Abstract
Increasing attention has been paid to single-atom catalysts (SACs) in heterogeneous catalysis because of their unique electronic properties, maximized atomic utilization efficiency, and potential to serve as a bridge between the heterogeneous and homogeneous catalysis. However, SACs can have limited advantages or even constrained applications for the reactions that require designated metallic states with multiple atoms or surface sites with metal–metal bonds. As a cross-dimensional extension to the concept of SACs, fully exposed cluster catalysts (FECCs) offer diverse surface sites formed by an ensemble of metal atoms, for the adsorption and transformation of reactants/intermediates. More importantly, FECCs have the advantage of maximized atom utilization efficiency. Thus, FECCs provide a novel platform to design effective and efficient catalysts for certain chemical processes. This outlook summarizes recent advances and proposes prospective research directions in the design of catalysts and characterizations of FECCs, together with potential challenges.
Short abstract
This outlook summarizes recent study on fully exposed cluster catalysts and proposes prospective research directions in the design and characterization of catalysts, together with potential challenges.
Introduction
Single-Atom Catalyst: The Advantages and Drawbacks
A large amount of industrial processes include catalytic transformation.1 Of these processes, metal-based catalytic material is one of the mostly used catalysts for effective production of fuels and chemicals.2 As the catalytic process is a surface reaction, the bulk atoms of metal-based catalysts are far less important than the surface sites during reaction. Therefore, the large size of metal catalysts limits the effective utilization of metallic components. This is of vital importance especially when a reaction requires expensive noble metal as the active component. To increase the utilization efficiency of metal and to reduce the cost of catalysts, highly dispersed metal catalysts have been developed, with high ratio of surface metal atoms.3−6 The dispersed metal catalysts with the highest atomic utilization efficiency are those with isolated metal centers. In homogeneous catalysis, they exist as mononuclei organometallic catalysts that are widely used nowadays. In heterogeneous catalysis, the catalysts with isolated metal sites,7−12 which are commonly referred to as single-atom catalysts (SACs) recently,13 have attracted growing attention. A SAC has discrete metal centers over the support, which could reach a theoretical metal dispersion of 100%.14−18 The fast development of SACs has raised the concept of highly dispersed metal catalysts to a new level. Besides the outstanding metal utilization efficiency and excellent catalytic performance in some reactions,12,19,20 the key advantage of SACs is that the coordination structure of isolated metal atoms can be precisely designed and controlled, and thus, the electronic structure and coordination geometry of catalytic active centers can be well depicted.20−22 This offers the possibility of understanding complicated heterogeneous catalysis at the atomic or molecular level.
However, the SAC is not a universal type of catalyst that could work for any heterogeneous catalytic reactions. The beauty of SACs is the presence of discrete metal centers that lead to full atomic utilization efficiency, but the drawback is that they cannot offer sites with multiple metal atoms. The collective activation of reactant/intermediates by the sites with multiple metal atoms is of vital importance for some catalytic reactions. The famous examples include the so-called C7 center over iron and the B5 center over Ru for N2 activation.23,24 These examples indicate that the engagement of a group of metal atoms with a designated spatial arrangement is a must for the effective activation of reactants. Moreover, as the metal1 center of the SAC normally anchors over the oxygen of an oxide support, it loses part of the metallic properties required for the efficient adsorption and activation of specific catalytic reactions, leading to attenuated reactivity of SACs.
The Concept of Fully Exposed Cluster Catalysts
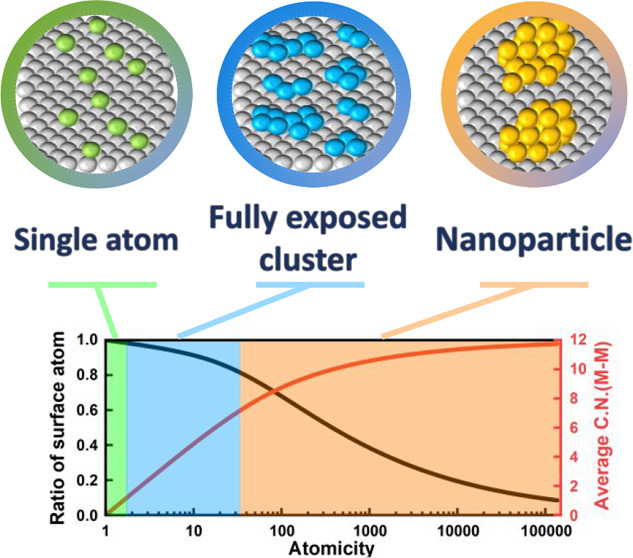
There is a type of catalyst, namely fully exposed cluster catalyst (FECC), which can not only provide the catalytic sites with multiple metal atoms but also maintain a full atomic utilization efficiency. FECC is so highly dispersed that all the metal atoms within it are available for the adsorption and transformation of reactants. Although there are unsupported FECCs (see the following section), in most cases, FECC refers to the clusters supported over certain substrates, such as metal oxides, carbon materials, zeolites, and metal carbides. As its stable metal loading can be higher than that of SAC, the FECC usually exhibits higher mass specific activity than the SAC, which is critically important for industrial applications. More importantly, supported FECCs exhibit two distinct features. The first one is its ultrasmall size, normally below 1 nm, which eliminates the presence of undesired bulk atoms (instead, nanoparticle catalysts have substantial amount of bulk atoms) and reduces the average coordination number of the metal atoms.25 The second one is a small contact angle with the support, forming a layered structure, which enhances the interaction between metal atoms and support, and increases the stability of the clusters.26 The special construct of FECCs creates abundant interfacial sites between metal atoms and substrates.27
Although the concept of FECCs was recently proposed,25,28 the catalysts matching this definition have been reported frequently in the literature as “subnanometer clusters”,29 “ultrasmall clusters”,30 and “clusters of low atomicity”.31 Indeed, with the full atomic utilization efficiency and special atomic construct, FECCs have great potential for the application in many catalytic reactions.
Characteristics of FECCs
The most important aspect of FECCs is that they possess an ensemble of metal atoms that offer a variety of structural possibilities and catalytic feasibilities. In Schemes 1 and 2, we illustrate the key features of the FECC. In Scheme 1, a fully exposed Pt cluster accommodated over the TiO2(111) surface is constructed. The FECC is composed of 13 Pt atoms, with 9 atoms in the first layer, in close contact with the titania substrate, and another four Pt atoms in the second layer (Scheme 1). As the metal cluster has strong electronic interaction with the oxide support,32,33 charge transfer from the interfacial Pt atoms to the titania support is anticipated. Indeed, for the Pt atoms in direct contact with titania, an increase in valence state was observed, although the amount of transferred charge depends on the exact location and the interaction with the oxide support. For those Pt atoms in the second layer, the metallic valence state is preserved (Scheme 1a), in accordance with recently reported results.34,35 The charge state of these adjacent metal atoms provides aeolotropic sites that may be required in the activation of specific reactants.36
Scheme 1. An Example of a Fully-Exposed Cluster Catalyst (i.e., Pt13 Cluster Loaded on the TiO2 Surface).
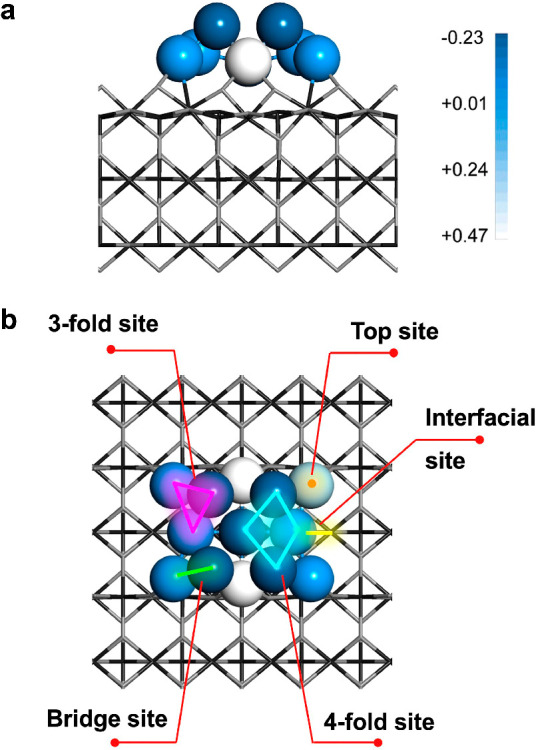
In the side (a) and top (b) view of the Pt13/TiO2 FECC, the level of color highlights the charge of Pt atom from Bader charge analysis. The bar on the top right marks the value of the charge, and the lighter color indicates a stronger charge transfer from the Pt atom to the substrates. Clearly, the charge of the Pt atoms in contact directly with oxide substrate are more positive, while those in the 2nd layer preserve more metallic characteristics.
