Abstract
BACKGROUND:
Malaria remains a leading transfusion associated infectious risk in endemic areas. However, the prevalence of malaria parasitemia has not been well characterized in blood donor populations. This study sought to determine the prevalence of Plasmodium in red blood cell (RBC) and whole blood (WB) units after the rainy season in Uganda.
METHODS AND MATERIALS:
Between May and July 2018, blood was collected from the sample diversion pouch of 1000 WB donors in Kampala and Jinja, Uganda. The RBC pellet from ethylenediamine tetraacetic acid (EDTA) anticoagulated blood was stored at −80°C until testing. DNA was extracted and nested PCR was used to screen samples at the genus level for Plasmodium, with positive samples further tested for species identification.
RESULTS:
Malaria parasitemia among asymptomatic, eligible blood donors in two regions of Uganda was 15.4%; 87.7% (135/154) of infections were with P. falciparum, while P. malariae and P. ovale were also detected. There were 4.3% of blood donors who had mixed infection with multiple species. Older donors (>30 years vs. 17–19 years; aPR = 0.31 [95% CI = 0.17–0.58]), females (aPR = 0.60 [95% CI = 0.42–0.87]), repeat donors (aPR = 0.44 [95% CI = 0.27–0.72]) and those donating near the capital city of Kampala versus rural Jinja region (aPR = 0.49 [95% CI = 0.34–0.69]) had a lower prevalence of malaria parasitemia.
CONCLUSIONS:
A high proportion of asymptomatic blood donors residing in a malaria endemic region demonstrate evidence of parasitemia at time of donation. Further research is needed to quantify the risk and associated burden of transfusion-transmitted malaria (TTM) in order to inform strategies to prevent TTM.
Nearly half of the world population resides in areas at risk of malaria transmission.1 In 2017, there were an estimated 219 million cases of malaria reported worldwide, with 92% of those cases occurring in Africa.2 Malaria is a mosquito-transmitted infection that infects red blood cells (RBCs) and is readily transfusion transmissible. Transfusion of Plasmodium-infected RBCs can result in transfusion-transmitted malaria (TTM). A recent meta-analysis reported that the global prevalence of malaria in healthy blood donors as determined by microscopy is 10.5%.3 Despite the burden of disease, routine screening for malaria is uncommon practice, whereby only seven countries in Africa report blood donor screening for malaria using either a peripheral smear or antigen diagnostic test.4 This highlights a need for more research to understand the extent of TTM in endemic countries, particularly sub-Saharan Africa where the majority of malaria infections occur.3
There are five parasite species that cause malaria in humans: Plasmodium falciparum, P. malariae, P. vivax, P. knowlesi, and P. ovale. P. ovale is further divided into two subspecies, P. ovale walikeri and P. ovale curtisi. The most virulent and prevalent species, P. falciparum, is estimated to cause 99.7% of infections in sub-Saharan Africa.2 In non-immune individuals, malaria typically causes a non-specific febrile illness, which may progress to severe or complicated disease in the absence of prompt and effective antimalarial treatment. Selected populations are particularly susceptible to severe malaria, such as women and children under 5 years of age. Severe anemia is one of the most common complications of malaria in sub-Saharan Africa, with an estimate of over a million cases occurring annually and accounting for an estimated 20% of P. falciparum hospitalizations.5 Blood transfusion is a critical treatment modality for severe anemia; consequently, the highest proportion of blood transfusions in sub-Saharan Africa are being directed to children for severe anemia due to malaria and malnutrition.6
Although malaria infection is dangerous to individuals lacking immunity, asymptomatic infections among adults living in endemic areas with clinical immunity are common.7 Asymptomatic parasitemia can persist in the blood for years or even decades following exposure.7,8 Pertinent to transfusion risk, Plasmodium spp can survive in processed, stored blood products for up to 18 days.9,10 Although a transfusion with a blood product that has been collected from a parasitemic donor may not cause severe disease in semi-immune patients, infection in a non-immune recipient can progress to severe or even fatal malaria.11
In Uganda, malaria is endemic in >95% of the country, yet data on the risk of TTM are lacking.12,13 Malaria transmission in Uganda is perennial in 90%−95% of the country, with annual peaks following the rainy seasons; the latter usually occur from March to May and August to October.14 In this study, multi-species nested polymerase chain reaction (PCR) was used to evaluate the overall and species-specific malaria parasitemia in asymptomatic Ugandan blood donors.
MATERIALS AND METHODS
Study setting
Blood donations in Uganda are collected by the Ugandan Blood Transfusion Services (UBTS) at 14 collection centers throughout the country, eight of which also function as regional blood banks that process and distribute units of blood (Fig. 1A). In addition to these main 14 collection centers, blood is collected at nearby communities using mobile clinics. The associated samples from those clinics are processed through one of the main centers. Currently, all (i.e., 100%) donations at UBTS are collected from voluntary non-remunerated donors. The blood donation process starts with the administration of a short questionnaire pertaining to general health, history of fever, or recent illness during the past 7 days. Following disinfection of the phlebotomy site on the donorʼs arm, approximately 30 mL of blood is drawn into a sample diversion pouch after which blood is collected into the main blood bag. Only the latter is intended for transfusion. Blood from the sample diversion pouch is used for serologic testing for human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and T. pallidum (syphilis) using the ARCHITECT (Abbott), in addition to determination of ABO and Rhesus (Rh) D blood groups using the NEO (Immucor). For this study, 3–4 mLs of ethylenediamine tetraacetic acid (EDTA) anticoagulated blood was collected from the sample diversion pouch for subsequent testing for malaria.
