Abstract
The expression of clock genes, regulating the synchronization of metabolic and behavioral processes with environmental light/dark cycles, is regulated by methylation and might be influenced by short-term exposure to airborne particulate matter (PM), especially in individuals that are hypersensitive to proinflammatory cues. The present study aimed to evaluate the effects of PM2.5 and PM10 on the methylation profile of the clock genes ARNTL, CLOCK, CRY1, CRY2, PER1, PER2, and PER3 in a population of 200 women with obesity. A significant association between PM10 exposure and the methylation of clock genes was found, namely, this was negative for PER2 gene and positive for the CLOCK, CRY1, CRY2, and PER3 genes. PM2.5 was negatively associated with methylation of PER2 gene and positively with methylation of CRY2 gene. Evidence was observed for effect modification from body mass index (BMI) regarding the PER1 gene: as PM2.5/10 increases, DNA methylation increases significantly for relatively low BMI values (BMI = 25), while it decreases in participants with severe obesity (BMI = 51). PM may therefore alter the epigenetic regulation of clock genes, possibly affecting circadian rhythms. Future studies are needed to clarify how alterations in clock gene methylation are predictive of disease development and how obesity can modulate the adverse health effects of PM.
Keywords: obesity, particulate matter, DNA methylation, clock genes, CLOCK, PER, ARNTL, CRY, circadian rhythms
1. Introduction
Nowadays, bad air quality is considered one of the major concerns for public health. Exposure to excessive concentrations of air pollutants has been implicated in the etiology and/or worsening of several pathological conditions, ranging from cardiovascular and neurological diseases to cancer [1,2,3]. It is now generally recognized that one of the main pathological mechanisms elicited by airborne pollutants, including particulate matter (PM), is based on the activation of a systemic inflammatory cascade [4,5]. After inhalation, fine PM deposits throughout the respiratory tract and damages the airway epithelium. This process promotes the recruitment of innate immune cells and the release of proinflammatory mediators, including cytokines and chemokines [6,7].
Obesity is an important susceptibility factor to the noxious effects started by PM exposure since this condition is characterized per se by a state of chronic low-grade inflammation and increased systemic oxidative stress [3,8,9]. Indeed, the excessive visceral fat accumulation that characterizes subjects with obesity, or even overweight, perturbs the metabolic homeostasis of adipocytes, leading to a large secretion of adipokines, proinflammatory factors that stimulate the production of oxygen free radicals by leukocytes [10,11,12]. Moreover, it has been hypothesized that the daily-inhaled air volume by subjects with obesity is greater than would be expected for normal-weight individuals, thus potentially resulting in an increased uptake of airborne PM [13,14]. Therefore, particulate air pollution is likely to act as an external proinflammatory trigger that exacerbates the pre-existing inflammatory status of subjects with obesity, making them hypersensitive to PM exposure.
Recently, it has been suggested that PM10, one of the principal components of particulate air pollution, could influence the methylation of the genes that regulate the circadian cycle [15,16]. Circadian rhythms are given by biochemical and behavioral oscillations that occur in about 24 h, in a coordinated way with external light/dark cycles. For the proper regulation of such daily fluctuations, both central and peripheral clocks are required. The hypothalamic suprachiasmatic nucleus (SCN), whose neurons receive the light stimulus directly from the retina, poses as the main circadian pacemaker that sends the signal to the peripheral clocks (gastrointestinal tract, adipose tissue, liver, muscles, heart, lungs, adrenal glands) and synchronizes the rhythmicity of all the cells in the body [17]. At the molecular level, clock genes are responsible for the maintenance of the daily oscillations that characterize many aspects of the cellular metabolism and program several physiological and behavioral processes, including the sleep/wake cycle, body temperature, hormonal secretion, locomotor activity, and eating behavior [18]. Due to their fundamental role in cellular physiology and metabolism, the expression of clock genes must be tightly regulated to avoid circadian misalignments. The cellular rhythmicity is given by a complex regulatory pathway, known as transcriptional and translational feedback loop (TTFL), which consists of positive and negative interconnected circuits. In the positive arm, the transcriptional activators CLOCK and ARNTL/BMAL1 form a heterodimer that binds to the Enhancer box (E-box) within the promoter region of genes belonging to the Period (PER1, PER2, PER3) and Cryptochrome (CRY1, CRY2) families, thus promoting their transcription [19]. In turn, PER and CRY proteins underpin a negative feedback mechanism: after translocating into the nucleus, they interact with the CLOCK:ARNTL complex and inhibit the transcription of their genes. This transcriptional repression results in a decrease in the levels of PER and CRY proteins, thus allowing the start of a new cycle [20,21].
A growing body of literature has been linking circadian misalignments to metabolic diseases. Sleep deprivation, night eating, and other unhealthy habits linked to circadian rhythm deregulation have negative consequences on weight gain and on the risk of developing weight-related pathologies [22,23]. Moreover, circadian rhythm disruption has been recently suggested to enhance inflammation and oxidative stress [24,25,26]. However, to date, the comprehensive mechanisms evoked by proinflammatory environmental factors on the epigenetic regulation of clock genes and the possible modifying role exerted by elevated BMI values remain largely unclear.
To investigate the potential role of overweight/obesity as a risk factor for circadian rhythm disruption in response to proinflammatory environmental triggers, we designed an epidemiological study investigating the effects of PM exposure on blood DNA methylation in a population of 200 women with an unhealthy body mass index (BMI), and therefore hypersusceptible due to their pre-existing, low-grade inflammatory status.
First, we evaluated whether exposure to airborne PM10 could alter the methylation of clock genes, potentially resulting in altered gene expression and circadian misalignments. In detail, we searched for an association between PM10 concentrations and the percentage of methylated CpGs in the promoter region of the clock genes ARNTL, CLOCK, CRY1, CRY2, PER1, PER2, and PER3, which play crucial roles in the regulation of the 24-h cellular rhythmicity. Then, we explored the role of the BMI as a variable that might influence the effect of PM on the methylation pattern of clock genes. Overall, this study provides new insights into the epigenetic outcomes of short-term PM exposure on the circadian CLOCK pathway in a population of hypersusceptible subjects.
2. Materials and Methods
2.1. Study Subjects
The present study was conducted on 200 women with overweight/obesity enrolled between 2010 and 2013 and randomly selected among the participants of the larger SPHERE study (ERC-2011-StG 282413), whose aim consists of investigating the molecular mechanisms evoked by PM exposure with a possible impact on human health [27]. All participants were residents in Lombardy at the time of enrollment, which was carried out at the Center for Obesity and Work (Department of Preventive Medicine, IRCCS Ca’ Granda—Ospedale Maggiore Policlinico) in Milan.
All subjects willing to participate in the study were asked to fill out a questionnaire to collect information about their eating habits and lifestyle, including current and past smoking habits, alcohol consumption, and physical activity. They were also required to indicate the place and date of birth, the residential address with characteristics of the house and traffic in the area, pathological and family history, educational qualifications, type and place of work.
Each candidate was subjected to measurements of weight, height, and abdominal circumference, and spirometry, electrocardiogram, and blood and urine tests were performed. BMI was calculated as the ratio between the subject’s weight (kg) and height squared (m2). According to the current guidelines [28], subjects who had a BMI between 25 and 29.9 kg/m2 were classified as overweight, whereas subjects with a BMI greater than or equal to 30 kg/m2 or higher were classified as obese.
