Abstract
Introduction
Over half of those hepatitis C virus (HCV)/HIV coinfected live in low-income and middle-income countries, and many remain undiagnosed or untreated. In 2016, Médecins Sans Frontières (MSF) established a direct-acting antiviral (DAA) treatment programme for people HCV/HIV coinfected in Myanmar. The purpose of our study was to evaluate the real-world cost and cost-effectiveness of this programme, and potential cost-effectiveness if implemented by the Ministry of Health (MoH).
Methods
Costs (patient-level microcosting) and treatment outcomes were collected from the MSF prospective cohort study in Dawei, Myanmar. A Markov model was used to assess cost-effectiveness of the programme compared with no HCV treatment from a health provider perspective. Estimated lifetime and healthcare costs (in 2017 US$) and health outcomes (in disability-adjusted life-years (DALYs)) were simulated to calculate the incremental cost-effectiveness ratio (ICER), compared with a willingness-to-pay threshold of per capita Gross Domestic Product in Myanmar ($1250). We evaluated cost-effectiveness with updated quality-assured generic DAA prices and potential cost-effectiveness of a proposed simplified treatment protocol with updated DAA prices if implemented by the MoH.
Results
From November 2016 to October 2017, 122 with HIV/HCV-coinfected patients were treated with DAAs (46% with cirrhosis), 96% (n=117) achieved sustained virological response. Mean treatment costs were $1229 (without cirrhosis) and $1971 (with cirrhosis), with DAA drugs being the largest contributor to cost. Compared with no treatment, the program was cost-effective (ICER $634/DALY averted); more so with updated prices for quality-assured generic DAAs (ICER $488/DALY averted). A simplified treatment protocol delivered by the MoH could be cost-effective if associated with similar outcomes (ICER $316/DALY averted).
Conclusions
Using MSF programme data, the DAA treatment programme for HCV among HIV-coinfected individuals is cost-effective in Myanmar, and even more so with updated DAA prices. A simplified treatment protocol could enhance cost-effectiveness if further rollout demonstrates it is not associated with worse treatment outcomes.
Keywords: health economics, HIV, viral hepatitis
Key questions.
What is already known?
Estimates show that implementing hepatitis C virus (HCV) screening and treatment programmes with generic direct-acting antivirals (DAAs) would be cost-saving within a 10-year period.
HCV treatment is likely cost-effective in low/middle-income country (LMIC) settings where DAAs are available at low costs.
What are the new findings?
Using Médecins Sans Frontières programme data, we found that compared with no treatment, HCV treatment with quality-assured DAA among HIV-coinfected individuals is cost-effective in Myanmar.
Access to affordable, quality-assured generic DAAs improved cost-effectiveness.
A simplified treatment protocol delivered by the Ministry of Health could be highly cost-effective among HIV/HCV-coinfected individuals if combined with an HCV screening programme.
What do the new findings imply?
A simplified treatment protocol could enhance cost-effectiveness if not associated with worse treatment outcomes.
National HCV programmes in Myanmar and similar LMIC settings should no longer consider DAA cost a barrier, but rather consider these data along with simplified models of care as a means to cure people with HCV infection and progress towards WHO HCV elimination goals.
Introduction
Among people living with hepatitis C virus (HCV) infection, coinfection with HIV can lead to accelerated liver cirrhosis, liver cancer and death compared with those with HCV monoinfection.1–3 Globally, an estimated 6.2% of people living with HIV show serological evidence of HCV antibody (2.3 million individuals), the majority residing in low/middle-income countries (LMICs).4 In Myanmar, an estimated 5.3% of the 222 000 HIV-infected individuals are HCV-seropositive,5–7 but in the rural Southern township of Dawei, HCV seroprevalence rises to 8% among people living with HIV (data unpublished), and as high as 23% among male HIV-infected fishermen.8
Promisingly, HCV treatment with new direct-acting antivirals (DAAs) is highly effective among HCV/HIV-coinfected individuals (>90% cure rate).9 Yet the previous high cost of DAAs restricted many individuals in LMIC settings from accessing treatment in these highest burdened areas.10 Few studies have evaluated the cost-effectiveness of HCV treatment in LMIC settings where healthcare management of liver disease and costs of providing DAA treatment differ dramatically from high-income countries. Existing evaluations are limited to theoretical analyses of DAA-containing regimens for HCV monoinfection in Egypt, India, Pakistan and Thailand; and have not evaluated real-world programme implementation costs or cost-effectiveness.11–14 Evaluating real-world HCV treatment programmes in low-income settings is critical to designing and implementing cost-effective HCV treatment programmes to achieve the global HCV elimination targets set by the WHO as it provides real data of current programmes which allow a better understanding of which components are driving cost and where cost-savings can be made.15
In 2016, Médecins sans Frontières (MSF) began a UNITAID-funded HCV treatment programme within an HIV cohort in Dawei, Myanmar using interferon (IFN)-free DAA-based regimens, and in 2018, obtained updated prices for quality-assured generic DAAs.16 With programmatic experience treating HCV/HIV-coinfected patients in Dawei, MSF subsequently proposed a simplified HCV treatment protocol as a potential HCV model of care that aligns with the 2017 Myanmar Ministry of Health (MoH) National Hepatitis Guidelines.
The purpose of this primary research, performed in collaboration with MSF, was to evaluate the cost of providing DAA treatment in the MSF programme and assess the cost-effectiveness of the programme compared with no treatment among HCV/HIV-coinfected patients in Myanmar. Additionally, we use these data to evaluate the potential cost-effectiveness of HCV treatment using generic DAAs and a simplified treatment protocol as proposed by MSF to the Myanmar MoH. To our knowledge, this is the first study to conduct a cost and cost-effectiveness analysis of a real-world HCV treatment programme for HIV-infected individuals in an LMIC.
