Abstract
Polyamines (PAs), such as spermidine (SPD) and spermine (SPM), are essential to promote cell growth, survival, proliferation and longevity. In the adult central nervous system (CNS), SPD and SPM are accumulated predominantly in healthy adult glial cells where PA synthesis is not present. To date, the accumulation and biosynthesis of PAs in developing astrocytes are not well understood. The purpose of the present study was to determine the contribution of uptake and/or synthesis of PAs using proliferation of neonatal astrocytes as an endpoint. We inhibited synthesis of PAs using α-difluoromethylornithine (DFMO; an inhibitor of the PA biosynthetic enzyme ornithine decarboxylase (ODC)) and inhibited uptake of PAs using trimer44NMe (PTI; a novel polyamine transport inhibitor). DFMO, but not PTI alone, blocked proliferation suggesting that PA biosynthesis was present. Furthermore, exogenous administration of SPD rescued cell proliferation when PA synthesis was blocked by DFMO. When both synthesis and uptake of PAs were inhibited (DFMO + PTI), exogenous SPD no longer supported proliferation. These data indicate that neonatal astrocytes synthesize sufficient quantities of PAs de novo to support cell proliferation, but are also able to import exogenous PAs. This suggests that the PA uptake mechanism is present in both neonates as well as in adults and can support cell proliferation in neonatal astrocytes when ODC is blocked.
Keywords: astrocyte, polyamines, α-difluoromethylornithine, ornithine decarboxylase, novel polyamine transport inhibitor (trimer44NMe)
Introduction
Polyamines (PAs) are essential to all living cells. They are low molecular weight, organic compounds containing two or more positively charged amine groups which can interact with negatively charged molecules such as RNA, DNA, phospholipids, ATP, and acidic proteins (Watanabe et al. 1991). PAs play multiple roles in cell proliferation, growth and survival (Pegg 2016), as well as, glial cell function (Skatchkov et al. 2014; Skatchkov et al. 2016) and increasing life span as a “longevity elixir” (Madeo et al. 2010; Skatchkov et al. 2014; Eisenberg et al. 2016; Pegg 2016; Madeo et al. 2018).
The role of PAs in cellular proliferation is one of their most prominent functions (Pegg 2016). PAs are available from endogenous synthesis (Pegg 2016) and exogenous nutrition sources (Larqué et al. 2007; Soda et al. 2013; Muñoz-Esparza et al. 2019) including PAs produced by the gut microbiome (Larqué et al. 2007; Handa et al. 2018; Muñoz-Esparza et al. 2019). In animals, ornithine is synthesized predominantly from arginine obtained through food (Morris 2007) and is converted to the first polyamine putrescine (PUT) by ornithine decarboxylase (ODC). PUT is subsequently converted to spermidine (SPD) and then spermine (SPM) by the enzymes spermidine synthase (SRM) and spermine synthase (SMS), respectively by adding 3-amino-propyl residues derived from decarboxylated S-adenosylmethionine. Alternatively, decarboxylation of arginine to agmatine by arginine decarboxylase (ADC) and subsequent conversion to PUT via the enzyme agmatinase (Fig. 1) is another way to produce PUT (the precursor of SPD and SPM).
Figure 1. Polyamine (PA) Synthesis and Catabolism.
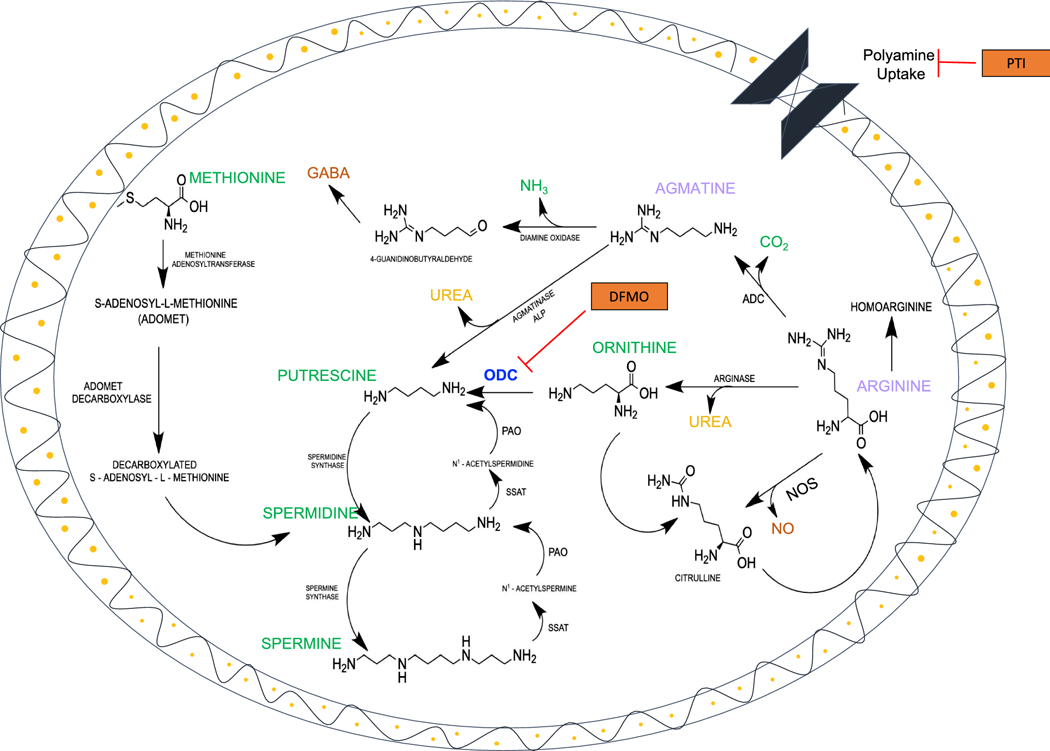
The PA, putrescine (PUT), is synthesized from L-ornithine by the enzyme (i) ornithine decarboxylase (ODC). Then, (ii) spermidine synthase with participation of (iii) decarboxylated S-adenosyl-L-methionine adds a 3-aminopropyl residue to PUT to form SPD. Finally, (iv) spermine synthase and decarboxylated S-adenosyl-L-methionine form SPM. The catabolism of PAs occurs via production of acetyl-PAs, aldehydes and hydrogen peroxide by the enzymes (v) spermidine/spermine-N1-acetyltransferase (SSAT) and polyamine oxidase (PAO). Alternatively, PAs may also be synthesized from L-arginine in two ways: (1) agmatine is produced from the amino acid arginine by decarboxylation using the enzymes (i) arginine decarboxylase (ADC) and then from agmatine by (ii) agmatinase (ALP) to make PUT; and (2) by the enzymes (i) arginase to make ornithine and then from ornithine by (ii) ODC to make PUT. Also, the enzyme nitric oxide synthase (NOS) produces an important neuroactive mediator “nitric oxide” (NO). In addition, the enzyme arginase produces an important antioxidant, urea. The neurotransmitter gamma amino butyric acid (GABA) is produced in astrocytes via guanidinobutyraldehyde and 4-guanidinobutyrate. We used α-difluoro-methylornithine (DFMO) an irreversible inhibitor of ODC and a trimer44NMe as a polyamine transport inhibitor (PTI) in primary cultured astrocytes derived from 1–3 day old rats to study the involvement of polyamine uptake and biosynthesis pathways in the proliferation of neonatal astrocytes.
As shown in Fig. 1, the PA catabolism pathway converts SPM and SPD into N1–acetylpolyamines due to the action of the enzyme spermine/spermidine N1 –acetyltransferase (SSAT). Increased release of acetylated PA from astrocytes was found in patients displaying HIV associated CNS disorders (Merali et al. 2014). Furthermore, the enzyme polyamine oxidase (PAO) facilitates catabolism of the higher polyamines (SPD and SPM) back into PUT (Pegg 2016) and abnormal PAO activity was found in an Alzheimer’s mouse model (Pietropaoli et al. 2018). The degradation pathway may be toxic to cells (Gossé et al. 2006; Skatchkov et al. 2014; Pegg 2016; Pietropaoli et al. 2018) due to release of reactive by-products (aldehydes and peroxide). These by-products can cause several CNS disorders (Skatchkov et al. 2014; Pegg 2016; Pietropaoli et al. 2018), unless cells are protected by cyanoglycosides such as procyanidins (Gossé et al. 2006).
