Abstract
Photosensitizers (PSs) inevitably release a large amount of energy in the form of fluorescence during photodynamic therapy (PDT). However, under the premise of satisfying fluorescence imaging, a large amount of energy is lost, which limits the efficiency of tumor therapy. Accordingly, in this study, we developed a new strategy (BDP-CR) using the single-molecule Förster resonance energy transfer (smFRET) mechanism to transfer part of the fluorescent energy into heat for combined PDT and photothermal therapy (PTT) featuring the “1 + 1 > 2” amplification effect. Under the 671 nm light irradiation, BDP-CR can produce singlet oxygen (1O2) for PDT based on the BDP moiety and also generate hyperthermia to achieve the PTT effect by exciting CR based on the smFRET effect, which effectively kills cancer cells both in vitro and in vivo. This strategy exhibits a broad absorption peak with strong light-harvesting ability, which improves photon utilization for treatment while realizing fluorescence imaging. Of note, owing to the smFRET effect, we achieve a combination treatment outcome at relatively low concentrations and light doses. Thus, we believe that this design concept will provide a new strategy for single-molecule FRET photosensitizers in combination therapy of cancer with potential clinical application prospects.
Short abstract
A new strategy (BDP-CR) using the smFRET mechanism to transfer part of the fluorescent energy into heat for combined PDT and PTT featuring the “1 + 1 > 2” amplification effect is presented.
Introduction
Phototherapy, involving photodynamic therapy (PDT) and photothermal therapy (PTT), has been acknowledged as one of the most representative therapeutic strategies for the treatment of cancer owing to its precision and noninvasiveness.1−4 For PDT, triplet photosensitizers (PSs) sensitize oxygen to generate high levels of reactive oxygen species (ROS) to kill cancer cells, which achieves tumor suppression through the apoptotic pathway.5−8 Thus, PDT is an oxygen-dependent process which limits its function owing to the hypoxic environment of the tumor.9,10 PTT is a typical light–heat energy conversion therapeutic strategy.11−13 PSs in the excited state return to the ground state through nonradiative transition with a release of heat, which induces local heat shock to kill tumor cells by the apoptosis and/or necrosis pathway without oxygen consumption.14,15 On the other hand, the low concentration and light dose of PS ensure its good biological safety in PDT.16−18 Comparatively, current PTT PSs exhibit strong absorption in the near-infrared (NIR) region, while the concentrations and light doses are relatively high.13 Clearly, the advantages and disadvantages of the two treatment methods are complementary. PDT can solve the problems of high concentrations and light doses in PTT, which improves treatment safety.19 Meanwhile, PTT can overcome the shortcomings of oxygen dependence in PDT to improve the holistic treatment effect.20 Hence, the combination of PDT and PTT is believed to be an alternative approach for improving the tumor treatment effect.21−24
Organic small-molecule PS exhibits excellent degradability, synthetic flexibility, and biocompatibility, which makes it suitable for in vivo cancer therapy.25−27 In most cases, the non-negligible radiative transition of PSs will release a large amount of energy in the form of fluorescence during the process of PDT.28,29 However, under the premise of satisfying fluorescence imaging, this part of the energy will undoubtedly impair nonradiative transition for generating heat, which limits the efficiency of tumor therapy. Reasonably using this part of energy so that it can be transferred to excite the photothermal part for PTT in an organic single-molecule system will improve photon utilization for treatment.30 In our previous work, we reported that the improvement of photon utilization could be achieved by hypoxia-induced treatment conversion from PDT to PTT.20 As an ideal alternative, single-molecule Förster resonance energy transfer (smFRET),31,32 one of the most promising organic molecular PS design strategies, has been applied to fluorescence imaging33,34 and fluorescence imaging-guided PDT.35,36 Motivated by these, we aimed to design a smFRET-based organic PS with in situ visualization for combined PDT and PTT.
Herein, for the first time, we report a smart molecular design method (BDP-CR) based on smFRET guidance, with an encouraging “1 + 1 > 2” effect for the combination of PDT and PTT of tumors. Diiododistyrylbodipy (BDP)37−40 and croconaine (CR)41,42 have been proven to be PDT and PTT PSs, respectively. Because the fluorescence of BDP and absorption of CR have an excellent spectral overlap, as a proof of concept, the BDP moiety was used as an energy donor to pair up with CR by click reaction to construct a smFRET system (Scheme 1). This new system broadens the spectral absorption range in the NIR region 600–850 nm, which improved the light harvesting ability. Under normoxic conditions, the absorbed photon energy is mainly released to sensitize oxygen to generate ROS for PDT and to excite the CR moiety via FRET for PTT. Under a severe hypoxic condition, photoenergy will be mainly used to kill cancer cells by O2-independent PTT. More significantly, owing to the intramolecular FRET effect, we achieve the synergistic therapy effect at relatively low concentrations and light doses, achieving a magnified effect of “1 + 1 > 2” both in vitro and in vivo. We believe that this design concept will provide a new strategy for small-molecule PSs in synergistic therapy for cancer.
Scheme 1. Schematic Illustration of the smFRET-Based Combination Phototherapy Mechanism (BDP-CR) as Well as Light-Triggered Cancer Cell Death.
Results and Discussion
Synthesis and Spectroscopic Properties
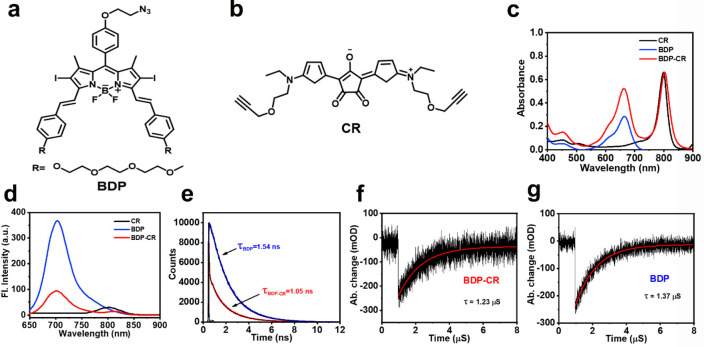
As shown in Scheme S1, BDP was connected to CR by a click reaction under mild conditions to form BDP-CR, and to better understand our concept, separate BDP and CR units were prepared as the controls (Figure 1a,b). The chemical structures of BDP-CR, BDP, CR, and all synthetic intermediates were fully confirmed by 1H NMR, 13C NMR, and ESI-MS analytical data (Figures S19–S32).