Methods
Setting and model of care
The MSF-Dawei HIV clinic was established in 2004, targeting patients in Dawei and the entire Thanintharyi division in Southern Myanmar. In 2014, MSF began screening all HIV-positive patients attending the MSF-Dawei HIV clinic (87% of HIV-positive patients in the region) for HCV, initially providing IFN-based treatment. In late 2016, a UNITAID-funded prospective cohort study evaluating IFN-free HCV regimens with DAAs was initiated in the clinic. Within the MSF-Dawei clinic, there were 73 local staff members and 2 expatriate staff. Data including patient characteristics, outcomes and costs were collected from this UNITAID study, which was part of a larger multicentre cohort study to evaluate the effectiveness and cost-effectiveness of HCV screening and treatment programmes in LMICs.17
We assessed costs and outcome data among chronically HCV-infected (HCV RNA-positive) patients from the MSF-Dawei HIV cohort initiated on IFN-free DAA treatment between November 2016 and October 2017. There were no restrictions on treatment eligibility by HCV disease stage or substance use criteria. Prior to initiation, patients underwent liver disease staging and testing for comorbidities. Patients were classified by METAVIR stage (F0, F1, F2, F3, F4) based on transient elastography with those classified as having cirrhosis (F4) if they had a liver stiffness measure of ≥11 kPa. Decompensated cirrhosis (DC) was defined as liver stiffness ≥11 kPa and Child-Pugh score ≥6 based on values for HCV/HIV-coinfected patients.18 All patients were screened for hepatocellular carcinoma (HCC) via abdominal ultrasound. Patients were treated with sofosbuvir+daclatasvir (SOF+DAC) without or with ribavirin (RBV) as per the 2015 European Association for the Study of the Liver recommendations.19 During treatment, patients returned every 2–4 weeks (or more frequently, if necessary) for routine clinical monitoring and biological testing (figure 1). Patients were evaluated for sustained virological response (SVR), defined as a negative HCV RNA test 12 or more weeks after the end of treatment. Patients who did not achieve SVR12 with DAAs were not retreated. Patients were considered as lost to follow-up if they did not return within 2 months after a scheduled appointment and were not noted as dead or transferred out. Intention-to-treat SVR rates were calculated that included patients who were lost to follow-up or died.
Figure 1.
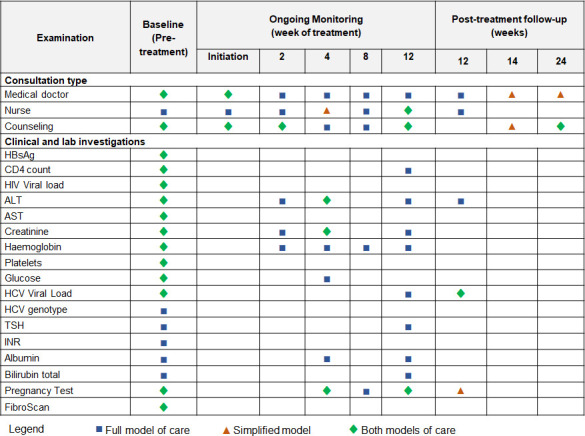
Treatment protocols for the MSF full model of care and simplified model of care for patients on a 12-week treatment regimen. Mandatory appointments shown, optional appointments excluded. ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; INR, international normalised ratio (coagulation test); MSF, Médecins Sans Frontières; TSH, thyroid stimulating hormone.
Costing methods
Overall costing approach
We performed a patient-level microcosting analysis of HCV treatment delivery from a programme provider’s perspective, incremental to the standard twice-yearly HIV visits. Data on costs were obtained from MSF’s financial records, receipts and price lists from a 12-month period (January 2017–December 2017), when the majority of the HCV-related costs were incurred. Records prior to 2017 were used to allocate a proportion of capital equipment costs obtained in previous years based on expected service lives estimated by interviewing local staff. Using an ingredients approach, patient-level resource use (in terms of type and frequency of visit) was combined with cost information for each patient interaction type. Patient-level data on number and type of visits, clinical examinations, laboratory investigations, treatment regimens and treatment outcomes were extracted from electronic medical records.20 Resources were valued from MSF financial records, invoices, price lists and additionally informed through interviews with key staff (finance, logistics, pharmacy manager, medical activity manager). We present costs stratified by HCV-related visit components, HCV-related lab costs, DAA costs and coordination costs, as described below. Results are presented in 2017 US$.
HCV-related visit components
HCV-related visits were classified as: (1) pre-treatment (2) on-treatment and (3) post-treatment as per the MSF protocol (figure 1). All HCV-related labs costs were excluded from visit costs and costed separately (see below). Each HCV visit included personnel time specific to the visit (patient-interacting and administrative time, determined by staff diaries), space/materials depending on which area of the clinic was used (laboratory, medical, counselling, pharmacy), and proportion of usage for HCV treatment. For each location, the visit cost incorporated recurrent costs (general personnel costs, medicines (excluding HCV), medical and laboratory supplies, non-medical supplies, transport operating costs, building rental and insurance, maintenance, utilities and bills, freight and clearance, travel and training) and capital costs (buildings, vehicles, medical equipment including FibroScan transient elastography machine, laboratory equipment including GeneXpert real-time PCR system, cold chain equipment, non-medical equipment, construction and rehabilitation, and furniture). Building space for each location visit was determined through site maps and visual inspection and allocated as HCV related by determining the proportion of all consultations which were HCV related from records. Personnel effort by visit type was determined by general staff category (coordination, nursing, medical doctor, individual counselling, pharmacy, registration, human resources, support staff), involvement in HCV-related activities and allocated to proportion of staff, budget, floor space or consultations. Group counselling for HCV treatment, in which patients shared HCV treatment experiences and served as a discussion group for treatment preparation (including counselling on HCV infection, transmission, encouragement for family testing, lifestyle, treatment and monitoring plan, and contact tracing to minimise loss to follow-up), was costed separately.
HCV-related laboratory costs
Costs of HCV-related laboratory investigations as per the MSF protocol (figure 1) were obtained from invoices and price lists.
