Abstract
Background
The secondary fracture prevention gap in the osteoporosis field has been previously described as a ‘crisis’. Closing this gap is increasingly important in the context of accumulating evidence showing that an incident fragility fracture is associated with an increased risk of subsequent fracture within 1–2 years, known as imminent fracture risk. The objective of this study was to use health services data to characterize the time between index fragility fractures occurring at different osteoporotic sites and subsequent fractures.
Methods
This retrospective observational study used de-identified health services data from the publicly funded healthcare system in Ontario, the largest province of Canada. Patients aged > 65 with an index fragility fracture occurring between 2011 and 2015 were identified from the ICES Data Repository using International Classification of Diseases (ICD)-10 codes. We examined median time to subsequent fragility fractures for osteoporotic fracture sites until the end of follow-up (2017). BMD assessment and use of osteoporosis therapies following index fracture were also characterized.
Results
Among 115,776 patients with an index fragility fracture, 17.8% incurred a second fragility fracture. Median time between index and second fracture occurring at any site was 555 days (interquartile range: 236–955). For each index fracture site examined, median time from index to second fracture was < 2 years. The proportion of patients with BMD assessment was 10.3% ≤1 year prior to and 16.4% ≤1 year post index fracture. The proportion of patients receiving osteoporosis therapy was 29.8% ≤1 year prior, 34.6% ≤1 year post, and 25.9% > 3 years post index fracture.
Conclusions
This cohort of Canadian patients aged > 65 years who experienced a fragility fracture at any site are at imminent risk of experiencing subsequent fracture within the next 2 years and should be proactively assessed and treated.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-021-04051-9.
Keywords: Osteoporosis, Fragility fracture, Subsequent fracture, Real-world data, Imminent fracture risk, Post fracture care, Secondary fracture prevention
Background
Fractures due to osteoporosis have been labelled as a public health crisis in part due to an increasing number of older adults at risk of fragility fractures and low rates of post-fracture management resulting in a large care gap across Europe and North America [1]. In Canada, osteoporosis is responsible for approximately 200,000 cases of fragility fractures annually [2–4] and the incidence of fragility fractures was estimated to be higher than that for myocardial infarction, stroke, and breast cancer combined [2, 3, 5, 6]. The burden of fragility fractures is high in adults aged over 65 years [7, 8] and the predicted increase in the number of older adults (eg, in Canada from 15% in 2011 to 25% in 2031) [9] is expected to result in a proportional increase in the number of fragility fractures in the next decades [10].
An incident fragility fracture is associated with an acute risk of subsequent fracture occurring within 1–2 years, known as an imminent fracture risk [11, 12]. When examining a 10-year period after an incident fracture, it was reported that the majority of subsequent fragility fractures tend to occur within the initial 2 years – 61% of subsequent fractures were reported to occur within the initial 2 years after a hip fracture, 54% after forearm fracture and 53% after humerus fracture (undefined anatomical location) [13]. Studies have shown that a fragility fracture occurring at any site within 1–2 years prior, including non-vertebral sites such as wrist and humerus (proximal or undefined anatomical location), was a better predictor of subsequent fracture risk than a more temporally distant fracture [11, 12, 14–21]. Recent clinical guidelines have therefore recommended urgent initiation of pharmacotherapy in osteoporotic adults who have sustained a fragility fracture at any osteoporotic site in the preceding 2 years [11].
In contrast to osteoporosis clinical practice, diagnosis of an incident event in patients with cardiovascular disease routinely results in urgent initiation of pharmacotherapy to prevent secondary events [22, 23]. In Canada, approximately 90% of patients receive antiplatelet therapy and other secondary prevention measures following acute coronary syndrome to prevent future events [24], whereas only 10–20% of patients receive pharmacotherapy following a fragility fracture [25–27]. Thus, recently published global calls to action have labelled the secondary prevention treatment gap a ‘crisis’ and urged for a shift in focus from managing osteoporosis to managing fragility fractures [28–30].
The objective of this study was to characterize imminent risk using a simple and novel approach, by describing the time to subsequent fracture after index fragility fractures of different sites. Proportions of patients receiving bone mineral density (BMD) assessment and osteoporosis medications pre and post index fracture were also described. A large Canadian patient cohort was drawn from Ontario, a province contributing to more than one-third of osteoporotic fractures occurring in Canada [4] with the aim to provide evidence supporting the urgency of closing the secondary fracture prevention gap as part of the existing calls to action.
Methods
Data sources and setting
This was a population-based retrospective database study conducted in Ontario, Canada. Health care encounters were recorded in multiple record-level, administrative datasets in the ICES Data Repository. Encrypted patient-specific identifiers (ICES-specific key number (IKN)) were used to link the administrative datasets [31]. The datasets include health services records for as many as 13 million people living in Ontario [32]. The primary databases used for this study are provided in Supplementary Table 1.
Study participants
Ontario residents aged > 65 with an index fragility fracture occurring between January 1, 2011 to March 31, 2015 were identified from healthcare records. The cohort was limited to those aged > 65 to collect medication data based on public drug coverage for at least 1 year prior to the index fracture. Data from 5 years prior to the index event, and up to March 31, 2017 were analysed (Supplementary Figure 1). Depending on when the index fracture occurred within the study period, the opportunity for follow up in this cohort ranged between 2 years (2015–2017) and 6 years (2011–2017). Fragility fractures were identified from hospital admissions, emergency and ambulatory care using International Classification of Diseases (ICD)-10 diagnostic codes for fracture as a main diagnosis or admitting diagnosis (see Supplementary Table 2). Patients were excluded from the cohort if they presented with non-osteoporotic fracture sites (ie, skull, face, hands, and feet) or fractures associated with a trauma code to maximize the probability that only fragility fractures were examined (see Supplementary Table 3) [33]. Patients were also excluded if they experienced a fragility fracture within 5 years prior to the index date to minimize the likelihood that examined outcomes were related to a recent fracture occurring prior to an index event.