Figure 1.
Chemical structures of (a) BDP and (b) CR. (c) Absorption spectra, (d) fluorescence spectra, and (e) fluorescence decay curves of BDP and BDP-CR. Nanosecond transient absorption decay traces of (f) BDP-CR and (g) BDP at 660 nm in DMSO, λex = 470 nm.
The UV–vis absorbance spectrum in Figure 1c shows that BDP-CR exhibited a double absorption peak, which demonstrated the common absorption characteristics of BDP and CR (the molar extinction coefficients were ca. 2 × 105 M–1 cm–1). Furthermore, BDP-CR achieved a much broader absorption of 600–850 nm (compared to 600–700 nm for BDP), which indicated a stronger light-harvesting ability compared to that of free BDP. Upon 671 nm excitation, the fluorescence of BDP-CR at 702 nm was greatly quenched (Φf = 5.2%) and was much weaker than that of BDP (Φf = 27%) (Figure 1d and Table 1). CR had almost no fluorescence under irradiation (Φf = 0.2%). Meanwhile, the fluorescence lifetime of BDP-CR was also shorter than that of BDP, which further confirmed that efficient energy transfer occurred in BDP-CR (Figure 1e). Moreover, the nanosecond transient absorption decay traces demonstrated that the triplet-state lifetime of BDP-CR was similar to that of BDP (Figure 1f,g), which indicated that the smFRET effect did not interfere with the distribution of the T1 state, and the T1 state of BDP-CR was mainly attributed to the energy acceptor unit BDP but not the CR moiety.
Table 1. Photophysical Properties of BDP-CR, BDP, and CRa.
| molecule | λabs (nm) | λem (nm) | ε (× 104 M–1 cm–1) | Φf (%) | ΦΔ (%) |
|---|---|---|---|---|---|
| BDP-CR | 665 + 804 | 702 | 17.3 (665 nm); 22.1 (804 nm) | 5.2 | 42.3 |
| BDP | 665 | 704 | 9.52 (665 nm) | 27 | 55.8 |
| CR | 796 | 802 | 22.1 (796 nm) | 0.2 | 0.85 |
λabs: absorption maximum wavelength (nm). λem: emission maximum wavelength (nm). ε: molar extinction coefficient (104 M–1 cm–1). Φf: fluorescence quantum yield. ΦΔ: singlet oxygen quantum yield.
Then, we used 1,3-diphenylisobenzofuran (DPBF) as a probe to test their 1O2 generation capacity. As shown in Figure 2a, the irradiation of a solution containing BDP-CR and DPBF with 671 nm light led to an apparent decrease in the DPBF absorbance intensity (λabs = 415 nm) over a period of 120 s, which confirmed that 1O2 was generated. According to the decay curves of DPBF, the 1O2 yield of BDP-CR (ΦΔBDP-CR) was calculated to be 42.3% using MB as the reference (ΦΔMB = 52% in DMSO)43 which was similar to that of BDP. However, there was no sign that 1O2 could be generated by CR (ΦΔCR = 0.85%) under identical experimental conditions (Figure 2b, Figure S1, and Table 1). The obtained result demonstrated that the 1O2 generation of BDP-CR was attributed to the BDP moiety. Hence, the connection of CR does not affect BDP-CR to generate 1O2, which can be used for killing tumor cells during PDT.
Figure 2.
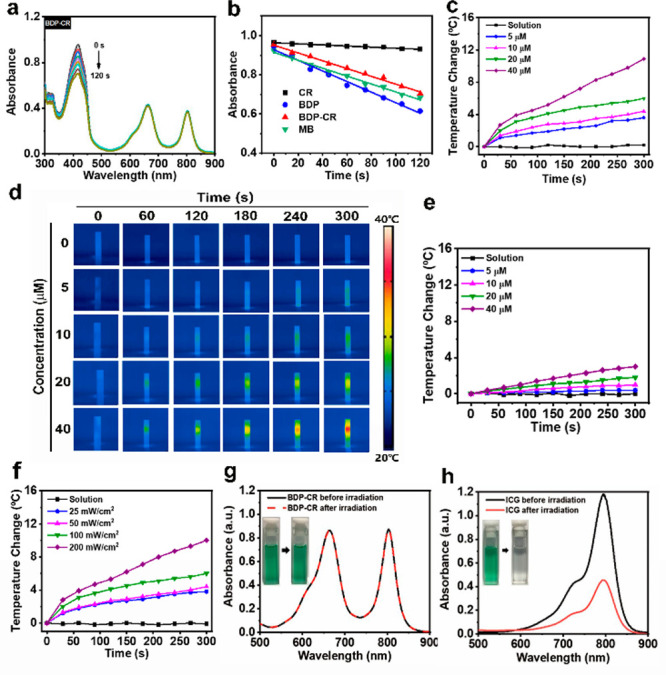
(a) Photodegradation curves of 1,3-diphenylisobenzofuran (DPBF) in the presence of BDP-CR under 671 nm irradiation. (b) DPBF absorbance change at 415 nm after the MB, CR, BDP, and BDP-CR were treated with 671 nm irradiation. Temperature change of (c) BDP-CR and (e) BDP in the PBS buffer solutions (0.01 M PBS, PBS/DMSO = 2:1 v/v, pH = 7.4) at various concentrations (0, 5, 10, 20, and 40 μM) under 100 mW/cm2 irradiation (671 nm). (d) Photothermal imaging of part c. (f) Photothermal heating curves for the change of temperature with time when BDP-CR (20 μM) was irradiated by 671 nm light at different power densities (0, 25, 50, 100, and 200 mW/cm2). (g) Absorption spectra of BDP-CR pre- and post-671 nm light irradiation (200 mW/cm2, 10 min). Insets are photographs of BDP-CR dispersion before (left) and after (right) light irradiation. (h) Absorption spectra of ICG solutions pre- and post-808 nm light irradiation (200 mW/cm2, 10 min). Insets are photographs of ICG solution before (left) and after (right) light irradiation.