DAA costs
Unit costs were determined from MSF invoices (online supplemental table S1). Patient-specific DAA costs were calculated based on observed length of treatment and treatment regimen.
bmjgh-2020-004181supp001.pdf (2MB, pdf)
Coordination costs
Per visit MSF coordination costs were included from the local coordination site (Dawei) and country coordination (Yangon) using a top-down method (see online supplemental material). For Dawei, HCV-related coordination costs were estimated through obtaining the remaining personnel, recurrent and capital costs associated with the HCV programme, after extracting specific costs attributable to direct HCV visits by type. For Yangon, coordination costs included the proportion of personnel effort attributed to the Dawei programme by staff type and non-personnel costs (eg, all HCV-related activities) and were allocated as a proportion of the total budget.
Cost-effectiveness methods
Disease progression model
We developed a compartmental, deterministic Markov model of liver disease progression in a closed cohort of diagnosed HCV/HIV-coinfected adults (figure 2), based on the liver disease distribution in the MSF cohort (online supplemental table S1). We simulated disease progression through each stage of HCV-related hepatic fibrosis (METAVIR stages F0, F1, F2, F3), compensated cirrhosis (CC, METAVIR F4), DC and HCC. Liver-related mortality was assumed to only occur from DC or HCC. The model did not include liver transplantation, as this is not commonly performed in Myanmar. The model was additionally stratified by treatment history and outcome (untreated, treated and cured, or treated and failed). Individuals with F3 or milder liver disease who were treated and achieved SVR were assumed not to have further liver fibrosis progression. Those with CC, DC or HCC who were treated and achieved SVR could progress to more severe liver disease states or liver-related death but at reduced rates. We assumed those who achieved SVR cannot be reinfected and those who did not achieve SVR with DAAs were not retreated. The model was developed in Matlab R2018a.
Figure 2.
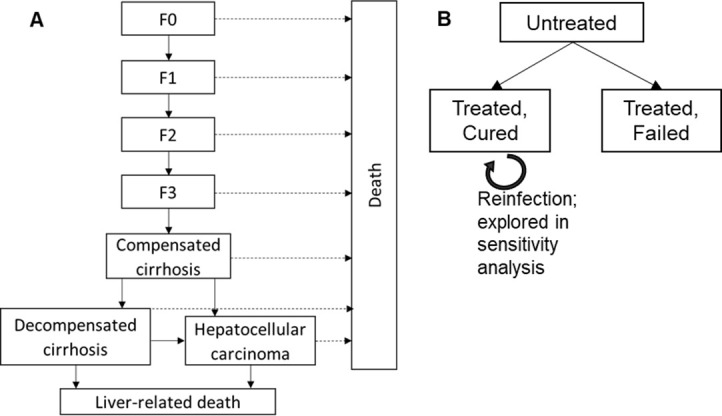
Schematic of Markov model showing (A) untreated chronic HCV disease progression by liver disease states and (B) stratification of the model by treatment. For those who are cured (achieve SVR), further liver disease progression is halted (if in stages F0–F3) or reduced compared with those who do not achieve SVR (if in stages CC, DC, HCC). F0–F3 are METAVIR hepatic fibrosis scores determined by transient elastography (<11.0 kPa); cirrhosis: METAVIR score ≥11.0 kPa; DC: METAVIR score ≥11.0 kPa and Child-Pugh score ≥6. HCC was determined by abdominal ultrasound. CC, compensated cirrhosis; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; SVR, sustained virological response.
Disease progression rates and mortality
Liver disease state transition probabilities (online supplemental table S3) were based on previous studies among HCV/HIV-coinfected individuals which suggest faster acceleration to more advanced hepatic fibrosis stages and mortality among HCV/HIV-coinfected individuals off antiretroviral therapy (ART) compared with HCV/HIV-coinfected individuals on ART.1 21–26 Background (non-HCV related) mortality rates were estimated given the CD4 count distribution, ART status of the cohort and estimated life expectancy based on mean age of the cohort weighted by sex27 (This information references 1.3 Background (non-HCV related) mortality rate calculation in the online supplemental text).
Costs
HCV treatment and routine HIV care and treatment costs were obtained through our patient-level analyses. Due to a lack of information available on patient access to care for advanced liver disease associated with HCV outside of the HIV clinic, for the baseline analysis we use estimates of HCV-related disease management costs from similar income settings (Cambodia), adjusted for Gross Domestic Product (GDP; online supplemental table S3). Patients who achieved SVR were assumed to remain in their disease stage and continue to accrue disease stage costs despite being cured.
Health utilities
Health outcomes were evaluated in disability-adjusted life-years (DALYs). Health disutilities for HIV and liver disease stages were obtained from the Global Burden of Disease (online supplemental table S3)28 and coinfection disutility values calculated as: [1−(1−HIV disability weight)×(1−HCV disability weight)].26
Cost-effectiveness analyses
We evaluated the cost-effectiveness of HCV treatment for HCV/HIV-infected individuals compared with no HCV treatment. We evaluated the following treatment protocol scenarios:
‘Observed MSF’: data from observed full MSF protocol from the implemented UNITAID HCV DAA study in 2016/2017, using 2017 DAA prices.
‘MSF updated DAA cost’: costs estimated from full MSF protocol, but with updated DAA prices based on the outcomes of the MSF HCV tender for quality-assured generic DAAs (reduces 12-weeks SOF+DCV from US$493 to US$120) negotiated after the study in 2018.
‘Simplified MoH’: we estimate costs of a simplified treatment protocol (as proposed by MSF to the Myanmar MoH after the study in 2018, figure 1) if implemented by the MoH. The simplified protocol reduced the number of visits and laboratory measurements and incorporated partial task-shifting from doctors to nurses. To represent implementation by the MoH, we also use local staff costs (26% less expensive than current staff costs), no MSF coordination costs, quality-assured generic DAA prices and updated HCV test costs (previously OraQuick rapid test, and now SD Bioline HCV rapid test resulting in a~US$5 reduction per test). We simulate cost-effectiveness of the proposed simplified protocol assuming the same SVR as observed with the full protocol.