In the nervous system, PA biosynthesis, distribution, and storage in glia and neurons has been widely studied in adults, without paying attention to developing glial cells (Laube and Veh 1997; Biedermann et al. 1998; Krauss et al. 2007; Peters et al. 2013; Madeo et al. 2018; Handa et al. 2018). It was highlighted that PA exchange is extremely important for normal function of the nervous system as well as in diseases (Bernstein and Muller 1999; Skatchkov et al. 2014; Madeo et al. 2018; Handa et al. 2018). However, little attention has been paid to glial cells (Skatchkov et al. 2016) and what happens to PA exchange during their early stages of development is still unknown.
To study this process in the critical days of cell development, we used primary cortical cultured astrocytes obtained from rats early in development (1–3 days old). We used PA biosynthesis and uptake blockers: α-difluoromethylornithine (DFMO; an irreversible inhibitor of ODC) and trimer44NMe (PTI; a novel polyamine transport inhibitor) (Muth et al. 2014). DFMO is an enzyme-activated irreversible inhibitor of ODC (Pegg et al. 1987; Wallace and Fraser 2004; Pegg 2016; Gitto et al. 2018; LoGiudice et al. 2018; Bae et al. 2018) and has been used as a chemo-preventative agent in cancer cells and in the clinic. DFMO is decarboxylated by ODC and covalently binds to ODC (Pegg et al. 1987). This irreversible inhibition results in block of PA synthesis with concomitant depletion of PUT, SPD, and SPM (albeit incomplete depletion of SPM) (Pegg et al. 1987). PTI has been shown to inhibit the import of SPD in DFMO treated Chinese Hamster Ovary cells and human pancreatic cancer cells and does not support cell growth and can outcompete the native PAs for cellular entry (Gitto et al. 2018).
We hypothesized that astrocytes from neonatal rats are capable of taking up exogenous PAs and may or may not synthesize PAs to support proliferation. In the present study, we used the PTI and DFMO to determine if astrocyte proliferation was supported by the PAs found in the extracellular space (via uptake) or by de novo synthesis of PAs utilizing the ODC biosynthetic pathway. Our results demonstrate ODC expression in primary cultured astrocytes obtained from neonatal rat brain. Furthermore, we show that astrocyte proliferation is supported by the synthesis of new PAs from the ornithine biosynthetic pathway. These cells, however, may also take up exogenous PAs to support proliferation, when PA biosynthesis is inhibited. In this regard, both PA biosynthesis and import are available to these growing cells to maintain homeostasis. Preliminary data were reported at the 49th Annual Meeting of the Society for Neuroscience (Malpica-Nieves et al. 2019).
2. Materials and Methods
2.1. Preparation of Primary Cortical Astrocyte Cultures:
Primary astrocyte cultures were prepared from the neocortex of 1–3 day old Sprague Dawley rats as previously described by our lab group (Rivera-Pagán et al. 2015). Briefly, brains were removed after decapitation and the meninges were stripped to avoid fibroblast contamination. Mixed glial cultures were plated in Dulbecco’s Modified Eagle Medium (DMEM) containing 25 mM glucose, 2 mM glutamine, 1 mM pyruvate, 10% fetal calf serum, and 100 U/mL penicillin/streptomycin. At 85–90% confluence, cultures were treated with 50 mM leucine methyl ester (LME) in growth medium (pH 7.4) for 60 min to eliminate microglia. LME is highly effective at eliminating microglia, compared to other cells. As we previously showed non-LME treated cultures and LME-treated cultures were immunostained for GFAP and counterstained with DAPI. The cultures contained only 8% non-GFAP positive cells after LME treatment while the non-treated by LME group had 39% non-GFAP positive cells. GFAP is a marker of astrocytes, therefore, these data demonstrate how our astrocyte culture procedure effectively decreases non-GFAP positive cells producing an enriched-astrocyte culture (Ferrer-Acosta et al. 2017). Cultures were then allowed to recover for at least 2 days in growth medium prior to experimentation. This protocol was approved by the Universidad Central del Caribe Institutional Animal Care and Use Committee.
2.2. DFMO and PTI:
α-Difluoromethylornithine (DFMO) is well known to inhibit polyamine synthesis and deplete PA content in cells (Dorhout et al. 1995; Koomoa et al. 2009; Koomoa et al. 2013). DFMO is an irreversible inhibitor of ODC (LoGiudice et al. 2018) generously provided by Dr. P. Woster (Medical University of South Carolina, Charleston, SC) to Dr. Otto Phanstiel. The trimer44NMe is a novel polyamine transport inhibitor (PTI), (Muth et al. 2014; Gitto et al. 2018; Malpica-Nieves et al. 2019) which was developed and synthesized in the laboratory of Dr. Otto Phanstiel at the University of Central Florida in Orlando, FL. PTI has been used in combination with DFMO to deplete PAs in cancer cells (Gitto et al. 2018) and it is expected that PTI alone would have no effect on PA content in astrocytes, but levels would be depleted when PTI and DFMO were co-administered. Both molecules were diluted using phosphate buffered saline solution for cells (CPBS) containing in mM: 133 NaCl, 2.7 KCl, 8.2 Na2HPO4 · 7H2O and 2.2 NaH2PO4, pH 7.4.
2.3. LIVE/DEAD Cell assay:
To determine the optimal concentrations for PTI and DFMO in our experiments, we performed a LIVE/DEAD cell assay according to manufacturer’s instructions (LIVE/DEAD® Viability/Cytotoxicity Kit for mammalian cells, ThermoFisher scientific, MA, USA, Cat. Number L3224). Astrocytes were plated at a density of 25,000 cells in each well of a Falcon 24 well plate (Corning NY, USA Cat. Number 353047). After 24 hours, astrocytes were treated with DFMO, PTI and SPD. The tested concentrations for PTI were 1, 3, 10, 30 and 100 μM, for DFMO were 0.5, 1, 3 and 5 mM and for SPD were 1, 10, and 100 μM. The LIVE/DEAD assay was performed 48 hours after treatment with PTI or DFMO using 4 μM EthD-1and 2 μM calcein-AM. Images were obtained by confocal microscopy using Alexa Fluor (488 nm) and Texas Red (543 nm) lasers and a 10x objective. Based on a qualitative assessment of the images, the concentrations selected for the proliferation test were the following: PTI 30 μM, DFMO 5 mM and SPD 10 μM. At these respective concentrations, the PTI, DFMO and SPD were non-toxic to cells.
2.4. Proliferation Test:
Cells were plated in 24 well dishes and the proliferation test was performed over the course of 9 days as detailed in Table 1. For cell counting, astrocytes were washed with 1000 μL of CPBS, then with 500 μL of 0.2X Trypsin in CPBS and incubated for 3 minutes at 37°C. The solution was removed, and cells were collected by repeated pipetting up and down with a micropipette containing DMEM. The cells were transferred to an Eppendorf tube and centrifuged at 15,000 RPM for 5 minutes to create a pellet. The supernatant was removed and 100μL of DMEM was added. Before the cells were counted, they were vortexed and further separated by up and down repetitions with the micropipette to break up any remaining cell clusters. Counting was performed using a TC20 cell counter (BioRad, Hercules, CA, USA).
Table 1.
Detailed methodology of the proliferation experiments.