Scheme 2. Illustration of Diverse Atomic Configurations of Pt13/TiO2FECC with the Same Atomicity (13 Pt Atoms).
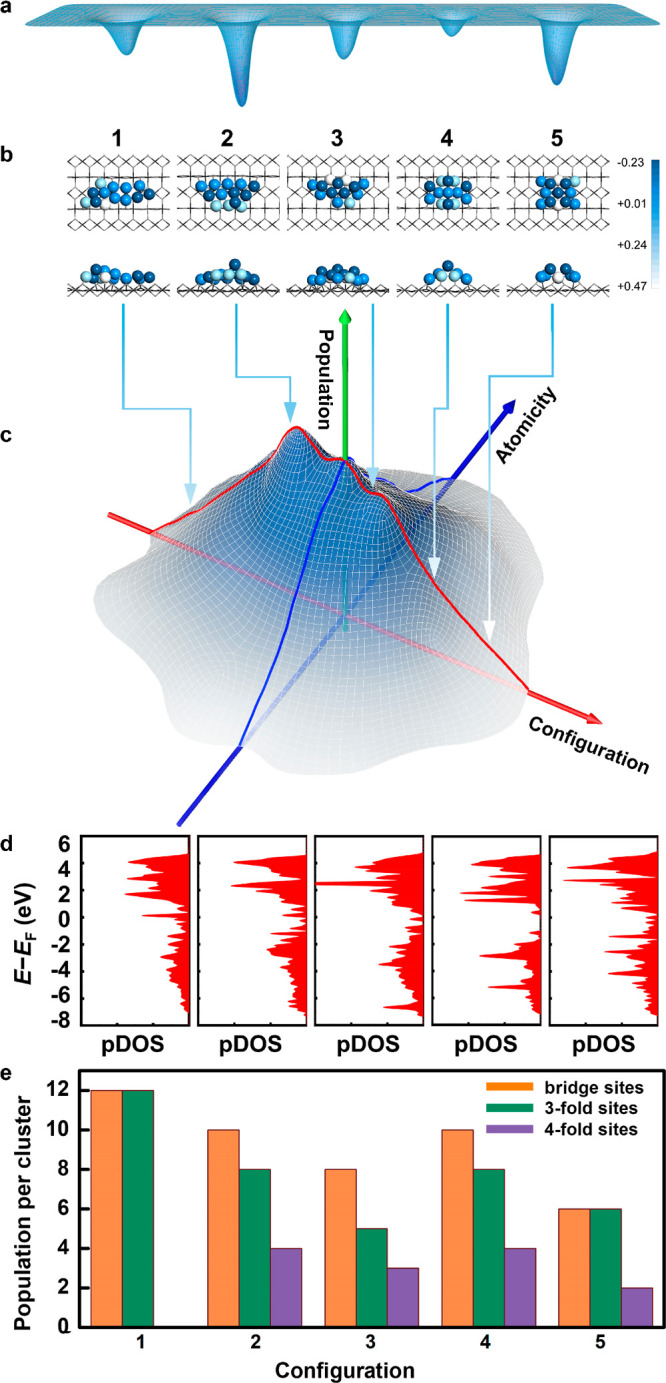
Five exemplified Pt13/TiO2 fully-exposed clusters with different relative energies (a) and atomic configurations (side-on and top views) (b); the level of color in (b) highlights the charge of Pt atom calculated through Bader charge analysis, with the lighter color indicating a stronger charge transfer from the Pt atom to the substrate. The bar on the right marks the value of the charge. (c) Population distribution towards the atomicity and atomic configuration of real fully-exposed Pt13/TiO2 cluster catalyst. (d) Projected density of state (pDOS) of the five Pt13/TiO2 fully-exposed clusters (corresponding to those in (b)). (e) The number of the collective metal sites (bridge, 3-fold, and 4-fold) of the five Pt13/TiO2 fully-exposed clusters shown in (b). Those of the top and interfacial sites were not taken into consideration. It should be noted that this example is set simply to illustrate the diversity in geometric and electronic structures of the cluster based on calculated structures, based on the DFT methods with much low tolerance in accuracy for geometry optimization.
Another feature of the FECC is that it provides diverse combinations of multiple metal atoms, and constructs rich surface sites for the adsorption and transformation of specific reactants/intermediates. For example, for the Pt13/TiO2 cluster catalyst (Scheme 1b), besides the top sites, it contains at least nine types of interfacial sites, six bridge sites, six 3-fold sites, and two 4-fold sites (Scheme 1b). In another word, it offers abundant feasibility of surface sites while keeping maximized atom utilization efficiency. In contrast, there are only the top and interfacial sites for the adsorption of substrate molecules over SACs. This shows the promise of FECCs in converting reactants that need the activation of collective metal sites, which is usually not offered by SACs. The rich surface-site diversity and the presence of neighboring surface sites may confer FECCs excellent reactivity and designated reaction paths toward certain products.
In an ideal situation, the FECC with identical atomicity (number of atoms) and configuration is highly anticipated. With well-defined atomic structures, the FECC can be used as model catalyst to investigate the structure-performance relationship, which is one of the most important but least clear aspects in catalysis research. The knowledge on this aspect will allow the rational design of effective catalysts for specific chemical processes. However, for a real FECC, although there are some recent attempts,37,38 the synthesis of a specific FECC with atomic precision and high degree of structural uniformity is still difficult. As shown in Scheme 2, we use an imaginary Pt13/TiO2 cluster catalyst as an example to illustrate the characteristics and complexity of real FECC. Although the aim is to fabricate a Pt cluster catalyst with exactly 13 Pt atoms, in a real synthetic process (e.g., wet chemistry methods or soft-landing methods), we will likely get the Pt cluster catalyst with a distribution of atomicity (Scheme 2c). This is similar to the size distribution of nanoparticle catalysts. Whether a narrower or a wider distribution in atomicity can be reached will depend on specific synthetic or post-treatment methods. It should also be noted that even a relatively uniform FECC were synthesized, it could still experience structural reconstruction during a reaction process especially under high temperature, which may lead to a wider distribution in atomicity.
The distribution in atomicity has an impact on catalytic performances (please refer to the recent excellent reviews related to this topic39−41). However, the atomic configuration (e.g., the arrangement of metal atoms on the surface of substrates and the types of neighboring surface sites in FECC) is more important. In a real FECC, the diversity in atomic configuration occurs unavoidably. Therefore, wide-distribution of atomicity and diversity of atomic configuration contribute to the overall heterogeneity of a real FECC (Scheme 2c). One of the aims of future research is to narrow the atomicity distribution and atomic configuration diversity in FECCs.
For the fully exposed Pt13 cluster on titania, there are more than 30 possible surface atomic configurations for a Pt cluster with exactly 13 atoms. Five of the possible configurations are shown in Scheme 2b. Though the five configurations possess different energy (Scheme 2a), all of them may exist on the oxide support as stable structures. It is clear that all of the metal cluster configurations, in the form of quasi-single layer to double layers, exhibit close contact with the oxide at relatively small contact angles. The interaction between metal atoms and support and the specific atomic configurations confer the FECC with the variation in apparent charge and electronic structure. The projected density of states (pDOS) on Pt 3d orbitals for five Pt13 cluster configurations are shown in Scheme 2d. Compared with bulk Pt particles with continuous valence band structure, the pDOS of Pt13 FECCs exhibits distinct features. The FECC shows two 3d distinct peaks and a narrow 3d electron band, indicating a noticeably different electron transfer from bulk metallic Pt. Each atomic configuration shows a unique d-band center in energy, indicating different reactivity among the clusters. Meanwhile, these FECCs possess different types of surface sites (Scheme 2e).