Due to the importance of reducing bias associated with obesity, we used people-first language (and we encourage scientific authors to do so), according to the standard recommendation of European Association for the Study of Obesity (EASO), The Obesity Society (TOS), and Obesity Canada (OC) [29,30,31,32].
Patients with oncological, cardiac, neurodegenerative, or other chronic pathologies were excluded. The subjects fitting the inclusion criteria signed a written informed consent for the donation of blood samples, approved by the Ethics Committee of the Fondazione Ca’Granda—Ospedale Maggiore Policlinico. The blood withdrawal was carried out for all subjects in the morning (9.00–10.30 a.m.) and after overnight fasting to avoid introducing confounding factors linked to the physiological daily fluctuations in the methylation of the clock genes [33].
2.2. Assessment of PM Exposure
PM concentrations were recorded by the Regional Environmental Protection Agency (ARPA Lombardia) through monitoring stations located throughout Lombardy and available online as daily means. For each subject, daily PM concentrations were assigned considering the values registered by the nearest station to their home address. Using ArcGIS® software (Esri), we geocoded each subject’s home address and each monitory station’s address. PM10 values were assigned according to each participant’s home address for the 6 days before blood sampling (from day −1 to day −6); instead, for the day of blood withdrawal (day 0) we considered the PM10 mean value registered by the three monitoring stations of Milan. Regarding PM2.5, as the presence of monitoring stations in the Lombardy area is very limited, we took into account the values registered in the city of Milan both for the day of blood sampling and the previous 6 days. In cases of incomplete series, missing values were attributed using an algorithm that integrated the annual average of the incomplete series and the PM concentrations of the nearest and more correlated monitors [34].
2.3. Sample Collection, DNA Extraction, and Bisulfite Treatment
Seven milliliters of whole blood were collected into EDTA tubes from each participant by venous phlebotomy. After centrifuging the blood tubes at 1200 g for 15 min to separate plasma, buffy coat, and erythrocytes, genomic DNA was extracted from the buffy coat fraction using the Wizard Genomic DNA Purification Kit (Promega; Madison, WI, USA) according to the manufacturer’s instructions. The concentration of the purified DNA was measured using the NanoDrop-1000 spectrophotometer (Thermo Fisher Scientific; Waltham, MA, USA). The DNA samples were plated at a concentration of 25 ng/µL in plates of 96 wells each and were treated with sodium bisulfite using the EZ-96 DNA Methylation-Gold™ Kit (Zymo Research; Irvine, CA, USA) following the manufacturer’s instructions. After elution, each DNA sample was divided into 10-µL aliquots using the Microlab STAR Automated Liquid Handling Workstation (Hamilton Company; Reno, NV, USA) and the plates were stored at −80 °C until use.
2.4. DNA Amplification and Pyrosequencing
Analysis of DNA methylation was performed via previously published methods with minor changes [15,35]. Briefly, 10 μL of bisulfite-treated template DNA was added with 25 μL of GoTaq Hot Start Green Master mix (Promega), 1 μL of forward primer (10 μM), and 1 μL of 5′ end-biotinylated reverse primer (10 μM) to set up a 50 μL PCR reaction. PCR cycling conditions and primer sequences are reported in Table 1.
Table 1.
Pyrosequencing assay information.
| Gene | Chromosome Position 1 |
CpG Sites | Primers: Forward (F) Reverse (R) Sequencing (S) |
Sequencing Length |
T° Annealing |
|
|---|---|---|---|---|---|---|
| ARNTL | chr11:13275818 -13278227 |
2 | F R S |
GGGGTTAGTTTGGGTAATAGAATTAG Bio-TAAACTCCCTAAATAAAAAAACAAC TTATTTTATTTTATTTTAGT |
38 bp | 54 °C |
| CLOCK | chr4:55547142 -55547530 |
2 | F R S |
TTTTTAGGAGATGGGAGAAGATGT Bio-TAAAAAATCCAAAAACCAAAAAAAA TTTTTTGTTAATATT |
28 bp | 51.5 °C |
| CRY1 | chr12:105617622 -105618592 |
3 | F R S |
TTTGTGAGGGAAGGTTTAGTTT Bio-AACAATTTCCAAACCCTCC TTTTTAAGGGTTATGAG |
27 bp | 56 °C |
| CRY2 | chr11:45846906 -45847578 |
4 | F R S |
TGTTTTTTGAGATTTGGTTTATTTT Bio- CCAAAACCCCTCTACCATTAACTA TGTTTTTTGAGATTTGGTTTATTTT |
33 bp | 54 °C |
| PER1 | chr17:8151724 -8152661 |
3 | F R S |
TAGGGTTAGGGATTGGAGAATAGA Bio-ACCCAAACAAAAAACACACTATC GGGTTAGGAGTGTAGATTTT |
27 bp | 52 °C |
| PER2 | chr2:238288036 -238291073 |
3 | F R S |
TGAGAAAGGTAGTATTTTTAAGG Bio-AAAACTCCACATACCCCACAC AGGAGGTTGTTTTGGGAGAT |
34 bp | 52 °C |
| PER3 | chr1:7784068 -7785195 |
3 | F R S |
TGTTTGTTATTGATTGTAAAGTGAG Bio-AATTTAAATCCCCCTTTCCCTAC TGTTTGTTATTGATTGTAAAGTGAG |
25 bp | 52 °C |
1 As reported by the UCSC Genome Browser, GRCh38/hg38 assembly.
The biotin molecule at the 5′ extremity of reverse primers was exploited to isolate a single DNA filament, which was subsequently used as a template for Pyrosequencing. The whole procedure was performed using the Pyromark® Gold Q96 kit (QIAGEN GmbH, Hilden, Germany). Briefly, after incubating 15 μL of PCR product with Streptavidin Sepharose HP beads (Amersham BioSciences Ltd., Little Chalfont, UK), the biotin-labeled single-stranded DNA was purified, washed, denatured with 0.2 M NaOH, and washed again using the Pyrosequencing Vacuum Prep Tool (QIAGEN). After elution, the purified DNA filament was briefly incubated in an Annealing mix containing the sequencing primer (0.3 μM), and the plates were then heated up to 85 °C. Pyrosequencing was performed with the PyroMark MD System (QIAGEN). CpG sites were queried within the promoter regions of the following genes: ARNTL, CLOCK, CRY1, CRY2, PER1, PER2, PER3.
The quantitative analysis of the methylation level at individual CpG positions within each gene’s promoter region was carried out using the Pyro Q-CpG software (Biotage, Uppsala, Sweden), which indicates the percentage of methylated cytosines out of the total number of cytosines (5-methyl-cytosine + unmethylated cytosines) at each CpG site of interest. Every sample was tested twice for each gene to guarantee the reproducibility of the experimental setting. Coefficients of variation for each assay are as follows: ARNTL = 0.2; CLOCK = 0.4; CRY1 = 0.3; CRY2 = 0.2; PER1 = 0.2; PER2 = 0.01; PER3 = 0.02.
2.5. Statistical Analysis
Standard descriptive statistics were performed for all variables. Continuous variables were expressed as the mean ± standard deviation (SD) or as the median with first-, and third-quartile (Q1–Q3), as appropriate. Categorical data were reported as frequencies with percentages.