The model was run for 100 years, with cost and utilities discounted at 3%/year. To account for parameter uncertainty, we performed a probabilistic sensitivity analysis, sampling 1000 parameter sets from parameter distributions (online supplemental table S3). We calculated the mean incremental cost-effectiveness ratio (ICER, mean incremental costs divided by mean incremental DALYs averted) for the intervention compared with no treatment. Interventions with an ICER less than a willingness-to-pay (WTP) threshold of one times per capita GDP of Myanmar (US$1250 in 2017) were considered cost-effective.29 30
One-way sensitivity analyses
We performed several one-way sensitivity analyses on the ICER for each of the ‘Observed MSF’, ‘MSF updated DAA cost’ and ‘Simplified MoH’ strategies compared with no treatment. We varied the discount rate (0% and 6% compared with 3% at baseline), time horizon (20 and 50 years vs 100 years at baseline), SVR rate (90% and 98% vs 96% at baseline), initial distribution of fibrosis stage (30% and 60% patients with cirrhosis vs 46% at baseline), HCV/HIV coinfection disutility values (lower and upper bounds vs mean values at baseline), transient elastography costs (cost in Cambodia observed with higher volume of use compared with Dawei: $4 compared with $115), reinfection among those who achieved SVR (5%/year vs 0% at baseline), no cost for care for all hepatic fibrosis stages (vs F0: $0; F1: $35; F2: $80; F3: $137; F4: $207; DC: $314; HCC: $378 at baseline), no coordination cost (vs $98 for patients without cirrhosis and $142 for patients with cirrhosis at baseline), and accelerated liver disease progression among patients with genotype (GT) 3 (HR: 1.31 for cirrhosis (95% CI 1.22 to 1.39); HR: 1.80 for HCC (95% CI 1.61 to 2.03)31; among 56% of patients). Additionally, for the ‘Simplified MoH’ strategy, we examine task-shifting to nurse-led care only during treatment, reducing overall physician interactions by 56% (nine visits vs four; and nurse interaction by 66% from six interactions to two) and examine equal SVR rates as observed in the MSF trial or those reduced to SVR rates to 70% in the event the simplified model results in reduced SVR.
HCV screening and treatment sensitivity analyses
Because screening occurred several years prior to the UNITAID intervention, our base case evaluates the cost-effectiveness of the DAA treatment programme only. For a sensitivity analysis, we explored the cost-effectiveness of a combined screening and treatment programme for the ‘Simplified MoH’ scenario compared with no screening and treatment across various HCV seroprevalences (0.5%–10%), reflecting likely geographical heterogeneity across Myanmar (see online supplemental information for details). We estimated associated screening costs based on testing yields for each prevalence scenario, assuming HCV antibody testing using the SD Bioline HCV rapid test (US$2.33) and GeneXpert HCV RNA test (US$21.09) with staff costs included.
Patient and public involvement
Study participants and the public were not involved in the design, conduct or reporting of this study. However, study findings will be disseminated through publications and presentations at conferences and other public events.
Results
Treatment outcomes
From November 2016 to October 2017, all 122 HIV-infected patients (mean age 43 years) who screened positive for HCV were treated with DAAs (56/122 (46%) with cirrhosis (CC or DC)). No HCC was detected among those treated or untreated. Roughly half of the treated cohort were GT3 (51%), followed by GT1 (46%) and GT6 (3%). Of these, 96% (n=117) achieved SVR. The majority of patients with cirrhosis (n=50; 89%) were treated with 24 weeks of SOF+DAC, but six were treated with 12 weeks of SOF+DAC+RBV resulting in lower costs (all six patients achieved SVR; GT1: n=1; GT3: n=4; GT6: n=1). One patient was previously treated with IFN-based HCV treatment (peg-IFN+RBV) prior to the availability of IFN-free DAA therapy and retreated with DAAs once available. Of those who did not achieve SVR (n=5), one died, one did not complete treatment and three completed treatment. There was no difference in SVR by liver fibrosis stage (online supplemental table S2).
Treatment delivery cost
The average cost of HCV treatment per patient was $1229 (95% CI $848 to $1829) for patients without cirrhosis and $1971 (95% CI $1307 to $2686) for patients with cirrhosis (online supplemental figure S1). Variations in cost were predominantly due to differences in durations of treatment and drug regimens, with minor differences in monitoring. DAA drug cost was the largest cost component and main driver of difference in cost by liver disease stage (without cirrhosis: $524 vs with cirrhosis: $1122; table 1). The second largest driver of cost was laboratory costs, with minimal difference by liver disease stage (without cirrhosis: $421 vs with cirrhosis: $437). Of these laboratory costs, transient elastography costs comprised $115, which was high because of the initial purchase price (~US$49 037) and relatively low usage (159 measurements in 2017). Visit costs were the third largest contributor to cost (breakdown by visit type in online supplemental table S4). Within the personnel component of visit costs, 61% of the personnel costs were due to physician costs (three local, one foreign), as the protocol incorporated physician-led treatment. Coordination costs were on average $98 per treatment for patients without cirrhosis and $142 per treatment for patients with cirrhosis (45% from Dawei, and 55% from Yangon; This corresponds to 1.1 Valuation of coordination costs in the online supplemental text).
Table 1.
Cost of HCV treatment by component type among HIV-infected individuals in Myanmar, with the ‘Observed MSF’ treatment protocol and proposed alternative protocols
| HCV visit costs per patient | HCV laboratory costs per patient | DAA costs per patient | HCV coordination costs per patient | Total HCV treatment costs per patient | |
| Observed MSF intervention* | |||||
| Non-cirrhotic | 186.60 (95% CI 158.65 to 292.94) | 420.80 (95% CI 194.80 to 718.94) | 523.53 (95% CI 411.60 to 663.81) | 97.60 (95% CI 82.74 to 153.66) |
1228.53 (95% CI 847.79 to 1829.35) |
| Cirrhotic | 270.47 (95% CI 225.45 to 419.17) | 436.73 (95% CI 251.62 to 697.04) | 1122.01 (95% CI 711.58 to 1349.37) | 141.84 (95% CI 118.20 to 220.15) | 1971.05 (95% CI 1306.85 to 2685.72) |
| MSF with updated DAA costs† | |||||
| Non-cirrhotic | 186.60 (95% CI 158.65 to 292.94) | 420.80 (95% CI 194.80 to 718.94) | 183.69 (95% CI 169.03 to 198.35) |
97.60 (95% CI 82.74 to 153.66) |
888.69 (95% CI 742.41 to 827.62) |
| Cirrhotic | 270.47 (95% CI 225.45 to 419.17) | 436.73 (95% CI 251.62 to 697.04) | 453.02 (95% CI 413.51 to 492.53) |
141.84 (95% CI 118.20 to 220.15) |
1302.06 (95% CI 1102.84 to 1219.49) |
| Simplified MoH‡ | |||||
| Non-cirrhotic | 80.92 | 216.33 | 120 | – | 417.25 |
| Cirrhotic | 89.54 | 271.25 | 240 | – | 600.79 |
*‘Observed MSF intervention’ presents summary data from observational study, including 2017 DAA prices.