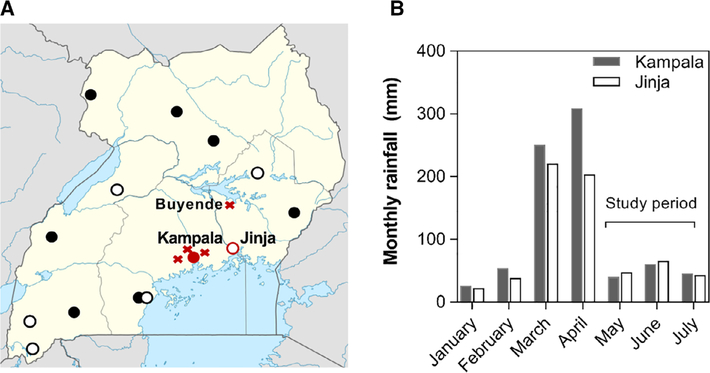
Fig. 1.
A map of Uganda Blood Transfusion Service blood processing and collection sites around the country and the monthly rainfall data for Uganda in 2018. A, a map showing the six blood collection (circle outline) and eight blood collection and processing centers (solid circle) across Uganda. The sites evaluated in this study were Kampala and Jinja in central Eastern Uganda (in red). The distance between Buyende and Jinja is ~60 miles. Regions that contributed at least 20 donor samples are indicated with an X. Wakiso (malaria prevalence, 15.0%), Mpigi (12.0%) and Mukono (10.3%) border Kampala and have higher malaria prevalence compared to samples collected at the UBTS headquarters in central Kampala (4.6%). Buyende (46.9%) had 64 donor samples that were sent to both Jinja (n = 34) and Kampala (n = 30) collection sites. B, a graph showing the rainfall data for 2018 with the 2 months preceding sample collection corresponding to the rainy season and the 3 months where samples were collected for malaria testing.
Sample collection
This cross-sectional study collected blood samples between May and July 2018, which were delivered weekly to the Makerere University-Global Health Uganda-Indiana University CHILD malaria laboratory in Kampala, where the samples were processed and later tested. Upon receipt, the samples were separated for storage of plasma for future studies and storage of red cell pellets for PCR and stored at −80°C until testing. Samples were collected from 2 of the 14 main collection centers: the UBTS headquarters in the central business district in Kampala, and from the Jinja collection center. Mobile clinics throughout communities near Kampala and Jinja districts were also used. Mobile clinics generally send the blood they collect to the closest fixed location. The Kampala center collects ~30% of the total blood supply throughout Uganda, while Jinja collects ~5%. The study was designed for the samples to be evenly distributed between two collection facilities that distribute blood in Kampala and Jinja. Using the donor unit numbers, deidentified donor information such as age, sex, region of collection, blood type, donor status (i.e., first-time donor or repeat donor), and serologic infectious disease test results was extracted from the donor database.
Rainfall data
Monthly cumulative rainfall data was obtained from the Uganda National Meteorological Authority (UNMA) for the months of January to July 2018 for Kampala and Jinja districts. The UNMA manages weather stations where they collect daily rainfall data using rain gauges. The daily data is then summed to obtain monthly total rainfall, as reported in mm (Fig. 1).15
PCR detection of malaria
DNA extraction was performed using the QIAamp DNA Blood Kit (Qiagen) using 200 uL of RBC pellet to yield approximately 60 uL of DNA eluted in buffer. To identify samples positive for malaria for subsequent species testing, nested PCR was used to screen at the genus level, targeting the small subunit 18S ribosomal RNA (ssrRNA) gene common to all Plasmodium species according to the Snounou protocol.16 As HotStarTaq (Qiagen) was used in the master-mix, modifications to the amplification conditions were made following optimization according to the manufacturerʼs instructions (Table S1, available as supporting information in the online version of this paper). Primers for Nest 1 were according to Krishna,17 and Nest 2 from Snounou (Table S2, available as supporting information in the online version of this paper).16,17 Total reaction volume (master-mix plus template) for all nests was 25 uL, amplified using the C1000 Touch Thermal Cycler (Bio-Rad). On each amplification plate, a known negative sample was used as a negative control, in addition to DNAse-free water as a no-template control. Positive commercial plasmid controls were used at the species level (BEI Resources). Samples that were inconclusive after genus-level screening were repeated using an increased amount of template in Nest 2.
Genus positive samples were evaluated for confirmatory species testing using species-specific primers (P. falciparum, P. malariae, P. ovale curtisi, P. ovale walikeri, P. vivax) for Nest 2 as previously described.16,18 All species-specific primers were checked for specificity using National Institute of Healthʼs (NIH) Primer-BLAST tool. Samples that were negative for species testing had Nest 2 repeated using an increased amount of template and cycles. Products were run using electrophoresis on 1% agarose gel prepared with tris-borate-EDTA (TBE) buffer, stained with ethidium bromide, and visualized with ultraviolet light. All genus positive samples were identified at the species level.
Statistical analyses
The primary outcome was the prevalence of Plasmodium spp. parasitemia, which included both single and mixed infection with P. falciparum, P. malariae, or P. ovale. Secondary outcomes included the species-specific prevalence of Plasmodium, and the prevalence of single and mixed infections. Overall donor characteristics were evaluated using descriptive statistics. Differences in donor characteristics and the study outcomes by location of static collection center were examined using Pearsonʼs χ2 tests.