Variables of interest and outcome measures
Outcome measures included rate of subsequent fractures and median time between index fractures occurring at different osteoporotic sites (i.e., hip, vertebral [clinical], wrist [distal radius, or both distal radius and ulna], clavicle/ribs/sternum, humerus, tibia/fibula/knee [including medial and lateral malleolus], pelvis, radius/ulna [proximal, midshaft, or distal ulna only], multisite, femur) and subsequent fractures, captured from index fracture until the end of the study period. To avoid double-counting, fracture codes of the same type that occurred within 3 months of each other were assumed to stem from the same fracture. Additional outcome measures included BMD assessment (by dual energy x-ray absorptiometry [DXA] only) and osteoporosis treatments prior to and following the index fracture. The following treatments licensed for use to treat osteoporosis in Canada were included in the analysis: bisphosphonates, denosumab, hormone replacement therapy (HRT), teriparatide and raloxifene. Calcium and vitamin D supplementation was not assessed as these data are not available in the administrative datasets of the ICES Data Repository. DXA-BMD assessment dates were obtained via Ontario Health Insurance Plan (OHIP) billing codes. For subsequent fractures and BMD assessment, all patients had a minimum 1-year of follow-up. Persistence of osteoporosis treatment was assessed and defined as time on any osteoporosis treatment during the study period and based on the number of days supplied with treatment.
Data synthesis and analysis
The number and proportion of patients with a subsequent fragility fracture and median time from index to subsequent fractures were described in the entire index fracture cohort. The number and proportion of patients with second fragility fractures were also reported in subgroups included in the assessment of BMD and osteoporosis medications. The proportion of patients undergoing BMD screening within 5 years prior and up to 5 years following the index fracture was obtained. The analysis of dispensed osteoporosis treatments included two time periods: (1) prior to and during the time of index event and (2) post index event. The first period included osteoporosis treatments dispensed within 1 year prior to, and at the time of the index event. Post index event dispensed osteoporosis treatments were assessed from 8 days post index fracture hospital discharge date up to 5 years post index fracture. To determine the proportion of patients with subsequent fractures, BMD assessments and osteoporosis treatment, the number of individuals in the specified year was divided by the number of individuals alive at the beginning of that year. Persistence was estimated using a cumulative incidence function adjusted for death as a competing risk; each patient contributed the time of observation from initiation to the end of osteoporosis treatment during the time frame starting from the date of the index fracture until the end of follow up. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement was used to report the findings from this study [34].
Results
Characteristics of the study cohort
The cohort included 115,776 patients with a fragility fracture (see Supplementary Figure 2). The median age was 81 years (IQR 74–87 years) and 72.3% of patients were women (Table 1). The most common sites of index fractures were the hip (27.3%, n = 31,613), wrist (15.4%, n = 17,859), and clavicle/ribs/sternum (12.6%, n = 14,559). Hip and clinical vertebral fractures vs non-hip non-vertebral fractures vs multisite fractures made up 34.0% (n = 39,334) vs 62.8% (n = 72,707) vs 3.2% (n = 3735) of all index fracture skeletal sites, respectively. The number of index fragility fractures occurring at any site annually ranged from 25,154 in 2011 to 29,385 in 2014. The most common types of comorbidities in this cohort were osteoarthritis (76.2%), diabetes (30.6%), and stroke or cerebrovascular events (30.3%). The proportion of patients on oral steroid treatment 1 year prior to index fracture was 2.9%.
Table 1.
Characteristics of the index fragility fracture cohort
| Characteristic | n (%)a |
|---|---|
| Total number of patients in cohort | 115,776 |
| Sex | |
| Women | 83,690 (72.3) |
| Men | 32,086 (27.7) |
| Age (years) | |
| Median (IQR) | 81 (74–87) |
| Mean (SD) | 80.4 (8.3) |
| 66–70 | 17,998 (15.5) |
| 71–75 | 17,847 (15.4) |
| 76–80 | 20,596 (17.8) |
| 81–85 | 24,119 (20.8) |
| ≥ 86 | 35,216 (30.4) |
| Index fracture by siteb | |
| Hip | 31,613 (27.3) |
| Wrist (distal radius, or both distal radius and ulna) | 17,859 (15.4) |