†‘MSF with updated DAA costs’ estimates costs with updated DAA prices for quality-assured generic DAAs negotiated in 2018.
‡‘Simplified MoH’ strategy estimates costs with generic DAAs and a proposed simplified protocol (figure 1), with local staff costs and no overheads. The 95% CIs are presented for the observed cost data reflecting patient variations in observed costs. For estimations of costs using updated cost data or simplified strategies, patients were assumed to adhere to the exact clinical schedule (see figure 1) and so no uncertainty is provided. Non-cirrhotic: METAVIR F0–F3, cirrhotic: F4 as measured by transient elastography.
DAA, direct-acting antiviral; HCV, hepatitis C virus; MoH, Ministry of Health; MSF, Médecins sans Frontières.
Updated quality-assured generic DAA costs were obtained after the end of our study ($120 for 12 weeks of SOF/DAC before MSF-overhead charges (online supplemental table S1). With these updated costs, the total estimated DAA costs when incorporating RBV (included in 54% of treatments) were $184 for 12 weeks, $453 for 24 weeks, reflecting variations in dose). With these costs, based on the observed treatment protocol, the total cost per treatment would be $889 for patients without cirrhosis and $1302 for patients with cirrhosis (table 1). In this scenario, the highest contributors to overall cost would be the laboratory and monitoring costs.
Cost-effectiveness of HCV treatment among HIV-infected individuals
The ‘Observed MSF’ treatment programme (mean treatment costs: $1229 (patients without cirrhosis), $1971 (patients with cirrhosis)) resulted in an average incremental cost of $2121 per patient treated including annual HIV care costs (online supplemental table S5), and 3.35 DALYs averted per patient. This led to a mean ICER of $634/DALY averted compared with no treatment, cost-effective compared with a WTP threshold of one times the per capita GDP of Myanmar ($1250) (table 2). In this analysis, 100% of the simulations fell under the WTP threshold.
Table 2.
Incremental cost-effectiveness of HCV treatment among HIV-infected individuals in Myanmar compared with no treatment, as observed and with proposed simplified protocols and newly negotiated DAA costs
| Strategy | Cost (US$ 2017) per capita | DALYs per capita | ICER mean | ||
| Total mean (95% CI) | Incremental mean compared with no treatment (95% CI) | Total mean (95% CI) | Incremental mean compared with no treatment (95% CI) | $/DALY averted compared with no treatment | |
| No treatment | 3991.71 (3133.86 to 4955.87) | – | 21.89 (20.77 to 22.92) | – | – |
| Observed MSF treatment programme* | 6112.72 (5019.45 to 7170.54) | 2121.01 (1885.59 to 2214.67) | 18.54 (17.48 to 19.50) | −3.35(−3.29 to −3.42) | 633.60 |
| MSF programme with updated DAA costs† | 5624.94 (4550.21 to 6738.39) | 1633.23 (1416.35 to 1782.52) | 18.54 (17.48 to 19.50) | −3.35 (−3.29 to −3.42) | 487.89 |
| Simplified MoH strategy‡ | 5050.30 (4009.81 to 6128.90) | 1058.59 (875.95 to 1173.03) | 18.54 (17.48 to 19.50) | −3.35 (−3.29 to −3.42) | 316.23 |
Estimates for interventions include cost of annual HIV care and treatment.
*‘Observed MSF intervention’ presents summary data from observational study, including 2017 DAA prices.
†‘MSF with updated DAA costs’ estimates costs with updated DAA prices for quality-assured generic DAAs negotiated in 2018.
‡‘Simplified MoH’ strategy estimates costs with generic DAAs and a proposed simplified protocol (figure 1), with local staff costs and no overheads.
DAA, direct-acting antiviral; DALYs, disability-adjusted life-years; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; MoH, Ministry of Health; MSF, Médecins sans Frontières.
The ‘MSF updated DAA cost’ analysis (with updated DAA prices, mean treatment cost $889 (patients without cirrhosis), $1302 (patients with cirrhosis)) produced a mean ICER of $488/DALY averted compared with no treatment, cost-effective under the WTP threshold (all simulations fell under the WTP threshold).
Finally, a ‘Simplified MoH’ strategy (also with cheaper drugs) could result in substantial reductions in treatment cost (patients without cirrhosis: $417, patients with cirrhosis: $601), and if resulting in equal treatment outcomes, could be highly cost-effective (mean ICER $316 DALY averted compared with no treatment, all simulations fell under the WTP threshold).
One-way sensitivity analyses
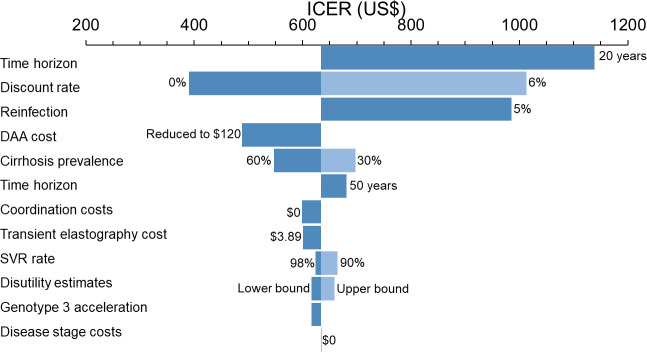
The ‘Observed MSF’ treatment programme remained cost-effective across all scenarios, if the discount rate was reduced to 0% or increased to 6%, there were no costs of care for hepatic fibrosis stages, coordination costs were excluded, GT3 patients were assumed to have accelerated liver disease progression, transient elastography costs were decreased, there was a time horizon of 20 or 50 years, they achieved a reduced SVR rate, there was different disutility estimates used, cirrhosis prevalence varied or reinfection rate was 5%/year (figure 3).