Donor characteristics associated with the prevalence of Plasmodium spp. were examined using modified Poisson regression with robust variance estimation.19 Adjusted prevalence ratios (aPR) and corresponding 95% confidence intervals (CI) were estimated from a multivariable model that included demographic variables determined to be important a priori and/or variables that were associated with Plasmodium spp. in univariable analyses (i.e., age group, sex, repeat donor status, location of static collection center, and a composite variable indicating reactivity for any other transfusion transmitted infections (TTI) examined, which included HIV, HBV, HCV, and syphilis). RhD status was not considered for inclusion in the multivariable model due to collinearity with location of static collection center. Since many donor characteristics and the prevalence of the outcome were substantially different by location of static collection center, a post-hoc stratified analysis was performed by location of the collection centers. In a supplemental analysis, factors associated with P. falciparum and P. malariae were separately examined in the overall study population using similar methods. Multivariable analyses used a complete-case analytic approach given that very few data were missing (<1%).
There were four regions outside of Jinja or Kampala that contributed at least 20 donor samples from mobile clinics: Buyende (n = 64), Mpigi (n = 25), Mukono (n = 29), and Wakiso (n = 20). Due to outlier prevalence data of a few mobile clinics that collected at least 20 units, these data are also presented separately.
Data were analyzed using R version 3.6.1. Two-tailed p values less than 0.05 were considered statistically significant.
Ethical approvals
Approval to conduct the study was obtained from the Uganda Cancer Institute Research Ethics Board, Kampala, Uganda and the Uganda National Council for Science and Technology as well as the Institutional Review Board at the Johns Hopkins University School of Medicine, Baltimore, MD, USA.
RESULTS
Description of donor characteristics and collection sites
This study evaluated 1000 samples from asymptomatic, eligible whole blood (WB) donors in Uganda between May and July 2018 from two collection centers in central (Kampala) and eastern (Jinja) Uganda. This period immediately followed the rainy season in March–April with rainfall data for each region shown in Fig. 1.
The mean age of donors was 25.4 years, 76.1% of blood donors were male, and 28.6% of samples were from repeat donors (Table 1). Overall, 25 donors (2.5%) were seroreactive for TTIs with 0.6% reactive for HIV, 0.6% reactive for syphilis, 0.8% reactive for HBV, and 0.8% for HCV. As designed, the distribution of samples was equal between the two static blood collection sites with Kampala accounting for 50.9% of donors and Jinja accounting for 49.1%. Donors who contributed specimens from the Jinja collection site were younger and less likely to be a repeat donor than donors from Kampala (p < 0.05 for both comparisons). Male donors predominated in Kampala; 68% of donors in Jinja were male compared to 84% in Kampala (p < 0.001).
TABLE 1.
Characteristics of blood donors evaluated for Plasmodium infection
| No. of participants (%) |
||||
|---|---|---|---|---|
| Donor characteristics | Overall (n = 1000) | Kampala (n = 509) | Jinja (n = 491) | p |
| Age group, years | ||||
| 17–19 | 302 (30.2) | 80 (15.7) | 222 (45.2) | |
| 20–24 | 301 (30.1) | 142 (27.9) | 159 (32.4) | <0.001 |
| 25–29 | 166 (16.6) | 106 (20.8) | 60 (12.2) | |
| ≥30 | 229 (22.9) | 179 (35.2) | 50 (10.2) | |
| Sex | ||||
| Male | 761 (76.1) | 427 (83.9) | 334 (68.0) | <0.001 |
| Female | 236 (23.6) | 79 (15.5) | 157 (32.0) | |
| Repeat donor | ||||
| No | 710 (71.0) | 339 (66.6) | 371(75.6) | 0.002 |
| Yes | 286 (28.6) | 166 (32.6) | 120 (24.4) | |
| Blood type | ||||
| O | 483 (48.3) | 235 (46.2) | 248 (50.5) | 0.662 |
| A | 271 (27.1) | 147 (28.9) | 124 (25.3) | |
| B | 201 (20.1) | 105 (20.6) | 96 (19.6) | |
| AB | 43 (4.3) | 21 (4.1) | 22 (4.5) | |
| RhD positive | ||||
| No | 41(4.1) | 31 (6.1) | 10 (2.0) | 0.003 |
| Yes | 958 (95.8) | 477 (93.7) | 481 (98.0) | |
| HIV positive | ||||
| No | 993 (99.3) | 503 (98.8) | 490 (99.8) | 0.173 |
| Yes | 6 (0.6) | 5 (1.0) | 1 (0.2) | |
| Syphilis positive | ||||
| No | 992 (99.2) | 503 (98.8) | 489 (99.6) | 0.281 |
| Yes | 6 (0.6) | 4 (0.8) | 2 (0.4) | |
| HCV positive | ||||
| No | 991 (99.1) | 503 (98.8) | 488 (99.4) | 0.496 |
| Yes | 8 (0.8) | 5 (1.0) | 3 (0.6) | |
| HBV positive | ||||
| No | 992 (99.2) | 504 (99.0) | 488 (99.4) | 0.510 |
| Yes | 8 (0.8) | 5 (1.0) | 3 (0.6) | |
| HBV or HCV positive | ||||
| No | 984 (98.4) | 499 (98.0) | 485 (98.8) | 0.478 |
| Yes | 15 (1.5) | 9 (1.8) | 6(1.2) | |
| Reactive for any TTI* | ||||
| No | 973 (97.3) | 490 (96.3) | 483 (98.4) | 0.083 |
| Yes | 25 (2.5) | 17 (3.3) | 8(1.6) | |
Notes: Percentages may not sum to 100% due to missing data (<1% for all variables). p values calculated using Pearson’s chi square tests comparing the frequency of a characteristic in Kampala versus Jinja.
Reactive serology for Syphilis, HIV, HBV, or HCV.
Abbreviations: HBV = hepatitis B; HCV = hepatitis C; HIV = human immunodeficiency virus; PR = prevalence ratio; Rh = Rhesus factor; TTI = transfusion transmitted infection.