| Clavicle/ribs/sternum | 14,559 (12.6) |
| Humerus | 13,237 (11.4) |
| Tibia/fibula/knee (including medial and lateral malleolus) | 10,894 (9.4) |
| Pelvis | 8328 (7.2) |
| Vertebral (clinical) | 7721 (6.7) |
| Radius/ulna (proximal, midshaft, or distal ulna only) | 4828 (4.2) |
| Multisite | 3735 (3.2) |
| Femur | 3002 (2.6) |
| Index fracture at any site by yearb | |
| 2011 | 25,154 |
| 2012 | 26,045 |
| 2013 | 27,969 |
| 2014 | 29,385 |
| 2015b | 7223 |
| Index fragility fracture treatment location | |
| Urban | 103,720 (89.6) |
| Rural | 10,626 (9.2) |
| Missing | 1430 (1.2) |
| Respiratory conditionsc | |
| Asthma | 17,538 (15.1) |
| COPD | 33,485 (28.9) |
| Inflammatory conditionsc | |
| RA | 4459 (3.9) |
| Psoriasis | 8076 (7.0) |
| SPA | 5084 (4.4) |
| Osteoarthritis | 88,223 (76.2) |
| Cancerc | 8390 (7.2) |
| Chronic kidney diseasec | 13,757 (11.9) |
| Diabetesc | 35,434 (30.6) |
| Vascular eventsc | |
| MI | 8175 (7.1) |
| Stroke or Cerebrovascular Events | 35,030 (30.3) |
| Dementiac | 24,092 (20.8) |
| OP treatmentd | |
| Any OP treatment | 32,757 (28.3) |
| Bisphosphonate | 29,030 (25.1) |
| Denosumab | 1578 (1.4) |
| HRT | 3597 (3.1) |
| Raloxifene | 656 (0.6) |
| Teriparatide | 0 (0) |
| Steroid used | 3340 (2.9) |
| Opioid used | 34,834 (30.1) |
Abbreviations: COPD chronic obstructive pulmonary disease, HRT hormone replacement therapy, IQR interquartile range, MI myocardial infarction, OP osteoporosis, RA rheumatoid arthritis, SD standard deviation, SPA spondyloarthritis
aValues reported as n (%) unless otherwise indicated; percent of total cohort (N = 115,776)
bIndex fragility fracture cases from January 1, 2011 to March 31, 2015
cTime frame for cancer was within 5 years prior to index date and for all other comorbidities any time prior to index date
dDispensed within one year prior to index fracture; assessed based on public coverage
Subsequent fractures
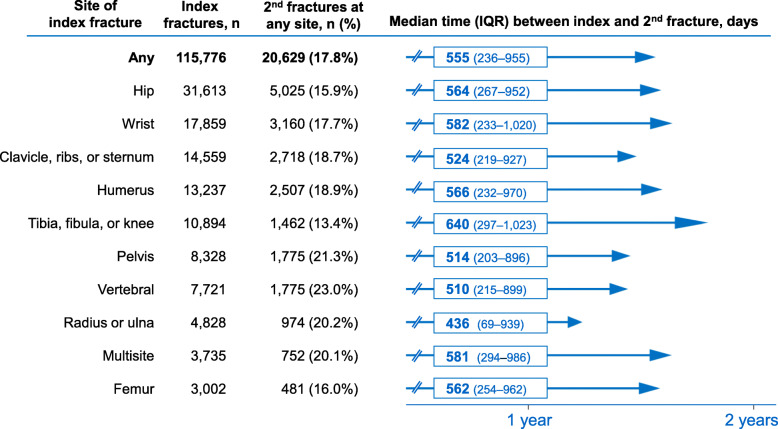
Among all patients in the cohort, 17.8% (n = 20,629) incurred a second fracture and 3.6% (n = 4197) incurred a third fracture over the study period. The median time between index and second fracture of any site was 555 days (interquartile range: 236–955; Fig. 1). When the median time was examined across any index fracture site, it was consistently < 2 years (< 730 days; Fig. 1). Furthermore, the proportion of patients experiencing a second fragility fracture was consistently > 15% across index fracture sites, except for index fractures of the tibia/fibula/knee (13.4%). The most common sites of second fracture were hip (27.8%, n = 5745), clavicle/sternum/ribs (11.9%, n = 2460), and wrist (10.9%, n = 2249), with the combination of hip and clinical vertebral fractures accounting for 36.7% (n = 7564) of second fractures (see Supplementary Figure 3).
Fig. 1.
Median time to second fragility fracture occurring at any site (by index fracture site). Number of index fractures, number and proportion of second fragility fractures at any site, and time to second fracture stratified by site of index fracture. Fracture sites are in descending order of number of index fractures. Abbreviations: IQR, interquartile range
Secondary fracture prevention management
The proportion of patients with BMD assessment was 10.3% within 1 year prior and 16.4% within 1 year post index fracture (Fig. 2a). The proportion of patients with BMD assessment post index fracture was the highest at 1-year post facture and decreased with time over 5 years after fracture (from 16.4 to 3.7%) (see Supplementary Figure 4a).
Fig. 2.
Proportion of fragility fracture patients undergoing BMD assessment ≤1 year prior to and ≤ 1 year post index fracture by: a sex; b age group; and c site of index fracture
BMD assessment post-fracture was more commonly performed in women (18.3%); 66–70 years age group (26.7%); and for patients with wrist index fracture site (27.4%) (Fig. 2a-c). Among these subgroups, patients in younger age groups or with index wrist fracture had lower absolute risk of second fracture relative to patients in older age groups or those with index fracture occurring at most of the other skeletal sites (Table 2). Further, patients with pelvis or multisite index fractures had among the lowest rates of BMD assessment post-index fracture and among the highest absolute risk of second fracture relative to other index fracture sites, while patients with hip or clavicle/ribs/sternum also had among the lowest BMD rates albeit the risk for subsequent fracture was not among the highest. Finally, men had lower rates of BMD assessment and also lower second fracture risk relative to women.
Table 2.