| Days | Experiment #1 (Fig. 4a) | Experiment #2 (Fig. 4b) | Experiment #3 (Fig. 5) |
|---|---|---|---|
| 0 | Plating of 25,000 astrocytes/well. | Plating of 25,000 astrocytes/well. | Plating of 25,000 astrocytes/well. |
| 1 | PTI (30μM) and DFMO (5mM) administration. | DFMO (5mM) and SPD (10 μM) administration. | PTI (30μM), DFMO (5mM) and SPD (10 μM) administration. |
| 2 | Cell counting (day 1 after treatment). | Cell counting (day 1 after treatment). | Cell counting (day 1 after treatment). |
| 3 | N/A | Replenishment of DFMO (5mM) and SPD (10 μM). | Replenishment of PTI (30μM), DFMO (5mM) and SPD (10 μM). |
| 4 | N/A | N/A | N/A |
| 5 | N/A | Replenishment of DFMO (5mM) and SPD (10 μM). | Replenishment of PTI (30μM), DFMO (5mM) and SPD (10 μM). |
| 6 | N/A | N/A | N/A |
| 7 | N/A | N/A | N/A |
| 8 | Cell counting (day 7 after treatment). | Cell counting (day 7 after treatment). | Cell counting (day 7 after treatment). |
2.5. SDS-PAGE and Western Blotting Analysis:
After 4 weeks in vitro, cultured cortical astrocytes prepared from 1–3 day old rats were harvested, pelleted, and resuspended in homogenization buffer as previously described (Rivera-Pagán et al. 2015). The homogenization buffer (pH 7.5) contained in mM: 20 Tris–HCl, 150 NaCl, 1.0 EDTA, 1.0 EGTA, 1.0 Phenylmethylsulfonyl Fluoride (PMSF), 1% Triton X-100 and an additional mixture of protease inhibitors (leupeptin, bestatin, pepstatin, and aprotinin) (Rivera-Pagán et al. 2015). As a positive control, we used a whole brain homogenate from a 2-day old rat. Western blotting was performed as previously described (Rivera-Pagán et al. 2015) using anti-ornithine decarboxylase (1:2000; Cat. #ab185690, Abcam, Cambridge, MA). We loaded 15 μg/mL of protein from each sample; whole brain and cultured astrocytes. Whole brain was used as a positive control and although no comparison was made for the relative amounts of ODC in whole brain vs astrocytes cultures, we used India ink staining for total protein to discern small differences in sample loading. Final detection was performed with the enhanced chemiluminescence methodology (SuperSignal® West Dura Extended Duration Substrate; Rockford, IL, USA) as described by the manufacturer, and the signal was assessed using a gel documentation system (ChemiDoc, Bio Rad). The image was obtained using the Image Lab software (Bio-Rad).
2.6. Statistical Analysis:
All statistical analyses were performed using the Statistical Package for the Social Sciences (IBM-SPSS, Chicago, IL, v.23.0 for Windows). Nonparametric Kruskal-Wallis test and Mann-Whitney Test were used to evaluate statistical differences between groups (Fig. 2). In addition, the Wilcoxon Signed Ranked Test was used to evaluate statistical difference within the groups between days 1 and 7 (Figs. 4 and 5). The P value used to determine statistical significance was < 0.05.
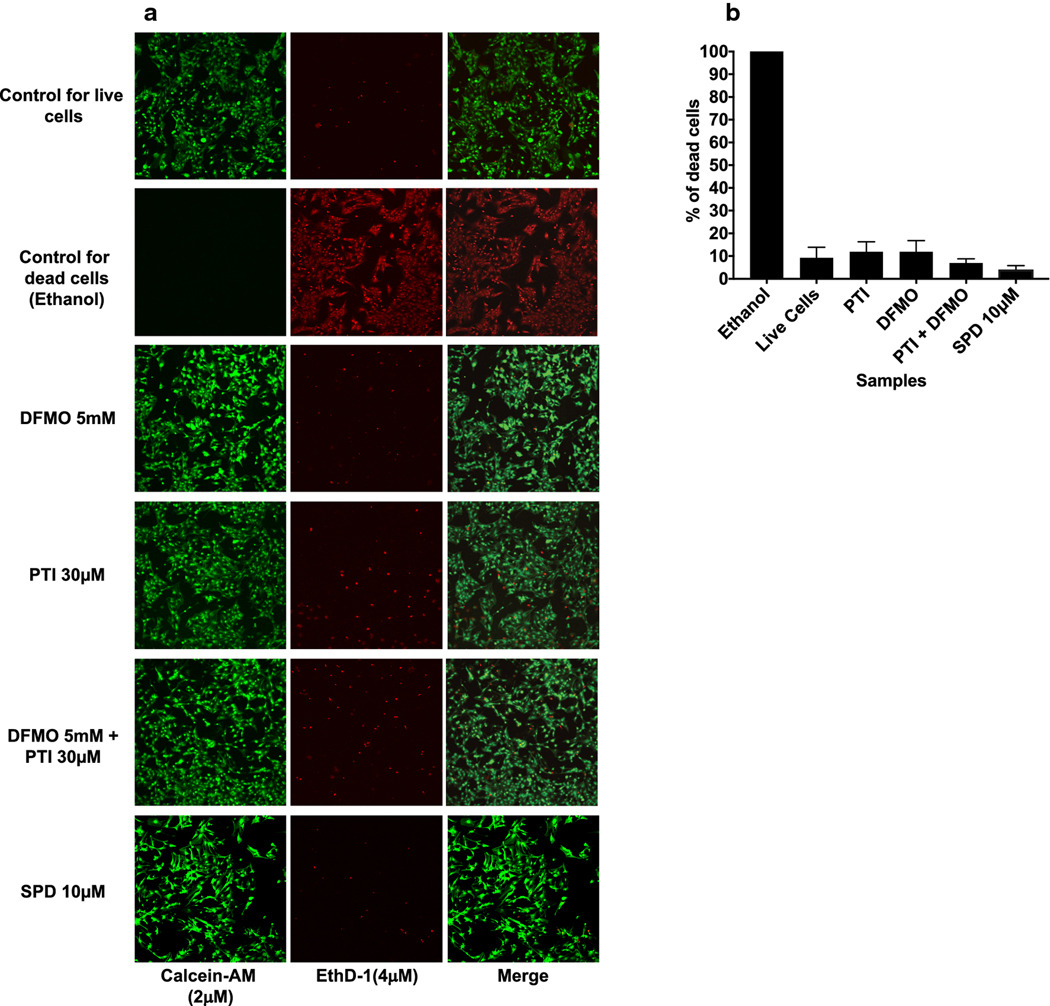
Figure 2. LIVE/DEAD Cell assay.
(a) Control for live cells (normal astrocytes without treatment) were incubated with a solution of EthD-1 (4 μM) and calcein-AM (2 μM) for 45 min at room temperature to determine the ratio of live cells (green) to dead cells (red) in typical astrocyte cultures (10X magnification was used for every image). Negative control astrocytes were incubated with 70% ethyl alcohol for 10 minutes at room temperature as a negative control for cell death. Then the cells were incubated with a solution of EthD-1 (4 μM) and calcein-AM (2 μM) for 45 minutes. The concentrations for DFMO, PTI and SPD were established from our previous dose response study (data not shown) and were DFMO (5 mM), PTI (30 μM) and SPD (10 μM). Astrocytes treated with DFMO, PTI, DFMO + PTI or SPD were incubated with a solution of EthD-1 (4 μM) and calcein-AM (2 μM) for 45 min at room temperature (n=18 in DFMO, PTI and DFMO+PTI and n=9 in SPD samples). (b) A summary of the percentage of dead cells in the various experimental groups. PTI (30 μM), DFMO (5 mM), PTI + DFMO and SPD (10 μM) do not cause significant cell death as compared to untreated astrocytes (denoted as live cells).
Figure 4a and 4b. Proliferation Assay Comparison.
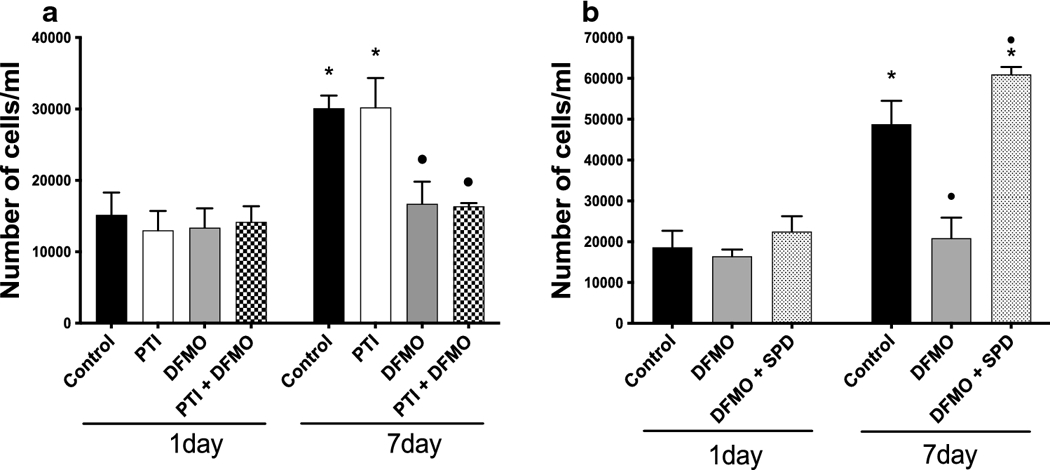
(a) 25,000 cells/well were plated and 24 hours later they were treated with PTI (30 μM), DFMO (5 mM) or both. Cells were treated for 1 and 7 days with PTI, DFMO or both and were counted on day 1 and day 7 after treatment. The media was never changed from day 0 (n=6 on each sample). (b) 25,000 cells/well were plated and 24 hours later they were treated with DFMO (5 mM) + Spermidine (SPD; 10 μM) or DFMO (5 mM) alone. Cells were incubated at 37°C for 1 and 7 days with DFMO + Spermidine and DFMO alone. On days 3 and 5, the cell culture media was changed to replenish with the same concentration of DFMO and Spermidine as on day 1. The control was also replenished with fresh media. Samples were counted on days 1 and 7 after treatment (n=6 on each sample). Asterisk (*) indicates statistical difference between the same treatment in 1 vs 7-days of proliferation (p<0.05; Wilcoxon Signed Ranks Test), whereas the circle (•) indicates statistical difference between the 7-day control and treated groups (p<0.05; Mann Whitney Test).