To estimate the effect of PM10 and PM2.5 exposure on clock gene methylation (CLOCK, ARNTL, CRY1, CRY2, PER1, PER2, PER3) we applied linear mixed-effects models adjusted for age, BMI, smoking habits, percentage of lymphocytes, run, CpG site, season, temperature, and humidity. We adopted a repeated-measures design as DNA methylation measurements were run in duplicate for each subject, and the Pyrosequencing-based DNA methylation analysis tested a variable number of CpG positions according to CpG density in the promoter assay. We also included the effects of CpG position and run in the models to account for variation in methylation estimates due to experimental sources of variation. An unstructured covariance structure was used to model within-subject errors. The Kenward–Roger approximation was used to estimate the degrees of freedom in the denominator.
To examine the potential modifier role of the BMI on the association between PM exposure and clock genes, we added an interaction term between BMI and exposure in each model. To produce the estimates and plots of the PM-clock gene’s relationship at selected BMIs, we entered the levels of PM into the equation along with the range of values for each clock gene, at the mean value of continuous covariate and selected level for categorical variables. The cut-offs selected for BMI were 1st percentile (BMI = 25 kg/m2) and 99th percentile (BMI = 51 kg/m2). Estimated effects are reported as percentage changes and confidence intervals (CI) associated with an increase of 10 µg/m3 in each pollutant, which corresponded to (exp(β)-1) × 100.
Normality and linearity assumption by graphical inspection and the best model selection was based on the minimization of the Akaike information criterion and maximization of the explained variance of the model. A p-value of 0.05 was considered statistically significant. Statistical analyses were performed with SAS software (version 9.4; SAS, Cary, NC, USA) and R software (version 4.0.3; The R Foundation, Vienna, Austria).
3. Results
3.1. Characteristics of the Study Population, PM Assessment, and DNA Methylation
As summarized in Table 2, the study population included 200 women aged 52.7 ± 12.9 years, who were recruited as part of the SPHERE study [27]. According to their BMI, 55 subjects (27.5%) had overweight (25 ≤ BMI < 30), 72 (36.0%) had obesity Class I (30 ≤ BMI < 35) m and 73 (36.5%) had obesity Classes II and III (BMI ≥ 35). About half of the participants (45.5%) were current (13%) or former (32.5%) smokers.
Table 2.
Characteristics of the study participants.
| Characteristics | Value | |
|---|---|---|
| Age (years ± SD) | 52.7 ± 12.9 | |
| BMI (kg/m2 ± SD) | 33.8 ± 5.5 | |
| Categorical BMI (number of subjects (%)) | ||
| 25 ≤ BMI < 30 (Overweight) | 55 (27.5%) | |
| 30 ≤ BMI < 35 (Obesity Class I) | 72 (36.0%) | |
| BMI ≥ 35 (Obesity Classes II and III) | 73 (36.5%) | |
| Smoking habits (number of subjects (%)) | ||
| Nonsmoker | 109 (54.5%) | |
| Ex-smoker | 65 (32.5%) | |
| Current smoker | 26 (13%) | |
| Percentage of lymphocytes (mean ± SD) | 30.9% ± 7.2% | |
| Season of enrollment (number of subjects (%)) | ||
| Winter | 57 (28.5%) | |
| Spring | 55 (27.5%) | |
| Summer | 28 (14.0%) | |
| Autumn | 60 (30.0%) | |
| Temperature (°C ±SD) | 69.8 ± 14.7 | |
| Humidity (% ±SD) | 12.7 ± 7.6 |
The mean daily levels of the individual PM10 and PM2.5 values (expressed as μg/m3) attributed to each study subject and evaluated within 1 day to 7 days before the subject recruitment are reported in Table 3: PM10 averaged from 48.0 (2 days before recruitment) to 54.8 ng/m3 (4 and 13 days before recruitment).
Table 3.
PM concentrations recorded by ARPA Lombardia monitoring stations.
| PM Size | Days before Blood Sampling |
Mean (μg/m3) |
SD | First Quartile (Q1) | Median (Q2) |
Third Quartile (Q3) |
|---|---|---|---|---|---|---|
| PM10 | Day 0 | 47.2 | 28.8 | 25.7 | 37.3 | 62.2 |
| Day −1 | 41.8 | 30.2 | 22.0 | 31.0 | 56.0 | |
| Day −2 | 39.8 | 27.2 | 21.0 | 31.0 | 52.0 | |
| Day −3 | 41.3 | 29.9 | 22.0 | 33.0 | 56.0 | |
| Day −4 | 43.6 | 28.4 | 23.0 | 36.0 | 60.0 | |
| Day −5 | 43.6 | 27.5 | 24.0 | 36.0 | 59.0 | |
| Day −6 | 42.9 | 26.7 | 24.0 | 36.0 | 53.0 | |
| Weekly mean | 42.8 | 22.9 | 26.7 | 36.4 | 54.0 | |
| PM2.5 | Day 0 | 32.9 | 23.5 | 16.0 | 25.8 | 45.0 |
| Day −1 | 30.9 | 22.6 | 14.0 | 24.0 | 43.0 | |
| Day −2 | 30.4 | 22.6 | 14.5 | 25.0 | 38.0 | |
| Day −3 | 32.2 | 26.5 | 13.0 | 25.0 | 44.0 | |
| Day −4 | 32.9 | 23.8 | 16.0 | 25.0 | 46.0 | |
| Day −5 | 31.2 | 21.0 | 14.3 | 25.3 | 41.5 | |
| Day −6 | 30.8 | 20.0 | 16.0 | 25.0 | 43.0 | |
| Weekly mean | 31.3 | 18.9 | 16.0 | 27.9 | 40.6 |
The average methylation values for the clock genes ARNTL, CLOCK, CRY1, CRY2, PER1, PER2, and PER3 obtained by Bisulphite-Pyrosequencing are shown in Table 4. All the values indicated are expressed in terms of percentages of 5-methylcytosine (%5mC).
Table 4.
Methylation values of clock genes.
| Gene | Mean (% mCpG) |
SD | First Quartile (Q1) | Median (Q2) |
Third Quartile (Q3) |
Min | Max |
|---|---|---|---|---|---|---|---|
| ARNTL | 1.1 | 0.7 | 1.1 | 0.8 | 1.3 | 0 | 7.2 |
| CLOCK | 1.9 | 1.6 | 1.2 | 0.8 | 2.7 | 0.3 | 7.5 |
| CRY1 | 2 | 1.5 | 1.7 | 1.1 | 2.5 | 0 | 10.3 |
| CRY2 | 1.2 | 0.5 | 1.2 | 1 | 1.4 | 0 | 3.7 |
| PER1 | 1.5 | 1 | 1.4 | 0.8 | 2.2 | 0 | 5.3 |
| PER2 | 78.7 | 3.5 | 79 | 76.9 | 80.8 | 60.5 | 86.7 |
| PER3 | 84.9 | 3.7 | 85.5 | 82.5 | 87.4 | 73.2 | 93.3 |
3.2. Association between PM and The Methylation of Clock Genes
We estimated the association between DNA methylation and exposure to PM10 measured on the day of blood collection (day 0), in the previous 6 days, and for the weekly average exposure. For each time lag, the association is expressed as a percent change in DNA methylation (% change) for 10 μg/m3 increments of PM10 (Supplementary Table S1).