Proportion of patients in the index fragility fracture cohort with second fracture occurring at any fracture site, by subgroups
| Subgroups (n)a | Second fracture, any fracture site % (n)b |
|---|---|
| By sex | |
| Women (83,690) | 19.3% (16,194) |
| Men (32,086) | 13.8% (4435) |
| By age category | |
| 66–70 (17,998) | 13.0% (2331) |
| 71–75 (17,847) | 15.3% (2737) |
| 76–80 (20,596) | 18.3% (3759) |
| 81–85 (24,119) | 20.4% (4911) |
| ≥ 86 (35,216) | 19.6% (6891) |
| By sex and age category | |
| Women < 75 years old (n = 22,544) | 14.7% (3312) |
| Women ≥75 years old (n = 61,146) | 21.1% (12,882) |
| Men < 75 years old (n = 9595) | 12.0% (1155) |
| Men ≥75 years old (n = 22,491) | 14.6% (3280) |
| By index fracture sitec | |
| Vertebral (7721) | 23.0% (1775) |
| Pelvis (8328) | 21.3% (1775) |
| Radius/ulna (4828) | 20.2% (974) |
| Multisite (3735) | 20.1% (752) |
| Humerus (13,237) | 18.9% (2507) |
| Clavicle/ribs/sternum (14,559) | 18.7% (2718) |
| Wrist (17,859) | 17.7% (3160) |
| Femur (3002) | 16.0% (481) |
| Hip (31,613) | 15.9% (5025) |
| Tibia/fibula/knee (10,894) | 13.4% (1462) |
a Subgroups included in the assessment of BMD and osteoporosis treatment
b Percent of respective subgroup
c Index fragility fracture cases from January 1, 2011 to March 31, 2015. Second fragility fracture cases from the date of index event to March 31, 2017. Reported from highest to lowest proportion of second fractures
Osteoporosis therapies
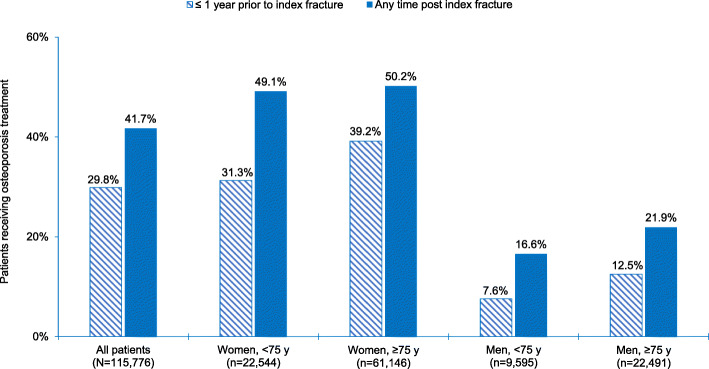
The proportion of patients receiving osteoporosis therapy was 29.8% within 1 year prior to and during the time of index fracture, 34.6% within 1 year post, and 25.9% during a period of > 3 years post index fracture. The highest proportion of patients receiving osteoporosis therapy at any time post index fracture (50.2%) or within 1 year post index fracture (42.8%) was among women aged ≥75 (Fig. 3; Supplementary Figure 4b). This subgroup was observed to also have the highest risk of second fracture observed relative to younger women or older/younger men (Table 2). Further, men had 2.3–3.0-fold lower rates of osteoporosis treatment post-index fracture relative to women (Fig. 3), yet their second fracture risk was only 1.2–1.4-fold lower. Of patients who were on osteoporosis therapy at the time of their index fracture, 73.4% (95% confidence interval (CI) 72.8–73.9%) were non-persistent (i.e. ended treatment) over the follow-up period.
Fig. 3.
Proportion of patients, by sex and age group, receiving any osteoporosis treatment within 1 year prior* to and during the time of index fragility fracture and at any time post index fracture. * ≤ 1 year prior period included osteoporosis treatments dispensed within 1 year prior to and during the time of index fracture, and also captured the period of 7 days post index fracture hospital discharge date (to reflect a potential delay in the dispensing of osteoporosis treatments prescribed at the time of the index event). Post index event dispensed osteoporosis treatments were assessed from 8 days post index fracture hospital discharge date until the end of study follow-up. Osteoporosis treatments examined in this cohort included bisphosphonates (alendronate, etidronate, risedronate, or zoledronic acid), denosumab, teriparatide, raloxifene, and HRT
Discussion
In this real-world cohort of patients aged > 65 with an index fragility fracture, 17.8% of patients (n = 20,629) sustained a second fracture during the follow-up period. Half of patients who incurred a second fracture, sustained it within 2 years after their index event; this timeframe was consistent across all index fracture sites examined. Hip and clinical vertebral fractures, which were previously reported to increase mortality in Canadian men and women by 2–3 fold [35], were found to make up 34.0% of index and 36.7% of second fractures in this cohort.
Our findings are supported by results of previous studies, where 18% of women ≥65 years and 11–12% of women ≥50 years sustained a second osteoporotic fracture within 2 years of an index fracture [21, 36, 37]. Studies examining fracture risk over time after an incident fracture have demonstrated a 2.7 to 5-fold higher fracture risk within 1–2 years post-fracture (ie, imminent risk) compared to 1.4 to 2-fold higher 10-year risk [12, 38]. The data highlighting high rates of subsequent fractures within 2 years post-fracture, as well as recommendations in recent international guidelines, support the need to recognize a fragility fracture at any site as an important risk factor for subsequent fracture [11, 13, 14, 28, 30].