Figure 5. Proliferation Assay Comparison.
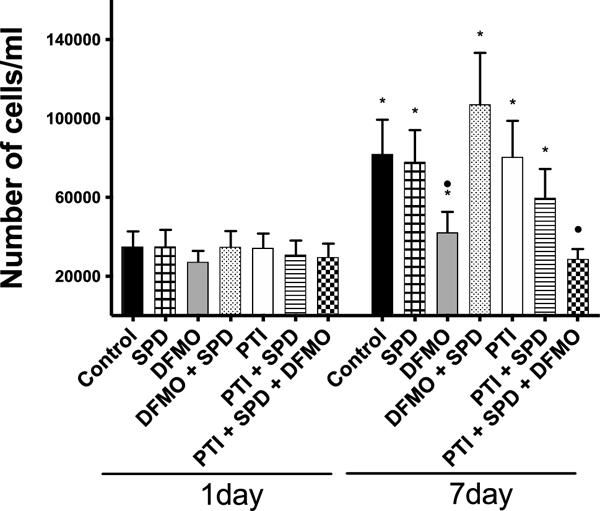
25,000 cells/well were plated and 24 hours later they were treated with DFMO (5 mM) + Spermidine (SPD; 10μM), PTI (30 μM) + Spermidine (SPD; 10 μM) and PTI (30 μM) + Spermidine (SPD; 10 μM) + DFMO (5 mM) or each treatment alone (n=10 on each sample). On days 3 and 5, the cell culture media was changed to replenish with the same concentration of DFMO, Spermidine and PTI as on day 1. Control was also replenished with fresh media. Samples were counted on days 1 or 7 after treatment. Asterisk (*) indicates statistical difference between the samples in 1 vs 7-day of proliferation, whereas the circle (•) indicates statistical difference between the samples in 7 day of proliferation and control (p<0.05; Wilcoxon Signed Ranks Test).
3. Results
3.1. Measurement of Cytotoxicity of Astrocytes in Response to DFMO and PTI
The concentration-dependent effects of DFMO (0–5 mM), PTI (0–100 μM) and SPD (0–100 μM) on cytotoxicity were assessed qualitatively using a Live/Dead cell assay. PTIs have been used in a variety of experiments with cancer cells and treatments for neuroblastoma and other neoplasias. The initial concentrations of DFMO, PTI and SPD used were based on previous published dose response studies in these cancer cells that produced a maximum biological response without causing toxicity to the cells (Gitto et al. 2018). The potential cytotoxic effects on cultured astrocytes have not been determined (Gitto et al. 2018). Based on our observations and qualitative analysis, we determined the optimal concentration of DFMO in astrocytes is 5 mM, PTI is 30 μM and SPD is 10 μM (data not shown). By qualitative analysis of the live/dead assay, it was seen that PTI, DFMO, the combination of both and SPD were comparable to control. PTI at higher concentration produced a change in the morphology of the cells causing a smudge-like appearance, which may indicate a cytotoxic effect of this concentration. SPD at 100 μM seemed to be cytotoxic to the cells and cells appeared with no projections (data not shown).
We next quantitatively assessed cytotoxic effects of the selected concentrations of DFMO, PTI and SPD (Fig. 2a, b) using the live/dead cell assay. The live and dead cells were quantified using Image J software. The control for dead cells was obtained by treating the cells with 70% ethanol for 30 min which resulted in 100% cell death (n=15). The untreated control group representing the percentages found in typical astrocyte cultures had 9.3 ± 4.7% (mean ± SEM, n=15) dead cells. The percentages of dead cells for the experimental groups was not significantly different from the untreated controls (denoted as live cells): PTI (11.9 ± 4.4%, n=15), DFMO (12.0 ± 4.9%, n=15), PTI and DFMO (7.0 ± 1.8%, n=15) and SPD (4.1 ± 1.0%, n=9). All groups had less than 15% dead cells including control.
3.2. Ornithine Decarboxylase Expression in Cultured Cortical Astrocytes.
Western blot analysis was performed to determine if primary cultured cortical astrocytes express ODC (Fig. 3a). We used whole brain as a positive control. ODC was expressed in both the positive control and in cultured astrocytes indicating that astrocytes cultured from neonatal rat cortex express the enzyme ODC, which facilitates the synthesis of the PA putrescine. Figure 3b shows the India ink stained membrane for total protein to discern small differences in protein loading.
Figure 3. Western blot analysis and India Ink staining of protein samples separated by SDS-PAGE.
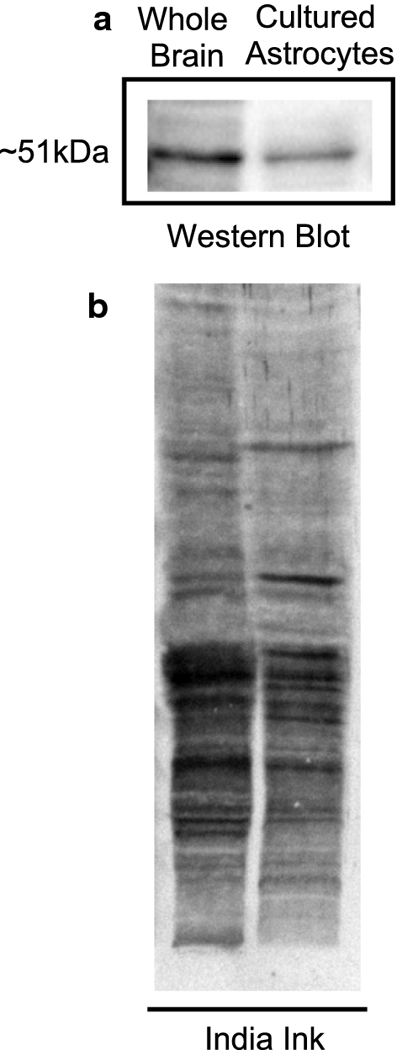
(a) Western Blot protein expression of ODC enzyme in primary cultured cortical astrocytes from 2-day old rats (4 weeks in vitro). As a control, we determined ODC expression in whole brain from a 2-day old rat. The molecular weight of ODC protein is 51kDa. (b) India ink stained membrane showing total protein.
3.3. Cell proliferation Assay; DFMO, but not PTI decreases astrocyte proliferation.
To assess the role of PA biosynthesis or uptake on astrocyte proliferation, we measured astrocyte proliferation 1 and 7 days after treatment with DFMO (5 mM), PTI (30 μM) or a combination of DFMO and PTI (Fig. 4a). Astrocytes were plated in 24 well plates and the number of cells/well on day 7 were compared with the number on day 1 after treatment. There were twice the number of cells for the untreated control group between day 1 and day 7 showing healthy proliferation. Treatment with DFMO prevented astrocyte proliferation, while treatment with PTI had no significant effect on cell proliferation, i.e., it is comparable to control. DFMO in combination with PTI also prevented astrocyte proliferation during 7 days of treatment. Indeed, there was no significant increase in cell numbers between day 1 and day 7 for the DFMO alone or the DFMO + PTI groups suggesting that de novo synthesis of PAs was necessary for cell proliferation.
3.4. Cell proliferation Assay; SPD rescued the decrease in astrocyte proliferation caused by DFMO.
To determine if exogenous PAs could rescue astrocyte proliferation when ODC and PA synthesis were blocked, we measured cell numbers 1 and 7 days after treatment with DFMO (5mM) or a combination of DFMO (5 mM) and the PA spermidine (SPD; 10 μM) (Fig 4b). Since, it has been reported that oxidases in the culture medium may degrade PAs, we exchanged the cell culture medium on days 3 and 5 after the initial administration with the same treatments added in day 1. The experimental paradigm is shown in Table 1. There was no statistical difference between the groups on day 1 after the treatment. As expected, there were approximately twice the numbers of astrocytes in the untreated control group between day 1 and day 7 and this proliferation was blocked by DFMO. Supplementing exogenous SPD to the cells increased the cell numbers significantly compared to DFMO group (Fig. 4b) suggesting that exogenous PAs can enter the astrocytes and support cell proliferation.