Figure 3.
Sensitivity analysis of the cost-effectiveness of the ‘Observed MSF’ model of care with 2017 DAA costs compared with no treatment. Costs shown in US$. The reduced FibroScan cost (US$3.89) scenario reflects the FibroScan cost estimated in similar income country setting with higher volume (GDP adjusted cost from Cambodia, US$2017; $4.31). Dark and light blue bars displayed when two values of a parameter were examined and resulted in ICER values lower and above the baseline ICER value (US$634). DAA, direct-acting antiviral therapy; GDP, Gross Domestic Product; ICER, incremental cost-effectiveness ratio; MSF, Médecins sans Frontières; SVR, sustained virological response (at 12 weeks).
The ‘MSF updated DAA cost’ and ‘Simplified MoH’ scenarios remained cost-effective for all sensitivity analyses (online supplemental figures S2 and S3). Furthermore, the ‘Simplified MoH’ scenario remained cost-effective with SVR rates of 70% (ICER: $372).
Screening and treatment sensitivity analyses
A combined screening and treatment programme among HIV-infected individuals implemented by the MoH using the ‘Simplified MoH’ strategy could be cost-effective at all HCV seroprevalences considered examined, including the lowest prevalence (0.5%; ICER: $489), below the national monoinfection estimate (2.7%) and the 8% observed among HIV-infected individuals in Dawei (ICER: $334; online supplemental figure S4).6 The ‘Simplified MoH’ strategy was cost-effective for HCV seroprevalences above 0.1% (ICER: $1152 at 0.1%; online supplemental figure S4).
Discussion
Main findings
Our study found that the DAA treatment programme among HCV/HIV-coinfected patients in Myanmar implemented by MSF is cost-effective, particularly with quality-assured generic DAAs. Moreover, a simplified model of care (proposed by MSF to the Myanmar MoH, incorporating fewer visits and task-shifting) implemented by the MoH with local staff could be highly cost-effective (ICER <$400/DALY averted compared with no treatment), if not associated with worse treatment outcomes, and could be cost-effective if combined with an HCV screening programme among HIV-infected individuals. These findings hold even with lower than observed (90%) SVR rates and reinfection rate was 5% per year. With quality-assured generic DAAs, treatment remained cost-effective even over 20-year time horizons.
The majority of treatment costs in our study were comprised of DAA costs in 2017, which were negotiated to lower prices after the study period in 2018 by the MSF Supply Centers and MSF Access campaign ($120 for 12-week course),16 underscoring the importance of generic competition to reduce drug prices and improve access to HCV treatment. The cost of transient elastography also contributed markedly to cost of treatment delivery because of the high purchase price and low annual use. These costs could be reduced if used in a higher volume clinic or if non-invasive methods for determining hepatic fibrosis were used (eg, Fibrosis-4 Index for Hepatic Fibrosis, aspartate aminotransferase-to-platelet ratio index or FibroSure).
Comparisons with existing literature
To our knowledge, our study is the first to evaluate the real-world costs and cost-effectiveness of DAA treatment in an implemented HCV treatment programme among HIV-infected individuals in a clinical setting in an LMIC. However, our study supports previous analyses indicating that HCV treatment is likely cost-effective in LMIC settings where DAAs are available at low costs. One study in Egypt found that implementing an HCV screening programme with IFN-based DAA therapy compared with no screening would be cost-effective.11 Compared with IFN-based therapy, IFN-free DAA therapy is superior in efficacy, has shorter treatment duration and better tolerability,32–35 yet in many settings historically more costly, though costs continue to fall.36 37 Similarly, two cost-effectiveness studies in India showed that implementing HCV screening and treatment with generic DAAs would be cost-saving within about a decade, but these studies did not use programmatic treatment delivery or outcome data.12 38 Importantly, none of these studies, ours included, incorporated data on access to healthcare, which may be low in LMICs, and therefore it is possible that treatment is less cost-effective than estimated if fewer medical costs are associated with untreated HCV infection. While our sensitivity analyses indicated that HCV treatment remained cost-effective with no cost of care for hepatic fibrosis stages, further work is warranted to assess real-world medical utilisation for liver disease in LMICs.
Strengths and limitations
The main strength of our study is that it was based on real-world programmatic costs and outcome data. However, as with all modelling studies, there were numerous uncertainties in the parameter values. Nevertheless, we incorporated these uncertainties in our analysis, conducted sensitivity analyses, and our results were generally robust to most of these uncertainties. First, no patients in our cohort received additional care for HCV within the MSF-Dawei HIV clinic, but it was unknown whether they received care at other medical facilities and so was not included in our analysis. Future work in this area is warranted to refine our estimates.
Second, we used published data on disease progression from other settings, while it is unclear whether these are truly generalisable to Myanmar. Our baseline analysis did not simulate differential disease progression by GT. Half our cohort was GT3, which has been associated with accelerated liver disease progression in HCV-monoinfected individuals,31 39 40 yet it is unclear if this is true in HCV/HIV coinfection. A sensitivity analysis incorporating accelerated disease progression among GT3 patients improved the cost-effectiveness.
Third, reinfection rates among HIV-infected individuals in Myanmar are unknown, however we note that our analyses with quality-assured generic DAA prices indicated that treatment was cost-effective even with reinfection rates of 5%.