We further examined the photothermal properties of BDP-CR by monitoring their temperature changes over time in PBS buffer solution (0.01 M PBS, PBS/DMSO = 2:1 v/v, pH = 7.4) under different power densities and at different concentrations. The temperature of the BDP-CR solution was increased by ca. 24 °C upon irradiation with a 671 nm light (100 mW/cm2) for 5 min. Furthermore, the solution temperature gradually increased with an increase of BDP-CR concentrations from 0 to 40 μM, as shown by IR thermal imaging (Figure 2c,d). The temperature increase of BDP-CR was much higher than that of BDP or CR after light irradiation (671 nm, 100 mW/cm2, 5 min) under the same concentration, yielding the “1 + 1 > 2” effect (Figure 2e, Figure S2). These data indicated that energy was transferred from BDP to CR to generate heat. The concentration of BDP-CR and light power density were considerably lower than other small-molecule photothermal reagents (ca. 500 μM and 1 W/cm2), which met the requirement of achieving a low concentration and light dose for PTT.10,44 Similarly, the temperature increase of the BDP-CR solution (20 μM) with a 671 nm light irradiation at different power densities from 0 to 200 mW/cm2 was considerably higher than those of BDP or CR (Figure 2f, Figure S3). Clearly, the smFRET effect of BDP-CR was critical for generating the photothermal effect, which indicated that BDP-CR could be used as a photothermal agent. The photothermal conversion efficiency (η) of BDP-CR was approximately 22.42%, which further indicated that an efficient photon utilization for heating was considerably improved by the smFRET effect (Figure S4).
Indocyanine green (ICG), which is a typical FDA-approved photothermal agent, was used to investigate the photostability of BDP-CR by checking their resistance against photobleaching. BDP-CR was irradiated with a 671 or 808 nm light (100 mW/cm2) for 5 min, while ICG was irradiated with an 808 nm light under the same conditions. Subsequently, the absorption spectra of BDP-CR and ICG pre- and postirradiation were measured. No absorption and color changes were observed for BDP-CR after 671 or 808 nm light irradiation, whereas the absorption of ICG considerably decreased, which was accompanied by the solution color change from green to colorless (Figure 2g,h and Figure S5). Furthermore, BDP-CR exhibited no degradation after five irradiation–cooling cycles (Figure S6). Both above-mentioned results confirmed the excellent photostability of BDP-CR.
In Vitro Application
The biocompatibility and synergistic treatment effect of BDP-CR were evaluated in vitro. The cancer cells’ uptake of BDP-CR and BDP was examined by fluorescence confocal microscopy. As expected, the red fluorescence of BDP-CR in 4T1 cells was weaker than that of BDP after 4 h of incubation, which was consistent with the result of solution tests, revealing that the fluorescence of BDP-CR was partially quenched (Figure S7). Subsequently, the subcellular localization shown in fluorescence images (Figure S8) revealed that BDP-CR overlapped well with the lysosomal staining agent LysoTracker (Pearson’s coefficient 0.86), which was considerably better than the mitochondria (0.27) and nucleus (0.02). Thus, these results demonstrated that BDP-CR was efficiently taken up by the cancer cells.
Next, the in vitro killing effect of cancer cells by BDP-CR under both normoxic and hypoxic conditions was tested using a methyl thiazolyl tetrazolium (MTT) assay. As shown in Figure 3a,d, all the cell viabilities were approximately 90% when the 4T1 cells were incubated with BDP-CR, BDP + CR (mixture of BDP and CR), BDP, or CR under both normoxic and hypoxic conditions in the absence of irradiation, respectively, indicating their negligible biological toxicity and excellent biocompatibility. After the cells were incubated with BDP-CR under normoxia with 671 nm light irradiation (100 mW/cm2) for 5 min, cell viability decreased to 30%, which was significantly lower than that of cells incubated with BDP, CR, or BDP + CR, which suggested a considerable improvement in the killing effect of cancer cells (Figure 3b). Under hypoxic conditions with 671 nm light irradiation, the cancer cell killing effect of BDP-CR was significantly better than that of cells incubated with BDP + CR, BDP, or CR, which demonstrated that BDP-CR had a considerable killing effect on cancer cells under hypoxia (Figure 3e). Similar results were also obtained in MCF-7, HeLa, and B16 cell lines under normoxia (Figure S9). These results revealed that the light utilization efficiency was improved owing to the smFRET effect, which clearly confirmed the superior “1 + 1 > 2” effect of BDP-CR. Subsequently, 4T1 cells were stained with propidium iodide (PI, red fluorescence) and calcein-AM (green fluorescence) to observe dead and live cells under both normoxic and hypoxic conditions. Clearly, nearly all cells treated with BDP-CR under 671 nm light irradiation showed strong red fluorescence revealed as dead cells, which was more than that treated with BDP under 671 nm light irradiation. In comparison, very weak red fluorescent signals and strong green fluorescence were observed in the other groups, which indicated live cells (Figure 3f). The excellent killing effect was consistent with other cancer cells under the same conditions (Figure S10). To further confirm the synergistic killing effect of BDP-CR, the cell viability of PDT or PTT was investigated separately. 4T1 cells were incubated with BDP-CR for 4 h and were then irradiated with a 671 nm light (100 mW/cm2) on ice to decrease the effect of PTT on cell viability. The results showed a 43.72 ± 2.52% cell survival based only on the PDT effect of the BDP-CR treatment. When 4T1 cells were pretreated with NAC (an effective ROS scavenger) and then incubated with BDP-CR under 671 nm light irradiation (i.e., the PDT effect was removed), the PTT effect resulted in 52.27 ± 1.92% cell survival. However, the cell viability owing to the synergistic PDT/PTT effect (i.e., cells treated with BDP-CR and irradiated with a 671 nm light without NAC pretreatment) decreased to only 30%, which achieved a better cancer cell killing effect of combination therapy (Figure 3c). These results demonstrated that a synergistic PDT/PTT mechanism was involved in the killing of cancer cells. To determine whether broadband absorption utilization facilitated the enhancement of the cell killing effect, we used 671 or 808 nm as the representative wavelengths of energy donors and acceptors, respectively. Interestingly, when 4T1 cells were exposed to an 808 nm light, a certain killing effect was exhibited on cancer cells, but cell viability was higher than irradiation with a 671 nm light under all concentrations (Figure S11). All of these results confirmed that our strategy considerably improved the killing effect in vitro by generating the photothermal effect to achieve the combined therapy.