3.5. Cell proliferation Assay; PTI inhibited the rescue of proliferation when used in combination with spermidine and DFMO.
To determine if PA uptake is the pathway whereby exogenous SPD rescues astrocyte proliferation when PA synthesis is blocked, we measured astrocyte cell numbers 1 and 7 days after treatment with the combination of DFMO (5mM) and PTI (30 μM) and the combination of DFMO (5mM), PTI (30 μM), and SPD (10 μM). For comparison, we treated the astrocytes with DFMO (5mM), PTI (30 μM) or SPD (10 μM) alone (Fig. 5) as well. As described above, the cell culture medium was replenished on days 3 and 5 for all groups. There was no statistical difference between the groups on day 1 after the treatment. Consistent with the results obtained in Fig. 4, DFMO prevented cell proliferation compared to control and this inhibitory effect was reversed by exogenous application of SPD (Fig. 5). In contrast, astrocyte cell numbers were not significantly different from control for the PTI and PTI + SPD experimental groups demonstrating that synthesis of PAs by astrocytes is sufficient to support cell proliferation. However, the increased proliferation response to exogenous SPD did not occur when astrocytes were treated with a combination of DFMO (to block synthesis) and PTI (to block uptake), suggesting that PTI blocks SPD uptake.
4. Discussion
Despite the discovery of PAs in the mid-20th century (Pegg 2016), their role in human health, nutrition and specifically in brain function and dysfunction has been only appreciated since the 1990s ( (Lopatin et al. 1994; Koh et al. 1995; Eisenberg et al. 2016; Nichols and Lee 2018; Madeo et al. 2018; Handa et al. 2018; Bae et al. 2018). Strong intracellular effects of PAs were found in neurons (Williams 1997; Burnashev 2005; Nichols and Lee 2018) and in glial cells (Biedermann et al. 1998; Kucheryavykh et al. 2008; Benedikt et al. 2012; Skatchkov et al. 2015). On the other hand, extracellular effects of PAs were much less studied: for example extracellular PAs facilitated neurons in the cortex (Rozov and Burnashev 1999) and PAs promoted neuronal regeneration (Noro et al. 2015) and synaptic function (Sigrist et al. 2014; Bhukel et al. 2017; Maglione et al. 2019) as well as helping to restore age-dependent (Gupta et al. 2013), fear-dependent (Signor et al. 2017) and trauma-dependent (Frühauf-Perez et al. 2018) memory impairments.
Intriguingly, SPD and SPM are found preferentially in glial cells but not in neurons: particularly in astrocytes, Bergmann glia (Laube and Veh 1997) and Müller glia (Biedermann et al. 1998; Skatchkov et al. 2000; Skatchkov et al. 2014) in the adult CNS. There is recent evidence that SPM/SPD act as endogenous modulators of the activity of glial cells via connexin (Cx) gap junctions (Benedikt et al. 2012), specifically glial Cx43 (Skatchkov et al. 2015) and inwardly rectifying potassium channels, Kir (Biedermann et al. 1998), specifically glial Kir4.1 channels (Kucheryavykh et al. 2008). This leads to the question of how SPD and SPM, which regulate many neuronal ion channels (Lopatin et al. 1994; Fakler et al. 1994; Nichols and Lopatin 1997; Huang and Moczydlowski 2001; Fleidervish et al. 2008; Reichenbach and Bringmann 2010; Nichols and Lee 2018) and glutamate receptors (Donevan and Rogawski 1995; Koh et al. 1995; Bowie and Mayer 1995; Williams 1997; Rozov and Burnashev 1999; Burnashev 2005) are in fact localized in neighboring glial cells? Since astrocytes outnumber neurons about 3.5 times in cortex and about 12.5 times in brainstem (Lent et al. 2012) the major PA exchange should be found in astrocytes via PA synthesis or uptake.
Surprisingly, the ADC-arginase pathway to produce PAs was found in neurons, but not in glia (Peters et al. 2013), and still neurons do not accumulate PAs (Laube and Veh 1997). Similarly, ODC and spermidine synthase (SRM) were also found in neurons, but not in adult astrocytic glial cells (Bernstein and Muller 1999; Krauss et al. 2007). Only during pathological conditions was ODC localized in some astrocytes (Bernstein and Muller 1999). Therefore, the accumulation of PAs in glial cells can probably be explained by taking up PAs from the extracellular space as has been shown for other cell types (Burns et al. 2009; Sala-Rabanal et al. 2013; Gamble et al. 2019). One of the ways was via the SLC22A-1,2–3 types of organic cation transporters (OCTs) which were shown to be PA transporters (Sala-Rabanal et al. 2013) and are expressed in astrocytes (Inazu et al. 2003; Cui et al. 2009). Furthermore, PAs could be stored in astrocyte vesicles by the SLC18B-1 vesicular transporter (Hiasa et al. 2014). Therefore, there are confusing reports about PA content, PA uptake, and the presence or absence of PA biosynthetic enzymes in different CNS cell types and there is an open question as to how healthy adult glial cells accumulate SPD and SPM, when they do not seem to contain the biosynthetic enzymes needed to synthesize these PAs. Additionally, there is very little evidence about the PA uptake and synthesis in neonatal astrocytes.
With the results of our experiments, we can conclude that DFMO alone or in combination with PTI prevents astrocyte proliferation during 7 days of treatment whereas PTI alone has no effect on astrocyte proliferation; i.e., it is comparable to control. This suggests that primary cultured astrocytes from 1–2-day old rats are able to synthesize their own PAs to support cell proliferation. Consistent with these findings, ODC expression was found in 4-week-old cultured cortical astrocytes. Similarly, Dot and colleagues (2002) found ODC activity in cultured cerebellar astrocytes, which peaked at 6 days in culture and declined up to 24 days in culture (which is the last time point examined). While inhibition of PA uptake was correlated with increased ODC activity at earlier time points, there was no difference in ODC activity in the presence or absence of PA uptake at later time points (Dot et al. 2002).
The use of PAs by astrocytes to promote cell proliferation is highly regulated by intracellular sources, since the presence of exogenous SPD alone is not sufficient to increase the proliferation of astrocytes. However, after ODC block the SPD added exogenously prevented the DFMO-induced block of astrocyte proliferation suggesting that astrocytes cultured from neonatal rats are capable of both (i) synthesizing sufficient quantities of PAs and (ii) are able to use the PTI sensitive PA transport system to support cell proliferation when synthesis is inhibited.
However, this is very different in adult CNS because immunohistochemistry studies have demonstrated that ODC is absent in astrocytes of healthy brain (Bernstein and Muller 1999), but PAs are predominantly present in adult astrocytes and other glial cells (Laube and Veh 1997). Also, PA content is undetectable or in very low concentration in neurons (Laube and Veh 1997; Bernstein and Muller 1999). Surprisingly, not only ODC but other PA biosynthesis enzymes are found in neurons rather than glial cells (Biedermann et al. 1998; Bernstein and Muller 1999; Krauss et al. 2007; Peters et al. 2013) in adults. This suggests that PAs enter glial cells through an uptake pathway that has not yet been characterized in detail.
Interestingly, in the immature retina, PAs and their biosynthetic enzymes are strongly expressed in practically all cell types including radial Müller glia and neurons in the rat retina during the first 3 days of the neonatal period (Rios et al. 2017) suggesting that synthesis in glial cells occurs during the early critical days of cell development (Rios et al. 2017; Malpica-Nieves et al. 2019). In adult and aging Müller glia, there is accumulation of SPD and SPM without its synthesis (Biedermann et al. 1998; Skatchkov et al. 2000) and its amount declines with age (Rios et al. 2017; Malpica-Nieves et al. 2019). Also, SPD and SPM synthases (Rios et al. 2017; Malpica-Nieves et al. 2019) are found in young progenitors and then are restricted to neurons in adults, but the number of neurons expressing SPD and SPM synthases also declines with age (Rios et al. 2017; Malpica-Nieves et al. 2019).