Fourth, although we used observational data for our main analysis (‘Observed MSF’), our analyses examining a simplified model of care as proposed by MSF to the MoH are theoretical. The MoH strategy assumed equal SVR rates and projected costs based on adherence to the planned visits and monitoring plan. The true cost of an MoH strategy is unknown and could be different between HCV treatment programmes implemented at the hospital versus clinic level, due to variation in staff and clinical monitoring costs. Real-world data on costs and treatment outcomes are required to confirm these findings, although our sensitivity analyses show that treatment with generic DAA costs was cost-effective when including lower SVR and higher treatment costs, indicating that it is likely that our results would hold in other settings with worse treatment outcomes. Furthermore, compared with no treatment, treatment with DAAs would likely remain cost-effective in other real-world settings even with lower SVR rates. Additionally, the MoH may be able to acquire DAAs at even lower prices than the updated DAA costs included in our analyses, if purchased in bulk, which would further increase cost-effectiveness.
Fifth, the Markov model that we developed to describe HCV disease progression simulated an average population behaviour and thus did not incorporate individual-level heterogeneity. Sixth, both the study and model did not account for retreatment after DAA failure as retreatment was not available during the study period and is currently not recommended by national guidelines. We note however that within the MSF cohort, select individuals have been eligible for retreatment since 2019.
Finally, our study was based on data from a cohort receiving care from an MSF clinic in a single, rural setting (Dawei, Myanmar), so it is unclear whether our results are generalisable to the country or if scaled up to the broader population of people living with HIV. Additionally, our treated cohort were all on ART with well-controlled HIV and had access to consistent HIV counselling which may have resulted in greater adherence levels and follow-up than in a larger healthcare system without available counselling. Integrating HCV treatment into existing ART programmes and HIV clinics may be an effective strategy to reach HCV/HIV-coinfected populations and should be considered. Additionally, we recognise the clinical and medical environments equivalent to that of MSF clinics, which offer intensive follow-up protocols and patient engagement, may not exist nationally. Non-MSF clinics in Myanmar may differ in resource allocation and availability such as human resources, logistics and supply chains, which may influence (1) access and support for patients seeking care and treatment, and (2) treatment adherence, patient retention and patient outcomes, which could impact cost-effectiveness.
Conclusion
In conclusion, we found the MSF treatment programme for HCV infection among HIV-infected individuals in Myanmar cost-effective, with the potential of being even more cost-effective when using a simplified protocol as long as this does not result in worse treatment outcomes. Access to affordable, quality-assured generic DAAs improved cost-effectiveness.
Practical implications
Given our cost-effectiveness projections, national programmes in Myanmar and similar settings should no longer consider DAA cost a barrier, but rather consider these data along with simplified models of care as a means to cure people with HCV infection and progress towards WHO HCV elimination goals. While this study evaluated the current HCV treatment programme implemented by MSF, these results can be informative to the MoH in Myanmar and other similar LMIC settings.
Acknowledgments
We thank Jessica Burry for providing comments that greatly contributed to the clarity and context of the manuscript.
Footnotes
Handling editor: Lei Si
Contributors: LKM—data collection, analysis, manuscript preparation, writing and editing. AC—data analysis, review and editing of the manuscript. KPS—field coordination, provided data and expert opinion, review and editing of the manuscript. DCJ—provided data and expert opinion, review and editing of the manuscript. J-MZ—provided key data, review and editing of the manuscript. AI—participated in conceptualisation and coordination of study, review and editing of the manuscript. AL—conceptualisation, coordination, oversight of the study, review and editing of the manuscript. AN—provided data, review and editing of the manuscript. JGW—conceptualisation of study design, analytical plan and guidance, review and editing of the manuscript. NM—conceptualisation of study design, analytical plan and guidance, review and editing of the manuscript. VLR and AW—review and editing of the manuscript. CM—review and editing of the manuscript. SMK—review and editing of the manuscript. SB—review and editing of the manuscript. RSG—review and editing of the manuscript; PV—conceptualisation and coordination of the study, study design, analytical plan, review and editing of the manuscript. NKM—conceptualisation and coordination of the study, study design, analytical plan, data collection, preparation, review and editing of the manuscript.
Funding: This study was funded by UNITAID grant no SPHQ14-LOA -217. LKM acknowledges support from the National Institute of Drug Abuse (NIDA), National Institutes of Health (NIH) (grant number T32 DA023356). PV acknowledges support from the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Evaluation of Interventions at the University of Bristol in partnership with Public Health England (PHE). NM acknowledges funding from NIAID and NIDA (grant number R01AI147490) and from the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH) funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK.
Disclaimer: The views in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, the Department of Health and Social Care or Public Health England.
Competing interests: NM has received unrestricted research grants and honoraria from Gilead and Merck, outside the submitted work. PV has received unrestricted research grants from Gilead, outside the submitted work.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the MSF Ethical Review Board and the Ethical Review Committee of the Department of Medical Research (Lower Myanmar).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Thein H-H, Yi Q, Dore GJ, et al. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 2008;22:1979–91. 10.1097/QAD.0b013e32830e6d51 [DOI] [PubMed] [Google Scholar]
- 2.Chen T-Y, Ding EL, Seage Iii GR, et al. Meta-Analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis 2009;49:1605–15. 10.1086/644771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lédinghen V, Barreiro P, Foucher J, et al. Liver fibrosis on account of chronic hepatitis C is more severe in HIV-positive than HIV-negative patients despite antiretroviral therapy. J Viral Hepat 2008;15:427–33. 10.1111/j.1365-2893.2007.00962.x [DOI] [PubMed] [Google Scholar]
- 4.Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016;16:797–808. 10.1016/S1473-3099(15)00485-5 [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS . HIV and AIDS estimates, Myanmar, 2017. [Google Scholar]
- 6.Lwin AA AK, Htun MM, Kyaw YY. Sero-Prevalence of hepatitis B and C viral infections in Myanmar: national and regional survey in 2015. Myanmar Health Sciences Research Journal 2017;29. [Google Scholar]
- 7.Martinello M, Amin J, Matthews GV, et al. Prevalence and disease burden of HCV coinfection in HIV cohorts in the Asia Pacific region: a systematic review and meta-analysis. AIDS Rev 2016;18:68–80. [PubMed] [Google Scholar]
- 8.Ousley J, Nesbitt R, Kyaw NTT, et al. Increased hepatitis C virus co-infection and injection drug use in HIV-infected fishermen in Myanmar. BMC Infect Dis 2018;18): :657. 10.1186/s12879-018-3558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciences. G. EPCLUSA (sofosbuvir and Velpatasvir) tablets, for oral use., in initial U.S. approval, 2016. [Google Scholar]
- 10.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013;58:1598–609. 10.1002/hep.26431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DD, Hutton DW, Raouf AA, et al. Cost-Effectiveness model for hepatitis C screening and treatment: implications for Egypt and other countries with high prevalence. Glob Public Health 2015;10:296–317. 10.1080/17441692.2014.984742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal R, Chen Q, Goel A, et al. Cost-Effectiveness of hepatitis C treatment using generic direct-acting antivirals available in India. PLoS One 2017;12:e0176503. 10.1371/journal.pone.0176503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim AG, Qureshi H, Mahmood H, et al. Curbing the hepatitis C virus epidemic in Pakistan: the impact of scaling up treatment and prevention for achieving elimination. Int J Epidemiol 2018;47:550–60. 10.1093/ije/dyx270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapol N, Lochid-Amnuay S, Teerawattananon Y. Economic evaluation of pegylated interferon plus ribavirin for treatment of chronic hepatitis C in Thailand: genotype 1 and 6. BMC Gastroenterol 2016;16:91. 10.1186/s12876-016-0506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . Global health sector strategy on viral hepatitis 2016-2021. Geneva, 2016. [Google Scholar]
- 16.Frontières MS. MSF secures generic hepatitis C treatment at $120 compared to $147,000 launch price tag, 2017. Available: https://msfaccess.org/msf-secures-generic-hepatitis-c-treatment-120-compared-147000-launch-price-tag [Accessed 21 May 2019].
