Abstract
Background.
Individual variability in tonic (resting) and phasic (reactivity) respiratory sinus arrhythmia (RSA) may underlie risk for dysregulated emotion and behavior, two transdiagnostic indicators that permeate most psychological disorders in youth. The interaction between tonic and phasic RSA may specify unique physiological profiles during the transition to adolescence. The current study utilized clinically referred youth (Mage = 12.03; s.d. = 0.92) to examine baseline RSA, RSA reactivity, and their interaction as predictors of dysregulated emotion and behavior in daily life.
Method.
Participants were 162 youth (47% female; 60% minority) in psychiatric treatment for any mood or behavior problem. RSA was assessed during three, 2-minute baselines and an 8-minute parent-child conflict discussion task. Dysregulated emotion and behavior were assessed during a 4-day ecological momentary assessment protocol that included 10 time-based prompts over a long weekend.
Results.
Greater RSA withdrawal to the conflict was associated with dysregulated basic emotion (sadness, anger, nervousness, stress) in daily life. Two distinct interactions also emerged, such that baseline RSA was related to dysregulated complex emotion (shame, guilt, loneliness, emptiness) and dysregulated behavior as a function of RSA reactivity to conflict. Lower baseline RSA and greater RSA withdrawal were associated with dysregulated complex emotion, while higher baseline RSA and greater RSA withdrawal were associated with dysregulated behavior.
Conclusions.
Findings point to physiological profiles that increase the risk of dysregulated emotion and behavior during the transition to adolescence. Excessive RSA withdrawal uniquely, and in combination with baseline RSA, increased risk for dysregulation in daily life, underscoring the role of autonomic stress responding as a risk factor for psychopathology.
Keywords: adolescence, aggression, emotion dysregulation, psychophysiology, RSA, transdiagnostic
Emotional and behavioral dysregulation (e.g. affective variability, reactive verbal and physical aggression) represent transdiagnostic indicators that permeate nearly all psychological disorders in youth (Kazdin, 2003; McLaughlin, Hatzenbuehler, Mennin, & Nolen-Hoeksema, 2011). Enhancing our understanding of their neurobiological underpinnings has the potential to elucidate key etiological mechanisms that could improve current interventions. Along these lines, individual variability in peripheral physiology, specifically variation in tonic (resting) and phasic (reactivity) respiratory sinus arrhythmia (RSA), may underlie risk for dysregulated emotion and behavior (Beauchaine & Bell, 2019). The transition to adolescence may be a particularly critical period for assessing this potential biomarker given normative increases in physiological reactivity and interpersonal conflict (Granic, Hollenstein, Dishion, & Patterson, 2003; Steinberg & Morris, 2001). However, few studies examine RSA during this sensitive developmental window. Moreover, tonic and phasic RSA are often examined independently as predictors of disorder-specific outcomes, despite evidence that their interaction may help specify unique physiological profiles of at-risk youth (Yaroslavsky, Bylsma, Rottenberg, & Kovacs, 2013; Yaroslavsky, Rottenberg, & Kovacs, 2014). The current study expands on previous literature using a transdiagnostic sample of clinically referred youth to examine baseline RSA, RSA reactivity to parent-child conflict, and their interaction as predictors of dysregulated emotion and behavior in daily life.
Dysregulated emotion and behavior as transdiagnostic indicators of psychopathology
Dysregulated emotion and behavior are multifaceted transdiagnostic constructs that reflect heightened reactivity to internal or external events (American Psychiatric Association, 2013). Dysregulated emotion has been defined as intense, variable, or prolonged affective episodes (Linehan, 1993). Some research suggests that affective variability – frequent and intense fluctuations in one’s emotional experience – may be a particularly important facet of dysregulated emotion, as variability in negative emotions (e.g. anger, sadness) has been associated with various forms of psychopathology (Houben, Van Den Noortgate, & Kuppens, 2015; Scott et al., 2020). Dysregulated behavior has been most commonly studied in youth in terms of discrete verbal and physical acts of aggression. Verbal and physical ‘reactive’ aggression – impulsive, emotionally-driven aggressive acts – have received growing attention in light of their transdiagnostic relevance and predictive utility (Card & Little, 2006; Schaeffer, Petras, Ialongo, Poduska, & Kellam, 2003). Notably, dysregulated emotion and behavior have been linked prospectively to worsening internalizing and externalizing symptoms in adolescence, providing support for the notion that these transdiagnostic indicators function to increase the risk for a wide range of psychopathology during this developmentally sensitive period (Card & Little, 2006; McLaughlin et al., 2011). These findings highlight the importance of continuing to examine these constructs among transdiagnostic samples of youth to improve our understanding of psychopathology as it develops along common and divergent pathways (Cicchetti & Rogosch, 1996).
RSA and risk for psychopathology
RSA refers to the amount of variability between heartbeats (i.e. inter-beat interval) that rhythmically fluctuates with respiration (Berntson et al., 1997). This non-invasive index is of particular interest, given its unique association with parasympathetic nervous system (PNS) function, specifically vagal control of the sinoatrial node (Berntson, Cacioppo, & Quigley, 1994), and its implicated role in self-regulation (Beauchaine, 2001; Porges, 2007). During rest, tonic PNS activation functions via the vagus nerve to slow heart rate and increase its variability (i.e. higher RSA), which facilitates energy and emotion regulation. During stress (e.g. perceived threat, environmental challenge), withdrawal of PNS control – a releasing of vagal control – results in increased heart rate and reduced RSA variability, allowing one to mobilize the metabolic resources needed to optimally respond to environmental demands (i.e. RSA reactivity; Porges, 2007). Consistent with this theoretical perspective, better mental health and functional outcomes are generally linked to higher tonic RSA, and to moderate RSA reactivity (specifically RSA withdrawal) during environmental challenges and stress (Graziano & Derefinko, 2013).
Individual variability in tonic and phasic RSA has been the focus of a growing body of literature aimed at identifying neurobiological indices of risk for psychopathology in youth (Beauchaine, 2015). Specifically, research in clinical and community samples shows that lower tonic RSA increases the risk for a wide range of psychiatric disorders, suggesting a reduced capacity for responsiveness to environmental demands (Beauchaine et al., 2019). Additionally, excessive RSA reactivity (i.e. greater RSA withdrawal relative to peers) in response to challenge and/or stress (i.e. passively viewing emotionally evocative stimuli) is linked to internalizing and externalizing pathology (Beauchaine et al., 2019; Fanti et al., 2019). While moderate RSA withdrawal is considered an adaptive and necessary response to stress, excessive withdrawal may be problematic, as it is associated with overly rapid mobilization of fight or flight responding (Porges, 2007). Although truly dangerous circumstances may require a more pronounced physiological reaction to enable necessary emotional and behavioral responses, it may be less contextually appropriate, and by extension maladaptive, to marshal a pronounced RSA response to everyday stressors or challenges, like interpersonal conflict.
