Abstract
Background:
In patients stabilized during hospitalization for acute decompensated heart failure (ADHF), initiation of sacubitril/valsartan compared with enalapril decreased the risk of cardiovascular death or rehospitalization for heart failure (HF) without increasing the risk of adverse events. It is unknown whether potentially high-risk subpopulations have a similar risk-benefit profile.
Methods:
PIONEER-HF was a multicenter, randomized, double-blind trial of in-hospital initiation of sacubitril/valsartan (n=440) vs. enalapril (n=441) in patients stabilized during hospitalization for ADHF. The composite of cardiovascular death or rehospitalization for HF was adjudicated. Safety outcomes included worsening renal function, symptomatic hypotension, and hyperkalemia. We evaluated heterogeneity in the effect of sacubitril/valsartan on these efficacy and safety outcomes in selected subgroups of clinical concern: patients with baseline SBP ≤118 mmHg (median) (n=448), baseline NT-proBNP >2701 pg/ml (median) (n=395), eGFR <60 ml/min/1.73 m2 (n=455), ≥1 additional hospitalization for HF within the prior year (n=343), admission to the ICU during the index hospitalization (n=96), inotrope use during the index hospitalization (n=68), and severe congestion (n=219).
Results:
The relative risk reduction in cardiovascular death or rehospitalization for HF with sacubitril/valsartan vs. enalapril was consistent across all high-risk subgroups (p-interaction=NS for each). The risks of worsening renal function, symptomatic hypotension, and hyperkalemia with sacubitril/valsartan vs. enalapril were also consistent in each high- vs. low-risk subgroup (p-interaction=NS for each).
Conclusions:
In high-risk subpopulations admitted for ADHF, treatment with sacubitril/valsartan after initial stabilization conferred a consistent reduction in cardiovascular death or rehospitalization for HF and was well-tolerated.
Introduction
Patients who are hospitalized for acute decompensated heart failure (ADHF) are at high risk for poor outcomes, including recurrent hospitalizations and death.1 Moreover, hospitalized heart failure (HF) patients may be more susceptible to therapy-related complications due to active modulation of diuretic therapy, fluctuating renal function, and concomitant dose adjustment of neurohormonal antagonists.2
Despite these potential risks, the PIONEER-HF trial (Comparison of Sacubitril/Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode, n=881) demonstrated that initiation of sacubitril/valsartan in patients with heart failure with reduced ejection fraction (HFrEF) who had achieved hemodynamic stability during hospitalization for ADHF resulted in a greater reduction in N-terminal pro-B type natriuretic peptide (NT-proBNP) as compared with enalapril.3 Furthermore, in an exploratory analysis, sacubitril/valsartan significantly reduced the clinical composite outcome of cardiovascular death or rehospitalization for HF through 8 weeks (hazard ratio 0.58, 95% confidence interval 0.39-0.87).4 Moreover, the efficacy and safety of sacubitril/valsartan appeared consistent irrespective of the maximum dose achieved.5
Owing to the trial inclusion criteria, PIONEER-HF enrolled a relatively sick population of patients with ADHF; nevertheless, there was a spectrum of clinical HF severity, and thus a spectrum of risk for the key efficacy and safety outcomes. Given uncertainty in the risk-benefit profile of in-hospital initiation of sacubitril/valsartan in patients at higher risk for complications, we assessed outcomes in selected subpopulations of clinical concern in PIONEER-HF.
Methods
Study Population
The study design and primary results of the PIONEER-HF trial have been previously published.3 In brief, PIONEER-HF trial was a prospective, multicenter, randomized, double-blind trial comparing the in-hospital initiation of sacubitril/valsartan vs. enalapril in patients with HFrEF who had achieved hemodynamic stability during hospitalization for ADHF (n=881).3 The key eligibility criteria included signs and symptoms of HF, left ventricular ejection fraction [LVEF] of 40% or less, and an NT-proBNP concentration ≥1600 pg/ml or B-type natriuretic peptide (BNP) concentration ≥400 pg/ml. Patients were enrolled between 24 hours and 10 days following initial presentation and were required to be hemodynamically stable, including a systolic blood pressure (SBP) ≥100 mmHg for at least 6 hours, with no increase in intravenous diuretic dosing or use of intravenous vasodilators during the preceding 6 hours, and no intravenous inotropes during the preceding 24 hours. All patients enrolled in PIONEER-HF signed written informed consent. The protocol was approved by institutional review boards at all participating sites. We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for further discussions.