- 17.Frontières MS. Ensuring access to the HCV treatment revolution for HCV/HIV co-infected patients in low and middle income countries 2013.
- 18.Dienstag JL, Ghany MG, Morgan TR, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology 2011;54:396–405. 10.1002/hep.24370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association for Study of Liver . EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015;63:199–236. 10.1016/j.jhep.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308:2584–93. 10.1001/jama.2012.144878 [DOI] [PubMed] [Google Scholar]
- 22.Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158:329–37. 10.7326/0003-4819-158-5-201303050-00005 [DOI] [PubMed] [Google Scholar]
- 23.Pineda JA, Romero-Gómez M, Díaz-García F, et al. Hiv coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology 2005;41:779–89. 10.1002/hep.20626 [DOI] [PubMed] [Google Scholar]
- 24.Merchante N, Girón-González JA, González-Serrano M, et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS 2006;20:49–57. 10.1097/01.aids.0000198087.47454.e1 [DOI] [PubMed] [Google Scholar]
- 25.López-Diéguez M, Montes ML, Pascual-Pareja JF, et al. The natural history of liver cirrhosis in HIV-hepatitis C virus-coinfected patients. AIDS 2011;25:899–904. 10.1097/QAD.0b013e3283454174 [DOI] [PubMed] [Google Scholar]
- 26.Martin NK, Devine A, Eaton JW, et al. Modeling the impact of early antiretroviral therapy for adults coinfected with HIV and hepatitis B or C in South Africa. AIDS 2014;28 Suppl 1:S35–46. 10.1097/QAD.0000000000000084 [DOI] [PubMed] [Google Scholar]
- 27.Organization, W.H . Life tables Myanmar, 2016. [Google Scholar]
- 28.Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the global burden of disease 2013 study. Lancet Glob Health 2015;3:e712–23. 10.1016/S2214-109X(15)00069-8 [DOI] [PubMed] [Google Scholar]
- 29.Tan-Torres Edejer T, . Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis, e.a. Geneva: WHO, 2003. [Google Scholar]
- 30.Bank W. GDP per capita, Myanmar, 2018. [Google Scholar]
- 31.Kanwal F, Kramer JR, Ilyas J, et al. Hcv genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. veterans with HCV. Hepatology 2014;60:98–105. 10.1002/hep.27095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and Velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015;373:2599–607. 10.1056/NEJMoa1512610 [DOI] [PubMed] [Google Scholar]
- 33.Chromy D, Mandorfer M, Bucsics T, et al. High efficacy of interferon-free therapy for acute hepatitis C in HIV-positive patients. United European Gastroenterol J 2019;7:507–16. 10.1177/2050640619835394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira VL, Tonin FS, Assis Jarek NA, et al. Efficacy of interferon-free therapies for chronic hepatitis C: a systematic review of all randomized clinical trials. Clin Drug Investig 2017;37:635–46. 10.1007/s40261-017-0521-4 [DOI] [PubMed] [Google Scholar]
- 35.Chou R, Hartung D, Rahman B, et al. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: a systematic review. Ann Intern Med 2013;158:114–23. 10.7326/0003-4819-158-2-201301150-00576 [DOI] [PubMed] [Google Scholar]
- 36.Gray E, O'Leary A, Kieran JA, et al. Direct costs of interferon-based and interferon-free direct-acting antiviral regimens for the treatment of chronic hepatitis C infection. J Viral Hepat 2016;23:677–86. 10.1111/jvh.12532 [DOI] [PubMed] [Google Scholar]
- 37.Lee AS, van Driel ML, Crawford DH. The cost of successful antiviral therapy in hepatitis C patients: a comparison of IFN-free versus IFN-based regimens at an individual patient level in Australia. Clinicoecon Outcomes Res 2017;9:595–607. 10.2147/CEOR.S146280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaillon A, Mehta SR, Hoenigl M, et al. Cost-Effectiveness and budgetary impact of HCV treatment with direct-acting antivirals in India including the risk of reinfection. PLoS One 2019;14:e0217964. 10.1371/journal.pone.0217964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan A, Patel K, Naggie S. Genotype 3 infection: the last stand of hepatitis C virus. Drugs 2017;77:131–44. 10.1007/s40265-016-0685-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Nicola S, Aghemo A, Rumi MG, et al. Hcv genotype 3: an independent predictor of fibrosis progression in chronic hepatitis C. J Hepatol 2009;51:964–6. 10.1016/j.jhep.2009.08.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2020-004181supp001.pdf (2MB, pdf)