In addition to the difference in electronic structure compared to bulk metal particle catalyst, FECC is different from single-atom catalyst, due to the presence of metal–metal bonds and active catalytic sites with multiple metal atoms. With the potential to possess narrowly distributed or even uniform atomic clusters in synthesis, FECCs would bring tremendous opportunities into future catalysis research.
FECC can be divided into two categories: supported and bare FECCs, with or without the presence of solid support. Solid support can significantly affect the catalytic performance and thus the investigation methods of FECCs. Interesting progress has been made over the bare FECCs,38 and we will focus on supported FECCs in this outlook. We will begin with methods of characterizing the structural properties of FECCs and then present a few representative experimental and theoretical studies of FECC, and finally provide our perspectives on this emerging area.
Characterization of FECCs
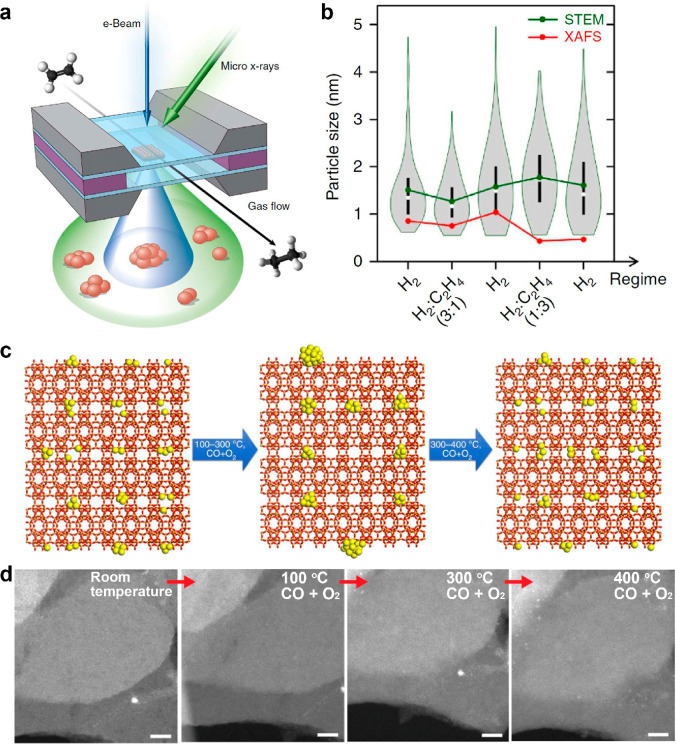
How can one determine in structure whether a catalyst is FECC, especially if a FECC can keep its unique structural feature under working conditions? To obtain the structural information on fully exposed clusters, modern microscopic and spectroscopic characterization techniques are essential. Moreover, the characterizations in operando/in situ modes can reveal the real state structural information on catalysts under working conditions, which have attracted more attention than ever before.42 The major geometric properties that we would like to detect for FECCs are their atomicity and atomic configuration. Statistically averaged properties can be obtained through spectroscopic methods, especially X-ray absorption spectroscopy (XAS), which provide average coordination property of metal atoms, leading to the information on average size of the clusters and their anchoring environment on supports. Besides, atomically resolved electron microscopy provides more detailed information about the geometry of metal clusters. By combining multiple operando/in situ characterization techniques, a thorough picture of the structural dynamics of catalysts can arise. An example here is illustrated by Frenkel et al., who performed combined imaging and spectroscopy measurements to probe the complex structural dynamics of supported FECC catalysts.43 As shown in Figure 1a, the evolution of Pt clusters in the catalytic hydrogenation of ethylene is monitored by operando XAFS and STEM simultaneously, and the result indicated a slight agglomeration of Pt clusters due to the switch of different reaction conditions (Figure 1b). This method can be applied to a wide range of mechanistic study of catalytic reactions. Another example is an in situ environmental transmission electron microscopy (ETEM) study of the structural evolution of MCM-22 confined subnanometric Pt species (Pt@MCM-22) under reactive atmospheres.44 As shown in Figure 1c, specifically, under the atmosphere of CO and O2, the confined atomically dispersed Pt species could agglomerate into Pt clusters or even small nanoparticles from room temperature to 100 °C. Such structure feature of Pt@MCM-22 remained constant from 100 to 300 °C. However, when the reaction temperature continuously increased from 300 to 400 °C, the Pt nanoparticles could disintegrate into highly dispersed Pt species and Pt clusters (Figure 1d).
Figure 1.
Monitoring structure evolution of FECCs under real reaction conditions. Schematic illustration of the combined operando XAS/STEM experimental setup (a) and corresponding experimental results of the structural dynamics of SiO2 supported Pt clusters during ethylene hydrogenation reaction (b).43 Reprinted with permission, copyright 2015, the authors. Schematic illustration (c) and in situ ETEM results (d) of the structural evolution of MCM-22 confined Pt species from room temperature to 100, 300, and 400 °C under CO + O2 reactive atmosphere. Scale bars in (d) are 10 nm.44 Reprinted with permission, copyright 2018, the authors.
In addition, other characterization methods such as diffuse reflection infrared Fourier transform spectroscopy (DRIFTS), near ambient pressure X-ray photoelectron spectroscopy (NAP-XPS), and inelastic neutron scattering (INS) can provide useful information about the structural dynamics and reaction mechanism of supported FECCs during the reaction.45−47 We believe that with the development of FECCs, more and more state-of-the-art techniques will be used to reveal the structural characteristics of FECCs at working reaction conditions.
Constructing FECCs on Various Supports
Designing and constructing stable atomic clusters is of the first step toward the catalysis by FECC. Anchoring FECC via chemical bonds to structurally stable materials is a common strategy in synthesis. We anticipate that various supports, including metal oxide, carbon-based materials (native and heteroatom-doped graphene, graphene oxide, CNTs, and nanodiamond), zeolite, metal carbide, can be used to accommodate FECCs. Special care needs to be taken in the synthetic process, as the precise control to keep the majority of metal species in the form of fully exposed clusters is not easy. It is usually desired to create energy barriers to keep the less-stable fully exposed clusters from transforming into other forms. For this purpose, one can employ promotive additives, an oxygen vacancy, and other surface defects like silanol nest in zeolite or surface sites induced by doping.
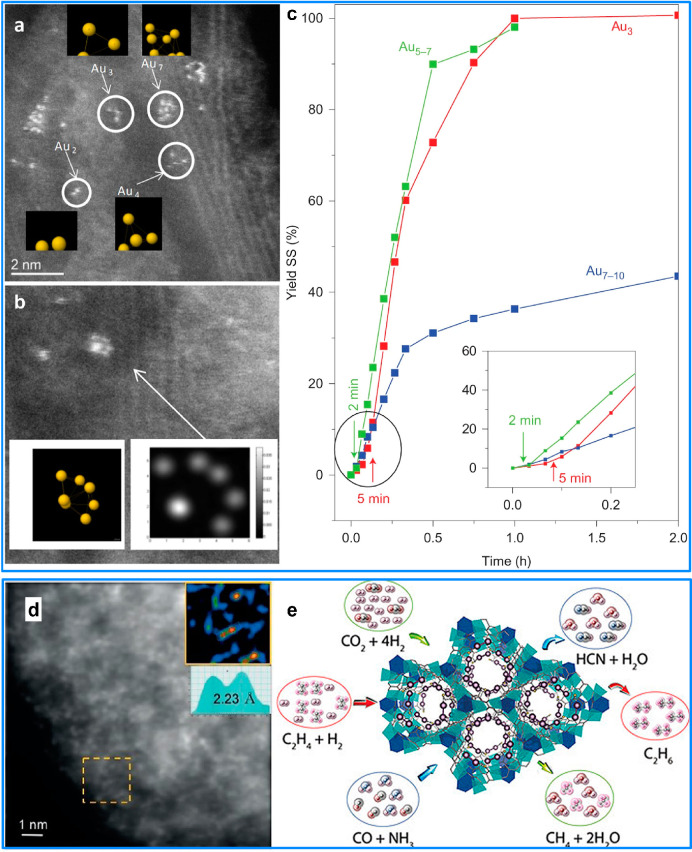
As an example, a series of multiwall carbon nanotubes (MWCNTs) supported Au clusters with low atomicity (Au3, Au5–7 and Au7–10) were successfully fabricated (Figure 2a,b). In the catalytic oxidation of thiophenol to disulfide, these fully exposed Au clusters catalysts exhibited superior catalytic activity (7.5 × 105 h–1 for Au5–7) (Figure 2c), which are even comparable to that of sulfhydryl oxidase enzymes. In contrast, both MWCNTs supported isolated Au atoms and large Au aggregates suffered from the lack of O2 activation or strong Au–S passivation, leading to far less activity.48 This work demonstrated that the FECC possesses unique catalytic behavior that could not be accomplished by other types of catalysts.