Subgroups
Three exploratory subgroups were defined in the primary analysis plan: (1) baseline SBP ≤118 mmHg (median) (n=448); (2) NT-proBNP concentration >2701 pg/ml at randomization (median) (n=395); and (3) estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 (n=455) at randomization.3 We defined four additional exploratory subgroups of patients at potentially high-risk for complications: (1) ≥1 additional hospitalization for HF within the prior year (n=343), (2) admission to the ICU during the index hospitalization (n=96), (3) use of inotropes during the index hospitalization (n=68), and (4) more severe congestion at presentation as determined by a Congestion Score ≥4 (n= 219). The components of the Congestion Score have been previously described (Supplemental Methods).6
Safety and Efficacy Outcomes
The efficacy outcome selected for this analysis was the composite of cardiovascular death or rehospitalization for HF. This outcome was adjudicated by an independent and blinded Clinical Endpoint Committee following completion of the trial.4 The high-risk subgroup analyses of time-averaged proportional change in NT-proBNP concentration from baseline through weeks 4 and 8 (primary efficacy outcome in the PIONEER-HF trial) have been reported previously.3
The key safety outcomes for this analysis were worsening renal function (an increase in serum creatinine of at least 0.5 mg/dl and a decrease in eGFR of at least 25%), symptomatic hypotension, and hyperkalemia (serum potassium of at least 5.5 mmol/L). We did not evaluate the incidence of angioedema in high-risk subgroups since only one patient treated with sacubitril/valsartan and six patients treated with enalapril developed angioedema during the 8-week trial follow-up period.
Statistical Analysis
To identify patients at the highest risk for cardiovascular death or rehospitalization for HF, we evaluated the univariate associations between the seven high-risk indicators and the composite efficacy outcome of cardiovascular death or rehospitalization for HF (regardless of treatment group assignment) using Cox proportional hazards regression analysis with the risk indicator as the independent variable. To identify patients at the highest risk for worsening renal function, symptomatic hypotension, and hyperkalemia, we evaluated the univariate associations between the seven high-risk indicators and each of the safety outcomes using logistic regression analysis.
We estimated the Kaplan-Meier (KM) cumulative event rates of cardiovascular death or rehospitalization for HF at 8 weeks in each subgroup. We used Cox regression analysis with a treatment by subgroup interaction term to test for heterogeneity in the efficacy of sacubitril/valsartan vs. enalapril on the outcome of cardiovascular death or rehospitalization for HF in each subgroup. We calculated the incidence of worsening renal function, symptomatic hypotension, and hyperkalemia through 8 weeks in patients treated with sacubitril/valsartan vs. enalapril. We used the Breslow-Day test to assess for heterogeneity in the safety of sacubitril/valsartan vs. enalapril on each safety outcome in each subgroup.
Results
Efficacy of Sacubitril/Valsartan in High-Risk Subgroups
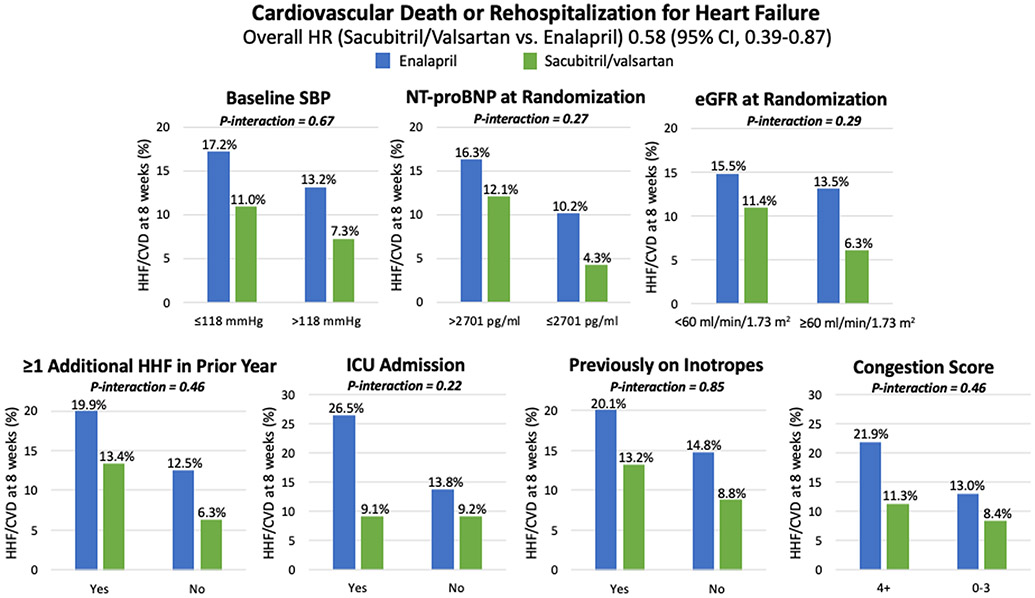
Patients with higher baseline NT-proBNP concentration (i.e., >2701 pg/ml), more severe clinical congestion during the index admission, and those with at least one prior hospitalization for HF during the preceding year had a significantly higher risk of cardiovascular death or rehospitalization for HF at 8 weeks (Supplemental Table 1). In each of these subgroups, as well as in the other preselected subgroups, treatment with sacubitril/valsartan vs. enalapril conferred a consistent reduction in cardiovascular death or rehospitalization for HF (p-interaction = NS) (Figure 1) as well as the individual components (Supplemental Table 2).