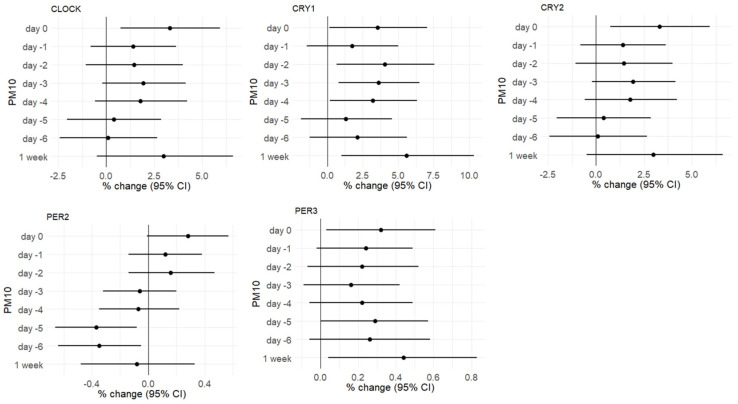
As illustrated in Figure 1, we observed a negative association between PM10 exposure and CpG methylation for the PER2 gene on days −5 and −6. Moreover, a significant positive association was observed for CRY1 on days 0, −2, −3, and −4; for CRY2 on day 0; for CLOCK on day −1 and for PER3 on day −5. Considering the weekly mean PM10 exposure, a positive association was observed for CRY1 and PER3. Conversely, the methylation status of CpG dinucleotides in the promoter region of ARNTL and PER1 was not significantly associated with PM10 concentrations.
Figure 1.
Plots showing the associations between short-term exposure to PM10 and the methylation of CLOCK, CRY1, CRY2, PER2, and PER3 genes from day 0 to day −6 (single days and weekly average). Methylation values are provided as percentage changes (% changes) in methylation associated with 10-µg/m3 increments, estimated by multivariable regression models adjusted for age, BMI, smoking habits, percentage of lymphocytes, Pyrosequencing run, CpG site, season, temperature, and humidity.
For the genes showing at least one significant association with a time lag, percentage changes (% changes) in methylation associated with 10-µg/m3 increments were reported.
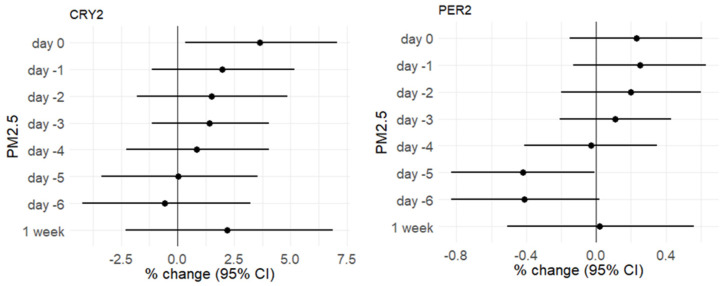
Regarding PM2.5, we observed that the methylation of CRY2 was positively associated with PM2.5 increments on day 0, while the association was significantly negative for PER2 on day −5 (Figure 2). Supplementary Table S2 reports the association coefficients for all the genes investigated.
Figure 2.
Plots showing the associations between short-term exposure to PM10 and the methylation of CRY2 and PER2 genes from day 0 to day −6. Methylation values are provided as percentage changes (% changes) in methylation associated with 10-µg/m3 increments, estimated by multivariable regression models adjusted for age, BMI, smoking habits, percentage of lymphocytes, Pyrosequencing run, CpG site, season, temperature, and humidity.
3.3. Effect of Obesity on The Relationship between PM and Methylation
As obesity/overweight is an important susceptibility factor to the toxic effects of PM, we tested the possible role of BMI as an effect modifier of the association between the exposure to PM10 and PM2.5 and clock gene methylation (Supplementary Table S3). A significant effect modification of the BMI was observed for CRY2 (on days −5 and −6 for PM10; on days 0 and −5 for PM2.5), PER1 (for both PM10 and PM2.5, on days 0, −1, −2, −5, −6 and on the weekly average), and PER2 (on day −6 for PM10). Thus, Table 5 and Table 6 report the association between PM exposure (PM10 in Table 5 and PM2.5 in Table 6) and the methylation of the CRY2, PER1, and PER2 genes for selected BMI values (BMI = 25 and BMI = 51).
Table 5.
Association between PM10 exposure and the methylation of the CRY2, PER1, and PER2 genes for selected BMI values. Significant associations (p-value < 0.05) are highlighted in bold characters.
| Methylation Genes Δ% (95% CI) p−Value | CRY2 | PER1 | PER2 | |||
|---|---|---|---|---|---|---|
| BMI = 25 | BMI = 51 | BMI = 25 | BMI = 51 | BMI = 25 | BMI = 51 | |
| PM10 Exposure | ||||||
| Day 0 | 5.4 (1.5; 9.4) | −1.2 (−6.8; 4.7) | 6.9 (3.3; 10.7) | −9.2 (−13.9; −4.2) | 0.5 (0.1; 1) | −0.2 (−0.9; 0.5) |
| 0.007 | 0.673 | <0.001 | <0.001 | 0.020 | 0.521 | |
| Day −1 | 3.2 (−0.1; 6.6) | −2 (−6.8; 3) | 4.7 (1.2; 8.3) | −8.4 (−12.5; −4.1) | 0.2 (−0.2; 0.6) | 0 (−0.6; 0.6) |
| 0.058 | 0.424 | 0.009 | 0.000 | 0.370 | 0.958 | |
| Day −2 | 3.2 (−0.9; 7.5) | −1.2 (−6.5; 4.4) | 2.7 (−1.4; 7) | −6.1 (−11; −1) | 0.5 (0; 1) | −0.3 (−1; 0.3) |
| 0.129 | 0.665 | 0.192 | 0.021 | 0.059 | 0.352 | |
| Day −3 | 1.8 (−2; 5.8) | 2.6 (−4.5; 10.2) | 1.2 (−2.7; 5.2) | −4.2 (−10.7; 2.7) | 0.2 (−0.3; 0.7) | −0.5 (−1.3; 0.4) |
| 0.351 | 0.481 | 0.557 | 0.224 | 0.470 | 0.266 | |
| Day −4 | 2 (−2.2; 6.4) | 1.5 (−5.3; 8.8) | 0.6 (−3.5; 5) | −1.8 (−8.2; 5.1) | 0.2 (−0.3; 0.8) | −0.6 (−1.4; 0.2) |
| 0.352 | 0.676 | 0.765 | 0.598 | 0.349 | 0.163 | |
| Day −5 | 4 (−0.3; 8.6) | −5.3 (−11.1; 0.8) | 3.8 (−0.3; 8) | −10.5 (−15.6; −5.1) | 0 (−0.5; 0.5) | −0.9 (−1.7; −0.2) |
| 0.068 | 0.089 | 0.070 | <0.001 | 0.985 | 0.011 | |
| Day −6 | 3.1 (−0.9; 7.2) | −4.4 (−9.4; 1) | 4.3 (0.5; 8.3) | −9.6 (−14; −5) | 0 (−0.4; 0.5) | −0.9 (−1.5; −0.2) |
| 0.134 | 0.110 | 0.026 | <0.001 | 0.842 | 0.009 | |
| 1 week | 6.2 (0.9; 11.7) | −2.5 (−9.6; 5.2) | 5.2 (0.2; 10.4) | −12.1 (−18.1; −5.7) | 0.3 (−0.3; 0.9) | −0.7 (−1.6; 0.1) |
| 0.021 | 0.506 | 0.043 | <0.001 | 0.318 | 0.100 | |
Table 6.