Malaria parasitemia in donors
The overall prevalence of malaria parasitemia in blood donors was 15.4%. Of those who tested positive, 87.7% (n = 135) were positive for P. falciparum, 31.2% (n = 48) for P. malariae, and 16.2% (n = 25) for P. ovale (Table 2). The overall prevalence of P. falciparum, P. malariae, and P. ovale was 13.5, 4.8, and 2.5% respectively. P. vivax was not detected in any donors. Among donors who tested positive for P. ovale, we identified 15 donors with P. ovale curtisi and 13 donors with P. ovale walikeri. Three donors with P. ovale were positive for both P. ovale species. Single infections were detected in 11.1% (n = 111) of donors accounting for 72.1% of infections, while 4.3% (n = 43) of donors had mixed infections and accounted for 27.9% of infections. The most common mixed infection was P. falciparum and P. malariae, which accounted for 53.5% of all mixed infections. There were 11 instances of triple infections (P. falciparum, P. malariae, and P. ovale) and two donors positive for P. falciparum, P. malariae, P. ovale curtisi, and P. ovale walikeri. There was a clear relationship between increased prevalence of non-falciparum species (P. malariae, 25.2%; P. ovale, 14.1%) in donors positive for P. falciparum compared to donors negative for P. falciparum (P. malariae, 1.6%; P. ovale, 0.7%).
TABLE 2.
Prevalence of Plasmodium in blood donors overall and by site
| No. of participants (%) |
||||
|---|---|---|---|---|
| Overall (n = 1000) | Kampala (n = 509) | Jinja (n = 491) | p | |
| Genus positive Species | 154 (15.4) | 43 (8.4) | 111 (22.6) | <0.001 |
| P. falciparum | 135 (13.5) | 38 (7.5) | 97 (19.8) | <0.001 |
| P. malariae | 48 (4.8) | 7(1.4) | 41 (8.4) | <0.001 |
| P. ovale | 25 (2.5) | 5(1.0) | 20 (4.1) | 0.002 |
| P. ovale curtisi | 15 (1.5) | 3 (0.6) | 12 (2.4) | 0.016 |
| P. ovale walikeri | 13 (1.3) | 3 (0.6) | 10 (2.0) | 0.043 |
| P. vivax | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Single infections | 111 (11.1) | 38 (7.5) | 73 (14.9) | <0.001 |
| P. falciparum | 93 (9.3) | 34 (6.7) | 59 (12.0) | 0.004 |
| P. malariae | 13 (1.3) | 3 (0.6) | 10 (2.0) | 0.043 |
| P. ovale | 5 (0.5) | 1 (0.2) | 4 (0.8) | 0.166 |
| P. vivax | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Mixed infections | 43 (4.3) | 5(1.0) | 38 (7.7) | <0.001 |
| P. falciparum + P. malariae | 23 (2.3) | 1 (0.2) | 22 (4.5) | <0.001 |
| P. falciparum + P. ovale | 8 (0.8) | 1 (0.2) | 7(1.4) | 0.029 |
| P. malariae + P. ovale | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0.326 |
| P. falciparum + P. malariae + P. ovale | 11 (1.1) | 2 (0.4) | 9(1.8) | 0.029 |
Notes: p values calculated using Pearson’s chi square tests comparing the prevalence of a given outcome in Kampala versus Jinja.
Table 2 also presents the site-specific prevalence of malaria parasitemia. The prevalence was 22.6% in samples originating from Jinja as compared to 8.4% in those samples from Kampala (p < 0.001). Jinja had a higher prevalence of all malaria species, including the P. ovale sub-species (p < 0.05 for all). Samples from Jinja were also more likely to have mixed infections (p < 0.001).
As blood donation occurs through mobile clinics in different regions and at designated collection centers, a more detailed assessment of malaria parasitemia was conducted by region. Of the samples that originated in the Jinja collection center, 450/491 (91.6%) were listed as coming from Jinja with the remaining samples coming from Buyende. For samples coming from Kampala, 389/508 (76.6%) of donations were in Kampala with at least 20 donations each coming from Mukono, Wakiso, Mpigi, and Buyende (Fig. 1). The prevalence of malaria parasitemia by region was as follows: Jinja (20.3%), Kampala (4.6%), Buyende (46.9%), Mpigi (12.0%), Mukono (10.3%), and Wakiso (15.0%). Buyende, a region North of Jinja that borders Lake Kyoga with the highest prevalence of malaria parasitemia, sent samples to each of the two static collection sites with 64.1% of Buyende samples sent to Jinja and 35.9% of Buyende samples sent to Kampala.
Correlates of malaria in blood donors
Correlates of malaria in the overall study population are shown in Table 3. In multivariable analysis, older donors (>30 years vs. 17–19 years; aPR = 0.31 [95% CI = 0.17–0.58]), female donors (aPR = 0.60, 95% CI = 0.42–0.87), repeat donors (aPR = 0.44 [95% CI = 0.27–0.72]) and donors with samples from the Kampala collection center (vs. Jinja; aPR = 0.49 [95% CI = 0.34–0.69]) had lower prevalence of malaria parasitemia (Table 3). In contrast, donors who tested positive for any other TTI had higher prevalence of malaria parasitemia (aPR = 1.86 [95% CI = 1.07–3.21]). Similar findings were observed when the data were stratified by-static collection site (Table 4), and when separately examining P. falciparum infection (Table S3, available as supporting information in the online version of this paper) and P. malariae (Table S4, available as supporting information in the online version of this paper) as separate individual outcomes.
TABLE 3.