Figure 2.
Constructing FECCs on different supports. (a,b) HAADF-STEM images of MWCNTs supported Au clusters and corresponding optimized configurations of Au clusters. (c) Oxidation of thiophenol to disulfide in the presence of O2 catalyzed by MWCNTs supported size-selected Au clusters.48 Reprinted with permission, copyright 2013, Springer Nature. AC-HAADF-STEM image (d) and catalytic applications (e) of thioether-functionalized MOF confined Pt02 clusters.30 Reprinted with permission, copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
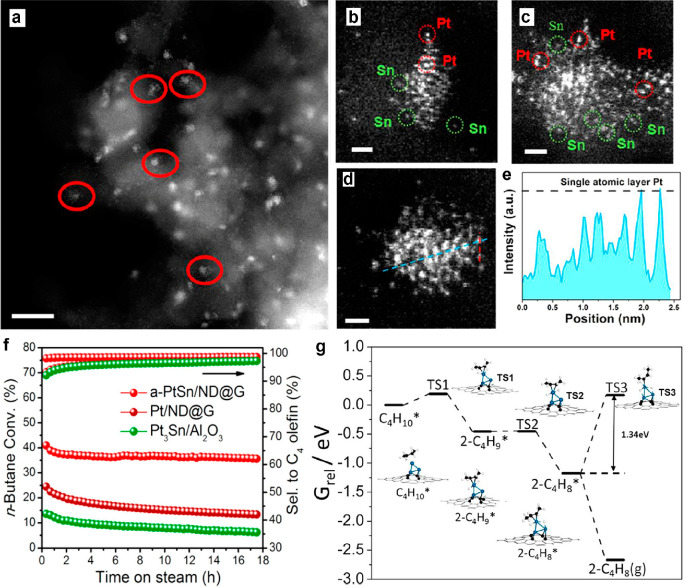
Metal–organic framework (MOF) can be used as the host for FECCs. For example, highly stable fully exposed Pt2 clusters were successfully fabricated homogeneously within the walls of a MOF material (Figure 2d,e).30 The fully exposed Pt catalyst has full atomic utilization of the metal center, which can lead to excellent reactivity. Indeed, the FECC catalysts showed good activity in various reactions including HCN production, CO2 methanation, alkene hydrogenations, and carbene-mediated reactions (Figure 2e). For catalysts operating at relatively high temperature, other strategies have been proposed to stabilize the fully exposed metal structures. Compared with MOF, zeolites have much higher thermal stability, which rendered them great possibilities as host materials to stabilize fully exposed metal clusters. Using siliceous MFI zeolite, Corma et al. successfully fabricated Pt quasi-FECC with superior stability for propane dehydrogenation at 600 °C.49,50 For the systems where stable FECC is challenging to fabricate, a second metal component can be added as a structural promoter to stabilize FECC. Recently, we reported a FECC of Sn-assisted Pt clusters (Figure 3).25 The bulk PtSn bimetallic catalyst is a conventional industrial catalyst for direct dehydrogenation of n-butane. However, it suffers from the deactivation induced by sintering of Pt3Sn alloy particles. Therefore, fabrication of highly dispersed Pt clusters with good stability is highly desired in this reaction. While isolated Pt center was demonstrated to have low activity in the dehydrogenation of butane, fully exposed Pt clusters isolated by Sn species anchored on nanodiamond@graphene support is highly active, selective, and stable toward C4 olefin production. Combining experimental results and DFT calculations, a typical Pt fully exposed cluster model supported on graphene has been proposed, with two Pt atoms anchored on the surface and another Pt atom uncoordinated. The mechanism study demonstrated that the undercoordinated Pt atom is the primary active site, and the high olefin selectivity stems from the easy desorption of n-butene product from the Pt3 clusters.
Figure 3.
Addition of bimetallic component as a strategy to stabilize FECC: direct dehydrogenation of n-butane over tin-assisted fully exposed Pt clusters stabilized on defect rich graphene.25 HAADF-STEM images of the a-PtSn/ND@G catalyst (a) showing the distribution of Pt clusters, scale bar = 10 nm, and (b-c) the atomic dispersion of Pt and Sn, scale bar = 0.5 nm. HAADF-STEM image of a-PtSn/ND@G (d) and the extracted line profiles (e) along the blue direction in (d), demonstrating the pronounced intensity difference between Pt and Sn, consistent well with their distinct atomic numbers (Z), together with the single-atomic-layer thickness of a typical PtSn cluster, scale bar = 0.5 nm. (f) Conversion and selectivity by time-on-stream during dehydrogenation of n-butane at 450 °C. Gas hour space velocity (GHSV) = 18 000 mL·gcat–1·h–1, nC4/H2 = 1:1 with He balance. (g) Gibbs free energy profile of direct butane dehydrogenation on the Pt3-Gr. Structures for intermediates and transition states from C4H10 to 2-C4H8 (T = 450 °C). Reprinted with permission, copyright 2019, American Chemical Society.
Utilizing the Structural Uniqueness
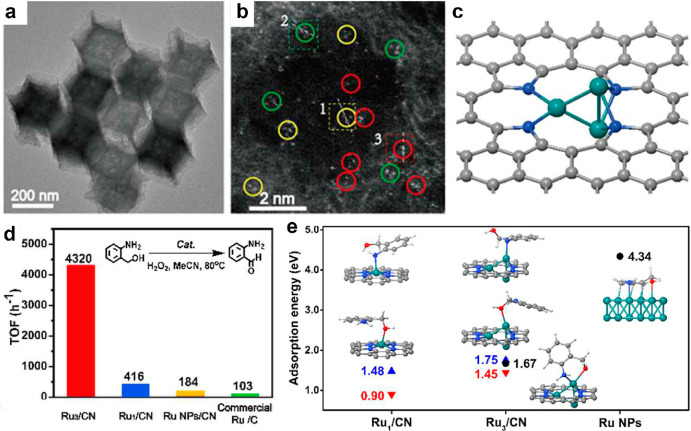
The key property of FECCs, as discussed earlier, is their unique electronic structure and the ability to provides the collective metal sites that required for large reactants or multireactants activation. In an interesting approach developed by Li and Wang et al., a Ru3 cluster stabilized by nitrogen species within molecular-scale cages of zeolitic imidazolate frameworks were obtained (Figure 4).51 The Ru FECC has an average Ru–Ru coordination number of 1.6 ± 0.5 and shows the trinuclei structure from the STEM observation (Figure 4a–c), indicating that a fully exposed Ru cluster with 3 atoms in average were successfully constructed. Catalytic studies revealed that the FECC structure possessed excellent activity toward selective oxidation of 2-aminobenzyl alcohol to 2-aminobenzaldehyde, surpassing that of Ru SAC or Ru nanoparticles (Figure 4d). DFT calculations showed that because of the limited atomicity of Ru3, the 2- aminobenzyl alcohol tends to be adsorbed with a moderate strength, whereas the strong adsorption between 2-aminobenzyl alcohol and Ru NPs would block the catalytic active sites. However, the Ru3 structure provides the optimal geometry where one Ru atom connects with both the hydroxyl and amino groups during the reaction, which is not possible for Ru1 because of limited bonding sites (Figure 4e). Thus, the Ru3 FECC exhibited better performance than the SAC or nanoparticle Ru catalysts.
Figure 4.