While tonic and phasic RSA have been studied as independent predictors of various forms of psychopathology, recent research highlights the importance of examining these indicators in combination to better understand within-individual changes to context as a risk factor for psychopathology. For example, studies examining internalizing disorders in youth have shown depression to be associated with the interaction between tonic RSA and RSA reactivity to an emotionally evocative film (Hinnant & El-Sheikh, 2009; Yaroslavsky et al., 2013, 2014). Specifically, this research points to a physiological profile characterized by low tonic RSA and excessive RSA withdrawal, a pattern of responding that may be associated with exaggerated emotional reactivity to environmental stressors and the use of maladaptive behavioral responses. Taken together, this work underscores the importance of examining within-individual interplay between tonic and phasic RSA as it applies to symptoms of broader dimensions of psychopathology.
Along these lines, there is a growing emphasis on examining associations between RSA and transdiagnostic endophenotypes, like dysregulated emotion and behavior. Research has documented associations between tonic RSA and RSA reactivity and dysregulation (Beauchaine & Bell, 2019). In line with the shift from discrete, disorder-specific outcomes to transdiagnostic indicators of psychopathology, the current study examined RSA as a predictor of dysregulated emotion and behavior in a transdiagnostic sample of clinically referred youth during the sensitive transition to adolescence. Moreover, the current study expands on prior literature, which has traditionally examined these constructs using laboratory-based assessments of discrete disorders, by capturing the frequency of these endophenotypes in daily life using an ecological momentary assessment (EMA) protocol.
Transition to adolescence as key developmental window
The transition to adolescence is characterized by normative increases in physiological reactivity that reflect substantial neurobiological changes (Steinberg & Morris, 2001). Research suggests that these changes are thought to underlie normative increases in dysregulated emotion and behavior. However, developmental changes in physiological reactivity appear to be amplified among at-risk youth and these amplifications occur during a time of heightened social stress and increases in interpersonal conflict, especially parent-child conflict (Granic et al., 2003; Steinberg & Morris, 2001). Combined, these factors may contribute to increased vulnerability for dysregulated emotion and behavior, intensifying risk for the range of psychopathology that also emerges during this developmental window (Spear, 2009). Examining physiological reactivity to stressors that reflect real-life, social experiences (e.g. parent-child conflict) during this sensitive developmental window may enhance our etiological understanding of risk for psychopathology. However, as noted above, the literature in this area has traditionally assessed physiological (RSA) reactivity while passively viewing emotionally evocative stimuli (see Cui et al., 2015; Woody et al., 2019 for exceptions), making it less clear how findings may generalize to developmentally salient stressors like parent-child conflict.
Current study
The primary goal of this study was to examine baseline RSA, RSA reactivity to parent-child conflict, and their interaction as predictors of dysregulated emotion and behavior during the critical transition to adolescence. Importantly, the current study utilized a transdiagnostic sample of clinically referred youth and examined these endophenotypes in daily life using an EMA protocol. This unique study design allowed for the first known empirical investigation to address these aims.
Methods
Sample
Participants were 162 youth (Mage = 12.03 years, s.d. = 0.92 years; 47% female; 60% racial/ethnic minority). Youth and their parents (88% biological mothers; Mage = 39.84; s.d. = 7.25; 94% female; 48% racial/ethnic minority) were recruited from pediatric primary care clinics within a large, Mid-Atlantic urban, academic hospital-based setting, and from ambulatory psychiatric treatment clinics in the same geographic region. All youth were receiving psychiatric treatment for any mood or behavior problem and were over-sampled for emotional reactivity based on the Affective Instability subscale from the Personality Assessment Inventory-Adolescent version (M = 13.05, s.d. = 2.90; scores >11 indicating clinical significance; Morey, 2007). This recruitment strategy was designed to capture a transdiagnostic sample of youth and diagnostic information can be found in Table 1. Any youth with an IQ estimate <70 (n = 5), not currently in treatment for a mood or behavior problem (n = 4), with an organic neurological medical condition (n = 1), diagnosed with an autism spectrum disorder or in a current manic or psychotic episode were excluded. All caregivers had legal custody and primary physical custody (>50% of the time). Caregivers reported having M = 3.24 children (s.d. = 1.68) and 49% reported living with their romantic partners. While 64% of households had at least one employed parent, 19% reported an annual household income between $20 000 and $39 000 and 31% reported annual income <$20 000. Additional demographic information is available upon request.
Table 1.
Sample prevalence of DSM-5 diagnoses
| Disorder | % with Diagnosis |
|---|---|
| Attention Deficit Hyperactivity Disorder | 62% |
| Oppositional Defiant Disorder | 45% |
| Borderline Personality Disorder | 33% |
| Major Depressive Disorder | 31% |
| Generalized Anxiety Disorder | 23% |
| Separation Anxiety Disorder | 19% |
| Conduct Disorder | 18% |
| Disruptive Mood Dysregulation Disorder | 17% |
| Social Phobia | 9% |
| Post-Traumatic Stress Disorder | 3% |
| Panic Disorder | 1% |
Note. Diagnostic information was obtained from child- and parent-report on the KSAD-S for all disorders except for borderline personality disorder, which was obtained from child- and parent-report on the CI-BPD.
Procedure
The present data were drawn from this longitudinal study where youth and their parents completed three assessments, each of which were every 9 months apart. Assessments included semi-structured diagnostic interviews, questionnaires, and a parent–child interaction task during which multiple physiological indices were continuously recorded. Following each laboratory assessment, youth and their parent also completed a 4-day EMA protocol. The current study focuses on the first assessment, specifically on RSA measured during the parent–child interaction task, and dysregulated emotion and behavior assessed during the EMA protocol. All study procedures were approved by the Human Research Protection Office (HRPO) and the Clinical and Translational Science Institute (CTSI) pediatric practice-based research network. Youth and their parents provided written informed consent and were compensated for their time.
Physiological reactivity to parent-child conflict
Youth RSA was assessed continuously as an index of parasympathetic function during three, 2-minute vanilla baselines (i.e. youth reading silently, youth listening to parent read, and youth thinking about a discussion topic), and an 8-minute parent-child conflict discussion task. Vanilla baselines were designed following best-practice recommendations (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992). For the conflict discussion, youth and their parent identified areas of disagreement using a 25-item questionnaire that included common areas of parent-child conflict (e.g. behavior toward siblings, internet/cell phone usage, behavior in school). If an area of conflict was endorsed, respondents rated frequency (1 = once in past month to 6 = more than once per day) and intensity (1 = not at all bad to 5 = extremely bad). Research assistants identified two conflict topics that were rated highly in terms of frequency (M = 5.23; s.d. = 1.13) and severity (M = 3.99; s.d. = 0.92) by both members of the dyad. Dyads were then asked to discuss topics with a goal of resolving disagreements in the future.