Donor characteristics associated with Plasmodium infection status in the overall study population
| Donor characteristics | Plasmodium prevalence, No. (%) | PR (95% CI) | p | Adjusted PR (95% CI) | p |
|---|---|---|---|---|---|
| Age group, years | |||||
| 17–19 | 79 (26.2) | ref | - | ref | - |
| 20–24 | 46 (15.3) | 0.58 (0.42–0.81) | 0.001 | 0.75 (0.54–1.05) | 0.094 |
| 25–29 | 17 (10.2) | 0.39 (0.24–0.64) | <0.001 | 0.55 (0.34–0.90) | 0.018 |
| ≥30 | 12 (5.2) | 0.20 (0.11–0.36) | <0.001 | 0.31 (0.17–0.58) | <0.001 |
| Sex | |||||
| Male | 125 (16.4) | ref | - | ref | - |
| Female | 28 (11.9) | 0.72 (0.49–1.06) | 0.096 | 0.60 (0.42–0.87) | 0.007 |
| Repeat donor | |||||
| No | 137 (19.3) | ref | - | ref | - |
| Yes | 17 (5.9) | 0.31 (0.19–0.50) | <0.001 | 0.44 (0.27–0.72) | 0.001 |
| Blood type | |||||
| O | 72 (14.9) | ref | - | - | - |
| A | 39 (14.4) | 0.97 (0.67–1.38) | 0.848 | - | - |
| B | 36 (17.9) | 1.20 (0.83–1.73) | 0.324 | - | - |
| AB | 7 (16.3) | 1.09 (0.54–2.22) | 0.808 | - | - |
| RhD positive | |||||
| No | 1 (2.4) | ref | - | - | - |
| Yes | 153 (16.0) | 6.55 (0.94–45.63) | 0.058 | - | - |
| Collection site | |||||
| Jinja | 111 (22.6) | ref | - | ref | - |
| Kampala | 43 (8.4) | 0.37 (0.27–0.52) | <0.001 | 0.49 (0.34–0.69) | <0.001 |
| Reactive for any TTI* | |||||
| No | 146 (15.0) | ref | - | ref | - |
| Yes | 8 (32.0) | 2.13 (1.18–3.85) | 0.012 | 1.86 (1.07–3.21) | 0.027 |
Notes: Prevalence ratios were estimated using a modified Poisson regression with robust variance estimation. The multivariable model included adjustment for age group, sex, repeat donor status, collection site, and reactivity for any TTI. RhD status was not included in the multivariable analysis due to collinearity.
Reactive serology for Syphilis, HIV, HBV, or HCV.
Abbreviations: HBV = hepatitis B; HCV = hepatitis C; HIV = human immunodeficiency virus; PR = prevalence ratio; Rh = Rhesus factor; TTI = transfusion transmitted infection.
TABLE 4.
Donor characteristics associated with Plasmodium infection stratified by collection location
| Donor characteristics | Plasmodium prevalence, No. (%) | PR (95% CI) | p | Adjusted PR (95% CI) | p |
|---|---|---|---|---|---|
| Jinja | |||||
| Age group, years | |||||
| 17–19 | 69 (31.1) | ref | - | ref | - |
| 20–24 | 25 (15.7) | 0.51 (0.34–0.76) | 0.001 | 0.59 (0.39–0.89) | 0.012 |
| 25–29 | 10 (16.7) | 0.54 (0.29–0.98) | 0.041 | 0.57 (0.32–1.03) | 0.062 |
| ≥30 | 7 (14.0) | 0.45 (0.22–0.92) | 0.029 | 0.45 (0.22–0.93) | 0.032 |
| Sex | |||||
| Male | 87 (26.0) | ref | - | ref | - |
| Female | 24 (15.3) | 0.59 (0.39–0.88) | 0.011 | 0.64 (0.43–0.97) | 0.033 |
| Repeat donor | |||||
| No | 98 (26.4) | ref | - | ref | - |
| Yes | 13 (10.8) | 0.41 (0.24–0.70) | 0.001 | 0.55 (0.32–0.96) | 0.036 |
| Blood type | |||||
| O | 52 (21.0) | ref | - | - | - |
| A | 28 (22.6) | 1.08 (0.72–1.62) | 0.720 | - | - |
| B | 27 (28.1) | 1.34 (0.90–2.00) | 0.151 | - | - |
| AB | 4 (18.2) | 0.87 (0.35–2.17) | 0.761 | - | - |
| RhD positive | |||||
| No | 1 (10.0) | ref | - | - | - |
| Yes | 110 (22.9) | 2.29 (0.35–14.79) | 0.385 | - | - |
| Reactive for any TTI* | |||||
| No | 107 (22.2) | ref | - | ref | - |
| Yes | 4 (50.0) | 2.26 (1.11–4.60) | 0.025 | 1.44 (0.72–2.88) | 0.299 |
| Kampala | |||||
| Age group, years | |||||
| 17–19 | 10 (12.5) | ref | - | ref | - |
| 20–24 | 21 (14.8) | 1.18 (0.59–2.38) | 0.638 | 1.09 (0.54–2.19) | 0.817 |
| 25–29 | 7 (6.6) | 0.53 (0.21–1.33) | 0.175 | 0.55 (0.22–1.39) | 0.201 |
| ≥30 | 5 (2.8) | 0.22 (0.08–0.63) | 0.005 | 0.23 (0.08–0.68) | 0.008 |
| Sex | |||||
| Male | 38 (8.9) | ref | - | ref | - |
| Female | 4(5.1) | 0.57 (0.21–1.55) | 0.270 | 0.40 (0.15–1.10) | 0.075 |
| Repeat donor | |||||
| No | 39 (11.5) | ref | - | ref | - |
| Yes | 4 (2.4) | 0.21 (0.08–0.58) | 0.002 | 0.31 (0.11–0.87) | 0.026 |
| Blood type | |||||
| O | 20 (8.5) | ref | - | - | - |
| A | 11 (7.5) | 0.88 (0.43–1.78) | 0.721 | - | - |
| B | 9 (8.6) | 1.01 (0.47–2.14) | 0.985 | - | - |
| AB | 3 (14.3) | 1.68 (0.54–5.19) | 0.368 | - | - |
| RhD positive | |||||
| No | 0 (0.0) | - | - | - | - |
| Yes | 43 (9.0) | - | - | - | - |
| Reactive for any TTI* | |||||
| No | 39 (8.0) | ref | - | ref | - |
| Yes | 5 (23.5) | 2.96 (1.19–7.33) | 0.019 | 2.58 (1.16–5.71) | 0.020 |
Notes: Prevalence ratios were estimated using a modified Poisson regression with robust variance estimation. For each collection site, the multivariable model included adjustment for age group, sex, repeat donor status, and reactivity for any TTI.