Structural and electronic uniqueness of FECCs. (a) TEM and (b) AC-HAADF-STEM images of Ru3/CN catalyst.51 (c) Schematic model of Ru3/CN. (d) Catalytic activity comparison between different Ru-based catalysts in catalytic oxidation of 2-aminobenzyl alcohol to 2-aminobenzaldehyde, the TOF were obtained at 20% conversion of 2-aminobenzyl alcohol. (e) Geometries of 2-aminobenzyl alcohol adsorbed on Ru3/CN (middle), Ru1/CN (left), and Ru NPs (right). The blue and red triangles and black circles mark the adsorption energies of the molecule bound via the amino group, the hydroxyl group, and both groups, respectively. Positive values represent exothermic adsorption. The gray, blue, red, white, and teal spheres in (c) and (e) represent C, N, O, H, and Ru atoms, respectively. Reprinted with permission, copyright 2017, American Chemical Society.
Conclusions and Perspectives
In summary, the FECC is a novel structure with unique properties and great potential in heterogeneous catalysis. The geometry and electronic structures of FECCs are different from those of bulk-like nanoparticles or SACs. The pseudotransitional state of FECCs has shown distinctive behavior in catalysis.
The pursuit of ideal heterogeneous catalysts leads to the effort toward developing catalysts with high atom utilization efficiency and unique geometric and electronic structures. Along this direction, the development of FECCs can provide significantly improved catalytic performances. The progress made so far is impressive, but the strategy to design and construct highly active and stable FECCs, especially those with well-defined structures and reaction paths, is attractive but challenging. With the growth of research efforts, we believe that it is possible to drive the catalysis of FECCs to its full potential. In perspective, here are a few research directions of FECC where we expect to significantly grow in the foreseeable future.
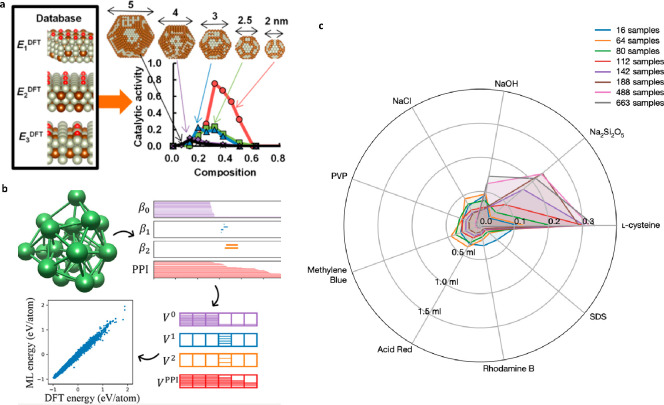
First, it is highly demanded to develop a standard evaluation process to systematically screen catalyst clusters in specific catalytic reactions, because the catalyst design should be based on the understanding of catalytic mechanisms. Apart from common experimental methods used in traditional catalysis, new approaches based on artificial intelligence (AI) to screen or optimize cluster catalysts are in development. Combining DFT calculation and machine learning, the catalyst with best performance can be found in an efficient manner with this bottom-up approach (Figure 5a,b).52−54 A modeling strategy for fully exposed cluster catalysts that can be widely implemented in various catalytic system is in exigent need. Furthermore, a mobile robotic chemist that can operate autonomously to perform catalytic experiments has been developed recently, and it has drastically enhanced the efficiency of searching for new catalysts in a top-up approach (Figure 5c).55 We should notice that as fully exposed clusters usually possess lower atomicity than that of nanoparticles reported, it would be easier to perform AI research based on DFT calculations.
Figure 5.
Combination of FECCs study with modern IT technology. (a) Predicting catalytic activity toward NO decomposition of RhAu nanoparticles by a DFT-aided machine-learning algorithm;53 Reprinted with permission, copyright 2020, American Chemical Society. (b) Topology-based machine learning strategy for lithium cluster structure prediction;54 Reprinted with permission, copyright 2017, American Chemical Society. (c) Radar plot showing the evolution of the robotic research on photocatalytic reactions; the scale denotes the fraction of maximum solution volume dispensed.55 Reprinted with permission, copyright 2020, the authors, under exclusive license to Springer Nature Limited.
Second, from the experimental side, the application of advanced characterization techniques can facilitate the understanding of structural properties of fully exposed clusters. Furthermore, conventional operando methods such as diffraction, EXAFS, and NAP-XPS could help to identify the structural and morphological properties of catalysts under working conditions. The recent development of atomically resolution chemical mapping, especially those coupled with environmental TEM,56,57 can empower us to get in-depth insights into the electronic structure of catalytic active state at the atomic level. By coupling the new mapping technique with the existing statistical characterization methods, it will provide a vivid picture of the FECC and its reaction process under working reaction conditions.
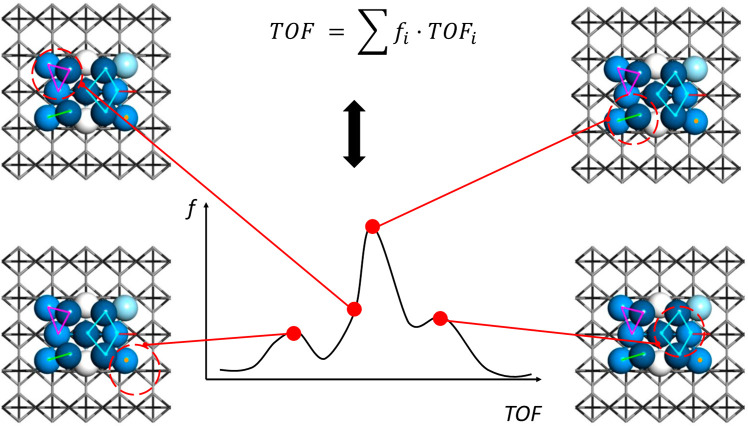
Third, although the distribution of the atomicity and atomic configuration of a real FECC are heterogeneous in nature, the key that governs the reactivity and selectivity of catalyst is the geometry and spatial assembling pattern of specific active sites (for example, 4-fold site or B5 center). Therefore, developing spectroscopic or transient kinetic methods to probe the nature and population of these active sites is especially important for FECC. The succuss in developing these methods, in return, will help the precise synthesis of highly active and narrowly distributed FECCs. For example, a powerful state-of-the-art tool is steady-state isotropic transient kinetic analysis (SSITKA). By this method, kinetic parameters of catalyst-surface reaction intermediates, such as concentration, site coverage, reactivity, and rate constants could be obtained.58 A combination of SSITKA and in situ DRIFTS may correlate surface coverage with species observed in DRIFTS, helping to discriminate between key intermediates and spectators species in catalysis. More importantly, deconvolution of SSITKA data could give a distribution of active sites in regards to their intrinsic turnover frequencies (TOFs)59 (Scheme 3). As FECC has limited sites on its surface, it is easy to identify the reactivity of different sites (i.e., different metal ensembles). Surely, monitoring different aspects of a catalyst including structural evolution, intrinsic activity, reactive species, electron transfer, and catalytic products simultaneously with various techniques would provide a comprehensive picture of a reaction and thus help to understand the mechanism and to design FECC with enhanced performance. Clearly, how to couple those sophisticated methods should be carefully strategized.
Scheme 3. Illustration of Distribution of Active Sites within One Single Fully Exposed Cluster (This Is Illustration Instead of Real Measured Data).
TOF refers to turn over frequency, and f represents the population ratio of a specific active site among all the sites.