Physiological recording and assessment
Electrocardiogram (ECG) signals were obtained from disposable Ag/Ag-Cl spot electrodes positioned in a modified lead-II configuration using Mindware BioLab software (MindWare Technologies, Ltd., Gahanna, OH). Two trained scorers visually inspected each recoded waveform (Berntson et al., 1997) and manually corrected artifacts using Mindware HRV 3.1.4 software (MindWare, Gahanna, OH). Any discrepancies arising in this process were resolved by consensus between first and second authors. The interbeat interval (IBI) series was resampled in equal 250 ms intervals, linearly detrended, and tapered using a Hanning window. Heart rate variability (HRV) was calculated using Fast Fourier transformation analysis of the IBI series, and RSA was defined as high-frequency heart rate variability (HF-HRV) associated with the log-transformed high frequency (HF) respiratory power band (0.12–0.50 Hz range; see Berntson et al., 1997).1 RSA analyses included a total of 154 youth with usable physiological data.2
RSA was estimated separately for each vanilla baseline (mean range = 6.55–6.83; s.d. range = 1.00–1.08) and for the conflict discussion task (mean = 6.87; s.d. = 0.90). To take advantage of the multiple baseline assessments, Confirmatory Factor Analysis (CFA) in MPlus version 8 (Muthén & Muthén, 2012) was employed to assess a latent baseline RSA construct using three baseline indicators. Overall model fit was acceptable (χ2 = 3.55, p > 0.05; CFI = 0.99; RMSEA = 0.13) and the average interitem correlation was 0.82 indicating a high degree of unidimensionality. To measure within-individual RSA reactivity, a difference score was calculated by subtracting RSA during baseline from RSA during the conflict discussion. Thus, negative scores reflect RSA withdrawal (reduced RSA during conflict relative to baseline), while positive scores reflect RSA augmentation (increased RSA during conflict relative to baseline). In this sample, mean RSA reactivity was slightly positive (mean = 0.31; s.d. = 0.64; range = −1.27–2.01), meaning the majority of those below the mean experienced RSA withdrawal, while all of those above the mean experienced RSA augmentation.
Dysregulated emotion and behavior in daily life
All youth completed a 4-day EMA protocol consisting of 10 time-based prompts over the course of a long weekend, with two of the days always including Saturday and Sunday (e.g. Friday: midday, nighttime; Saturday–Sunday: morning, midday, nighttime; Monday: midday, nighttime). Youth were prompted to complete assessments via a ‘beep’ on a study-provided phone. Compliance was high, with the average youth completing 91.1% of assessments.
Dysregulated emotion
At each assessment (n = 10), participants rated a series of negative emotions on a 4-point Likert scale (i.e. 0 = not at all; 1 = a little; 2 = a medium amount; 3 = a lot). Specifically, participants responded to the prompt ‘In the past 15 minutes, how much have you felt…’ (1) ‘sad (blue, unhappy),’ (2) ‘angry (mad),’ (3) ‘nervous (worried, uneasy),’ (4) ‘stressed,’ (5) ‘ashamed,’ (6) ‘guilty,’ (7) ‘lonely,’ and (8) ‘empty.’ To capture emotional variability or dysregulation, the mean squared successive difference (MSSD) was calculated for each negative emotion. MSSD assesses the difference between emotional intensity at consecutive assessments (e.g. sadness at T2–sadness at T1; sadness at T3–sadness at T2) to capture the magnitude of change (i.e. stronger emotional ups-and-downs) while taking temporal dependency into account (Trull et al., 2008). Higher MSSD indicates a more variable or dysregulated emotional profile, representative of larger moment-to-moment fluctuations in intensity and heightened reactivity to internal or external events.
MSSD calculations for each of the eight emotions were used as manifest indicators of a latent dysregulated emotion construct. To determine the appropriate measurement model for dysregulated emotion, we conducted exploratory and confirmatory factor analyses in MPlus. Manifest indicators (MSSD for each emotion) were entered into an exploratory factor analysis (EFA) with an oblique rotation (i.e. promax), which allows all items to load on all factors. The number of factors extracted was determined by several criteria (e.g. strength of loadings, number of eigenvalues >1.0, interpretability of the solution). In line with theoretical and empirical distinctions (Frijda, 1986; Ortony & Turner, 1990), a 2-factor solution emerged as optimal. Variability in sadness, anger, nervousness and stress loaded on one factor (dysregulated basic emotion; range of loadings: 0.414–0.914) and variability in shame, guilt, loneliness, and emptiness loaded on a second factor (dysregulated complex emotion; range of loadings: 0.579–0.639). A confirmatory factor analysis (CFA) using the two identified emotion factors confirmed good model fit: χ2 = 27.725, p = 0.03; CFI = 0.97; RMSEA = 0.07.
Dysregulated behavior
At the end of each of the 4 days, participants completed an end-of-day assessment: ‘When you were feeling your worst, did you let your feelings out by….’: (1) ‘crying/screaming,’ (2) ‘yelling at someone,’ or (3) ‘hitting, slamming or punching someone or something.’ Youth could endorse any of the above and a count score (range = 0–4) was generated for each behavior reflecting the number of days each behavior was endorsed (i.e. 0 = did not engage in behavior on any day, 4 = engaged in behavior every day). Nearly half of the sample reported verbal aggression at least once (crying/screaming (41.2%), yelling at someone (43.8%)), and 20.3% reported physical aggression. Counts for each behavior were used as manifest indicators of the latent dysregulated behavior construct. The small number of indicators necessitated the examination of a one-factor solution. Overall model fit was good (χ2 = 0.659, p > 0.05; CFI = 1.00; RMSEA < 0.0001) and the average interitem correlation was 0.35 which reflects sufficient unidimensionality for self-reported behavioral constructs (Clark & Watson, 1995).
Covariates
Demographic variables
Participant age, gender (0 = male; 1 = female) and minority status (0 = Caucasian; 1 = racial/ethnic minority) were included in analyses.
Stimulant use
All participants completed a questionnaire that included current medication (e.g. stimulant) usage and 18% reported taking stimulants on the day of study participation. Given the influence of stimulants on heart rate, a dichotomized construct (0 = no stimulant usage; 1 = same-day stimulant usage) was included in analyses.
Data analytic strategy
Using SPSS version 25 (SPSS, Inc., Chicago, IL), preliminary analyses included obtaining descriptive statistics, and examining bivariate correlations between all study variables using extracted latent scores for primary predictors and outcomes. Primary study hypotheses were tested in MPlus version 8 (Muthén & Muthén, 2012), and age, gender, minority status, and same-day stimulant use were included as covariates. Our model was estimated using maximum likelihood with robust standard errors (MLR) and included all primary predictors (baseline RSA, RSA reactivity, and their interaction)3 and outcomes (dysregulated basic emotion, dysregulated complex emotion, dysregulated behavior) (see Fig. 1)4. All significant interactions were probed and plotted for interpretation using RSA values ±1 s.d. above and below the mean. Fraley’s (2018) online utility for parsing the two-way interactions was used to assess simple slopes and regions of significance.
Fig. 1.