From our results, we can conclude that PAs are critical to support cell proliferation and they can be obtained either: (i) intracellularly via synthesis de novo or (ii) extracellularly by an uptake pathway. PA production in the cell is highly regulated and this regulation involves antizyme (AZ). When PA levels are high, these proteins are able to regulate themselves at the translational level, inhibiting ODC and exerting a negative control on PA uptake in the cell. AZ binds to ODC subunits and targets them for ubiquitin independent degradation by the 26S proteasome. To regulate PA metabolism even further, the cells contain an antizyme inhibitor (AZI) which is an ODC-related protein and helps negate AZ function (Kahana 2009; Reddy 2015; Ramos-Molina et al. 2018).
In our study, we used DFMO and PTI, first to test the block of PA synthesis with concomitant depletion of PUT, SPD, and SPM and second to inhibit the uptake of SPD in DFMO treated astrocytes and found that DFMO, but not PTI alone, blocked proliferation. This suggests that PA biosynthesis was present. Furthermore, exogenous administration of SPD rescued cell proliferation when PA synthesis was blocked by DFMO. A similar effect was found in human colorectal cancer cells (Corral and Wallace 2020). When both synthesis and uptake of PAs were inhibited (DFMO + PTI), exogenous SPD no longer supported proliferation. Surprisingly, there was no difference in astrocyte proliferation for control cells and those receiving exogenously applied SPD (see Figure 5). This could, in part, be due to activating the AZ system in astrocytes and reducing synthesis of endogenous PAs.
Taken together, our data indicate that neonatal astrocytes synthesize sufficient quantities of PAs de novo to support cell proliferation but are also able to import exogenous PAs. This suggests that the PA uptake mechanism is present in both neonates as well as in adults and can support cell proliferation in neonatal astrocytes when ODC is blocked.
The identity of uptake mechanism(s) that mediate the distribution and fluxes of PAs in astrocytes is currently unknown. Recently, Gamble et al. (2019) showed that the MYCN gene in neuroblastoma cells directly increased PA synthesis and promoted neuroblastoma cell proliferation but when DFMO was used it resulted in increased PA uptake by SLC3A2 protein expression but not glial SLC22A1,2,3. Using AMXT-1501 as a transport inhibitor (Burns et al. 2009) prevented or delayed tumor development in neuroblastoma-prone mice and extended survival in rodent models of established tumors. These findings suggest that combining block of synthesis and transport (DFMO + AMXT 1501) might be an effective strategy for treating neuroblastoma.
Biochemical characterization of PA uptake in cerebellar astrocytes has shown that uptake is dependent upon (i) the membrane potential, but (ii) is not mediated by co-transport with sodium (Dot et al. 2000). Therefore we suggest two potential pathways for PA uptake in astrocytes: (i) transporters and (ii) large channels (Skatchkov et al. 2014). It is known that the PA transport may occur via SLC22A organic cation transporters (OCTs) (Sala-Rabanal et al. 2013) expressed in astrocytes (Inazu et al. 2003) and large connexin 43 hemichannels (Cx43 HC) also expressed in astrocytes (Bennett et al. 2003; Benedikt et al. 2012). Both depend on the hyperpolarized membrane potential of astrocytes (Skatchkov et al. 2016). Since both are present in astrocytes, they allow the transfer of PAs in and out of astrocytes (Dot et al. 2000; Dot et al. 2002; Benedikt et al. 2012; Skatchkov et al. 2014; Hiasa et al. 2014; Skatchkov et al. 2016), making these potential pathways for PA uptake. Because OCTs and Cx43 hemichannels operate under different conditions, both pathways may be involved in PA uptake into glial cells particularly when PA synthesis is absent (such as in adult and specifically in aging brain). There have been reports supportive of a transmembrane protein and/or an endocytosis process for PA uptake in other cell types and this area has been reviewed (Poulin et al. 2012).
5. Conclusions
In conclusion our data indicate that cortical astrocytes cultured from neonatal rats synthesize sufficient quantities of PAs de novo to support cell proliferation, but can also take up exogenous PAs to support proliferation when synthesis is inhibited or blocked. Recent data highlight the role of dysfunctional PA biosynthesis in cancer (Gamble et al. 2019), brain toxicity (Pietropaoli et al. 2018) and the role of PAs in autophagy, memory, and lifespan (Sigrist et al. 2014; Madeo et al. 2018). The regulation of PA synthesis (Pegg et al. 1987; LoGiudice et al. 2018) and transport (Wallace and Fraser 2004; Sala-Rabanal et al. 2013; Muth et al. 2014) are also being investigated for anti-cancer applications (Pegg et al. 1987; Meyskens and Gerner 1999; Wallace and Fraser 2004; Muth et al. 2014; LoGiudice et al. 2018) and PAs are key players in cell transfection techniques for gene delivery (Amini et al. 2013). Understanding how normal brain cells respond to dysfunctional PA metabolism (and to therapies which target PA metabolism) may open new approaches to treat brain dysfunctions, disease, and aging. These insights are crucial for improved control of distinct pathological diseases such as HIV Associated Neurocognitive Disorder (HAND), seizures, stroke, Alzheimer’s disease, and other related diseases where PAs play a key role (Bernstein and Muller 1999; Skatchkov et al. 2016; Pegg 2016; Laube and Bernstein 2017; Madeo et al. 2018).
Acknowledgments
Declarations:
1. Funding:
This work was supported by the following funding sources: The National Institutes of Health grants: R15-NS116478, RO1-NS065201, R25-GM110513 and U54-MD007587 and Department of Education grant: P031S130068.
3. Ethics approval:
This study was performed following NIH Guidelines for the care and use of laboratory animals. All experiments were approved by the Universidad Central del Caribe Institutional Animal Care and Use Committee.
Footnotes
2. Conflicts of interest/Competing interests
The authors of this manuscript declare no conflicts of interest.
4. Consent to participate:
Not applicable.
5. Consent for publication
Not applicable.
6. Availability of data and material:
All data generated or analyzed during this study are included in this published article.
7. Code availability
Not applicable.
References:
- Amini R, Jalilian FA, Abdullah S, Veerakumarasivam A, Hosseinkhani H, Abdulamir AS, Domb AJ, Ickowicz D, Rosli R (2013) Dynam- ics of PEGylated-dextran-spermine nanoparticles for gene delivery to leukemic cells. Appl Biochem Biotechnol 170:841–853. 10.1007/s12010-013-0224-0 [DOI] [PubMed] [Google Scholar]
- Bae D-H, Lane DJR, Jansson PJ, Richardson DR (2018) The old and new biochemistry of polyamines. Biochim Biophys Acta BBA— Gen Subj 1862:2053–2068. 10.1016/j.bbagen.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Benedikt J, Inyushin M, Kucheryavykh YV, Rivera Y, Kucheryavykh LY, Nichols CG, Eaton MJ, Skatchkov SN (2012) Intracellular polyamines enhance astrocytic coupling. NeuroReport 23:1021–1025. 10.1097/WNR.0b013e32835aa04b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MV, Contreras JE, Bukauskas FF, Saez JC (2003) New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 26:610–617. 10.1016/j.tins.2003.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein HG, Muller M (1999) The cellular localization of the l-ornithine decarboxylase/polyamine system in normal and diseased central nervous systems. Prog Neurobiol 57:485–505. 10.1016/s0301-0082(98)00065-3 [DOI] [PubMed] [Google Scholar]
- Bhukel A, Madeo F, Sigrist SJ (2017) Spermidine boosts autophagy to protect from synapse aging. Autophagy 13:444–445. 10.1080/15548627.2016.1265193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann B, Skatchkov SN, Brunk I, Bringmann A, Pannicke T, Bernstein HG, Faude F, Germer A, Veh R, Reichenbach A (1998) Spermine/spermidine is expressed by retinal glial (Muller) cells and controls distinct K+ channels of their membrane. Glia 23:209–220. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15:453–462. 10.1016/0896-6273(95)90049-7 [DOI] [PubMed] [Google Scholar]