Fourth, new methods will be developed to precisely control the synthesis of clusters with narrow atomicity distribution or even full uniformity. Here, the challenges include anchoring of the clusters (by wet chemistry methods or soft-landing of presynthesized fully exposed cluster with well-defined structure) on the solid support, the removal of the stabilizing ligands, and the prevention of possible sintering of the clusters into larger particles remains in the pretreatment or reaction processes.60,61 Among them, how to manipulate the precise synthesis while maintaining the structural stability during reaction is an interesting but challenging topic for scientists. Moreover, introducing alternative composition to the clusters, including bimetallic structure and interface modification, will have a major impact on the catalytic performance. However, we need to point out that though the FECCs provide rich distribution of active sites, which contains possibilities for reactions that are difficult to proceed with conventional catalysts, it should also be noted that the variety is not always benefitial for catalytic reactions, as the side reactions might also accelerate. Actually, this problem is not solely a problem of FECCs, NPs also possess a variety of surface sites, even SAC sites are not homogeneously distributed on the support (it has different coordination environments). For heterogeneous catalysts, although we are aiming at narrowing the site distribution, we have to, in most times, live with heterogeneity. Indeed, a wide distribution of surface sites might not always facilitate the designated reactions. While we believe that narrowing the site distribution will restrain the diverging products distribution, the construct of multimetal sites or metal ensemble will always increase the complexity of the surface sites. However, for some of these reactions, this is a must, although it increased the site distribution as well. Therefore, the key of the balance is to fabricate FECCs with a preferential distribution of desired active sites.
The developments in synthesis, characterization, and application of fully exposed cluster catalysts are of significant interests. The concept of reducing the noble metal usage while keeping or even enhancing the function of catalyst is surely of great industrial value. Nevertheless, whether FECC can make an impact at the industrial scale remains to be explored, which will demand the joint efforts from both scientists and engineers.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFB0602200, 2016YFA0204100), the National Natural Science Foundation of China (21725301, 21821004, 91645115, 91845201, 21961160722), the Liaoning Revitalization Talents Program XLYC1907055 and the Sinopec China. Ding Ma acknowledges support from the Tencent Foundation through the XPLORER PRIZE.
The authors declare no competing financial interest.
References
- Hawker S. The Wide Scope of Catalysis Fine Chemicals through Heterogeneous Catalysis. Platin Met Rev. 2001, 45 (4), 154. [Google Scholar]
- Boudart M. Heterogeneous Catalysis by Metals. J. Mol. Catal. 1985, 30 (1–2), 27–38. 10.1016/0304-5102(85)80014-6. [DOI] [Google Scholar]
- Lu S. L.; Hu Y. M.; Wan S.; McCaffrey R.; Jin Y. H.; Gu H. W.; Zhang W. Synthesis of Ultrafine and Highly Dispersed Metal Nanoparticles Confined in a Thioether-Containing Covalent Organic Framework and Their Catalytic Applications. J. Am. Chem. Soc. 2017, 139 (47), 17082–17088. 10.1021/jacs.7b07918. [DOI] [PubMed] [Google Scholar]
- Zhao G. X.; Liu H. M.; Ye J. H. Constructing and controlling of highly dispersed metallic sites for catalysis. Nano Today 2018, 19, 108–125. 10.1016/j.nantod.2018.02.013. [DOI] [Google Scholar]
- Tang C. H.; Surkus A. E.; Chen F.; Pohl M. M.; Agostini G.; Schneider M.; Junge H.; Beller M. A Stable Nanocobalt Catalyst with Highly Dispersed CoNx Active Sites for the Selective Dehydrogenation of Formic Acid. Angew. Chem., Int. Ed. 2017, 56 (52), 16616–16620. 10.1002/anie.201710766. [DOI] [PubMed] [Google Scholar]
- Dong C.; Li Y.; Cheng D.; Zhang M.; Liu J.; Wang Y.-G.; Xiao D.; Ma D. Supported Metal Clusters: Fabrication and Application in Heterogeneous Catalysis. ACS Catal. 2020, 10 (19), 11011–11045. 10.1021/acscatal.0c02818. [DOI] [Google Scholar]
- Raja R.; Sankar G.; Thomas J. M. Designing a molecular sieve catalyst for the aerial oxidation of n-hexane to adipic acid. Angew. Chem., Int. Ed. 2000, 39 (13), 2313–2316. . [DOI] [PubMed] [Google Scholar]
- Dugal M.; Sankar G.; Raja R.; Thomas J. M. Designing a heterogeneous catalyst for the production of adipic acid by aerial oxidation of cyclohexane. Angew. Chem., Int. Ed. 2000, 39 (13), 2310–2313. . [DOI] [PubMed] [Google Scholar]
- Thomas J. M.; Greaves G. N.; Sankar G.; Wright P. A.; Chen J. S.; Dent A. J.; Marchese L. On the Nature of the Active-Site in a Coapo-18 Solid Acid Catalyst. Angew. Chem., Int. Ed. Engl. 1994, 33 (18), 1871–1873. 10.1002/anie.199418711. [DOI] [Google Scholar]
- Thomas J. M.; Raja R.; Lewis D. W. Single-site heterogeneous catalysts. Angew. Chem., Int. Ed. 2005, 44 (40), 6456–6482. 10.1002/anie.200462473. [DOI] [PubMed] [Google Scholar]
- Kyriakou G.; Boucher M. B.; Jewell A. D.; Lewis E. A.; Lawton T. J.; Baber A. E.; Tierney H. L.; Flytzani-Stephanopoulos M.; Sykes E. C. H. Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations. Science 2012, 335 (6073), 1209–1212. 10.1126/science.1215864. [DOI] [PubMed] [Google Scholar]
- Zhai Y. P.; Pierre D.; Si R.; Deng W. L.; Ferrin P.; Nilekar A. U.; Peng G. W.; Herron J. A.; Bell D. C.; Saltsburg H.; Mavrikakis M.; Flytzani-Stephanopoulos M. Alkali-Stabilized Pt-OHx Species Catalyze Low-Temperature Water-Gas Shift Reactions. Science 2010, 329 (5999), 1633–1636. 10.1126/science.1192449. [DOI] [PubMed] [Google Scholar]
- Qiao B. T.; Wang A. Q.; Yang X. F.; Allard L. F.; Jiang Z.; Cui Y. T.; Liu J. Y.; Li J.; Zhang T. Single-atom catalysis of CO oxidation using Pt-1/FeOx. Nat. Chem. 2011, 3 (8), 634–641. 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- Jones J.; Xiong H. F.; Delariva A. T.; Peterson E. J.; Pham H.; Challa S. R.; Qi G. S.; Oh S.; Wiebenga M. H.; Hernandez X. I. P.; Wang Y.; Datye A. K. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353 (6295), 150–154. 10.1126/science.aaf8800. [DOI] [PubMed] [Google Scholar]
- Fei H. L.; Dong J. C.; Feng Y. X.; Allen C. S.; Wan C. Z.; Volosskiy B.; Li M. F.; Zhao Z. P.; Wang Y. L.; Sun H. T.; An P. F.; Chen W. X.; Guo Z. Y.; Lee C.; Chen D. L.; Shakir I.; Liu M. J.; Hu T. D.; Li Y. D.; Kirkland A. I.; Duan X. F.; Huang Y. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal 2018, 1 (1), 63–72. 10.1038/s41929-017-0008-y. [DOI] [Google Scholar]