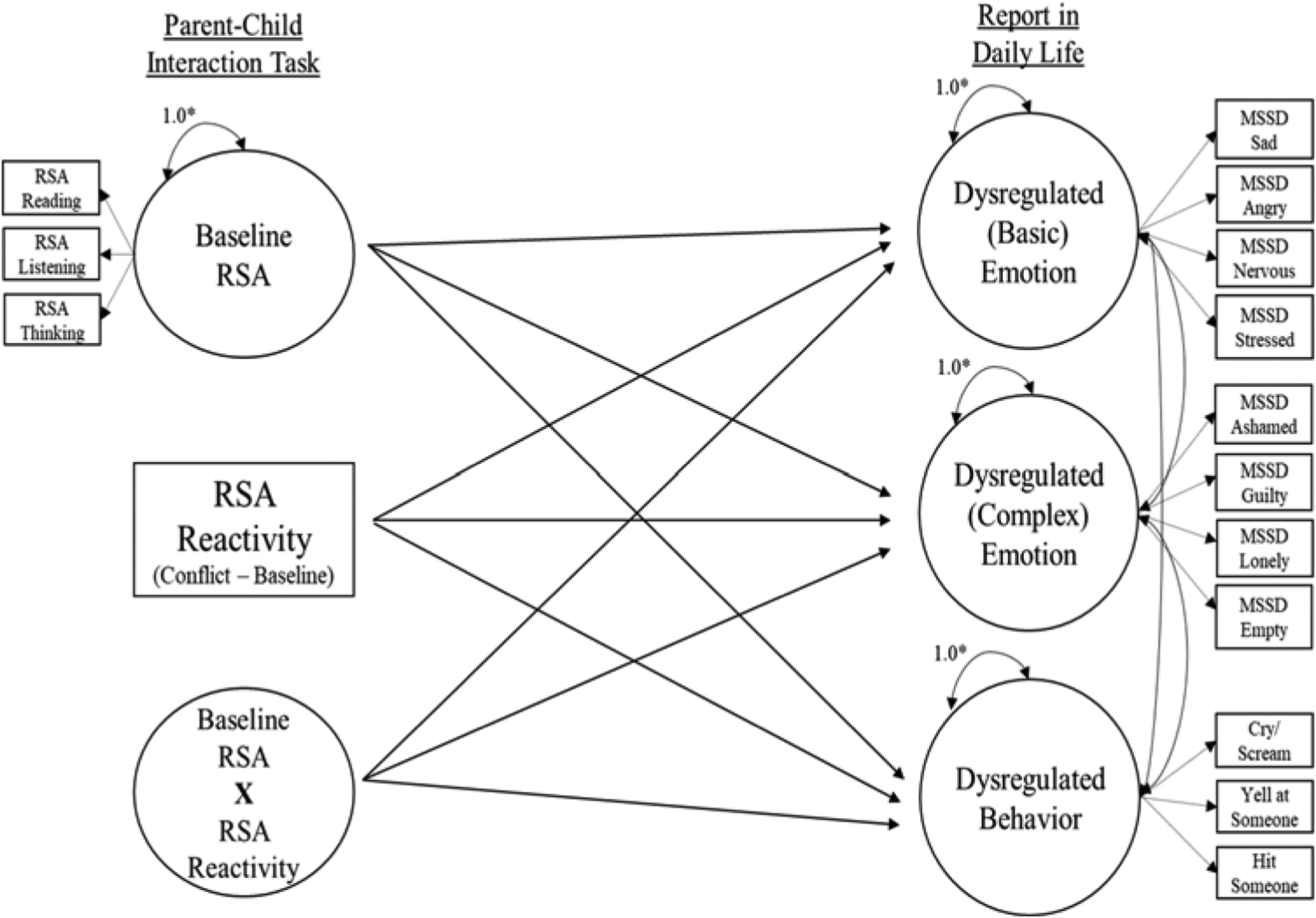
Conceptual model for testing associations between baseline RSA, RSA reactivity, and baseline RSA × RSA reactivity as predictors of dysregulated emotion and behavior in daily life.
Results
Descriptive statistics and bivariate correlations
Table 2 provides bivariate correlations for all study variables. Male youth were more likely to take stimulant medication on the day of the laboratory assessment. Female youth reported higher levels of dysregulated basic and complex emotion in daily life. Minority youth displayed higher baseline RSA. Higher baseline RSA was associated with RSA reactivity (greater withdrawal) and with more dysregulated behavior in daily life. Dysregulated basic and complex emotion were positively correlated, and higher levels of both were associated with dysregulated behavior in daily life.
Table 2.
Bivariate correlations among all study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age | |||||||||
| 2. Gender | 0.187* | ||||||||
| 3. Minority status | −0.138 | −0.099 | |||||||
| 4. Same day stimulant use | −0.039 | −0.251** | −0.121 | ||||||
| 5. Mean heart rate | −0.161* | −0.163* | −0.035 | 0.214** | |||||
| 6. Baseline RSA | −0.139 | 0.036 | 0.171* | −0.069 | −0.577** | ||||
| 7. RSA reactivity | 0.148 | −0.027 | −0.053 | −0.053 | 0.152† | −0.299* | |||
| 8. Dysregulated (basic) emotion | 0.064 | 0.229** | −0.128 | 0.010 | −0.091 | 0.122 | −0.107 | ||
| 9. Dysregulated (complex) emotion | 0.073 | 0.323** | −0.067 | −0.055 | −0.079 | 0.023 | −0.097 | 0.744** | |
| 10. Dysregulated behavior | −0.095 | 0.031 | 0.132 | 0.034 | −0.160† | 0.194* | −0.034 | 0.226** | 0.177* |
RSA, respiratory sinus arrhythmia.
Significant associations are bolded,
p < 0.01,
p < 0.05,
p = 0.05.
RSA as a predictor of dysregulated emotion and behavior in daily life
Table 3 depicts results controlling for the effects of gender, minority status, and same-day stimulant use. RSA reactivity to parent-child conflict was associated with dysregulated basic emotion in daily life, with greater RSA withdrawal predicting higher levels of dysregulated basic emotion. There was also a significant interaction between baseline RSA and RSA reactivity to parent-child conflict in the prediction of dysregulated complex emotion and behavior in daily life.5
Table 3.
Associations between baseline RSA, RSA reactivity, and baseline RSA × RSA reactivity, and dysregulated emotion and behavior in daily life
| Dysregulated (basic) emotion | Dysregulated (complex) emotion | Dysregulated behavior | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | s.e. | p | β | s.e. | p | β | s.e. | p | |
| Baseline RSA | 0.093 | 0.129 | 0.471 | −0.086 | 0.066 | 0.192 | 0.231 | 0.124 | 0.062 |
| RSA reactivity | −0.174 | 0.070 | 0.013 | −0.113 | 0.062 | 0.070 | −0.018 | 0.105 | 0.866 |
| Baseline × Reactivity | −0.102 | 0.099 | 0.304 | 0.123 | 0.051 | 0.016 | −0.227 | 0.100 | 0.023 |
RSA, respiratory sinus arrhythmia.
Note: Significant associations are bolded and represent effects after controlling for age, gender, minority status, and same-day stimulant use.
Post-hoc probing of these interactions revealed two distinct patterns of moderation. Simple slopes analyses showed that baseline RSA was related to dysregulated complex emotion (b = −0.30, s.e. = 0.07, p = 0.003) and dysregulated behavior (b = 0.62, s.e. = 0.25, p = 0.015) as a function of RSA withdrawal (−1 s.d.); in contrast, baseline RSA was unrelated to dysregulated complex emotion (b = 0.12, s.e. 0.15, p > 0.40) and dysregulated behavior (b = −0.13, s.e. = 0.18, p > 0.45) as a function of RSA augmentation (+1 s.d.). Region of significance analyses indicated that lower baseline RSA and greater RSA withdrawal to parent-child conflict were associated with dysregulated complex emotion in daily life (Fig. 2) whereas higher baseline RSA and greater RSA withdrawal to parent-child conflict were associated with dysregulated behavior in daily life (Fig. 3).