Association between PM2.5 exposure and the methylation of the CRY2, PER1, and PER2 genes for selected BMI values. Significant associations (p-value < 0.05) are highlighted in bold characters.
| Methylation Genes Δ% (95% CI) p-Value | CRY2 | PER1 | PER2 | |||
|---|---|---|---|---|---|---|
| BMI = 25 | BMI = 51 | BMI = 25 | BMI = 51 | BMI = 25 | BMI = 51 | |
| PM10 exposure | ||||||
| Day 0 | 8 (3; 13.2) | −4.4 (−11.6; 3.4) | 9.7 (4.8; 14.8) | −10.1 (−16.6; −3) | 0.7 (0.1; 1.2) | −0.7 (−1.6; 0.2) |
| 0.002 | 0.258 | <0.001 | 0.006 | 0.019 | 0.144 | |
| Day −1 | 2.9 (−1.2; 7.2) | 0.1 (−6.4; 7.1) | 5.8 (0.9; 10.8) | −8.3 (−14.5; −1.7) | 0.4 (−0.1; 0.9) | 0 (−0.9; 0.8) |
| 0.168 | 0.969 | 0.019 | 0.014 | 0.100 | 0.932 | |
| Day −2 | 3.1 (−1.9; 8.4) | −2.3 (−9.6; 5.4) | 3.9 (−1.3; 9.3) | −8.5 (−15; −1.5) | 0.5 (−0.1; 1.1) | −0.5 (−1.4; 0.4) |
| 0.221 | 0.542 | 0.141 | 0.019 | 0.103 | 0.303 | |
| Day −3 | 2.6 (−1.5; 7) | −1.2 (−8.9; 7.2) | 2.3 (−1.9; 6.8) | −5 (−12.3; 3) | 0.5 (−0.1; 1) | −0.7 (−1.7; 0.3) |
| 0.217 | 0.768 | 0.288 | 0.215 | 0.080 | 0.167 | |
| Day −4 | 1.3 (−4.2; 7) | −1 (−9.1; 7.7) | 1.5 (−4; 7.2) | −0.3 (−8.1; 8.2) | 0.3 (−0.3; 1) | −0.7 (−1.7; 0.3) |
| 0.656 | 0.813 | 0.604 | 0.941 | 0.334 | 0.170 | |
| Day −5 | 4.2 (−1.5; 10.2) | −8.7 (−16.3; −0.5) | 4.6 (−0.7; 10.3) | −11.9 (−18.7; −4.5) | −0.1 (−0.7; 0.6) | −1.2 (−2.2; −0.2) |
| 0.148 | 0.038 | 0.090 | 0.002 | 0.828 | 0.018 | |
| Day −6 | 2.8 (−2.8; 8.7) | −6.5 (−13.3; 0.8) | 6.3 (0.8; 12.2) | −11.3 (−17.2; −5) | 0 (−0.7; 0.6) | −1 (−1.9; −0.2) |
| 0.327 | 0.079 | 0.026 | 0.001 | 0.966 | 0.021 | |
| 1 week | 5.9 (−0.6; 12.7) | −4.6 (−13.4; 5) | 7.4 (1; 14.1) | −12.7 (−20.4; −4.4) | 0.5 (−0.2; 1.3) | −0.9 (−2; 0.2) |
| 0.074 | 0.332 | 0.023 | 0.004 | 0.184 | 0.115 | |
Interestingly, by applying this statistical model we observed PM-induced changes in DNA methylation for genes such as PER1, whose methylation level was not associated to PM exposure according to the previous model.
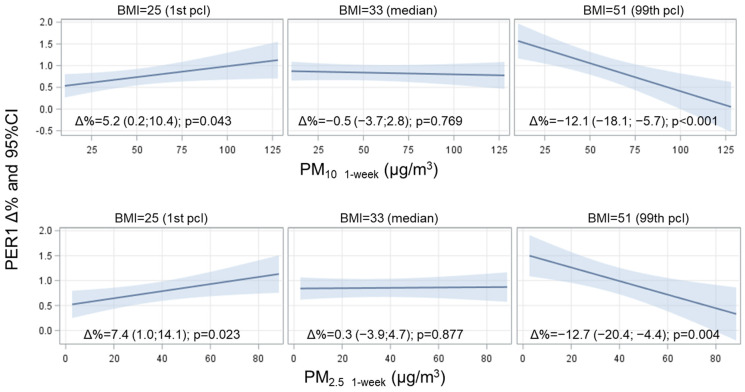
Considering the p-value for the study week, PER1 is the only gene whose methylation change induced by both PM10 and PM2.5 is influenced by the BMI.
Figure 3 reports the effect of the BMI on the relationship between PM weekly averages and PER1 methylation: for low BMI values (BMI = 25 kg/m2, 1st percentile) DNA methylation increases significantly as PM2.5/10 increases; instead, for high BMI values (BMI = 51 kg/m2, 99th percentile), a decrease in methylation is observed as PM10 increases. Median BMI values (BMI = 33 kg/m2) are also indicated and are not associated with significant changes in methylation.
Figure 3.
Interaction effect of PM and BMI on PER1 methylation level. The strength of the association between PM and PER1 at three selected levels of BMI (1st, 50th, and 99th percentile) is presented. Estimates were calculated from multivariate models adjusted for age, BMI, smoking habits, percentage of lymphocytes, Pyrosequencing run, CpG site, season, temperature, and humidity. Adjusted delta percent changes (Δ%) are reported for 10 µg/m3 increases in PM concentration. The p-values for the interaction term with BMI were p = 0.001 for PM10, and p < 0.001 for PM2.5. To produce the estimate, we entered the levels of PM into the equation along with the range of values for PER1, at the mean value of continuous covariate, and selected levels for categorical variables.
4. Discussion
In the present study, conducted in a population of subjects with overweight/obesity, we observed an association between PM exposure measured in the week before the blood drawing and the methylation of circadian cycle genes (i.e., CLOCK, CRY1, CRY2, PER1, PER2, and PER3). In particular, we found that increments of PM10 concentrations were associated to the hypermethylation of the CLOCK, CRY1, CRY2, and PER3 genes and to the hypomethylation of PER2. In contrast, PER1 and ARNTL methylation levels appeared not to be correlated to PM10 levels. Regarding PM2.5, we reported an association with DNA methylation, which was positive for CRY2 and negative for PER2.
Recently, it has been hypothesized that atmospheric particulate matter can affect the regulation of circadian rhythms, resulting in the desynchronization of both central and peripheral clocks [24,36]. However, the molecular mechanisms underlying the association between PM exposure and circadian cycle disruption have not been completely clarified.