Reactive serology for Syphilis, HIV, HBV, or HCV.
Abbreviations: HBV = hepatitis B; HCV = hepatitis C; HIV = human immunodeficiency virus; PR = prevalence ratio; Rh = Rhesus factor; TTI = transfusion transmitted infection.
DISCUSSION
Malaria remains a frequent—albeit neglected—transfusion-associated complication in malaria endemic countries. In this study, we evaluated the prevalence of malaria parasitemia in Ugandan blood donors by nested PCR. We report a prevalence of malaria parasitemia of 15.4%, whereby P. falciparum was detected in 87.7% of positive samples. Mixed infections with multiple Plasmodium species were common, occurring in 27.9% of positive samples. The prevalence was reduced in donors over 25 years of age, females, and those who donated near Kampala, while the risk of malaria was increased in donors who screened positive for other TTIs. These data suggest that efforts to screen donors based on a history of illness is not sufficient to reduce the risk of TTM from blood collected from asymptomatic donors. Alternative strategies to reduce the burden of malaria (e.g., pathogen reduction technologies, malaria chemoprevention in recipients) should be considered.
The results are consistent with our current understanding of malaria throughout Africa and also in Uganda. In a meta-analysis, malaria prevalence among healthy blood donors was 21% by microscopy and 36% by molecular methods.3 In a study in Ghana, Plasmodium genome prevalence was 50% by nucleic acid testing.20 Prevalence varies significantly across Uganda due to diverse landscapes, with areas of high elevation (both Kampala and Jinja are at ~4000 feet elevation) and minimal transmission that are prone to epidemics, and communities near swamps and wetlands that have some of the highest transmission intensities reported in sub-Saharan Africa, at >1500 infective bites per person per year.21 Parasite prevalence, as detected by microscopy, was reported as 19% in children under 5 years of age according to the 2014–2015 Uganda Malaria Indicator Survey.22 The same survey reported a malaria prevalence in Kampala of 0.4%.22 Jinja district is a peri-urban area near Lake Victoria about 80 kilometers east of Kampala and is considered a low to medium transmission area with an annual entomological inoculation rate between 2.8% and 6% and parasite prevalence >7%.14,21 Prevalence in children detected by blood smear in this area is around 6%−7%.14,23 Data from the 2014–2015 Uganda Malaria Indicator Survey show that four UBTS collection facilities are in areas with prevalence less than 10% in children by blood smear, seven are in areas with prevalence between 10%−19%, two are in areas between 20%−29%, and one is in an area with >30% prevalence.22 Prevalence data reported by blood smear are likely an under-estimate due to lack of sensitivity of microscopy compared to PCR.24 UBTS has 14 collection centers throughout the country, with 8 serving as regional blood banks where blood is tested, processed, and distributed. As a result, blood may be collected from an area of high malaria transmission and distributed to communities in lower transmission areas, putting the population at greater risk for TTM and severe or recurrent malaria.
Asymptomatic malaria infections are common in endemic countries due to the development of clinical, but not sterile, immunity.7 Individuals with recurrent exposure to malaria develop a protective acquired immune response resulting in largely asymptomatic or pauci-symptomatic infections, and low parasitemia.7 The blood donor system in Uganda relies on self-reported history of fever to screen for illness, but this strategy is insufficient in the context of asymptomatic malaria when, by definition, fever is not present. Potential screening options for malaria include antigen detection using rapid diagnostic tests (RDTs), direct visualization of malaria parasites by blood smear, or more sensitive nucleic acid testing methods like PCR or loop-mediated isothermal amplification. However, a highly sensitive assay is likely needed since even low-level parasitemia can be infectious.25
The testing for this study was performed using nested PCR, a sensitive and specific method enabling detection of low-level infections, such as the ones seen in asymptomatic adults, and the differentiation of species. Molecular tests like PCR can identify as few as 1–5 parasite per uL of blood and can therefore identify infections that less sensitive tests may miss.24 However, because of cost, the need for highly trained staff and sophisticated facilities equipped to prevent contamination, PCR is not practical for malaria screening in low-resource settings. The most commonly used method for malaria screening in sub-Saharan Africa is microscopy by blood smear. Microscopy is relatively inexpensive but is a time consuming and laborious process that relies on the availability of highly trained individuals and appropriate equipment. In addition, microscopy lacks sensitivity to detect low-level infections with the average microscopist able to detect about 100 parasites per uL of blood.24 RDTs are relatively quick and simple to perform, with comparable sensitivity to microscopy, however, they are less reliable in detecting non-falciparum infections.24 While RDTs are likely the most practical malaria screening tool for blood donors, deferral of malaria positive donors in high prevalence areas may further exacerbate shortages in the blood supply. Approximately 40 countries in sub-Saharan Africa fail to meet the WHOʼs donation goal of 10 units per 1000 population.26 Implementation of malaria screening would reduce the ability for endemic countries to meet current blood needs, thus leading to increased morbidity and mortality.