Fig. 2.
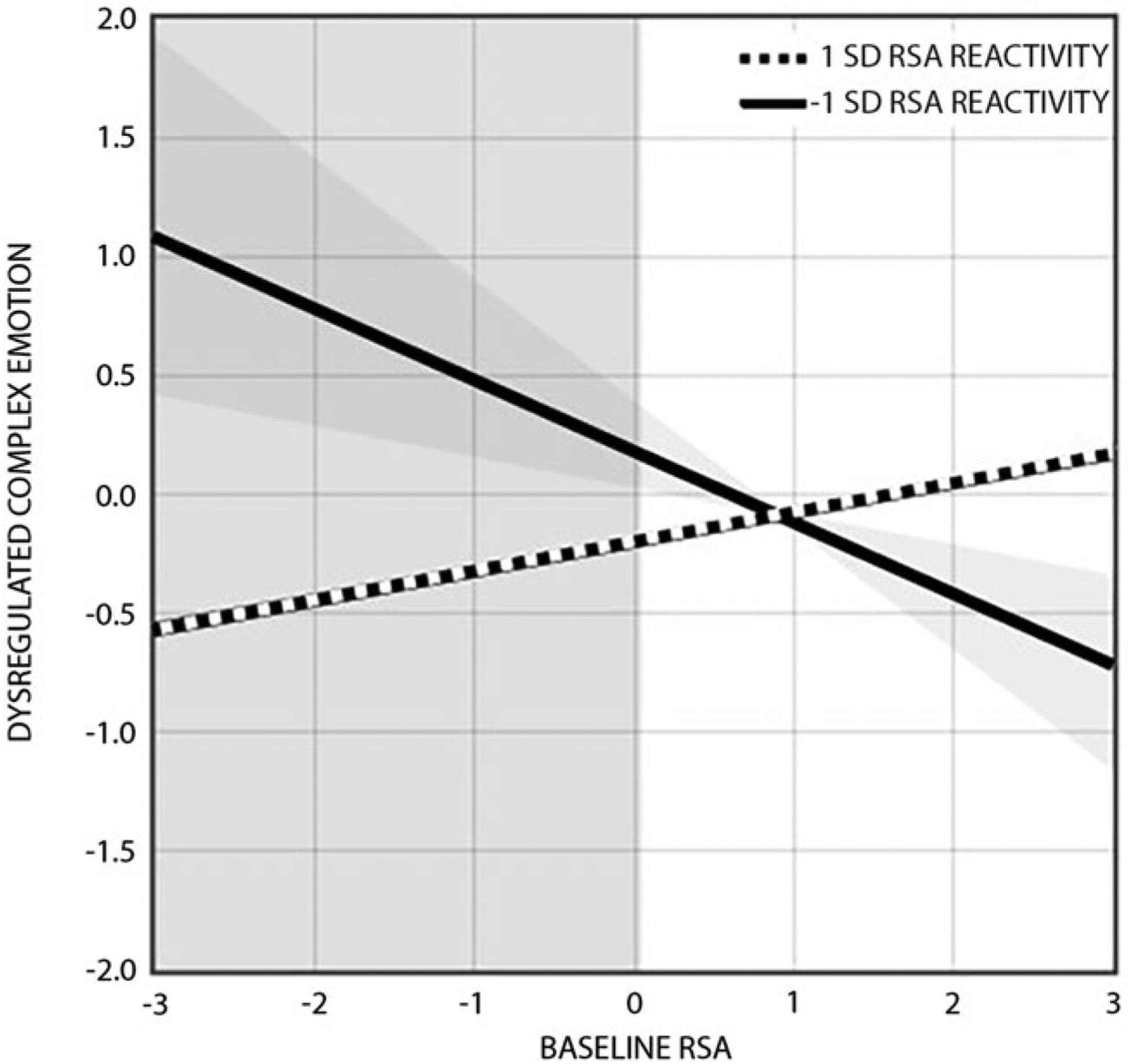
RSA reactivity to parent-child conflict moderates the effect of baseline RSA on dysregulated complex emotion in daily life. Effects are shown at ±1 s.d. above and below the mean on RSA reactivity (mean = 0.31; s.d. = 0.64), with negative values reflecting RSA withdrawal and positive values reflecting RSA augmentation. Simple slopes analyses revealed that baseline RSA was related to dysregulated complex emotion as a function of RSA withdrawal (−1 s.d.: b = −0.30, s.e. = 0.07, p = 0.003), but not as a function of RSA augmentation (+1 s.d.: b = 0.12, s.e. = 0.15, p > 0.40). The overlapping shaded areas represent the point beyond which baseline RSA values (< 0.04; sample range = −1.27–2.01) predict dysregulated complex emotion for youth with greater RSA withdrawal (<−0.19; sample range = −1.83–2.83).
Fig. 3.
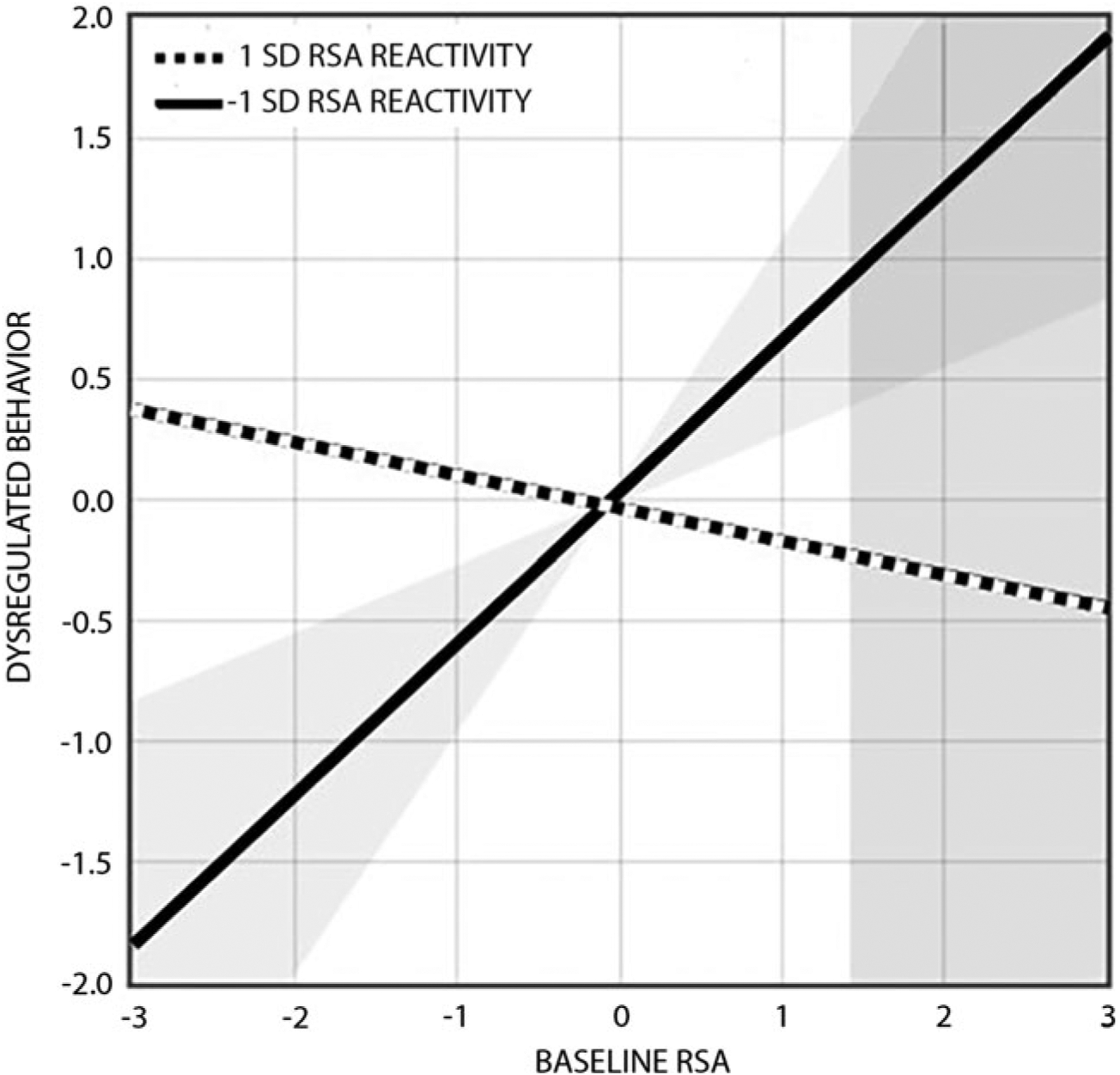
RSA reactivity to parent-child conflict moderates the effect of baseline RSA on dysregulated behavior in daily life. Effects are shown at ±1 s.d. above and below the mean on RSA reactivity (mean = 0.31; s.d. = 0.64), with negative values reflecting RSA withdrawal and positive values reflecting RSA augmentation. Simple slopes analyses revealed that baseline RSA was related to dysregulated behavior as a function of RSA withdrawal (−1 s.d.: b = 0.62, s.e. = 0.25, p = 0.015), but not as a function of RSA augmentation (+1 s.d.: b = −0.13, s.e. = 0.18, p > 0.45). The overlapping shaded areas represent the point beyond which baseline RSA values (>1.42; sample range = −1.27–2.01) predict dysregulated behavior for youth with greater RSA withdrawal (<−0.09; sample range = −1.83–2.83).
Discussion
The current study is the first to examine baseline RSA, RSA reactivity to parent-child conflict, and their interaction as predictors of dysregulated emotion and behavior in daily life. Importantly, these associations were examined using a transdiagnostic sample of clinically referred youth during the transition to adolescence, a particularly sensitive period characterized by normative increases in physiological reactivity and interpersonal conflict (Granic et al., 2003; Steinberg & Morris, 2001). Results demonstrated that youth with greater RSA withdrawal to parent-child conflict reported greater dysregulated basic emotion (sadness, anger, nervousness, stress) in daily life. Additionally, two interactions emerged such that baseline RSA was related to dysregulated complex emotion and dysregulated behavior as a function of RSA reactivity to conflict. Specifically, for youth experiencing greater RSA reactivity to parent-child conflict, lower baseline RSA was associated with dysregulated complex emotion (shame, guilt, loneliness, emptiness) in daily life while higher baseline RSA was associated with dysregulated behavior in daily life. Importantly, these effects were demonstrated within a multivariate structural equation model in which all possible associations between predictors and outcomes were examined simultaneously.