A first potential mechanism could derive from the capacity of airborne particulate to stimulate the production of highly reactive free radicals and increase oxidative stress. Since CLOCK and ARNTL are sensitive to the intracellular redox state [37], this may justify the effect of a pro-oxidative agent such as PM on cellular circadian pathways. Accordingly, 24-h oscillations of H2O2 levels seem to have a modulatory effect on the activity of the CLOCK protein via cysteine oxidation [38]. Secondly, the fine and ultrafine PM may influence the epigenetic regulation of clock genes by stimulating the release of microvesicles by the lung epithelium and inflammatory cells [27]. Indeed, the SPHERE study demonstrated that the miRNA cargo of extracellular vesicles varies depending on PM concentrations, which could therefore regulate the levels of intracellular gene expression [39]. Additionally, the inflammatory response induced by PM could impinge upon the transcription of clock genes. In this regard, it has been demonstrated that the inducible transcription factor NF-κB, after being activated in response to inflammatory triggers, binds to the promoters of the PER and CRY clock repressors, thus inhibiting their expression [40]. This observation could be in agreement with our findings reporting that PM10 exposure associates with increased methylation of CRY1, CRY2, and PER3. Although there is mounting evidence on the capacity of PM to alter the expression of clock genes [41,42], the possible consequences of PM-induced methylation changes remain largely unclear. It has been reported that altered DNA methylation profiles in clock genes are often coupled to aberrant expression patterns, with possible implications in pathological conditions [43]. In addition, in the present work we only considered methylation occurring in promoter CpGs, while also non-CpG methylation of DNA can provide a mechanism for regulating gene expression by directly affecting the binding of transcription factors [44]. However, it is worth considering that DNA methylation is only one of the mechanisms that control the expression of circadian genes [45,46] and PM could modulate other molecular changes with a stronger impact on the regulation of clock genes rather than DNA methylation.
Moreover, to date, only a few studies have focused on PM-induced methylation changes occurring within the promoter region of circadian genes. To our knowledge, investigated methylation outcomes include cardiovascular disease [15], fetal development [18], and intervertebral disc degeneration [47]. Therefore, due to the gaps in the current knowledge, it is still difficult to speculate about the possible role of altered methylation patterns on circadian misalignments.
Our study was based on a population of women with overweight/obesity and it did not include any normal-weight subjects. Notably, the median value of the BMI in this population, corresponding to 33 kg/m2, is higher than the Italian average (25 kg/m2, referred to females) [48]. Hence, this difference between the study group and the overall population could hamper the generalization of our findings: indeed, the BMI is considered as an important determinant of hypersusceptibility when evaluating the biological mechanisms induced by exposure to particulate matter [49].
In this regard, our data indicate that the BMI significantly changes the association between PM exposure and the methylation of the CRY2, PER1, and PER2 genes, suggesting that the BMI acts as an effect modifier. The modifying role of the BMI was particularly evident for PER1, especially because no significant methylation changes were observed in response to PM increases for this gene. More in detail, we reported that PM2.5/10 increments correspond to an increase in DNA methylation for low BMI values (BMI = 25, 1st percentile), while it markedly decreases in participants with severe obesity (BMI = 51, 99th percentile). To the best of our knowledge, PER1 methylation levels have not been related to obesity or other metabolic alterations/disturbances. Although the overall methylation status of clock genes has been associated with glucose metabolism and dietary intake, no significant effect has been observed for DNA methylation changes in the promoter regions of individual genes, including PER1 [50,51]. Moreover, the methylation level of PER1 has been reported to be uncoupled to gene expression [52] and to be strongly sex-dependent [53,54], so this finding remains difficult to interpret.
Epigenetic mechanisms are strongly involved in the development of obesity and obesity-related health effects since altered methylation profiles of genes involved in inflammation, adipogenesis, and glucose metabolism have been described in individuals with overweight/obesity [51,55]. Considering that many aspects of cellular metabolism are under circadian control, it is likely that deregulating the expression of genes encoding key components of the core molecular clock machinery can have pronounced effects on both peripheral and central metabolic regulatory processes. As an example, lipid synthesis and accumulation are dependent on ARNTL expression levels in adipocytes [56].
DNA methylation signatures at circadian genes have been associated with obesity [51,57]; however, it is not clear whether these alterations are implicated in the etiology of obesity, or if they are a mere molecular consequence of other deregulated pathways. Interestingly, the methylation of clock genes has been found to change in response to dietary interventions [58,59], enforcing the hypothesis that energy metabolism and cellular rhythmicity are regulated reciprocally. In this complex interplay, it is conceivable that PM-induced DNA methylation changes occurring in the promoter region of clock genes might be modulated by the BMI, taken as an indicator of a healthy/unbalanced metabolic status.
The present study must be interpreted taking into account both strengths and limitations. First, due to the limited number of study subjects, it is possible that the associations observed were due to chance. In this regard, we applied a statistical model that took into account several potential confounders, all of which were considered as independent variables, to reduce bias and chance findings. However, the findings need to be replicated in larger groups of subjects, possibly including subjects with a BMI < 25 and males, in order to allow generalizability of the findings. Another possible limitation concerns the attribution of PM2.5 concentrations to the study population. Since the number of available PM2.5-recording monitoring stations was much lower than the number of PM10 monitoring stations, the PM2.5 values assigned to the participants may not fully mirror the real concentrations experienced by study subjects. However, since the data obtained are generally in agreement with those relative to PM10 (of which a large percentage is made of particles with an aerodynamic diameter <2.5 µm in the study area), it is unlikely that this could affect the interpretation of our results.
In the future, it will be useful to assess whether the association between PM and the methylation of circadian genes exist also in normal-weight individuals and, since PM fuels inflammation, whether there is an association between the methylation of clock genes and inflammatory disease development. Furthermore, it would be interesting to evaluate the effect exerted by other components of air pollution, such as ozone, nitrogen, and carbon oxides, as they could be implicated in modulating the detected changes in DNA methylation.
5. Conclusions
This study highlights the association between the exposure to atmospheric particulate matter and clock gene methylation. In addition, our data suggest that BMI could be a susceptibility factor capable of altering the effect of PM on the considered DNA methylation outcomes. Further studies will be necessary to unravel a possible impact of methylation changes on the expression of clock genes and the regulation of circadian rhythmicity, as well as the role of obesity as a potential risk factor conferring hypersensitivity to PM.
Acknowledgments
We thank the Occupational Medicine Medical Residents for their help in examining and recruiting study subjects. We are grateful to the nurses of the “Medicina del Lavoro” Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Enrico Radice, Raquel Cacace and Barbara Marinelli for database development and preparation, Elisabetta Angiolino from the Regional Environmental Protection Agency (ARPA), and the volunteers who participated in the study.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/18/3/1122/s1, Table S1: Associations between short-term exposure to PM10 and clock methylation genes, Table S2: Associations between short-term exposure to PM2.5 and clock methylation genes, Table S3: Significance of the interaction term testing the modifier role of BMI, on the association between PM exposure and clock methylation genes.