Figure 3.
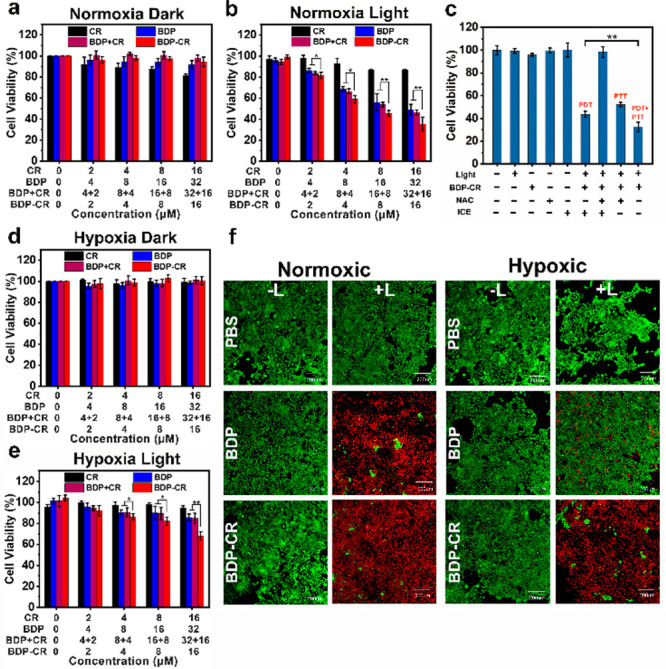
Ex vivo survival rate of 4T1 cells after incubation with CR, BDP, BDP + CR, and BDP-CR at different concentrations in the dark (a, d) or with 671 nm light (100 mW/cm2) irradiation (b, e) for 5 min under normoxia and hypoxia conditions. (c) Cytotoxicity evaluation of 4T1 cells with BDP-CR treatment under different conditions in normoxia. (f) Calcein AM and propidium iodide costaining fluorescence imaging in 4T1 cells under normoxia and hypoxia conditions. Scale bar: 200 μm. Statistical significance: *P< 0.05 and **P< 0.01.
Then, we investigated the mechanism of photoinduced cytotoxicity. To verify that BDP-CR can produce 1O2 at the cellular level, 2,7-dichlorofluorescein diacetate (DCFH-DA) was used as the probe to detect cellular ROS production. An obvious intracellular green fluorescence was detected in the presence of BDP-CR with light irradiation, which indicated effective 1O2 generation. When the phototherapy group was pretreated with NAC, almost no fluorescence was observed, suggesting that produced ROS was 1O2 (Figure 4a). The obtained result demonstrated that light irradiation of BDP-CR induced an effective 1O2 production, which indicated the capability for PDT in vitro. Recently, several studies have reported that phototherapy can induce intracellular organelles’ disruption by triggering cancer cell apoptosis.45−47 Because of the lysosomal localization property, acridine orange (AO) was used as the lysosomal integrity indicator to investigate the BDP-CR-mediated lysosome destruction. A massive red fluorescence of AO was observed in the “Control”, “BDP-CR”, and “Light” groups, which indicated that lysosomes were still intact. However, the integrity of lysosomes was considerably destroyed, as identified by the AO red fluorescence that disappeared after incubation with BDP-CR and 671 nm light irradiation (Figure 4b). Therefore, the lysosome integrity was severely disrupted by BDP-CR phototherapy and subsequently resulted in cancer cell apoptosis and death.
Figure 4.
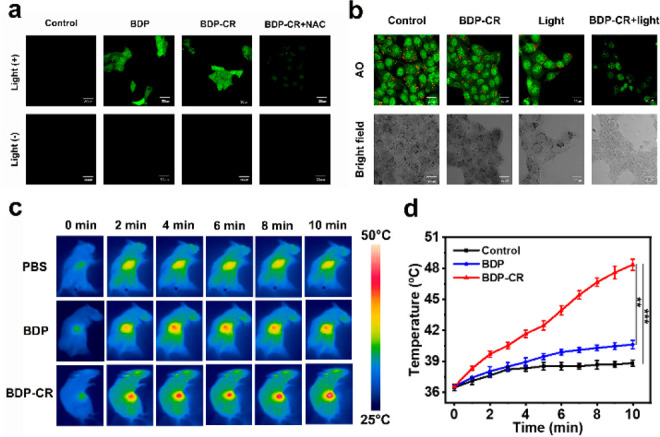
(a) Evaluation of 1O2 generation in 4T1 cells. (b) CLSM images of AO staining for lysosomal integrity. (c) IR thermal images of 4T1 tumor-bearing mice following 671 nm laser (0.3 W/cm2) irradiation at different time points. The laser exposure was performed at 4 h after injection of 100 μL of BDP-CR, BDP, or PBS. (d) Temperature changes at the tumor sites as a function of the 671 nm laser (0.3 W/cm2) irradiating time, which is the quantitative data of part c. Scale bars: 30 μm. Error bars, mean ± SD (n = 4), **P < 0.01, ***P< 0.001 determined by the Student’s t test.