RSA reactivity was uniquely related to dysregulated emotion, namely variability in the intensity of basic emotions like anger, sadness, and anxiety. Youth with greater RSA withdrawal to parent-child conflict were characterized by more extreme fluctuations in basic emotions in daily life. This is consistent with previous research linking large reductions in RSA during passive, emotionally evocative tasks to symptoms of internalizing and externalizing psychopathology (Beauchaine & Thayer, 2015) and extends this work by illustrating a similar physiological response in the context of a more developmentally salient and ecologically-valid stressor (i.e. parent-child conflict). Additionally, our findings suggest that variability in the intensity of anger, sadness, and anxiety might explain the association between excessive RSA withdrawal and internalizing and externalizing disorders, in line with assertions that RSA reactivity underlies emotional lability specifically (Beauchaine, 2001; Beauchaine & Bell, 2019). While RSA withdrawal is a normative stress response that enables attention allocation and the mobilization of metabolic resources (Porges, 2007), research suggests that the adaptiveness of this response is non-linear, and largely context-dependent (see Davis, Brooker, & Kahle, 2020). Because of its association with the deployment of defensive action (i.e. fight or flight responding), excessive RSA withdrawal may be less adaptive within the context of everyday stressors, like interpersonal conflict, and may lower one’s threshold for experiencing dysregulated emotion (Porges, 2007). Overall, our findings suggest that a strong autonomic response to the conflict may serve as a marker for intense emotional ups and downs in daily life and indicate risk for various forms of psychopathology during the transition to adolescence.
We also observed two distinct interactions. First, youth with lower baseline RSA and greater RSA withdrawal to parent-child conflict reported extreme variability in the intensity of complex emotion (shame, guilt, loneliness, emptiness) in daily life. This atypical pattern of RSA responding was one of the two profiles previously identified as a marker of depression risk (Hinnant & El-Sheikh, 2009; Yaroslavsky et al., 2013, 2014). The resemblance of our complex emotion results to findings in depression risk is not surprising, given that dysregulation of emotions like shame, guilt, and loneliness has been linked to internalizing disorders (American Psychiatric Association, 2013). This combination of low tonic RSA and excessive RSA withdrawal may have a synergistic impact on cardiac arousal that functions to deplete regulatory capacity, with vagal control over heart rate both chronically low and further removed under stress. Reduced tonic RSA is associated with increased sympathetic arousal (Porges, 2007; Porges, Doussard-Roosevelt, & Maiti, 1994), and excessive RSA reactivity to conflict has been linked with sustained attention to emotional stimuli (Woody et al., 2019); together, these processes might exaggerate the potency of emotional stimuli and impede the ability to access more adaptive executive resources.