Pathogen reduction technology (PRT) refers to a variety of approaches (e.g., nanofiltration, solvent detergent treatment, photochemical inactivation, etc.) that may be used to treat the blood product globally. Given that PRT is effective across different classes of pathogens, it has the advantage of mitigating risk of established TTIs as well as emerging and re-emerging agents.27,28 Germane to TTM, photochemical inactivation has shown efficacy for TTM.25 The data reported herein suggest PRT could have a substantial impact, particularly if targeted to high prevalence areas. However, PRT is not as effective when there is a high parasite burden.25 Additional studies are needed to assess the feasibility, efficacy, and cost of implementing PRT in low-income countries across multiple populations.
Treatment with anti-malarials, either added to blood packs in vitro, given to all transfusion recipients, or to targeted high-risk groups, is another potential approach to reduce the rate of TTM. A study in Malawi found that administration of 3 months of malaria chemoprevention following discharge from the hospital in children admitted with severe malarial anemia, prevented 40% of deaths or hospital admissions due to recurrence of severe anemia or severe malaria.29 An expanded trial in Kenya and Uganda is currently underway to further investigate the efficacy of malaria chemoprevention as post-discharge management of severe anemia.30 The value of these studies of prophylactic malaria chemoprevention extend to TTM. Chemoprevention would reduce re-infections with malaria in children who receive blood transfusion; as such, it could also reduce the burden of recurrent anemia due to malaria, which in turn would favorably impact the need for repeated transfusions. Chloroquine prophylaxis has been widely used for children, but the high cost of artemisinates has limited the expansion of the programs. A cost–benefit analysis evaluating the cost of prophylactic treatment combined with risks of drug exposure, non-adherence, and drug resistance has yet to be undertaken.
There are a number of limitations of this study. The study evaluated donors over a 3-month period following the rainy season, when the prevalence of malaria is usually highest. A longer sampling period could provide greater insight into seasonal changes in malaria prevalence. While the study included two different collection sites in Uganda with estimates on prevalence from mobile collection clinics to provide further information on regional differences, the data are not representative of the entire country. An expanded study including random sampling from each collection site would provide further information regarding regional differences in malaria prevalence. The Uganda Blood Transfusion Service only collects limited demographic data among their blood donors. Additional information on occupation, education, and history of malaria would have strengthened the multivariate analyses. We do not have information on whether the malaria infected units were transfused and whether they resulted in TTM. Further, we did not evaluate malaria by microscopy or RDT, so we cannot comment on the number of infections that may have-been identified in donors if malaria screening was implemented at the time of donation. It is also uncertain as to how molecular evidence of infection (i.e., submicroscopic parasitemia) manifests clinically or correlates with a clinical outcome. We also did not quantitate the parasitemia to understand the burden of disease. While the precise sensitivity of our assay was not determined, the use of nested PCR for the detection of malaria allowed us to detect low density infections, with typical parasite thresholds for molecular testing between 1–5 parasites per uL of blood. We were able to differentiate between malaria species and identify a surprising number of mixed infections with some donors having quadruple infections. We show that both P. ovale curtisi and P. ovale walikeri co-circulate in Uganda with both sub-species observed in single donors.
The present study of two primary regions in Uganda demonstrates that malaria is common in donor blood occurring in 15.4% donors and ranged from 4.6 to 46.9% by region despite efforts to screen for illness prior to donation. Additional research is needed to understand the impact of donor malaria parasitemia and TTM. As the prevalence of malaria parasitemia remains high in Uganda and malaria is an important determinant of severe anemia requiring transfusion, efforts to evaluate PRT and/or public health policies related to malaria chemoprevention in recipients are likely needed to reduce the burden of TTM in Uganda.
Supplementary Material
Table S1. Summary of amplification conditions
Table S2. Summary of primers
Table S3. Donor characteristics associated with P. falciparum infection in the overall study population.
Table S4. Donor characteristics associated with P. malariae infection in the overall study population.
ACKNOWLEDGMENTS
We would like the thank the staff members at UBTS for assisting with sample collection and database extraction, the Chandy John lab (Katrina Co, Dibyadyuti Datta, and Giselle Lima-Cooper) and Victor Asua for technical support, and to the members of the GHUIU CHILD Lab (Ronald Wasswa, Geoffrey Situma, Ivan Mufumba, and Carol Kazinga) for processing and testing the samples.
Financial support: This work was supported in part by the U.S. Department of Defense Peer Reviewed Medical Research Program (W81XWH1810742 to A.A.R.T) and the National Institute of Allergy and Infectious Diseases (R01AI128779 to A.A.R.T and T32AI102623 to E.U.P.).