Author Contributions
Conceptualization, V.B. and A.C.P.; Methodology, L.T., P.M., F.S., L.F.; Formal Analysis, S.I.; Investigation, V.B., M.R., M.B.; Subjects’ enrollment: L.V.; Writing—Original Draft Preparation, P.M.; Writing—Review and Editing, P.M., L.F., M.R., V.B.; Supervision, V.B., A.C.P.; Funding Acquisition, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by EU Programme “Ideas”, European Research Council (ERC-2011-StG 282,413 to V.B.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the ethics committee “Comitato Etico—Milano Area 2” of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20,122 Milan, Italy (approval number 1425).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study is available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schraufnagel D.E., Balmes J.R., Cowl C.T., De Matteis S., Jung S.-H., Mortimer K., Perez-Padilla R., Rice M.B., Riojas-Rodriguez H., Sood A., et al. Air Pollution and Noncommunicable Diseases. Chest. 2019;155:417–426. doi: 10.1016/j.chest.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoek G., Krishnan R.M., Beelen R., Peters A., Ostro B., Brunekreef B., Kaufman J.D. Long-term air pollution exposure and cardio- respiratory mortality: A review. Environ. Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamanaka R.B., Mutlu G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. (Lausanne) 2018;9 doi: 10.3389/fendo.2018.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji X., Zhang Y., Li G., Sang N. Potential Role of Inflammation in Associations between Particulate Matter and Heart Failure. Curr. Pharm. Des. 2018;24:341–358. doi: 10.2174/1381612824666180110150550. [DOI] [PubMed] [Google Scholar]
- 5.Tang H., Cheng Z., Li N., Mao S., Ma R., He H., Niu Z., Chen X., Xiang H. The short- and long-term associations of particulate matter with inflammation and blood coagulation markers: A meta-analysis. Environ. Pollut. 2020;267:115630. doi: 10.1016/j.envpol.2020.115630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Grove K.C., Provoost S., Brusselle G.G., Joos G.F., Maes T. Insights in particulate matter-induced allergic airway inflammation: Focus on the epithelium. Clin. Exp. Allergy. 2018;48:773–786. doi: 10.1111/cea.13178. [DOI] [PubMed] [Google Scholar]
- 7.Cooper D.M., Loxham M. Particulate matter and the airway epithelium: The special case of the underground? Eur. Respir. Rev. 2019;28:190066. doi: 10.1183/16000617.0066-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormack M.C., Belli A.J., Kaji D.A., Matsui E.C., Brigham E.P., Peng R.D., Sellers C., Williams D.L., Diette G.B., Breysse P.N., et al. Obesity as a susceptibility factor to indoor particulate matter health effects in COPD. Eur. Respir. J. 2015;45:1248–1257. doi: 10.1183/09031936.00081414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong G.H., Qian Z., Liu M.-M., Wang D., Ren W.-H., Fu Q., Wang J., Simckes M., Ferguson T.F., Trevathan E. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: The Seven Northeastern Cities study. Int. J. Obes. 2013;37:94–100. doi: 10.1038/ijo.2012.125. [DOI] [PubMed] [Google Scholar]
- 10.Manna P., Jain S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015;13:423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unamuno X., Gómez-Ambrosi J., Rodríguez A., Becerril S., Frühbeck G., Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018;48:e12997. doi: 10.1111/eci.12997. [DOI] [PubMed] [Google Scholar]
- 12.Atawia R.T., Bunch K.L., Toque H.A., Caldwell R.B., Caldwell R.W. Mechanisms of obesity-induced metabolic and vascular dysfunctions. Front. Biosci. Landmark. 2019;24:890–934. doi: 10.2741/4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brochu P., Bouchard M., Haddad S. Physiological Daily Inhalation Rates for Health Risk Assessment in Overweight/Obese Children, Adults, and Elderly. Risk Anal. 2014;34:567–582. doi: 10.1111/risa.12125. [DOI] [PubMed] [Google Scholar]
- 14.Weichenthal S.A., Godri Pollitt K., Villeneuve P.J. PM2.5, oxidant defence and cardiorespiratory health: A review. Environ. Health. 2013;12:40. doi: 10.1186/1476-069X-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantone L., Tobaldini E., Favero C., Albetti B., Sacco R.M., Torgano G., Ferrari L., Montano N., Bollati V. Particulate Air Pollution, Clock Gene Methylation, and Stroke: Effects on Stroke Severity and Disability. Int. J. Mol. Sci. 2020;21:3090. doi: 10.3390/ijms21093090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nawrot T.S., Saenen N.D., Schenk J., Janssen B.G., Motta V., Tarantini L., Cox B., Lefebvre W., Vanpoucke C., Maggioni C., et al. Placental circadian pathway methylation and in utero exposure to fine particle air pollution. Environ. Int. 2018;114:231–241. doi: 10.1016/j.envint.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Rosenwasser A.M., Turek F.W. Neurobiology of Circadian Rhythm Regulation. Sleep Med. Clin. 2015;10:403–412. doi: 10.1016/j.jsmc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Panda S. Circadian physiology of metabolism. Science. 2016;354:1008–1015. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang R.-C. The discoveries of molecular mechanisms for the circadian rhythm: The 2017 Nobel Prize in Physiology or Medicine. Biomed. J. 2018;41:5–8. doi: 10.1016/j.bj.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albrecht U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron. 2012;74:246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagannath A., Taylor L., Wakaf Z., Vasudevan S.R., Foster R.G. The genetics of circadian rhythms, sleep and health. Hum. Mol. Genet. 2017;26:R128–R138. doi: 10.1093/hmg/ddx240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCuen-Wurst C., Ruggieri M., Allison K.C. Disordered eating and obesity: Associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Ann. N. Y. Acad. Sci. 2018;1411:96–105. doi: 10.1111/nyas.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang J., Sundar I.K., Yao H., Sellix M.T., Rahman I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014;28:176–194. doi: 10.1096/fj.13-232629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheiermann C., Kunisaki Y., Frenette P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H., Huang L., Zhao J., Chen S., Liu J., Li G. The circadian clock and inflammation: A new insight. Clin. Chim. Acta. 2021;512:12–17. doi: 10.1016/j.cca.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Bollati V., Iodice S., Favero C., Angelici L., Albetti B., Cacace R., Cantone L., Carugno M., Cavalleri T., De Giorgio B., et al. Susceptibility to particle health effects, miRNA and exosomes: Rationale and study protocol of the SPHERE study. BMC Public Health. 2014;14:1137. doi: 10.1186/1471-2458-14-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weir C.B., Jan A. BMI Classification Percentile And Cut Off Points. StatPearls Publishing; Treasure Island, FL, USA: 2019. [PubMed] [Google Scholar]
- 29.Ramos Salas X., Alberga A.S., Cameron E., Estey L., Forhan M., Kirk S.F.L., Russell-Mayhew S., Sharma A.M. Addressing weight bias and discrimination: Moving beyond raising awareness to creating change. Obes. Rev. 2017;18:1323–1335. doi: 10.1111/obr.12592. [DOI] [PubMed] [Google Scholar]
- 30.Puhl R., Suh Y. Health Consequences of Weight Stigma: Implications for Obesity Prevention and Treatment. Curr. Obes. Rep. 2015;4:182–190. doi: 10.1007/s13679-015-0153-z. [DOI] [PubMed] [Google Scholar]
- 31.Albury C., Strain W.D., Brocq S.L., Logue J., Lloyd C., Tahrani A. The importance of language in engagement between health-care professionals and people living with obesity: A joint consensus statement. Lancet Diabetes Endocrinol. 2020;8:447–455. doi: 10.1016/S2213-8587(20)30102-9. [DOI] [PubMed] [Google Scholar]