The second interaction found higher baseline RSA and greater RSA withdrawal to parent-child conflict to predict dysregulated behavior (i.e. verbal and physical reactive aggression) in daily life, suggesting this profile increases risk for extreme behavioral expressions of dysregulation. This is consistent with research linking high resting RSA and excessive RSA reactivity to reactive forms of aggression (Zhang & Gao, 2015), and suggests that suddenly removing vagal control from a system ‘at rest’ might signal the presence of threat. This is in line with theoretical conceptualizations of reactive aggression as a threat response that occurs at the height of a freeze-flight-fight defense cascade (Gray, 1987). While this finding appears to stand in contrast to literature linking low tonic RSA to psychopathology (Beauchaine et al., 2019; Fanti et al., 2019), it echoes the work demonstrating a robust link between low resting heart rate and aggression across development (Jennings, Pardini, & Matthews, 2017; Ortiz & Raine, 2004). Although we failed to find a main effect of baseline RSA on dysregulation in daily life, the high inverse correlation between heart rate and RSA suggests future work may seek to disentangle these associations. Taken together, a nuanced picture emerges, whereby youth with the low-baseline-plus-excessive-withdrawal combination experienced more intense (complex) emotional upheavals, an endophenotype that is more consistent with internalizing pathology. By contrast, youth characterized by high-baseline-plus-excessive-withdrawal were more likely to display extreme behavioral dysregulation like verbal and physical reactive aggression, an endophenotype more consistent with externalizing pathology.6 Combined findings suggest that, among disordered youth, baseline RSA may be neither universally ‘adaptive’ or ‘maladaptive’ on its own, and instead underscore the importance of also assessing one’s physiological response to stress. Along these lines, youth with greater RSA withdrawal to conflict showed extreme fluctuations of basic emotions, an endophenotype shared across internalizing and externalizing pathologies. This echoesresearch suggesting that high rates of comorbidity among internalizing andexternalizing disorders are associated with greater RSA withdrawal than either disorder alone (Calkins, Graziano, & Keane, 2007; Pang & Beauchaine, 2013). Taken together, these findings highlight the importance of continuing to examine the within individual interplay of tonic and phasic RSA among transdiagnostic samples of youth.
Findings from the current study should be considered within the context of several limitations. First, the current study used a transdiagnostic sample of clinically referred youth, limiting our ability to make any definitive conclusions about the extent to which the physiological profiles identified in the current sample generalize to community samples of typically developing youth. While this reflects our primary aim to examine associations between physiological profiles and transdiagnostic indicators of psychopathology as they manifest in daily life and echoes research highlighting the importance of assessing these association within clinical populations (see Graziano & Derefinko, 2013), future inclusion of a comparison group subjected to the same experimental tasks would be useful. Second, while several important covariates (i.e. age, gender, minority status, stimulant medication use) were included in the model, our study was not adequately powered to examine potential moderation and future work may assess how these factors influence findings. Additionally, while our assessment of peripheral physiology expands on previous laboratory-based assessments (i.e. passive viewing of emotionally evocative stimuli) by utilizing dyadic interactions, we recognize this approach limits internal validity and acknowledge the potential benefits of utilizing standardized methods across studies (Davis et al., 2020). To balance the need for internal and external validity we standardized many aspects of the interaction. First, vanilla baselines were comprised of uniform tasks (e.g. reading, listening, thinking) in attempt to provide a more stable and reliable comparison condition (Jennings et al., 1992), and allow a more precise estimate of physiological reactivity during the experimental task of interest. Second, discussion topics were selected from a standard list based on frequency and severity ratings, and the length of discussion was consistent across dyads; however, dyadic scripts were not standardized to promote spontaneously conversation, maximizing ecological validity. Finally, our physiological assessment did not explicitly measure respiration; thus, we cannot determine whether respiration influenced our findings. Additionally, although we focused on RSA as an indicator of parasympathetic function, we understand that other autonomic influences are likely at play. Future studies may seek to investigate alternative physiological indices (i.e. impedance cardiography) in isolation and in conjunction with RSA to more comprehensively examine autonomic processes and their relationship to psychopathology.
The current study broadens our understanding of RSA as a biomarker for dysregulated emotion and behavior. Specifically, results point to excessive RSA withdrawal to conflict as a risk factor for extreme fluctuations in basic emotions like anger, sadness, and anxiety in daily life. Furthermore, findings bridge a gap in the literature by examining the interaction between baseline RSA and RSA reactivity in a transdiagnostic sample of pre-adolescent youth. Results highlight two unique physiological profiles, both characterized by excessive RSA withdrawal to conflict, that increase risk for dysregulated complex emotion and dysregulated behavior in daily life. These physiological profiles were identified in clinically referred youth and suggest that intervention strategies targeting reactivity to stress, specifically interpersonal conflict, may be particularly useful in helping to reduce symptoms associated with dysregulated emotion and behavior in this population. Finally, our findings highlight the potential importance of assessment and intervention during the critical transition to adolescence, a vulnerable developmental period before severe forms of psychopathology become entrenched.
Acknowledgements.
We are grateful to all the families who took part in this study, and to the MoodY study team, which includes interviewers and their supervisors, data managers, student workers, and volunteers. This study was supported by grants awarded to Dr. Stephanie Stepp and Dr. Amy Byrd from the National Institute on Mental Health (R01 MH101088, F32 MH110077). Additional funding from the National Institute of Health also supported this work (T32 MH018951, K01 MH109859).
Footnotes
Conflict of interest. None.
This bandwidth was selected based on initial inspection of the data to accommodate the nontrivial number of participants (n = 53 adolescents, 32.7%) whose peak respiratory frequency during one or more tasks fell at or above 0.40 Hz (the more typical upper limit of the high frequency respiratory band).
A total of eight youths were excluded due to unusable or unacceptable RSA data. Five youth were lost to equipment failure, and one youth was excluded from analyses due to a pronounced arrhythmia that resulted in an unreliable RSA estimate. Two additional youth were excluded because their RSA values were >3 standard deviations below the mean.
In accordance with previous literature, both baseline RSA and RSA reactivity were included as independent predictors in our model. This allowed us to evaluate the contribution of within-individual change in RSA (i.e. reactivity) over and above baseline RSA (and vice versa) on outcomes of interest.
Because our model included a latent interaction, numerical integration was required for estimation. χ2 and related fit statistics are only available when means, variances, and covariances are sufficient for model estimation, which is not the case with numerical integration. Thus, χ2 and related fit statistics cannot be generated (Muthén & Muthén, 2012).
Given debate about the degree to which RSA is truly a product of vagal activity versus an artifact of heart rate (HR; e.g. Monfredi et al., 2014), and recent research highlighting the potential nontrivial implications of examining RSA in isolation (i.e. without accounting for HR), we followed state of the art recommendations by de Geus, Gianaros, Brindle, Jennings and Berntson (2019). Specifically, all models were re-run using: (1) HR instead of RSA; (2) HR as a covariate; and (3) RSA adjusted for HR. Regarding (1), there were no significant associations with dysregulated emotion or behavior in daily life; regarding (2 and 3), results mirrored those reported above, suggesting that the present findings can be interpreted as effects of relatively unconfounded, or largely vagally-mediated influences on RSA.
Supplemental analyses were conducted to clarify whether our intermediate behavioral phenotypes (i.e. dysregulated emotion and behavior in daily life) were differentially associated with increased risk for specific dimensions of psychopathology. Specifically, we examined associations between daily reports of dysregulated emotion and behavior, and indices of internalizing and externalizing symptomatology using child- and parent-report on Achenbach’s Youth Self-Report and Child Behavior Checklist (Achenbach & Rescorla, 2001) while controlling for covariates. Dysregulated complex emotion was uniquely associated with higher internalizing symptoms (β = 0.366 (0.117), p = 0.002), while dysregulated behavior was uniquely related to externalizing symptoms (β = 0.312 (0.102), p = 0.003), in line with our interpretation of the interaction findings.