- Qu Y. T.; Li Z. J.; Chen W. X.; Lin Y.; Yuan T. W.; Yang Z. K.; Zhao C. M.; Wang J.; Zhao C.; Wang X.; Zhou F. Y.; Zhuang Z. B.; Wu Y.; Li Y. D. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat. Catal 2018, 1 (10), 781–786. 10.1038/s41929-018-0146-x. [DOI] [Google Scholar]
- Lu Y. B.; Wang J. M.; Yu L.; Kovarik L.; Zhang X. W.; Hoffman A. S.; Gallo A.; Bare S. R.; Sokaras D.; Kroll T.; Dagle V.; Xin H. L.; Karim A. M. Identification of the active complex for CO oxidation over single-atom Ir-on-MgAl2O4 catalysts. Nat. Catal 2019, 2 (2), 149–156. 10.1038/s41929-018-0192-4. [DOI] [Google Scholar]
- Malta G.; Kondrat S. A.; Freakley S. J.; Davies C. J.; Lu L.; Dawson S.; Thetford A.; Gibson E. K.; Morgan D. J.; Jones W.; Wells P. P.; Johnston P.; Catlow C. R. A.; Kiely C. J.; Hutchings G. J. Identification of single-site gold catalysis in acetylene hydrochlorination. Science 2017, 355 (6332), 1399–1402. 10.1126/science.aal3439. [DOI] [PubMed] [Google Scholar]
- Yang X. F.; Wang A. Q.; Qiao B. T.; Li J.; Liu J. Y.; Zhang T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46 (8), 1740–1748. 10.1021/ar300361m. [DOI] [PubMed] [Google Scholar]
- Lin L. L.; Zhou W.; Gao R.; Yao S. Y.; Zhang X.; Xu W. Q.; Zheng S. J.; Jiang Z.; Yu Q. L.; Li Y. W.; Shi C.; Wen X. D.; Ma D. Low-temperature hydrogen production from water and methanol using Pt/alpha-MoC catalysts. Nature 2017, 544 (7648), 80–83. 10.1038/nature21672. [DOI] [PubMed] [Google Scholar]
- Duchesne P. N.; Li Z. Y.; Deming C. P.; Fung V.; Zhao X. J.; Yuan J.; Regier T.; Aldalbahi A.; Almarhoon Z.; Chen S. W.; Jiang D. E.; Zheng N. F.; Zhang P. Golden single-atomic-site platinum electrocatalysts. Nat. Mater. 2018, 17 (11), 1033–1039. 10.1038/s41563-018-0167-5. [DOI] [PubMed] [Google Scholar]
- Lin L. L.; Yao S. Y.; Gao R.; Liang X.; Yu Q. L.; Deng Y. C.; Liu J. J.; Peng M.; Jiang Z.; Li S. W.; Li Y. W.; Wen X. D.; Zhou W.; Ma D. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation. Nat. Nanotechnol. 2019, 14 (4), 354–361. 10.1038/s41565-019-0366-5. [DOI] [PubMed] [Google Scholar]
- Strongin D. R.; Carrazza J.; Bare S. R.; Somorjai G. A. The Importance of C-7 Sites and Surface-Roughness in the Ammonia-Synthesis Reaction over Iron. J. Catal. 1987, 103 (1), 213–215. 10.1016/0021-9517(87)90109-6. [DOI] [Google Scholar]
- Honkala K.; Hellman A.; Remediakis I. N.; Logadottir A.; Carlsson A.; Dahl S.; Christensen C. H.; Norskov J. K. Ammonia synthesis from first-principles calculations. Science 2005, 307 (5709), 555–558. 10.1126/science.1106435. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y.; Deng Y. C.; Cai X. B.; Chen Y. L.; Peng M.; Jia Z. M.; Jiang Z.; Ren P. J.; Yao S. Y.; Xie J. L.; Xiao D. Q.; Wen X. D.; Wang N.; Liu H. Y.; Ma D. Tin-Assisted Fully Exposed Platinum Clusters Stabilized on Defect-Rich Graphene for Dehydrogenation Reaction. ACS Catal. 2019, 9 (7), 5998–6005. 10.1021/acscatal.9b00601. [DOI] [Google Scholar]
- Perini A.; Jacucci G.; Martin G. Cluster Free-Energy in the Simple-Cubic Ising-Model. Phys. Rev. B: Condens. Matter Mater. Phys. 1984, 29 (5), 2689–2697. 10.1103/PhysRevB.29.2689. [DOI] [Google Scholar]
- Yao S. Y.; Zhang X.; Zhou W.; Gao R.; Xu W. Q.; Ye Y. F.; Lin L. L.; Wen X. D.; Liu P.; Chen B. B.; Crumlin E.; Guo J. H.; Zuo Z. J.; Li W. Z.; Xie J. L.; Lu L.; Kiely C. J.; Gu L.; Shi C.; Rodriguez J. A.; Ma D. Atomic-layered Au clusters on alpha-MoC as catalysts for the low-temperature water-gas shift reaction. Science 2017, 357 (6349), 389–393. 10.1126/science.aah4321. [DOI] [PubMed] [Google Scholar]
- Jeong H.; Lee G.; Kim B. S.; Bae J.; Han J. W.; Lee H. Fully Dispersed Rh Ensemble Catalyst To Enhance Low-Temperature Activity. J. Am. Chem. Soc. 2018, 140 (30), 9558–9565. 10.1021/jacs.8b04613. [DOI] [PubMed] [Google Scholar]
- Vajda S.; Pellin M. J.; Greeley J. P.; Marshall C. L.; Curtiss L. A.; Ballentine G. A.; Elam J. W.; Catillon-Mucherie S.; Redfern P. C.; Mehmood F.; Zapol P. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 2009, 8 (3), 213–216. 10.1038/nmat2384. [DOI] [PubMed] [Google Scholar]
- Mon M.; Rivero-Crespo M. A.; Ferrando-Soria J.; Vidal-Moya A.; Boronat M.; Leyva-Perez A.; Corma A.; Hernandez-Garrido J. C.; Lopez-Haro M.; Calvino J. J.; Ragazzon G.; Credi A.; Armentano D.; Pardo E. Synthesis of Densely Packaged, Ultrasmall Pt-2(0) Clusters within a Thioether-Functionalized MOF: Catalytic Activity in Industrial Reactions at Low Temperature. Angew. Chem., Int. Ed. 2018, 57 (21), 6186–6191. 10.1002/anie.201801957. [DOI] [PubMed] [Google Scholar]
- Li L.; Wang Y. C.; Vanka S.; Mu X. Y.; Mi Z. T.; Li C. J. Nitrogen Photofixation over III-Nitride Nanowires Assisted by Ruthenium Clusters of Low Atomicity. Angew. Chem., Int. Ed. 2017, 56 (30), 8701–8705. 10.1002/anie.201703301. [DOI] [PubMed] [Google Scholar]
- Li S. W.; Xu Y.; Chen Y. F.; Li W. Z.; Lin L. L.; Li M. Z.; Deng Y. C.; Wang X. P.; Ge B. H.; Yang C.; Yao S. Y.; Xie J. L.; Li Y. W.; Liu X.; Ma D. Tuning the Selectivity of Catalytic Carbon Dioxide Hydrogenation over Iridium/Cerium Oxide Catalysts with a Strong Metal-Support Interaction. Angew. Chem., Int. Ed. 2017, 56 (36), 10761–10765. 10.1002/anie.201705002. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Mei S.; Yuan K.; Wang D. J.; Liu H. C.; Yan C. H.; Zhang Y. W. Low-Temperature CO2Methanation over CeO2-Supported Ru Single Atoms, Nanoclusters, and Nanoparticles Competitively Tuned by Strong Metal-Support Interactions and H-Spillover Effect. ACS Catal. 2018, 8 (7), 6203–6215. 10.1021/acscatal.7b04469. [DOI] [Google Scholar]
- Chen A. L.; Yu X. J.; Zhou Y.; Miao S.; Li Y.; Kuld S.; Sehested J.; Liu J. Y.; Aoki T.; Hong S.; Camellone M. F.; Fabris S.; Ning J.; Jin C. C.; Yang C. W.; Nefedov A.; Woll C.; Wang Y. M.; Shen W. J. Structure of the catalytically active copper-ceria interfacial perimeter. Nat. Catal 2019, 2 (4), 334–341. 10.1038/s41929-019-0226-6. [DOI] [Google Scholar]
- Beniya A.; Higashi S.; Ohba N.; Jinnouchi R.; Hirata H.; Watanabe Y. CO oxidation activity of non-reducible oxide-supported mass-selected few-atom Pt single-clusters. Nat. Commun. 2020, 11 (1), 1888. 10.1038/s41467-020-15850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deelen T. W.; Mejia C. H.; de Jong K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal 2019, 2 (11), 955–970. 10.1038/s41929-019-0364-x. [DOI] [Google Scholar]