Thereafter, we adopted annexin V-FITC/propidium iodide (PI) reagents as fluorescent agents to investigate the cell apoptosis and death pathway (Figure S12). AV staining (green fluorescence) indicated the early stage of cell apoptosis or necrosis, and PI staining (red fluorescence) of the nucleus indicated late apoptotic or dead cells. After incubating 4T1 cells with BDP-CR or BDP (10 μM) for 4 h, no fluorescence was observed, demonstrating that cells were alive. Inversely, intense green and red fluorescence was observed from the BDP-CR and BDP treated cells after 671 nm light irradiation, which indicated that most cancer cells underwent late apoptosis or necrosis states. Similar results were observed in an annexin V-FITC/PI apoptosis assay by flow cytometry (Figure S13). The apoptosis or necrosis ratios for cells treated with BDP plus 671 nm light irradiation and BDP-CR plus 671 nm light irradiation were 43.5% and 90%, respectively. Simultaneously, no killing effect was observed for 4T1 cells treated with light or photosensitizers alone (cell viability >98%). Thus, we demonstrated that the tumor cell inhibition ability of BDP-CR with the combination of PDT and PTT was higher than that of BDP with single-mode PDT, suggesting the considerable improvement in killing efficacy of cancer cells by the smFRET effect.
In Vivo Application
Based on the promising in vitro results, we further explored the feasibility of BDP-CRin vivo for tumor therapy. After directly injecting BDP and BDP-CR, 4T1 tumor-bearing BALB/c mice were observed by fluorescence imaging. The fluorescence intensity of BDP-CR at the tumor gradually increased and reached the maximum at ca. 4 h postinjection, which was similar to BDP (Figure S14). To verify the photothermal effects of different agents, three tumor mouse model groups, “PBS + 671 nm light”, “BDP + 671 nm light”, and “BDP-CR + 671 nm light”, were injected with PBS, BDP, or BDP-CR, respectively. Then, 4 h (according to fluorescence imaging) after injection, the tumors for each group were continuously irradiated with a 671 nm light (0.3 W/cm2) for 10 min, and the treatment process was recorded by a thermal imaging camera every minute (Figure 4c,d). The obtained results indicated that the temperature of tumors in the “BDP-CR + 671 nm light” group rapidly increased to a much higher temperature attributing to the smFRET effect. Negligible temperature variation (ΔT ≈ 5 °C) was observed for the tumors in “BDP + 671 nm light” groups. The temperature of blank PBS (pH 7.4) buffer barely changed. Actually, the temperature not exceeding 42 °C was unable to efficiently kill tumor cells by the photothermal effect.48 Clearly, BDP-CR demonstrated an effective in vivo photothermal conversion for PTT.
To verify the antitumor effect of BDP-CRin vivo, 4T1 tumor-bearing mice were divided into eight treatment groups, including “PBS”, “PBS + 671 nm light”, “CR”, “BDP”, “BDP-CR”, “BDP + 671 nm light”, “BDP-CR + 808 nm light”, and “BDP-CR + 671 nm light.” Then, mice in the eight groups were directly injected with different reagents. The in vivo antitumor effects of different treatments were evaluated according to the change in tumor volume over 21 days (calculated from the next day after treatments). The 4T1 tumor-bearing mice from the “PBS + 671 nm light”, “BDP + 671 nm light”, and “BDP-CR + 671 nm light” groups were irradiated with a 671 nm light (0.3 W/cm2) for 10 min. As shown in Figure 5a, the “PBS + 671 nm light” mice showed a tumor growth of ca. 9-fold, which was similar to that of the PBS group. Owing to their negligible dark toxicities, the “CR”, “BDP”, and “BDP-CR” groups also exhibited no tumor suppression. On the 21st day after irradiation, the tumor volume increased by 4-fold in the “BDP + 671 nm light” group owing to the single PDT effect. The “BDP-CR + 671 nm light” group could reduce tumor volume by treatment and successfully inhibited tumors with only one treatment, which indicated the excellent synergistic treatment effect by the smFRET effect (Figure S15). Furthermore, tumors in the “BDP-CR + 808 nm light” group achieved a certain degree of tumor inhibition through a single PTT effect, whereas the “BDP-CR + 671 nm light” group completely inhibited the tumor growth, which indicated that the superb in vivo synergistic therapy outcome of BDP-CR could be obtained by broadband absorption as a result of the smFRET effect (Figure 5a,d and Figure S16). Clearly, different treatment effects can be seen from the average tumor weights, which showed the excellent antitumor effect (ca. 0.7-fold) of BDP-CR combined with light irradiation (Figure 5b). In addition, no changes in body weight were observed for any groups (Figure 5c).
Figure 5.

(a) Relative tumor volume (tumor volume divided by initial volume) in various mouse groups. (b) Tumor weight for mice in different treatment groups. (c) Changes in the weights of mice in different groups over time. (d) Images of mice and tumors with different treatments at 21 days post-treatment. (e) H&E stained tumor sections from the indicated treatment groups and ROS measurement via DCFH-DA staining in tumor sections. Scale bars = 100 μm. Different groups: (I) PBS; (II) PBS + 671 nm light; (III) CR; (IV) BDP; (V) BDP-CR; (VI) BDP+ 6 71 nm light; (VII) BDP-CR + 808 nm light; and (VIII) BDP-CR + 671 nm light. Data are shown as mean ± SD (n = 5), ***P < 0.001, ****P < 0.0001 determined by the Student’s t test.