Our study findings also provide evidence for lack of secondary fracture prevention showing only 16.4% of patients in this cohort undergoing BMD assessment within one-year post-index fracture, which is recommended to help re-assess fracture risk post-fracture [39]. BMD assessment has also been associated with an increased use of osteoporosis therapies and reduction in the rate of hip fractures [40, 41]. Wrist index fracture was the site associated with the highest rates of post-fracture BMD assessment, but was not associated with as high of a risk for subsequent fractures relative to many other index fracture sites. As such, increased awareness among practitioners is needed to recognize fractures at all other osteoporotic sites (except ankle) as a prompt for BMD assessment, as much as, and even more so, than wrist fracture. Although patients in older age groups or with hip index fractures were also associated with lower BMD post-fracture assessment rates relative to their counterparts, the need for post-fracture BMD assessment for the purposes of fracture risk re-assessment is not as high in these groups of patients, because hip fracture alone or older age plus a history of fracture are indicators of high fracture risk independent of BMD [7, 13]. Osteoporosis therapies, which reduce the risk of vertebral and non-vertebral fractures [7, 42, 43] were dispensed ≤1 year prior and during the time of index fracture in 29.8% of patients and in 41.7% of patients any time post index-fracture [24, 44]. Although, based on our data, it was not possible to estimate 10-year FRAX fracture risk for all patients in this cohort, it could be estimated for women aged ≥75. All women aged ≥75 were potentially at high fracture risk in this cohort (based on FRAX calculator inputs of age 75, BMI 25–31 kg/m2 and a history of fragility fracture) [45], and should have initiated therapy according to the 2010 Osteoporosis Canada guidelines [7]. However, only 50.2% of women aged ≥75 received therapy over the study follow-up, compared to 39.2% within 1 year prior and during the time of index fracture. Furthermore, recent guidelines recommend urgent initiation of treatment in all adults who have sustained a fragility fracture in the preceding 2 years to help prevent subsequent fractures [11, 13, 43]. Finally, when considering post-fracture treatment gap in relation to subsequent fracture risk, men were observed to have a larger discrepancy between these two outcomes relative to women, and based on these data more awareness is needed among clinical practitioners to recognize a fragility fracture as a disease event requiring appropriate secondary prevention in men.
In our cohort, approximately 73% of patients discontinued osteoporosis treatment by the end of the follow up period. This is higher than rates reported in a recent study of Canadian women and men recruited as part of a Fracture Liaison Service (FLS); 1- and 2-year non-persistence rates were 34 and 46%, respectively [46]. Considering the decline in treatment rates and persistence observed with time after fracture, it is important to note the benefits of long-term osteoporosis treatment highlighted in recent literature [43, 47, 48].
The secondary fracture prevention gap described here may have been influenced by several factors documented in recent studies including: insufficient communication from the fracture clinic informing family doctors of their patient incurring a fragility fracture and of high fracture risk (if present) [49]; not incorporating initiation of osteoporosis treatment into discharge order sets following hip fracture [50]; deprioritization of osteoporosis management over other chronic diseases in primary care potentially due in part to underestimation of the consequences of fragility fractures on morbidity and mortality in elderly people [51]; lack of urgency around secondary fracture prevention by utilizing 10 year fracture risk instead of imminent fracture risk [7]; the overreliance on densitometric osteoporosis diagnosis thresholds (BMD T-score of ≤ − 2.5) for therapy initiation rather than history of fracture [52, 53]; lack of guidance surrounding the benefit of osteoporosis treatment and the risk of rare adverse events from these treatments (ie, atypical femoral fractures and osteonecrosis of the jaw; < 80 per 100,000 person-years) [7, 54]; and overestimated concerns of these rare events by other specialties (i.e., dentists concerned with osteonecrosis of the jaw [54, 55]). Current efforts are urgently needed to help address the secondary prevention care gap and its contributors, as part of new guidelines development, advocacy measures, and other initiatives (eg, FLS) [11, 13, 53, 54, 56].
This study examined patients aged > 65 on the provincial public drug benefit program and close to one-third of patients were aged ≥86, which limited the generalizability of the results to younger Canadians. By excluding patients who had another fracture 5 years prior to their index event, but not beyond those 5 years, the cohort was potentially biased towards an older population. There may be an underestimation of the number of fractures in this cohort, particularly non-hip, considering that only the ‘Most Responsible Diagnosis’ and ‘Pre-Admit Comorbidity’ were used to identify index fractures. Vertebral fractures were likely underestimated considering that two-thirds are typically silent [57, 58]. Medication rates may have been underestimated, since only medications covered through the provincial public drug program were captured. Medications without public coverage during this study period (i.e. denosumab for post-menopausal women with osteoporosis prior to 2012; denosumab for men with osteoporosis) or with restricted reimbursement criteria (i.e. teriparatide) may have been reimbursed through private insurance plans. As in prior healthcare database research, the determination of fragility fracture was based on the exclusion of high-trauma ICD codes and not independent adjudication [33], which may have inaccurately represented the number of fragility fractures in this cohort.
Conclusion
Patients aged > 65 who have suffered a fragility fracture at any skeletal site are at imminent risk of experiencing subsequent fracture within the next 2 years and should be proactively assessed and treated for osteoporosis [7, 11, 40, 43, 59], considering the large secondary fracture prevention gap highlighted in this retrospective study, in addition to prior research on osteoporosis management in Canada [25–27].