- Yan H.; Lin Y.; Wu H.; Zhang W. H.; Sun Z. H.; Cheng H.; Liu W.; Wang C. L.; Li J. J.; Huang X. H.; Yao T.; Yang J. L.; Wei S. Q.; Lu J. L. Bottom-up precise synthesis of stable platinum dimers on graphene. Nat. Commun. 2017, 8, 1070. 10.1038/s41467-017-01259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. Y.; Yang K. R.; Wang Z. C.; Yan X. X.; Cao S. F.; Ye Y. F.; Dong Q.; Zhang X. Z.; Thorne J. E.; Jin L.; Materna K. L.; Trimpalis A.; Bai H. Y.; Fakra S. C.; Zhong X. Y.; Wang P.; Pan X. Q.; Guo J. H.; Flytzani-Stephanopoulos M.; Brudvig G. W.; Batista V. S.; Wang D. W. Stable iridium dinuclear heterogeneous catalysts supported on metal-oxide substrate for solar water oxidation. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (12), 2902–2907. 10.1073/pnas.1722137115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty I.; Pradeep T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117 (12), 8208–8271. 10.1021/acs.chemrev.6b00769. [DOI] [PubMed] [Google Scholar]
- Du Y. X.; Sheng H. T.; Astruc D.; Zhu M. Z. Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties. Chem. Rev. 2020, 120 (2), 526–622. 10.1021/acs.chemrev.8b00726. [DOI] [PubMed] [Google Scholar]
- Chen Z. W.; Chen L. X.; Yang C. C.; Jiang Q. Atomic (single, double, and triple atoms) catalysis: frontiers, opportunities, and challenges. J. Mater. Chem. A 2019, 7 (8), 3492–3515. 10.1039/C8TA11416A. [DOI] [Google Scholar]
- Vendelbo S. B.; Elkjaer C. F.; Falsig H.; Puspitasari I.; Dona P.; Mele L.; Morana B.; Nelissen B. J.; van Rijn R.; Creemer J. F.; Kooyman P. J.; Helveg S. Visualization of oscillatory behaviour of Pt nanoparticles catalysing CO oxidation. Nat. Mater. 2014, 13 (9), 884–890. 10.1038/nmat4033. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zakharov D.; Zhao S.; Tappero R.; Jung U.; Elsen A.; Baumann P.; Nuzzo R. G.; Stach E. A.; Frenkel A. I. Complex structural dynamics of nanocatalysts revealed in Operando conditions by correlated imaging and spectroscopy probes. Nat. Commun. 2015, 6, 7583. 10.1038/ncomms8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. C.; Zakharov D. N.; Arenal R.; Concepcion P.; Stach E. A.; Corma A. Evolution and stabilization of subnanometric metal species in confined space by in situ TEM. Nat. Commun. 2018, 9, 574. 10.1038/s41467-018-03012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Doronkin D. E.; Zhao Z. Y.; Plessow P. N.; Jelic J.; Detlefs B.; Pruessmann T.; Studt F.; Grunwaldt J. D. Photothermal Catalysis over Nonplasmonic Pt/TiO2 Studied by Operando HERFD-XANES, Resonant XES, and DRIFTS. ACS Catal. 2018, 8 (12), 11398–11406. 10.1021/acscatal.8b03724. [DOI] [Google Scholar]
- Carosso M.; Vottero E.; Lazzarini A.; Morandi S.; Manzoli M.; Lomachenko K. A.; Ruiz M. J.; Pellegrini R.; Lamberti C.; Piovano A.; Groppo E. Dynamics of Reactive Species and Reactant-Induced Reconstruction of Pt Clusters in Pt/Al2O3 Catalysts. ACS Catal. 2019, 9 (8), 7124–7136. 10.1021/acscatal.9b02079. [DOI] [Google Scholar]
- Ruano D.; Cored J.; Azenha C.; Perez-Dieste V.; Mendes A.; Mateos-Pedrero C.; Concepcion P. Dynamic Structure and Subsurface Oxygen Formation of a Working Copper Catalyst under Methanol Steam Reforming Conditions: An in Situ Time-Resolved Spectroscopic Study. ACS Catal. 2019, 9 (4), 2922–2930. 10.1021/acscatal.8b05042. [DOI] [Google Scholar]
- Corma A.; Concepcion P.; Boronat M.; Sabater M. J.; Navas J.; Yacaman M. J.; Larios E.; Posadas A.; Lopez-Quintela M. A.; Buceta D.; Mendoza E.; Guilera G.; Mayoral A. Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity. Nat. Chem. 2013, 5 (9), 775–781. 10.1038/nchem.1721. [DOI] [PubMed] [Google Scholar]
- Liu L. C.; Lopez-Haro M.; Lopes C. W.; Li C. G.; Concepcion P.; Simonelli L.; Calvino J. J.; Corma A. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nat. Mater. 2019, 18 (8), 866–873. 10.1038/s41563-019-0412-6. [DOI] [PubMed] [Google Scholar]
- Fortea-Perez F. R.; Mon M.; Ferrando-Soria J.; Boronat M.; Leyva-Perez A.; Corma A.; Herrera J. M.; Osadchii D.; Gascon J.; Armentano D.; Pardo E. The MOF-driven synthesis of supported palladium clusters with catalytic activity for carbene-mediated chemistry. Nat. Mater. 2017, 16 (7), 760–766. 10.1038/nmat4910. [DOI] [PubMed] [Google Scholar]
- Ji S. F.; Chen Y. J.; Fu Q.; Chen Y. F.; Dong J. C.; Chen W. X.; Li Z.; Wang Y.; Gu L.; He W.; Chen C.; Peng Q.; Huang Y.; Duan X. F.; Wang D. S.; Draxl C.; Li Y. D. Confined Pyrolysis within Metal-Organic Frameworks To Form Uniform Ru-3 Clusters for Efficient Oxidation of Alcohols. J. Am. Chem. Soc. 2017, 139 (29), 9795–9798. 10.1021/jacs.7b05018. [DOI] [PubMed] [Google Scholar]
- Hayden B. E. Particle Size and Support Effects in Electrocatalysis. Acc. Chem. Res. 2013, 46 (8), 1858–1866. 10.1021/ar400001n. [DOI] [PubMed] [Google Scholar]
- Chen X.; Chen D.; Weng M. Y.; Jiang Y.; Wei G. W.; Pan F. Topology-Based Machine Learning Strategy for Cluster Structure Prediction. J. Phys. Chem. Lett. 2020, 11 (11), 4392–4401. 10.1021/acs.jpclett.0c00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnouchi R.; Asahi R. Predicting Catalytic Activity of Nanoparticles by a DFT-Aided Machine-Learning Algorithm. J. Phys. Chem. Lett. 2017, 8 (17), 4279–4283. 10.1021/acs.jpclett.7b02010. [DOI] [PubMed] [Google Scholar]
- Burger B.; Maffettone P. M.; Gusev V. V.; Aitchison C. M.; Bai Y.; Wang X.; Li X.; Alston B. M.; Li B.; Clowes R.; Rankin N.; Harris B.; Sprick R. S.; Cooper A. I. A mobile robotic chemist. Nature 2020, 583 (7815), 237–241. 10.1038/s41586-020-2442-2. [DOI] [PubMed] [Google Scholar]
- Dou J.; Sun Z. C.; Opalade A. A.; Wang N.; Fu W. S.; Tao F. Operando chemistry of catalyst surfaces during catalysis. Chem. Soc. Rev. 2017, 46 (7), 2001–2027. 10.1039/C6CS00931J. [DOI] [PubMed] [Google Scholar]
- Tao F.; Crozier P. A. Atomic-Scale Observations of Catalyst Structures under Reaction Conditions and during Catalysis. Chem. Rev. 2016, 116 (6), 3487–3539. 10.1021/cr5002657. [DOI] [PubMed] [Google Scholar]
- Ledesma C.; Yang J.; Chen D.; Holmen A. Recent Approaches in Mechanistic and Kinetic Studies of Catalytic Reactions Using SSITKA Technique. ACS Catal. 2014, 4 (12), 4527–4547. 10.1021/cs501264f. [DOI] [Google Scholar]
- Shannon S. L.; Goodwin J. G. Characterization of Catalytic Surfaces by Isotopic-Transient Kinetics during Steady-State Reaction. Chem. Rev. 1995, 95 (3), 677–695. 10.1021/cr00035a011. [DOI] [Google Scholar]
- Fernandez E.; Boronat M. Sub nanometer clusters in catalysis. J. Phys-Condens Mat 2019, 31 (1), 013002. [DOI] [PubMed] [Google Scholar]
- Tyo E. C.; Vajda S. Catalysis by clusters with precise numbers of atoms. Nat. Nanotechnol. 2015, 10 (7), 577–588. 10.1038/nnano.2015.140. [DOI] [PubMed] [Google Scholar]