To evaluate the biosafety and lack of side effects of phototherapy agents, the major organs of each group, including heart, liver, spleen, lung, kidney, and tumor tissues, were prepared for hematoxylin and eosin (H&E) staining. No obvious damage or toxicity was observed in the tissues except for the tumors, demonstrating the biosafety of the agents for normal tissues (Figure S17). However, the tumor slices of “BDP + 671 nm light”, “BDP-CR + 808 nm light”, and “BDP-CR + 671 nm light” groups showed an obvious necrosis, with that for the “BDP-CR + 671 nm light” group being more conspicuous (Figure 5e). The hemolysis properties of BDP-CR also indicated that it had excellent biological safety (Figure S18). 2,7-Dichlorodi-hydrofluorescein diacetate (DCFH-DA) was used for measuring intratumoral ROS production in these treated mice, which showed a considerable green fluorescence in the tumors of the “BDP-CR + 671 nm light” group. This result confirmed that BDP-CR could generate a large amount of 1O2 after light irradiation at the tumor site for PDT. Therefore, the smFRET-guided synergistic PDT and PTT mechanism for tumor treatment was successfully verified, indicating its potential clinical application significance.
Conclusion
In summary, we developed a new strategy for designing a smart PS based on the smFRET mechanism for tumor PDT and PTT combined therapy with an encouraging amplification effect of “1 + 1 > 2.” This new system considerably broadens the spectral absorption range in the NIR region, which improves the light harvesting ability. After 671 nm light irradiation, BDP-CR exhibits an excellent 1O2 generation capability, and part of the fluorescence energy is converted into heat at relatively low concentrations and light doses. Definitely, this strategy allows more absorbed energy to be used for treatment, which considerably improves the treatment effect as well as overcomes insufficient photon utilization efficiency of traditional photodynamic PSs and the limitation of oxygen dependence in PDT. Both in vitro and in vivo experiments achieved a considerable improvement in antitumor effect through the smFRET effect compared with single BDP. Furthermore, the mice were observed to be in good physiological condition after the treatment. Therefore, we strongly believe that this new PS design strategy has enormous potential significance for clinical cancer phototherapy.
Acknowledgments
This work was financially supported by the National Science Foundation of China (21925802, 21878039, 21808028, 22022803, 22078046), the NSFC-Liaoning United Fund (U1908202), and National Key Research and Development Plan (2018AAA0100301).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.0c01551.
Reagents, instruments, and methods; full synthetic and characterization details; additional spectral and in vitro data; in vivo fluorescence and photothermal imaging; and tumor treatment (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Xie Z.; Fan T.; An J.; Choi W.; Duo Y.; Ge Y.; Zhang B.; Nie G.; Xie N.; Zheng T.; Chen Y.; Zhang H.; Kim J. S. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. 10.1039/D0CS00215A. [DOI] [PubMed] [Google Scholar]
- He Z.; Zhao L.; Zhang Q.; Chang M.; Li C.; Zhang H.; Lu Y.; Chen Y. An Acceptor-Donor-Acceptor Structured Small Molecule for Effective NIR Triggered Dual Phototherapy of Cancer. Adv. Funct. Mater. 2020, 30, 1910301. 10.1002/adfm.201910301. [DOI] [Google Scholar]
- Zhang L.; Wang D.; Yang K.; Sheng D.; Tan B.; Wang Z.; Ran H.; Yi H.; Zhong Y.; Lin H.; Chen Y. Mitochondria-Targeted Artificial “Nano-RBCs” for Amplified Synergistic Cancer Phototherapy by a Single NIR Irradiation. Adv. Sci. 2018, 5, 1800049. 10.1002/advs.201800049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S.; Tan X.; Fang S.; Wang Y.; Liu T.; Wang X.; Yuan Y.; Sun H.; Qi Q.; Shi C. Mitochondria-Targeted Small-Molecule Fluorophores for Dual Modal Cancer Phototherapy. Adv. Funct. Mater. 2016, 26, 2826–2835. 10.1002/adfm.201600159. [DOI] [Google Scholar]
- Deng W.; McKelvey K. J.; Guller A.; Fayzullin A.; Campbell J. M.; Clement S.; Habibalahi A.; Wargocka Z.; Liang L.; Shen C.; Howell V. M.; Engel A. F.; Goldys E. M. Application of Mitochondrially Targeted Nanoconstructs to Neoadjuvant X-ray-Induced Photodynamic Therapy for Rectal Cancer. ACS Cent. Sci. 2020, 6, 715–726. 10.1021/acscentsci.9b01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S. S.; Zhang L.; Zhang X. Z. An ATP-Regulated Ion Transport Nanosystem for Homeostatic Perturbation Therapy and Sensitizing Photodynamic Therapy by Autophagy Inhibition of Tumors. ACS Cent. Sci. 2019, 5, 327–340. 10.1021/acscentsci.8b00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Li M.; Sun W.; Fan J.; Du J.; Peng X. An estrogen receptor targeted ruthenium complex as a two-photon photodynamic therapy agent for breast cancer cells. Chem. Commun. 2018, 54, 7038–7041. 10.1039/C8CC03786H. [DOI] [PubMed] [Google Scholar]