References
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA school-age forms & profiles Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- American Psychiatric Association. (2013). The diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author. [Google Scholar]
- Beauchaine TP (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13(2), 183–214. doi: 10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, & Bell ZE. (2019). Respiratory sinus arrhythmia as a transdiagnostic biomarker of emotion dysregulation. In Beauchaine TP, & Crowell SE (Eds.), The Oxford handbook of emotion dysregulation. New York: Oxford University Press. [Google Scholar]
- Beauchaine TP, Bell Z, Knapton E, McDonough-Caplan H, Shader T, & Zisner A (2019). Respiratory sinus arrhythmia reactivity across empirically based structural dimensions of psychopathology: A meta-analysis. Psychophysiology, 56(5), e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, & Thayer JF (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2), 338–350. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1994). Autonomic cardiac control. I. Estimation and validation from pharmacological blockades. Psychophysiology, 31(6), 572–585. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Thomas Bigger J Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, … Stone PH (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, & Keane SP (2007). Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology, 74(2), 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card N, & Little TD (2006). Proactive and reactive aggression in childhood and adolescence: A meta-analysis of differential relations with psychosocial adjustment. International Journal of Behavioral Development, 30, 466–480. doi: 10.1111/j.1467-8624.2008.01184.x. [DOI] [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8, 597–600. doi: 10.1017/s0954579400007318. [DOI] [Google Scholar]
- Clark LA, & Watson D (1995). Constructing validity: Basic issues in objective scale development. Psychological Assessment 7(3), 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Morris AS, Harrist AW, Larzelere RE, Criss MM, & Houltberg BJ (2015). Adolescent RSA responses during an anger discussion task: Relations to emotion regulation and adjustment. Emotion (Washington, D.C.), 15(3), 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EL, Brooker RJ, & Kahle S (2020). Considering context in the developmental psychobiology of self-regulation. Developmental Psychobiology, 62 (4), 423–435. [DOI] [PubMed] [Google Scholar]
- de Geus EJ, Gianaros PJ, Brindle RC, Jennings JR, & Berntson GG (2019). Should heart rate variability be “corrected” for heart rate. Biological, quantitative, and interpretive considerations. Psychophysiology, 56(2), e13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti KA, Eisenbarth H, Goble P, Demetriou C, Kyranides MN, Goodwin D, … Cortese S (2019). Psychophysiological activity and reactivity in children and adolescents with conduct problems: A systematic review and meta-analysis. Neuroscience Biobehavioral Reviews, 100, 98–107. [DOI] [PubMed] [Google Scholar]
- Fraley C (2018). Probing interactions in differential susceptibility research. Retrieved from https://www.yourpersonality.net/interaction/ros3.pl.
- Frijda N (1986). The emotions. Cambridge University Press [Google Scholar]
- Granic I, Hollenstein T, Dishion TJ, & Patterson GR (2003). Longitudinal analysis of flexibility and reorganization in early adolescence: A dynamic systems study of family interactions. Developmental Psychology, 39(3), 606–617. Retrieved from 12760527. [DOI] [PubMed] [Google Scholar]
- Gray JA (1987). The psychology of fear and stress. Cambridge, England: Cambridge University Press. [Google Scholar]
- Graziano P, & Derefinko K (2013). Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology, 94(1), 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnant JB, & El-Sheikh M (2009). Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. Journal of Abnormal Child Psychology, 37(8), 1049. [DOI] [PubMed] [Google Scholar]
- Houben M, Van Den Noortgate W, & Kuppens P (2015). The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychological Bulletin, 141(4), 901–930. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, & Johnson P (1992). Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology, 29(6), 742–750. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Pardini DA, & Matthews KA (2017). Heart rate, health, and hurtful behavior. Psychophysiology, 54(3), 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE (2003). Psychotherapy for children and adolescents. Annual Review of Psychology, 54, 253–276. Retrieved from 12185210. [DOI] [PubMed] [Google Scholar]
- Linehan MM (1993). Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford press. [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, Mennin DS, & Nolen-Hoeksema S (2011). Emotion dysregulation and adolescent psychopathology: A prospective study. Behaviour Research and Therapy, 49(9), 544–554. doi: 10.1016/j.brat.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfredi O, Lyashkov AE, Johnsen A-B, Inada S, Schneider H, Wang R, … Lakatta EG (2014). Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension, 64(6), 1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey LC (2007). Personality assessment inventory (PAI). Psychological Assessment Resources. [Google Scholar]
- Muthén BO, & Muthén LK (2012). Mplus user’s guide (7th ed.). Los Angeles, CA: Muthen and Muthen. [Google Scholar]
- Ortiz J, & Raine A (2004). Heart rate level and antisocial behavior in children and adolescents: A meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 43(2), 154–162. [DOI] [PubMed] [Google Scholar]
- Ortony A, & Turner TJ (1990). What’s basic about basic emotions?. Psychological Review, 97(3), 315. [DOI] [PubMed] [Google Scholar]
- Pang KC, & Beauchaine TP (2013). Longitudinal patterns of autonomic nervous system responding to emotion evocation among children with conduct problems and/or depression. Developmental Psychobiology, 55(7), 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagalperspective. BiologicalPsychology, 74(2), 116–143. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, & Maiti AK (1994). Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development, 59(2–3), 167–186. [PubMed] [Google Scholar]
- Schaeffer CM, Petras H, Ialongo N, Poduska J, & Kellam S (2003). Modeling growth in boys’ aggressive behavior across elementary school: Links to later criminal involvement, conduct disorder, and antisocial personality disorder. Developmental Psychology, 39(6), 1020–1035. Retrieved from 14584982. [DOI] [PubMed] [Google Scholar]
- Scott LN, Victor SE, Kaufman EA, Beeney JE, Byrd AL, Vine V, … Stepp SD (2020). Affective dynamics across internalizing and externalizing dimensions of psychopathology. Clinical Psychological Science, 8(3), 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2009). Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21(1), 87–97. doi: 10.1017/s0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, & Morris AS (2001). Adolescent development. Journal of Cognitive Education and Psychology, 2(1), 55–87. doi: 10.1891/194589501787383444. [DOI] [Google Scholar]
- Trull TJ, Solhan MB, Tragesser SL, Jahng S, Wood PK, Piasecki TM, & Watson D (2008). Affective instability: Measuring a core feature of borderline personality disorder with ecological momentary assessment. Journal of Abnormal Psychology, 117(3), 647. [DOI] [PubMed] [Google Scholar]
- Woody ML, James K, Foster CE, Owens M, Feurer C, Kudinova AY, & Gibb BE (2019). Children’s sustained attention to emotional facial expressions and their autonomic nervous system reactivity during parent-child interactions. Biological Psychology, 142, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Bylsma LM, Rottenberg J, & Kovacs M (2013). Combinations of resting RSA and RSA reactivity impact maladaptive mood repair and depression symptoms. Biological Psychology, 94(2), 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, & Kovacs M (2014). Atypical patterns of respiratory sinus arrhythmia index an endophenotype for depression. Development and Psychopathology, 26(4pt2), 1337–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, & Gao Y (2015). Interactive effects of social adversity and respiratory sinus arrhythmia activity on reactive and proactive aggression. Psychophysiology, 52(10), 1343–1350. [DOI] [PubMed] [Google Scholar]


