Thin Films of Carbon Nanotubes, Graphene, and Metallic Nanostructures. Adv. Mater. 2011, 23 (13), 1482–1513. 10.1002/adma.201003188. [DOI] [PubMed] [Google Scholar]
- Ellmer K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photonics 2012, 6 (12), 809–817. 10.1038/nphoton.2012.282. [DOI] [Google Scholar]
- Sannicolo T.; Lagrange M.; Cabos A.; Celle C.; Simonato J. P.; Bellet D. Metallic Nanowire-Based Transparent Electrodes for Next Generation Flexible Devices: a Review. Small 2016, 12 (44), 6052–6075. 10.1002/smll.201602581. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhang L.; Yu L.; Jiao Z.; Xie H.; Lou X. W.; Sun X. W. A bi-functional device for self-powered electrochromic window and self-rechargeable transparent battery applications. Nat. Commun. 2014, 5, 4921. 10.1038/ncomms5921. [DOI] [PubMed] [Google Scholar]
- Lao-atiman W.; Julaphatachote T.; Boonmongkolras P.; Kheawhom S. Printed Transparent Thin Film Zn-MnO2 Battery. J. Electrochem. Soc. 2017, 164 (4), A859–A863. 10.1149/2.1511704jes. [DOI] [Google Scholar]
- Nagai H.; Hara H.; Enomoto M.; Mochizuki C.; Honda T.; Takano I.; Sato M. Synchronous Electrochromism of Lithium Ion Battery with Chemically Fabricated Transparent Thin Films. Funct. Mater. Lett. 2013, 6 (2), 1341001. 10.1142/S1793604713410014. [DOI] [Google Scholar]
- Navarrete-Astorga E.; Rodriguez-Moreno J.; Martin F.; Sanchez L.; Cruz-Yusta M.; Schrebler R.; Dalchiele E. A.; Ramos-Barrado J. R. Optical semitransparent silver nanostructured layer electrode toward semitransparent lithium ion batteries. Thin Solid Films 2018, 653, 4–12. 10.1016/j.tsf.2018.02.041. [DOI] [Google Scholar]
- Ding E.-X.; Hussain A.; Ahmad S.; Zhang Q.; Liao Y.; Jiang H.; Kauppinen E. I. High-performance transparent conducting films of long single-walled carbon nanotubes synthesized from toluene alone. Nano Res. 2020, 13 (1), 112–120. 10.1007/s12274-019-2581-7. [DOI] [Google Scholar]
- Li X. S.; Zhu Y. W.; Cai W. W.; Borysiak M.; Han B. Y.; Chen D.; Piner R. D.; Colombo L.; Ruoff R. S. Transfer of Large-Area Graphene Films for High-Performance Transparent Conductive Electrodes. Nano Lett. 2009, 9 (12), 4359–4363. 10.1021/nl902623y. [DOI] [PubMed] [Google Scholar]
- Lipomi D. J.; Lee J. A.; Vosgueritchian M.; Tee B. C. K.; Bolander J. A.; Bao Z. A. Electronic Properties of Transparent Conductive Films of PEDOT:PSS on Stretchable Substrates. Chem. Mater. 2012, 24 (2), 373–382. 10.1021/cm203216m. [DOI] [Google Scholar]
- Wang L.; He X.; Li J.; Gao J.; Fang M.; Tian G.; Wang J.; Fan S. Graphene-coated plastic film as current collector for lithium/sulfur batteries. J. Power Sources 2013, 239, 623–627. 10.1016/j.jpowsour.2013.02.008. [DOI] [Google Scholar]
- Taberna P. L.; Mitra S.; Poizot P.; Simon P.; Tarascon J. M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5 (7), 567–573. 10.1038/nmat1672. [DOI] [PubMed] [Google Scholar]
- Suzuki Y.; Kami K.; Watanabe K.; Watanabe A.; Saito N.; Ohnishi T.; Takada K.; Sudo R.; Imanishi N. Transparent cubic garnet-type solid electrolyte of Al2O3-doped Li7La3Zr2O12. Solid State Ionics 2015, 278, 172–176. 10.1016/j.ssi.2015.06.009. [DOI] [Google Scholar]
- Nair J. R.; Imholt L.; Brunklaus G.; Winter M. Lithium Metal Polymer Electrolyte Batteries: Opportunities and Challenges. Electrochem. Soc. Interface 2019, 28 (2), 55–61. 10.1149/2.F05192if. [DOI] [Google Scholar]
- Tanio N.; Koike Y. What Is the Most Transparent Polymer?. Polym. J. 2000, 32 (1), 43–50. 10.1295/polymj.32.43. [DOI] [Google Scholar]
- Ngai K. S.; Ramesh S.; Ramesh K.; Juan J. C. A review of polymer electrolytes: fundamental, approaches and applications. Ionics 2016, 22 (8), 1259–1279. 10.1007/s11581-016-1756-4. [DOI] [Google Scholar]
- Pat S.; Ozen S.; Yudar H. H.; Korkmaz S.; Pat Z. The transparent all-solid-state rechargeable micro-battery manufacturing by RF magnetron sputtering. J. Alloys Compd. 2017, 713, 64–68. 10.1016/j.jallcom.2017.04.169. [DOI] [Google Scholar]
- Liu J.-g.; Ni H.-j.; Wang Z.-h.; Yang S.-y.; Zhou W.-f. Colorless and transparent high-temperature-resistant polymer optical films–current status and potential applications in optoelectronic fabrications. Optoelectronics—Materials and Devices 2015, 57–81. 10.5772/60432. [DOI] [Google Scholar]
- Sun Y.; Kong L.; Khan H. A.; Pecht M. G. Li-ion Battery Reliability – A Case Study of the Apple iPhone®. IEEE Access 2019, 7, 71131–71141. 10.1109/ACCESS.2019.2918401. [DOI] [Google Scholar]
- Wang M.; Vecchio D.; Wang C.; Emre A.; Xiao X.; Jiang Z.; Bogdan P.; Huang Y.; Kotov N. A. Biomorphic structural batteries for robotics. Sci. Robot. 2020, 5 (45), eaba1912. 10.1126/scirobotics.aba1912. [DOI] [PubMed] [Google Scholar]
- Wang M.; Emre A.; Tung S.; Gerber A.; Wang D.; Huang Y.; Cecen V.; Kotov N. A. Biomimetic Solid-State Zn2+ Electrolyte for Corrugated Structural Batteries. ACS Nano 2019, 13 (2), 1107–1115. 10.1021/acsnano.8b05068. [DOI] [PubMed] [Google Scholar]
- Lu N.; Kim D.-H. Flexible and Stretchable Electronics Paving the Way for Soft Robotics.. Soft Robotics 2014, 1 (1), 53–62. 10.1089/soro.2013.0005. [DOI] [Google Scholar]