- Li X.; Kim C. Y.; Lee S.; Lee D.; Chung H. M.; Kim G.; Heo S. H.; Kim C.; Hong K. S.; Yoon J. Nanostructured Phthalocyanine Assemblies with Protein-Driven Switchable Photoactivities for Biophotonic Imaging and Therapy. J. Am. Chem. Soc. 2017, 139, 10880–10886. 10.1021/jacs.7b05916. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Liang H.; Jiang P.; Zhang K. Y.; Liu S.; Yang T.; Zhao Q.; Yang L.; Lv W.; Yu Q.; Huang W. Multifunctional Phosphorescent Conjugated Polymer Dots for Hypoxia Imaging and Photodynamic Therapy of Cancer Cells. Adv. Sci. 2016, 3, 1500155. 10.1002/advs.201500155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. N.; Qi S.; Kim S.; Kwon N.; Kim G.; Yim Y.; Park S.; Yoon J. An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enhanced Photodynamic Therapy under Hypoxia. J. Am. Chem. Soc. 2019, 141, 16243–16248. 10.1021/jacs.9b09220. [DOI] [PubMed] [Google Scholar]
- Jung H. S.; Lee J. H.; Kim K.; Koo S.; Verwilst P.; Sessler J. L.; Kang C.; Kim J. S. A Mitochondria-Targeted Cryptocyanine-Based Photothermogenic Photosensitizer. J. Am. Chem. Soc. 2017, 139, 9972–9978. 10.1021/jacs.7b04263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y.; Li M.; Xiong T.; Zhao X.; Du J.; Fan J.; Peng X. A Single Molecule Drug Targeting Photosensitizer for Enhanced Breast Cancer Photothermal Therapy. Small 2020, 16, 1907677. 10.1002/smll.201907677. [DOI] [PubMed] [Google Scholar]
- Xi D.; Xiao M.; Cao J.; Zhao L.; Xu N.; Long S.; Fan J.; Shao K.; Sun W.; Yan X.; Peng X. NIR Light-Driving Barrier-Free Group Rotation in Nanoparticles with an 88.3% Photothermal Conversion Efficiency for Photothermal Therapy. Adv. Mater. 2020, 32, 1907855. 10.1002/adma.201907855. [DOI] [PubMed] [Google Scholar]
- Jung H. S.; Verwilst P.; Sharma A.; Shin J.; Sessler J. L.; Kim J. S. Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. 10.1039/C7CS00522A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Chang J.; Shi M.; Pan W.; Li N.; Tang B. A Dual-Targeted Organic Photothermal Agent for Enhanced Photothermal Therapy. Angew. Chem., Int. Ed. 2019, 58, 1057–1061. 10.1002/anie.201811273. [DOI] [PubMed] [Google Scholar]
- Cui D.; Huang J.; Zhen X.; Li J.; Jiang Y.; Pu K. A Semiconducting Polymer Nano-prodrug for Hypoxia-Activated Photodynamic Cancer Therapy. Angew. Chem., Int. Ed. 2019, 58, 5920–5924. 10.1002/anie.201814730. [DOI] [PubMed] [Google Scholar]
- Miao X.; Hu W.; He T.; Tao H.; Wang Q.; Chen R.; Jin L.; Zhao H.; Lu X.; Fan Q.; Huang W. Deciphering the intersystem crossing in near-infrared BODIPY photosensitizers for highly efficient photodynamic therapy. Chem. Sci. 2019, 10, 3096–3102. 10.1039/C8SC04840A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Kolemen S.; Yoon J.; Akkaya E. U. Activatable Photosensitizers: Agents for Selective Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1604053. 10.1002/adfm.201604053. [DOI] [Google Scholar]
- Tang Q.; Cheng Z.; Yang N.; Li Q.; Wang P.; Chen D.; Wang W.; Song X.; Dong X. Hydrangea-structured tumor microenvironment responsive degradable nanoplatform for hypoxic tumor multimodal imaging and therapy. Biomaterials 2019, 205, 1–10. 10.1016/j.biomaterials.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Long S.; Li M.; Cao J.; Li Y.; Guo L.; Sun W.; Du J.; Fan J.; Peng X. Oxygen-Dependent Regulation of Excited-State Deactivation Process of Rational Photosensitizer for Smart Phototherapy. J. Am. Chem. Soc. 2020, 142, 1510–1517. 10.1021/jacs.9b11800. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Yang X. Q.; Wei J. S.; Li X.; Wang H.; Zhao Y. D. Intelligent gold nanostars for in vivo CT imaging and catalase-enhanced synergistic photodynamic & photothermal tumor therapy. Theranostics 2019, 9, 5424–5442. 10.7150/thno.33015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W.; Yung B.; Huang P.; Chen X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. 10.1021/acs.chemrev.7b00258. [DOI] [PubMed] [Google Scholar]
- Feng J.; Yu W.; Xu Z.; Wang F. An intelligent ZIF-8-gated polydopamine nanoplatform for in vivo cooperatively enhanced combination phototherapy. Chem. Sci. 2020, 11, 1649–1656. 10.1039/C9SC06337D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Gong N.; Li Y.; Lu Q.; Wang X.; Li J. Atomic-Level Nanorings (A-NRs) Therapeutic Agent for Photoacoustic Imaging and Photothermal/Photodynamic Therapy of Cancer. J. Am. Chem. Soc. 2020, 142, 1735–1739. 10.1021/jacs.9b11553. [DOI] [PubMed] [Google Scholar]
- Tian R.; Sun W.; Li M.; Long S.; Li M.; Fan J.; Guo L.; Peng X. Development of a novel anti-tumor theranostic platform: a near-infrared molecular upconversion sensitizer for deep-seated cancer photodynamic therapy. Chem. Sci. 2019, 10, 10106–10112. 10.1039/C9SC04034J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M.; Zhao S.; Liu W.; Lee C. S.; Zhang W.; Wang P. Photosensitizers for Photodynamic Therapy. Adv. Healthcare Mater. 2019, 8, 1900132. 10.1002/adhm.201900132. [DOI] [PubMed] [Google Scholar]
- Li M.; Long S.; Kang Y.; Guo L.; Wang J.; Fan J.; Du J.; Peng X. De Novo Design of Phototheranostic Sensitizers Based on Structure-Inherent Targeting for Enhanced Cancer Ablation. J. Am. Chem. Soc. 2018, 140, 15820–15826. 10.1021/jacs.8b09117. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Yang Y.; Yu Y.; Guo S.; Wang W.; Zhu S. A cyanine-derivative photosensitizer with enhanced photostability for mitochondria-targeted photodynamic therapy. Chem. Commun. 2019, 55, 13542–13545. 10.1039/C9CC06157F. [DOI] [PubMed] [Google Scholar]