Supplementary Information
Additional file 1: Supplementary Table 1. Primary databases used for the study. Supplementary Table 2. Diagnosis Codes for Fragility Fractures. Supplementary Table 3. Diagnosis Codes for Trauma Codes. Supplementary Figure 1. Study schema. Supplementary Figure 2. Flow Diagram of patients included in study. Supplementary Figure 3. Number and proportion of patients with second fragility fractures by site of second fracture. Supplementary Figure 4. Proportion of patients with (a) BMD assessment, over a period of 5 years prior to and post fracture, by sex and (b) receiving any osteoporosis treatment, by sex and age group, over time*
Acknowledgements
This study made use of de-identified data from the ICES Data Repository, managed by ICES with support from its funders and partners: Canada’s Strategy for Patient-Oriented Research (SPOR), the Ontario SPOR Support Unit, the Canadian Institutes of Health Research and the Government of Ontario. The opinions, results, and conclusions reported are those of the authors. No endorsement by ICES or any of its funders or partners is intended or should be inferred. Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI. Medical writing support was provided by Khalid Siddiqui and Kyle Willms of Amaris Consulting UK Ltd., and funded by Amgen Canada Inc. The authors would like to thank Arpit Chhabra and Erika Martire for their support on the manuscript.
Abbreviations
- BMD
bone mineral density
- FLS
Fracture Liaison Services
- FRAX
Fracture assessment tool
- ICD
International classification of diseases
- IKN
ICES-specific key number
Authors’ contributions
JDA, JPB, ES, J-ET, MR, MP, PM-D, NB, and LS contributed to design of the study, review and interpretation of the data, and drafting and review of the manuscript. VB and ADB contributed to the review and interpretation of the data, and drafting and review of the manuscript. The author(s) read and approved the final manuscript.
Funding
This study was funded by Amgen Canada Inc. including design of the study and analysis and interpretation of data. Data collection was performed by ICES and sponsored by Amgen Canada Inc. Medical writing support was sponsored by Amgen Canada Inc.
Availability of data and materials
The data that support the findings of this study are available from ICES but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available [https://www.ices.on.ca/Data-and-Privacy/ICES-data] [32]. Data are however available from the authors upon reasonable request and with permission of ICES.
Ethics approval and consent to participate
The study protocol was approved by the Advarra Institutional Review Board. De-identified aggregate level data was provided from ICES following patient-level analysis.
Consent for publication
Not applicable.
Competing interests
JDA has received consulting fees from Amgen and Eli Lilly; received research funding from Amgen, Pfizer, and BMS; served on the speaker’s bureau for Amgen; served on boards for the International Osteoporosis Foundation and Ontario Rheumatology Association. JPB has received consulting fees and honoraria from Amgen and Servier; received research funding from Mereo BioPharma, Radius Health and Servier; served on speakers’ bureau for Amgen. ES has received consulting fees from Amgen. J-ET has received consulting fees, research funding and honoraria from Allergan, AstraZeneca, Amgen, CSL Behring, Janssen, Novo Nordisk, Sage, Assurex/Myriad, Edwards Lifesciences, Pfizer, Roche, Merck, GlaxoSmithKline, Evidera, PCDI, CADTH. VB has received honoraria from Amgen and have served on Amgen Ad Boards. AB has received consulting fees, research funding and honoraria from Amgen, Bristol Myers Squibb, Janssen, AstraZeneca, Novartis, Pfizer, Bayer, Lilly, Boehringer Ingelheim, Sanofi, Valeant. LS, NB, PM-D, MP and MR are employees of and own stock in Amgen.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roux C, Briot K. The crisis of inadequate treatment in osteoporosis. Lancet Rheumatol. 2020;2(2):E110–E1E9. doi: 10.1016/S2665-9913(19)30136-5. [DOI] [PubMed] [Google Scholar]
- 2.Leslie WD, O'Donnell S, Lagace C, Walsh P, Bancej C, Jean S, et al. Population-based Canadian hip fracture rates with international comparisons. Osteoporos Int. 2010;21(8):1317–1322. doi: 10.1007/s00198-009-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Osteoporosis Canada . Make the FIRST break the LAST with Fracture Liason Services: Appendix B: Fracture incidence and costs by province. 2013. [Google Scholar]
- 5.Canadian Cancer Society/National Cancer Institute of Canada . Canadian Cancer Statistics 2007. 2007. [Google Scholar]
- 6.Public Health Agency of Canada. Tracking heart disease and stroke in Canada 2009. Available from: https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/publicat/2009/cvd-avc/pdf/cvd-avs-2009-eng.pdf. Accessed 18 Dec 2020.
- 7.Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182(17):1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oden A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int. 2015;26(9):2243–2248. doi: 10.1007/s00198-015-3154-6. [DOI] [PubMed] [Google Scholar]
- 9.Statistics Canada . Population Projections for Canada, Provinces and Territories 2009 to 2036. 2010. [Google Scholar]
- 10.Jaglal S, Cameron C, Croxford R, MacKay C. Ontario Osteoporosis Strategy -Provincial Performance Data for Osteoporosis Management 2017. Available from: https://www.osteostrategy.on.ca/wp-content/uploads/Final-OP-Provincial-Performance-Status-Report-Apr-2017-1.pdf. Accessed 18 Dec 2020.