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
CONFLICT OF INTEREST
RS, HD, DKB, EM, RK, EMB, IL, HH and AT are co-investigators or principal investigators on a Mirasol clinical trial funded by the US Department of Defense assessing the efficacy of pathogen reduction.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.World Health Organization. Fact sheet about malaria [monograph on the internet]. 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/malaria
- 2.WHOS recommended citation: World Malaria Report 2018. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 3.Ahmadpour E, Foroutan-Rad M, Majidiani H, et al. Transfusion-transmitted malaria: a systematic review and meta-analysis. Open Forum Infect Dis 2019;6:ofz283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHOS recommended citations: World Health Organization. (2017). The 2016 global status report on blood safety and availability. World Health Organization. https://apps.who.int/iris/handle/10665/254987. License: CC BY-NC-SA 3.0 IGO [Google Scholar]
- 5.Taylor T, Olola C, Valim C, et al. Standardized data collection for multi-center clinical studies of severe malaria in African children: establishing the SMAC network. Trans R Soc Trop Med Hyg 2006;100:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapko JB, Mainuka P, Diarra-Nama AJ. Status of blood safety in the WHO African region: report of the 2006 survey. Brazzaville: World Health Organization. Regional Office for Africa; 2009. [Google Scholar]
- 7.Laishram DD, Sutton PL, Nanda N, et al. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J 2012;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce-Chwatt LJ. Transfusion malaria. Lancet 1985;2:271. [DOI] [PubMed] [Google Scholar]
- 9.WHOS recommended citation:World Health Organization. (2019). Screening donated blood for transfusion-transmissible infections: recommendations. World Health Organization. https://apps.who.int/iris/handle/10665/44202. [PubMed] [Google Scholar]
- 10.Chattopadhyay R, Majam VF, Kumar S. Survival of Plasmodium falciparum in human blood during refrigeration. Transfusion 2011;51:630–5. [DOI] [PubMed] [Google Scholar]
- 11.Mungai M, Tegtmeier G, Chamberland M, et al. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med 2001;344:1973–8. [DOI] [PubMed] [Google Scholar]
- 12.Yeka A, Gasasira A, Mpimbaza A, et al. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop 2012; 121:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owusu-Ofori A, Parry C, Bates I. Transfusion-transmitted malaria in countries where malaria is endemic: a review of the literature from sub-Saharan Africa. Clin Infect Dis 2010;51: 1192–8. [DOI] [PubMed] [Google Scholar]
- 14.Kamya MR, Arinaitwe E, Wanzira H, et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg 2015;92:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okullo AE, Matovu JKB, Ario AR, et al. Malaria incidence among children less than 5 years during and after cessation of indoor residual spraying in Northern Uganda. Malar J 2017;16:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snounou G, Singh B. Nested PCR analysis of Plasmodium parasites. Methods Mol Med 2002;72:189–203. [DOI] [PubMed] [Google Scholar]
- 17.Krishna S, Bharti PK, Chandel HS, et al. Detection of mixed infections with Plasmodium spp. by PCR, India, 2014. Emerg Infect Dis 2015;21:1853–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuehrer HP, Noedl H. Recent advances in detection of Plasmodium ovale: implications of separation into the two species Plasmodium ovale wallikeri and Plasmodium ovale curtisi. J Clin Microbiol 2014;52:387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 20.Freimanis GL, Owusu-Ofori S, Allain JP. Hepatitis B virus infection does not significantly influence Plasmodium parasite density in asymptomatic infections in Ghanaian transfusion recipients. PLoS One 2012;7:e49967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okello PE, Van Bortel W, Byaruhanga AM, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg 2006;75:219–25. [PubMed] [Google Scholar]
- 22.Recommended citation from DHS: Uganda Bureau of Statistics - UBOS and ICF International. 2015. Uganda Malaria Indicator Survey 2014–15. Kampala, Uganda: UBOS and ICF International. [Google Scholar]
- 23.Nankabirwa JI, Yeka A, Arinaitwe E, et al. Estimating malaria parasite prevalence from community surveys in Uganda: a comparison of microscopy, rapid diagnostic tests and polymerase chain reaction. Malar J 2015;14:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erdman LK, Kain KC. Molecular diagnostic and surveillance tools for global malaria control. Travel Med Infect Dis 2008;6:82–99. [DOI] [PubMed] [Google Scholar]
- 25.Allain JP, Owusu-Ofori AK, Assennato SM, et al. Effect of Plasmodium inactivation in whole blood on the incidence of blood transfusion-transmitted malaria in endemic regions: the African Investigation of the Mirasol System (AIMS) randomised controlled trial. Lancet 2016;387:1753–61. [DOI] [PubMed] [Google Scholar]
- 26.Weimer A, Tagny CT, Tapko JB, et al. Blood transfusion safety in sub-Saharan Africa: a literature review of changes and challenges in the 21st century. Transfusion 2019;59:412–27. [DOI] [PubMed] [Google Scholar]
- 27.Tobian AAR, Hume HA. Quest for the holy grail: pathogen reduction in low-income countries. Transfusion 2018;58:836–9. [DOI] [PubMed] [Google Scholar]
- 28.Ware AD, Jacquot C, Tobian AAR, et al. Pathogen reduction and blood transfusion safety in Africa: strengths, limitations and challenges of implementation in low-resource settings. Vox Sang 2018;113:3–12. [DOI] [PubMed] [Google Scholar]
- 29.Phiri K, Esan M, van Hensbroek MB, et al. Intermittent preventive therapy for malaria with monthly artemether–lumefantrine for the post-discharge management of severe anaemia in children aged 4–59 months in southern Malawi: a multicentre, randomised, placebo-controlled trial. Lancet Infect Dis 2012; 12:191–200. [DOI] [PubMed] [Google Scholar]
- 30.Kwambai TK, Dhabangi A, Idro R, et al. Malaria chemoprevention with monthly dihydroartemisinin-piperaquine for the post-discharge management of severe anaemia in children aged less than 5 years in Uganda and Kenya: study protocol for a multi-centre, two-arm, randomised, placebo-controlled, superiority trial. Trials 2018;19:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of amplification conditions
Table S2. Summary of primers
Table S3. Donor characteristics associated with P. falciparum infection in the overall study population.
Table S4. Donor characteristics associated with P. malariae infection in the overall study population.