- Zou J.; Yin Z.; Wang P.; Chen D.; Shao J.; Zhang Q.; Sun L.; Huang W.; Dong X. Photosensitizer synergistic effects: D-A-D structured organic molecule with enhanced fluorescence and singlet oxygen quantum yield for photodynamic therapy. Chem. Sci. 2018, 9, 2188–2194. 10.1039/C7SC04694D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.; Zou J.; Zhang J.; Sun Y.; Dong X.; Zhang Q. An anthracene functionalized BODIPY derivative with singlet oxygen storage ability for photothermal and continuous photodynamic synergistic therapy. J. Mater. Chem. B 2019, 7, 3303–3309. 10.1039/C9TB00180H. [DOI] [Google Scholar]
- Hohlbein J.; Craggs T. D.; Cordes T. Alternating-laser excitation: single-molecule FRET and beyond. Chem. Soc. Rev. 2014, 43, 1156–1171. 10.1039/C3CS60233H. [DOI] [PubMed] [Google Scholar]
- Uphoff S.; Holden S. J.; Le Reste L.; Periz J.; van de Linde S.; Heilemann M.; Kapanidis A. N. Monitoring multiple distances within a single molecule using switchable FRET. Nat. Methods 2010, 7, 831–836. 10.1038/nmeth.1502. [DOI] [PubMed] [Google Scholar]
- Wu L.; Huang C.; Emery B. P.; Sedgwick A. C.; Bull S. D.; He X. P.; Tian H.; Yoon J.; Sessler J. L.; James T. D. Forster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. 10.1039/C9CS00318E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L.; Lin W.; Zheng K.; Zhu S. FRET-Based Small-Molecule Fluorescent Probes: Rational Design and Bioimaging Applications. Acc. Chem. Res. 2013, 46, 1462–1473. 10.1021/ar300273v. [DOI] [PubMed] [Google Scholar]
- Li M.; Xiong T.; Du J.; Tian R.; Xiao M.; Guo L.; Long S.; Fan J.; Sun W.; Shao K.; Song X.; Foley J. W.; Peng X. Superoxide Radical Photogenerator with Amplification Effect: Surmounting the Achilles’ Heels of Photodynamic Oncotherapy. J. Am. Chem. Soc. 2019, 141, 2695–2702. 10.1021/jacs.8b13141. [DOI] [PubMed] [Google Scholar]
- Huang L.; Li Z.; Zhao Y.; Yang J.; Yang Y.; Pendharkar A. I.; Zhang Y.; Kelmar S.; Chen L.; Wu W.; Zhao J.; Han G. Enhancing Photodynamic Therapy through Resonance Energy Transfer Constructed Near-Infrared Photosensitized Nanoparticles. Adv. Mater. 2017, 29, 1604789. 10.1002/adma.201604789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamkaew A.; Lim S. H.; Lee H. B.; Kiew L. V.; Chung L. Y.; Burgess K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. 10.1039/C2CS35216H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudet A.; Burgess K. BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- Erbas S.; Gorgulu A.; Kocakusakogullari M.; Akkaya E. U. Non-covalent functionalized SWNTs as delivery agents for novel Bodipy-based potential PDT sensitizers. Chem. Commun. 2009, 4956–4958. 10.1039/b908485a. [DOI] [PubMed] [Google Scholar]
- Huang L.; Li Z.; Zhao Y.; Zhang Y.; Wu S.; Zhao J.; Han G. Ultralow-Power Near Infrared Lamp Light Operable Targeted Organic Nanoparticle Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 14586–14591. 10.1021/jacs.6b05390. [DOI] [PubMed] [Google Scholar]
- Spence G. T.; Hartland G. V.; Smith B. D. Activated photothermal heating using croconaine dyes. Chem. Sci. 2013, 4, 4240–4244. 10.1039/c3sc51978c. [DOI] [Google Scholar]
- Tang L.; Zhang F.; Yu F.; Sun W.; Song M.; Chen X.; Zhang X.; Sun X. Croconaine nanoparticles with enhanced tumor accumulation for multimodality cancer theranostics. Biomaterials 2017, 129, 28–36. 10.1016/j.biomaterials.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Mirenda M.; Strassert C. A.; Dicelio L. E.; San Roman E. Dye-polyelectrolyte layer-by-layer self-assembled materials: molecular aggregation, structural stability, and singlet oxygen photogeneration. ACS Appl. Mater. Interfaces 2010, 2, 1556–1560. 10.1021/am100195v. [DOI] [PubMed] [Google Scholar]
- Lin H.; Gao S.; Dai C.; Chen Y.; Shi J. A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. 10.1021/jacs.7b07818. [DOI] [PubMed] [Google Scholar]
- Tian J.; Zhou J.; Shen Z.; Ding L.; Yu J. S.; Ju H. A pH-activatable and aniline-substituted photosensitizer for near-infrared cancer theranostics. Chem. Sci. 2015, 6, 5969–5977. 10.1039/C5SC01721A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J.; Ding L.; Xu H. J.; Shen Z.; Ju H.; Jia L.; Bao L.; Yu J. S. Cell-specific and pH-activatable rubyrin-loaded nanoparticles for highly selective near-infrared photodynamic therapy against cancer. J. Am. Chem. Soc. 2013, 135, 18850–18858. 10.1021/ja408286k. [DOI] [PubMed] [Google Scholar]
- Chakrabortty S.; Agrawalla B. K.; Stumper A.; Vegi N. M.; Fischer S.; Reichardt C.; Kogler M.; Dietzek B.; Feuring-Buske M.; Buske C.; Rau S.; Weil T. Mitochondria Targeted Protein-Ruthenium Photosensitizer for Efficient Photodynamic Applications. J. Am. Chem. Soc. 2017, 139, 2512–2519. 10.1021/jacs.6b13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Fan X.; Li L.; Yang Y.; Nuernisha A.; Xue D.; He C.; Qian J.; Hu Q.; Chen H.; Liu J.; Huang W. Semiconducting Polymer Nanoparticles as Theranostic System for Near-Infrared-II Fluorescence Imaging and Photothermal Therapy under Safe Laser Fluence. ACS Nano 2020, 14, 2509–2521. 10.1021/acsnano.0c00043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.