- 11.Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological Management of Osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595–1622. doi: 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- 12.Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E, et al. Imminent risk of fracture after fracture. Osteoporos Int. 2017;28(3):775–780. doi: 10.1007/s00198-016-3868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanis JA, Harvey NC, McCloskey E, Bruyere O, Veronese N, Lorentzon M, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31(1):1–12. doi: 10.1007/s00198-019-05176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux C, Briot K. Imminent fracture risk. Osteoporos Int. 2017;28(6):1765–1769. doi: 10.1007/s00198-017-3976-5. [DOI] [PubMed] [Google Scholar]
- 15.Huntjens KM, Kosar S, van Geel TA, Geusens PP, Willems P, Kessels A, et al. Risk of subsequent fracture and mortality within 5 years after a non-vertebral fracture. Osteoporos Int. 2010;21(12):2075–2082. doi: 10.1007/s00198-010-1178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maravic M, Briot K, Roux C, College Francais d, Medecins R. Burden of proximal humerus fractures in the French National Hospital Database. Orthop Traumatol Surg Res. 2014;100(8):931–934. doi: 10.1016/j.otsr.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Clinton J, Franta A, Polissar NL, Neradilek B, Mounce D, Fink HA, et al. Proximal humeral fracture as a risk factor for subsequent hip fractures. J Bone Joint Surg Am. 2009;91(3):503–511. doi: 10.2106/JBJS.G.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schousboe JT, Fink HA, Taylor BC, Stone KL, Hillier TA, Nevitt MC, et al. Association between self-reported prior wrist fractures and risk of subsequent hip and radiographic vertebral fractures in older women: a prospective study. J Bone Miner Res. 2005;20(1):100–106. doi: 10.1359/JBMR.041025. [DOI] [PubMed] [Google Scholar]
- 19.Adachi JD, Berger C, Barron R, Weycker D, Anastassiades TP, Davison KS, et al. Predictors of imminent non-vertebral fracture in elderly women with osteoporosis, low bone mass, or a history of fracture, based on data from the population-based Canadian Multicentre Osteoporosis Study (CaMos) Arch Osteoporos. 2019;14(1):53. doi: 10.1007/s11657-019-0598-x. [DOI] [PubMed] [Google Scholar]
- 20.Giangregorio LM, Leslie WD, Manitoba Bone Density P Time since prior fracture is a risk modifier for 10-year osteoporotic fractures. J Bone Miner Res. 2010;25(6):1400–1405. doi: 10.1002/jbmr.35. [DOI] [PubMed] [Google Scholar]
- 21.Balasubramanian A, Zhang J, Chen L, Wenkert D, Daigle SG, Grauer A, et al. Risk of subsequent fracture after prior fracture among older women. Osteoporos Int. 2019;30(1):79–92. doi: 10.1007/s00198-018-4732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitchett DH, Goodman SG, Leiter LA, Lin P, Welsh R, Stone J, et al. Secondary prevention beyond hospital discharge for acute coronary syndrome: evidence-based recommendations. Can J Cardiol. 2016;32(7 Suppl):S15–S34. doi: 10.1016/j.cjca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Ma TT, Wong ICK, Man KKC, Chen Y, Crake T, Ozkor MA, et al. Effect of evidence-based therapy for secondary prevention of cardiovascular disease: systematic review and meta-analysis. PLoS One. 2019;14(1):e0210988. doi: 10.1371/journal.pone.0210988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell A, Hill MD, Herman RJ, Girard M, Cohen E, Canadian RoAfCHRSC Management of atherothrombotic risk factors in high-risk Canadian outpatients. Can J Cardiol. 2009;25(6):345–351. doi: 10.1016/S0828-282X(09)70088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bessette L, Ste-Marie LG, Jean S, Davison KS, Beaulieu M, Baranci M, et al. The care gap in diagnosis and treatment of women with a fragility fracture. Osteoporos Int. 2008;19(1):79–86. doi: 10.1007/s00198-007-0426-9. [DOI] [PubMed] [Google Scholar]
- 26.Papaioannou A, Giangregorio L, Kvern B, Boulos P, Ioannidis G, Adachi JD. The osteoporosis care gap in Canada. BMC Musculoskelet Disord. 2004;5:11. doi: 10.1186/1471-2474-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papaioannou A, Kennedy CC, Ioannidis G, Gao Y, Sawka AM, Goltzman D, et al. The osteoporosis care gap in men with fragility fractures: the Canadian multicentre Osteoporosis study. Osteoporos Int. 2008;19(4):581–587. doi: 10.1007/s00198-007-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binkley N, Blank RD, Leslie WD, Lewiecki EM, Eisman JA, Osteoporosis in Crisis BJP. It's time to focus on fracture. J Bone Miner Res. 2017;32(7):1391–1394. doi: 10.1002/jbmr.3182. [DOI] [PubMed] [Google Scholar]
- 29.Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364–376. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 30.Dreinhofer KE, Mitchell PJ, Begue T, Cooper C, Costa ML, Falaschi P, et al. A global call to action to improve the care of people with fragility fractures. Injury. 2018;49(8):1393–1397. doi: 10.1016/j.injury.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 31.ICES. Working with ICES data 2020. Available from: https://www.ices.on.ca/Data-and-Privacy/ICES-data/Working-with-ICES-Data. Accessed 18 Dec 2020.
- 32.ICES. ICES data 2020. Available from: https://www.ices.on.ca/Data-and-Privacy/ICES-data. Accessed 18 Dec 2020.
- 33.Nikitovic M, Wodchis WP, Krahn MD, Cadarette SM. Direct health-care costs attributed to hip fractures among seniors: a matched cohort study. Osteoporos Int. 2013;24(2):659–669. doi: 10.1007/s00198-012-2034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ioannidis G, Papaioannou A, Hopman WM, Akhtar-Danesh N, Anastassiades T, Pickard L, et al. Relation between fractures and mortality: results from the Canadian multicentre Osteoporosis study. CMAJ. 2009;181(5):265–271. doi: 10.1503/cmaj.081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinedo-Villanueva R, Charokopou M, Toth E, Donnelly K, Cooper C, Prieto-Alhambra D, et al. Imminent fracture risk assessments in the UK FLS setting: implications and challenges. Arch Osteoporos. 2019;14(1):12. doi: 10.1007/s11657-019-0569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banefelt J, Akesson KE, Spangeus A, Ljunggren O, Karlsson L, Strom O, et al. Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos Int. 2019;30(3):601–609. doi: 10.1007/s00198-019-04852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ. Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis. 2009;68(1):99–102. doi: 10.1136/ard.2008.092775. [DOI] [PubMed] [Google Scholar]
- 39.Osteoporosis Canada. Quality Standards for Fracture Liaison Services in Canada 2015. Available from: https://www.osteoporosis.ca/wp-content/uploads/Final-Quality-Standards-March-2015-English.pdf. Accessed 18 Dec 2020.