- Burnashev N (2005) Dynamic Modulation of AMPA Receptor-Mediated Synaptic Transmission by Polyamines in Principal Neurons. Focus on “Polyamines Modulate AMPA Receptor- Dependent Synaptic Response in Immature Layer V Pyramidal Neurons” J Neurophysiol 93:2371. 10.1152/jn.01297.2004 [DOI] [PubMed] [Google Scholar]
- Burns MR, Graminski GF, Weeks RS, Chen Y (2009) OPA receptor-mediated synaptic transmission by polyamines in ptent polyamine transport inhibitors for use in combination with a polyamine biosynthesis inhibitor. J Med Chem 52:1983–1993. 10.1021/jm801580w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral M, Wallace HM (2020) Upregulation of polyamine transport in human colorectal cancer cells. Biomolecules 10:499. 10.3390/biom10040499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K (2009) The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci USA 106:8043–8048. 10.1073/pnas.0900358106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA (1995) Intracellular polyamines mediate inward rectification of Ca2+-permeable a-amino-3-hydroxy- 5-methyl-4- isoxazolepropionic acid receptors. Proc Natl Acad Sci USA 92(20):9298–9302. 10.1073/pnas.92.20.9298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorhout B, te Velde RJ, Ferwerda H, Kingma AW, de Hoog E, Muskiet FA (1995) In vivo effects of 4-amidinoindan-1-one 2’-amidinohydrazone (CGP 48664A) and alpha-difluoromethylornithine (DFMO) on L1210 growth, cell-cycle phase distribution and polyamine contents. Int J Cancer 62:738–742. 10.1002/ijc.2910620615 [DOI] [PubMed] [Google Scholar]
- Dot J, Lluch M, Blanco I, Rodríguez-Alvarez J (2000) Polyamine uptake in cultured astrocytes: characterization and modulation by protein kinases. J Neurochem 75:1917–1926. 10.1046/j.1471-4159.2000.0751917.x [DOI] [PubMed] [Google Scholar]
- Dot J, Danchev N, Blanco I, Rodriguez-Alvarez J (2002a) Polyamine uptake is necessary for a normal biochemical maturation of astrocytes in culture. Neuro Report 13:1083–1087. 10.1097/00001756-200206120-00022 [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Büttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M, Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, de Angelis MH, Moustafa T, Haemmerle G, Mayr M, Willeit P, von Frieling-Salewsky M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA, Mühlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F (2016) Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 22:1428–1438. 10.1038/nm.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Br Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Hargpersberg JP (1994) A structural determinant of differential sensitivity of cloned inward rectifier K + channels to intracellular spermine. FEBS Lett 356:199–203. 10.1016/0014-5793(94)01258-X [DOI] [PubMed] [Google Scholar]
- Ferrer-Acosta Y, Gonzalez-Vega MN, Rivera-Aponte DE, Martinez-Jimenez SM, Martins AH (2017) Monitoring astrocyte reactivity and proliferation in vitro under ischemic-like conditions. J Vis Exp 128:55108. 10.3791/55108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish IA, Libman L, Katz E, Gutnick MJ (2008) Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc Natl Acad Sci 105:18994–18999. 10.1073/pnas.0803464105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühauf-Perez PK, Temp FR, Pillat MM, Signor C, Wendel AL, Ulrich H, Mello CF, Rubin MA (2018) Spermine protects from LPS-induced memory deficit via BDNF and TrkB activation. Neurobiol Learn Mem 149:135–143. 10.1016/j.nlm.2018.02.012 [DOI] [PubMed] [Google Scholar]
- Gamble LD, Purgato S, Murray J, Xiao L, Yu DMT, Hanssen KM, Giorgi FM, Carter DR, Gifford AJ, Valli E, Milazzo G, Kamili A, Mayoh C, Liu B, Eden G, Sarraf S, Allan S, Di Giacomo S, Flemming CL, Russell AJ, Cheung BB, Oberthuer A, London WB, Fischer M, Trahair TN, Fletcher JI, Marshall GM, Ziegler DS, Hogarty MD, Burns MR, Perini G, Norris MD, Haber M (2019) Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma. Sci Transl Med 11:477: eaau1099. 10.1126/scitranslmed.aau1099 [DOI] [PubMed] [Google Scholar]
- Gitto SB, Pandey V, Oyer JL, Copik AJ, Hogan FC, Phanstiel O, Altomare DA (2018) Difluoromethylornithine combined with a polyamine transport inhibitor is effective against gemcitabine resistant pancreatic cancer. Mol Pharm 15:369–376. 10.1021/acs.molpharmaceut.7b00718 [DOI] [PubMed] [Google Scholar]
- Gossé F, Roussi S, Guyot S, Schoenfelder A, Mann A, Bergerat JP, Seiler N, Raul F (2006) Potentiation of apple procyanidin-triggered apoptosis by the polyamine oxidase inactivator MDL 72527 in human colon cancer-derived metastatic cells. Int J Oncol 29(2):423–428. 10.3892/ijo.29.2.423 [DOI] [PubMed] [Google Scholar]
- Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koe-mans TS, Kramer JM, Liu KS, Schroeder S, Stunnenberg HG, Sinner F, Magnes C, Pieber TR, Dipt S, Fiala A, Schenck A, Schwaerzel M, Madeo F, Sigrist SJ (2013) Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci 16:1453–1460. 10.1038/nn.3512 [DOI] [PubMed] [Google Scholar]
- Handa AK, Fatima T, Mattoo AK (2018) Polyamines: bio-molecules with diverse functions in plant and human health and disease. Front Chem 6:10. 10.3389/fchem.2018.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa M, Miyaji T, Haruna Y, Takeuchi T, Harada Y, Moriyama S, Yamamoto A, Omote H, Moriyama Y (2014) Identification of a mammalian vesicular polyamine transporter. Sci Rep 4:6836. 10.1038/srep06836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CJ, Moczydlowski E (2001) Cytoplasmic polyamines as permeant blockers and modulators of the voltage-gated sodium channel. Biophys J 80:1262–1279. 10.1016/S0006-3495(01)76102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inazu M, Takeda H, Matsumiya T (2003) Expression and functional characterization of the extraneuronal monoamine transporter in normal human astrocytes. J Neurochem 84:43–52. 10.1046/j.1471-4159.2003.01566.x [DOI] [PubMed] [Google Scholar]
- Kahana C (2009) Antizyme and antizyme inhibitor, a regulatory tango. Cell Mol Life Sci CMLS 66:2479–2488. 10.1007/s00018-009-0033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Burnashev N, Jonas P (1995) Block of native Ca2+− permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol 84:43–52. 10.1113/jphysiol.1995.sp020813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomoa D-LT, Borsics T, Feith DJ, Coleman CC, Wallick CJ, Gamper I, Pegg AE, Bachmann AS (2009) Inhibition of S-adenosylmethionine decarboxylase by inhibitor SAM486A connects polyamine metabolism with p53-Mdm2-Akt/protein kinase B regulation and apoptosis in neuroblastoma. Mol Cancer Ther 8:2067–2075. 10.1158/1535-7163.MCT-08-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomoa D, Geerts D, Lange I, Koster J, Pegg A, Feith D, Bachmann AS (2013) DFMO/eflornithine inhibits migration and invasion downstream of MYCN and involves p27Kip1 activity in neuroblastoma. Int J Oncol 42:1219–1228. 10.3892/ijo.2013.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Weiss T, Langnaese K, Richter K, Kowski A, Veh RW, Laube G (2007) Cellular and subcellular rat brain spermidine syn- thase expression patterns suggest region-specific roles for poly- amines, including cerebellar pre-synaptic function. J Neurochem 103:679–693. 10.1111/j.1471-4159.2007.04770.x [DOI] [PubMed] [Google Scholar]
- Kucheryavykh YV, Shuba YM, Antonov SM, Inyushin MY, Cubano L, Pearson WL, Kurata H, Reichenbach A, Veh RW, Nichols CG, Eaton MJ (2008) Complex rectification of Muller cell Kir currents. Glia 56:775–790. 10.1002/glia.20652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larque E (2007) Biological significance of dietary polyamines. Nutrition 23:87–95. 10.1016/j.nut.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Laube G, Veh RW (1997) Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia 19:171ex. [DOI] [PubMed] [Google Scholar]
- Laube G, Bernstein H-G (2017) Agmatine: multifunctional arginine metabolite and magic bullet in clinical neuroscience? Biochem J 474(15):2619–2640. 10.1042/BCJ20170007 [DOI] [PubMed] [Google Scholar]
- Lent R, Azevedo FA, Andrade-Moraes CH, Pinto AV (2012) How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur J Neurosci 35:1–9. 10.1111/j.1460-9568.2011.07923.x [DOI] [PubMed] [Google Scholar]