- 32.Rubino F., Puhl R.M., Cummings D.E., Eckel R.H., Ryan D.H., Mechanick J.I., Nadglowski J., Ramos Salas X., Schauer P.R., Twenefour D., et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 2020;26:485–497. doi: 10.1038/s41591-020-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim A.S.P., Srivastava G.P., Yu L., Chibnik L.B., Xu J., Buchman A.S., Schneider J.A., Myers A.J., Bennett D.A., De Jager P.L. 24-Hour Rhythms of DNA Methylation and Their Relation with Rhythms of RNA Expression in the Human Dorsolateral Prefrontal Cortex. PLoS Genet. 2014;10:e1004792. doi: 10.1371/journal.pgen.1004792. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Giorgio C., Alessandro D.M., di Menno B., Daniela D., Marco I., Carmelo N., Gaetano S., Giuseppe V., Achille M. Evaluation of the temporal variation of air quality in Rome, Italy, from 1999 to 2008. Ann. Ist. Super. Sanita. 2010;46 doi: 10.4415/ANN_10_03_04. [DOI] [PubMed] [Google Scholar]
- 35.Bollati V., Baccarelli A., Hou L., Bonzini M., Fustinoni S., Cavallo D., Byun H.-M., Jiang J., Marinelli B., Pesatori A.C., et al. Changes in DNA Methylation Patterns in Subjects Exposed to Low-Dose Benzene. Cancer Res. 2007;67:876–880. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Li R., Chen R., Gu W., Zhang L., Gu J., Wang Z., Liu Y., Sun Q., Zhang K., et al. Ambient fine particulate matter exposure perturbed circadian rhythm and oscillations of lipid metabolism in adipose tissues. Chemosphere. 2020;251:126392. doi: 10.1016/j.chemosphere.2020.126392. [DOI] [PubMed] [Google Scholar]
- 37.Rutter J. Regulation of Clock and NPAS2 DNA Binding by the Redox State of NAD Cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 38.Man A.W.C., Xia N., Li H. Circadian Rhythm in Adipose Tissue: Novel Antioxidant Target for Metabolic and Cardiovascular Diseases. Antioxidants. 2020;9:968. doi: 10.3390/antiox9100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pergoli L., Cantone L., Favero C., Angelici L., Iodice S., Pinatel E., Hoxha M., Dioni L., Letizia M., Albetti B., et al. Extracellular vesicle-packaged miRNA release after short-term exposure to particulate matter is associated with increased coagulation. Part. Fibre Toxicol. 2017;14:32. doi: 10.1186/s12989-017-0214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong H.K., Maury E., Ramsey K.M., Perelis M., Marcheva B., Omura C., Kobayashi Y., Guttridge D.C., Barish G.D., Bass J. Requirement for NF-κB in maintenance of molecular and behavioral circadian rhythms in mice. Genes Dev. 2018;32:1367–1379. doi: 10.1101/gad.319228.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song P., Li Z., Li X., Yang L., Zhang L., Li N., Guo C., Lu S., Wei Y. Transcriptome Profiling of the Lungs Reveals Molecular Clock Genes Expression Changes after Chronic Exposure to Ambient Air Particles. Int. J. Environ. Res. Public Health. 2017;14:90. doi: 10.3390/ijerph14010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li R., Wang Y., Chen R., Gu W., Zhang L., Gu J., Wang Z., Liu Y., Sun Q., Zhang K., et al. Ambient fine particulate matter disrupts hepatic circadian oscillation and lipid metabolism in a mouse model. Environ. Pollut. 2020;262:114179. doi: 10.1016/j.envpol.2020.114179. [DOI] [PubMed] [Google Scholar]
- 43.Joska T.M., Zaman R., Belden W.J. Regulated DNA methylation and the circadian clock: Implications in cancer. Biology. 2014;3:560–577. doi: 10.3390/biology3030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin J., Lian T., Gu C., Yu K., Gao Y.Q., Su X.D. The effects of cytosine methylation on general transcription factors. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto-Uchida Y., Izawa J., Nishimura A., Hattori A., Suzuki N., Hirayama J. Post-translational Modifications are Required for Circadian Clock Regulation in Vertebrates. Curr. Genom. 2019;20:332–339. doi: 10.2174/1389202919666191014094349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X., Tian G., Li Z., Zheng L. The Crosstalk Between miRNA and Mammalian Circadian Clock. Curr. Med. Chem. 2015;22:1582–1588. doi: 10.2174/0929867322666150227155009. [DOI] [PubMed] [Google Scholar]
- 47.Numaguchi S., Esumi M., Sakamoto M., Endo M., Ebihara T., Soma H., Yoshida A., Tokuhashi Y. Passive cigarette smoking changes the circadian rhythm of clock genes in rat intervertebral discs. J. Orthop. Res. 2016;34:39–47. doi: 10.1002/jor.22941. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization . Global Status Report on Noncommunicable Diseases 2014. World Health Organization; Geneva, Switzerland: 2015. [DOI] [PubMed] [Google Scholar]
- 49.Bonzini M., Pergoli L., Cantone L., Hoxha M., Spinazzè A., Del Buono L., Favero C., Carugno M., Angelici L., Broggi L., et al. Short-term particulate matter exposure induces extracellular vesicle release in overweight subjects. Environ. Res. 2017;155:228–234. doi: 10.1016/j.envres.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng H., Zhu Y., Goldberg J., Vaccarino V., Zhao J. DNA methylation of five core circadian genes jointly contributes to glucose metabolism: A gene-set analysis in monozygotic twins. Front. Genet. 2019;10 doi: 10.3389/fgene.2019.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramos-Lopez O., Samblas M., Milagro F.I., Riezu-Boj J.I., Crujeiras A.B.B., Martinez J.A., Project M. Circadian gene methylation profiles are associated with obesity, metabolic disturbances and carbohydrate intake. Chronobiol. Int. 2018;35:969–981. doi: 10.1080/07420528.2018.1446021. [DOI] [PubMed] [Google Scholar]
- 52.Hsu M.C., Huang C.C., Choo K.B., Huang C.J. Uncoupling of promoter methylation and expression of Period1 in cervical cancer cells. Biochem. Biophys. Res. Commun. 2007;360:257–262. doi: 10.1016/j.bbrc.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 53.Bigini E.G., Chasens E.R., Conley Y.P., Imes C.C. DNA methylation changes and improved sleep quality in adults with obstructive sleep apnea and diabetes. BMJ Open Diabetes Res. Care. 2019;7:707. doi: 10.1136/bmjdrc-2019-000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jansen E.C., Dolinoy D., Peterson K.E., O’Brien L.M., Chervin R.D., Cantoral A., Tellez-Rojo M.M., Solano-Gonzalez M., Goodrich J. Adolescent sleep timing and dietary patterns in relation to DNA methylation of core circadian genes: A pilot study of Mexican youth. Epigenetics. 2020;00:1–14. doi: 10.1080/15592294.2020.1827719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wahl S., Drong A., Lehne B., Loh M., Scott W.R., Kunze S., Tsai P.-C., Ried J.S., Zhang W., Yang Y., et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–86. doi: 10.1038/nature20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimba S., Ishii N., Ohta Y., Ohno T., Watabe Y., Hayashi M., Wada T., Aoyagi T., Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. USA. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samblas M., Milagro F.I., Mansego M.L., Marti A., Martinez J.A. PTPRS and PER3 methylation levels are associated with childhood obesity: Results from a genome-wide methylation analysis. Pediatr. Obes. 2018;13:149–158. doi: 10.1111/ijpo.12224. [DOI] [PubMed] [Google Scholar]
- 58.Samblas M., Milagro F.I., Gómez-Abellán P., Martínez J.A., Garaulet M. Methylation on the Circadian Gene BMAL1 Is Associated with the Effects of a Weight Loss Intervention on Serum Lipid Levels. J. Biol. Rhythm. 2016;31:308–317. doi: 10.1177/0748730416629247. [DOI] [PubMed] [Google Scholar]
- 59.Engin A. Circadian rhythms in diet-induced obesity. Adv. Exp. Med. Biol. 2017;960:19–52. doi: 10.1007/978-3-319-48382-5_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study is available upon request to the corresponding author.