- 40.Wang P, Li Y, Zhuang H, Yu H, Cai S, Xu H, et al. Influence of bone densitometry on the anti-osteoporosis treatment after fragility hip fracture. Aging Clin Exp Res. 2019;31(10):1525–1529. doi: 10.1007/s40520-018-1094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medical Advisory S Utilization of DXA Bone mineral densitometry in Ontario: an evidence-based analysis. Ont Health Technol Assess Ser. 2006;6(20):1–180. [PMC free article] [PubMed] [Google Scholar]
- 42.Bouxsein ML, Eastell R, Lui LY, Wu LA, de Papp AE, Grauer A, et al. Change in Bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34(4):632–642. doi: 10.1002/jbmr.3641. [DOI] [PubMed] [Google Scholar]
- 43.Jin YZ, Lee JH, Xu B, Cho M. Effect of medications on prevention of secondary osteoporotic vertebral compression fracture, non-vertebral fracture, and discontinuation due to adverse events: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2019;20(1):399. doi: 10.1186/s12891-019-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Austin PC, Tu JV, Ko DT, Alter DA. Factors associated with the use of evidence-based therapies after discharge among elderly patients with myocardial infarction. CMAJ. 2008;179(9):901–908. doi: 10.1503/cmaj.080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centre for Metabolic Bone Diseases. Fracture Risk Assessment Tool 2011. Available from: https://www.sheffield.ac.uk/FRAX/tool.aspx?country=19. Accessed 18 Dec 2020.
- 46.Senay A, Fernandes JC, Delisle J, Morin SN, Perreault S. Persistence and compliance to osteoporosis therapy in a fracture liaison service: a prospective cohort study. Arch Osteoporos. 2019;14(1):87. doi: 10.1007/s11657-019-0633-y. [DOI] [PubMed] [Google Scholar]
- 47.Fink HA, MacDonald R, Forte ML, Rosebush CE, Ensrud KE, Schousboe JT, et al. Long-term drug therapy and drug discontinuations and holidays for Osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171(1):37–50. doi: 10.7326/M19-0533. [DOI] [PubMed] [Google Scholar]
- 48.Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 49.Frost DW, Toubassi D, Detsky AS. Rethinking the consultation process: optimizing collaboration between primary care physicians and specialists. Can Fam Physician. 2012;58(8):825–828. [PMC free article] [PubMed] [Google Scholar]
- 50.Bauer DC. Osteoporosis treatment after hip fracture: bad news and getting worse. JAMA Netw Open. 2018;1(3):e180844. doi: 10.1001/jamanetworkopen.2018.0844. [DOI] [PubMed] [Google Scholar]
- 51.Salminen H, Piispanen P, Toth-Pal E. Primary care physicians' views on osteoporosis management: a qualitative study. Arch Osteoporos. 2019;14(1):48. doi: 10.1007/s11657-019-0599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leslie WD, Seeman E, Morin SN, Lix LM, Majumdar SR. The diagnostic threshold for osteoporosis impedes fracture prevention in women at high risk for fracture: a registry-based cohort study. Bone. 2018;114:298–303. doi: 10.1016/j.bone.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Lewiecki EM, Binkley N, Bilezikian JP, Treated Osteoporosis I. Still Osteoporosis. J Bone Miner Res. 2019;34(4):605–606. doi: 10.1002/jbmr.3671. [DOI] [PubMed] [Google Scholar]
- 54.Brown JP, Morin S, Leslie W, Papaioannou A, Cheung AM, Davison KS, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician. 2014;60(4):324–333. [PMC free article] [PubMed] [Google Scholar]
- 55.Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American dental association council on scientific affairs. J Am Dent Assoc. 2011;142(11):1243–1251. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 56.Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for C. Economic Aspects of O et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ., 3rd Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7(2):221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 58.Ross PD. Clinical consequences of vertebral fractures. Am J Med. 1997;103(2A):30S–42S. doi: 10.1016/S0002-9343(97)90025-5. [DOI] [PubMed] [Google Scholar]
- 59.Beaudoin C, Jean S, Moore L, Gamache P, Bessette L, Ste-Marie LG, et al. Number, location, and time since prior fracture as predictors of future fracture in the elderly from the general population. J Bone Miner Res. 2018;33(11):1956–1966. doi: 10.1002/jbmr.3526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Primary databases used for the study. Supplementary Table 2. Diagnosis Codes for Fragility Fractures. Supplementary Table 3. Diagnosis Codes for Trauma Codes. Supplementary Figure 1. Study schema. Supplementary Figure 2. Flow Diagram of patients included in study. Supplementary Figure 3. Number and proportion of patients with second fragility fractures by site of second fracture. Supplementary Figure 4. Proportion of patients with (a) BMD assessment, over a period of 5 years prior to and post fracture, by sex and (b) receiving any osteoporosis treatment, by sex and age group, over time*
Data Availability Statement
The data that support the findings of this study are available from ICES but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available [https://www.ices.on.ca/Data-and-Privacy/ICES-data] [32]. Data are however available from the authors upon reasonable request and with permission of ICES.