- LoGiudice N, Le L, Abuan I, Leizorek Y, Roberts S (2018) Alpha- difluoromethylornithine, an irreversible inhibitor of polyamine biosynthesis, as a therapeutic strategy against hyperproliferative and infectious diseases. Med Sci 6(1):12. 10.3390/medsci6010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG (1994) Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372(6504):366–369. 10.1038/372366a0 [DOI] [PubMed] [Google Scholar]
- Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G (2010) Spermidine: a novel autophagy inducer and longevity elixir. Autophagy 6:160–162. 10.4161/auto.6.1.10600 [DOI] [PubMed] [Google Scholar]
- Madeo F, Eisenberg T, Pietrocola F, Kroemer G (2018) Spermidine in health and disease. Science 359: eaan2788. 10.1126/science.aan2788 [DOI] [PubMed] [Google Scholar]
- Maglione M, Kochlamazashvili G, Eisenberg T, Rácz B, Michael E, Toppe D, Stumpf A, Wirth A, Zeug A, Müller FE, Moreno- Velasquez L, Sammons RP, Hofer SJ, Madeo F, Maritzen T, Maier N, Ponimaskin E, Schmitz D, Haucke V, Sigrist SJ (2019) Spermidine protects from age-related synaptic alterations at hippocampal mossy fiber-CA3 synapses. Sci Rep 9:19616. 10.1038/s41598-019-56133-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpica-Nieves CJ, Rivera-Aponte DE, Tejeda-Bayron FA, Rios D, Zayas-Santiago A, Phanstiel O, Veh RW, Eaton MJ, Skatchkov SN (2019) The involvement of polyamine uptake and synthesis pathways in proliferation of neonatal astrocytes. Neuroscience Meeting Planner Chicago: Society for Neuroscience Program No. 204.14 [Google Scholar]
- Merali S, Barrero CA, Sacktor NC, Haughey NJ, Datta PK, Lang- ford D, Khalili K (2014) Polyamines: predictive biomarker for HIV-associated neurocognitive disorders. J AIDS Clin Res 5(6):1000312. 10.4172/2155-6113.1000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens FL, Gerner EW (1999) Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res Off J Am Assoc Cancer Res 5:945–951 [PubMed] [Google Scholar]
- Morris SM (1609S) Arginine metabolism: boundaries of our knowledge. J Nutr 137:1602S–1609S. 10.1093/jn/137.6.1602S [DOI] [PubMed] [Google Scholar]
- Muñoz-Esparza NC, Latorre-Moratalla ML, Comas-Basté O, Toro- Funes N, Veciana-Nogués MT, Vidal-Carou MC (2019) Polyamines in Food. Front Nutr 6:108. 10.3389/fnut.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth A, Madan M, Archer JJ, Ocampo N, Rodriguez L, Phanstiel O (2014) Polyamine transport inhibitors: design, synthesis, and combination therapies with difluoromethylornithine. J Med Chem 57:348–363. 10.1021/jm401174a [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN (1997) Inward rectifier potassium channels. Annu Rev Physiol 59:171–191. 10.1146/annurev.physiol.59.1.171 [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lee S-J (2018) Polyamines and potassium channels: a 25-year romance. J Biol Chem 293(48):18779–18788. 10.1074/jbc.TM118.003344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noro T, Namekata K, Kimura A, Guo X, Azuchi Y, Harada C, Nakano T, Tsuneoka H, Harada T (2015) Spermidine promotes retinal ganglion cell survival and optic nerve regeneration in adult mice following optic nerve injury. Cell Death Dis 6(4):e1720. 10.1038/cddis.2015.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE (2016) Functions of polyamines in mammals. J Biol Chem 291(29):14904–14912. 10.1074/jbc.R116.731661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE, McGOVERN KA, Wiest L (1987) Decarboxylation of a-difluoromethylornithine by ornithine decarboxylase. Biochem J 241:305–307. 10.1042/bj2410305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters D, Berger J, Langnaese K, Derst C, Madai VI, Krauss M, Fis- cher KD, Veh RW, Laube G (2013) Arginase and arginine decarboxylase—where do the putative gatekeepers of polyamine synthesis reside in rat brain? PLoS ONE 8(6):e66735. 10.1371/journal.pone.0066735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaoli S, Leonetti A, Cervetto C, Venturini A, Mastrantonio R, Baroli G, Persichini T, Colasanti M, Maura G, Marcoli M, Mariottini P, Cervelli M (2018) Glutamate excitotoxicity linked to spermine oxidase overexpression. Mol Neurobiol 55:7259–7270. 10.1007/s12035-017-0864-0 [DOI] [PubMed] [Google Scholar]
- Poulin R, Casero RA, Soulet D (2012) Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids 42:711–723. 10.1007/s00726-011-0987-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Molina B, Lambertos A, Peñafiel R (2018) Antizyme Inhibitors in Polyamine Metabolism and Beyond: Physiopathological Implications. Med Sci 6(4), 89. 10.3390/medsci6040089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VP (2015) Chapter 2—fluorinated compounds in enzyme-catalyzed reactions. In: Reddy VP (ed) Organofluorine compounds in biology and medicine. Elsevier, Amsterdam, pp 29–57. 10.1016/C2010-0-64780-8 (ISBN 978-0-444-53748-5). [DOI] [Google Scholar]
- Reichenbach A, Bringmann A (2010) Müller cells in the healthy and diseased Retina. Sringer, New York, pp 417 (ISBN 978-1-4419-1672-3) [DOI] [PubMed] [Google Scholar]
- Rios D, Malpica-Nieves C, Yomarie R, Veh RW, Eaton MJ, Zayas-Santiago A, Skatchkov SN 2017. Changes in polyamines expression in the Rat Retina with Aging. Annual biomedical research conference for minority students (ABRCMS), Phoenix [Google Scholar]
- Rivera-Pagán AF, Rivera-Aponte DE, Melnik-Martínez KV, Zayas- Santiago A, Kucheryavykh LY, Martins AH, Cubano LA, Skatchkov SN, Eaton MJ (2015) Up-regulation of trek-2 potassium channels in cultured astrocytes requires de novo protein synthesis: relevance to localization of trek-2 channels in astrocytes after transient cerebral ischemia. PLoS ONE 10:e0125195. 10.1371/journal.pone.0125195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N (1999) Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401:594–598. 10.1038/44151 [DOI] [PubMed] [Google Scholar]
- Sala-Rabanal M, Li DC, Dake GR, Kurata HT, Inyushin M, Skatchkov SN, Nichols CG (2013) Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol Pharm 10:1450–1458. 10.1021/mp400024d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signor C, Girardi BA, Lorena Wendel A, Frühauf PKS, Ulrich H, Mello CF, Rubin MA (2017) Spermidine improves the persistence of reconsolidated fear memory and neural differentiation in vitro: involvement of BDNF. Neurobiol Learn Mem 140:82–91. 10.1016/j.nlm.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Carmona-Gutierrez D, Gupta VK, Bhukel A, Mertel S, Eisenberg T, Madeo F (2014) Spermidine-triggered autophagy ameliorates memory during aging. Autophagy 10:178–179. 10.4161/auto.26918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatchkov SN, Eaton MJ, Krusek J, Veh RW, Biedermann B, Bringmann A, Pannicke T, Orkand RK, Reichenbach A (2000) Spatial distribution of spermine/spermidine content and K(+)-current rectification in frog retinal glial (Muller) cells. Glia 31:84–90. [DOI] [PubMed] [Google Scholar]
- Skatchkov SN, Woodbury-Fariña MA, Eaton M (2014) The role of glia in stress: polyamines and brain disorders. Psychiatr Clin 37:653–678. 10.1016/j.psc.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatchkov SN, Bukauskas FF, Benedikt J, Inyushin M, Kucheryavykh YV (2015) Intracellular spermine prevents acid-induced uncoupling of Cx43 gap junction channels. Neuro Report 26:528–532. 10.1097/WNR.0000000000000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatchkov SN, Antonov SM, Eaton MJ (2016) Glia and glial polyamines. Role in brain function in health and disease. Biochem Mosc Suppl Ser Membr Cell Biol 10:73–98. 10.1134/S1990747816010116 [DOI] [Google Scholar]
- Soda K, Kano Y, Chiba F, Koizumi K, Miyaki Y (2013) Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 8:e64357. 10.1371/journal.pone.0064357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace HM, Fraser AV (2004) Inhibitors of polyamine metabolism: review article. Amino Acids 26(4):353–365. 10.1007/s00726-004-0092-6 [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K (1991) Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem 266(31):20803–20809 [PubMed] [Google Scholar]
- Williams K (1997) Modulation and block of ion channels: a new biology of polyamines. Cell Signal 9:1–13. 10.1016/s0898-6568(96)00089-7 [DOI] [PubMed] [Google Scholar]