Figure 1. Composite of cardiovascular death or rehospitalization for heart failure in high- vs. low-risk subgroups through 8 weeks.
The relative risk reduction in cardiovascular death or rehospitalization for heart failure with sacubitril/valsartan vs. enalapril was consistent in all high-risk subgroups. CI indicates confidence interval; CVD, cardiovascular death; eGFR, estimated glomerular filtration rate; HHF, hospitalization for heart failure; HR, hazard ratio; ICU, intensive care unit; m2, meter-squared; min, minute; ml, milliliter; mmHg, millimeters of mercury; NT-proBNP, N-terminal pro-B type natriuretic peptide; pg, picograms; SBP, systolic blood pressure.
Safety of Sacubitril/Valsartan in High-Risk Subgroups
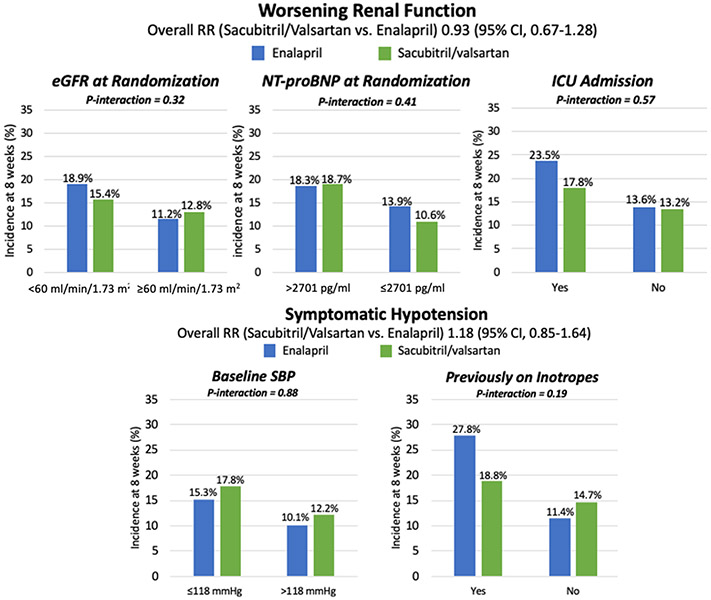
Patients with lower baseline eGFR (i.e., <60 ml/min/1.73 m2), higher baseline NT-proBNP concentration, and those admitted to the ICU during the index hospitalization each had a significantly increased risk of worsening renal function irrespective of treatment group assignment (Supplemental Table 1); however, the risk of worsening renal function with sacubitril/valsartan as compared with enalapril was consistent in all subgroups (Figure 2 and Supplemental Table 3).
Figure 2. Selected relevant safety outcomes in high- vs. low-risk subgroups through 8 weeks.
The rates of worsening renal function were consistent with sacubitril/valsartan vs. enalapril in the high-risk subgroups of patients with lower baseline eGFR, higher NT-proBNP, and those admitted to the ICU during the index hospitalization. The rates of symptomatic hypotension were consistent with sacubitril/valsartan vs. enalapril in the high-risk subgroups of patients with low baseline SBP and those on inotropes during the index hospitalization. CI indicates confidence interval; eGFR, estimated glomerular filtration rate; HF, heart failure; ICU, intensive care unit; m2, meter-squared; min, minute; ml, milliliter; mmHg, millimeters of mercury; NT-proBNP, N-terminal pro-B type natriuretic peptide; pg, picograms; RR, risk ratio; SBP, systolic blood pressure.
The overall risk of symptomatic hypotension was higher in patients with baseline SBP ≤118 mmHg (i.e., median) as compared to those with baseline SBP above the median (16.5% vs. 11.2%; p=0.02), but there was no difference in symptomatic hypotension risk with sacubitril/valsartan among patients with low vs. high SBP (p-interaction=0.88) (Figure 2). In a sensitivity analysis using SBP ≤110 mmHg to define low vs. high SBP, there was again no heterogeneity in symptomatic hypotension with sacubitril/valsartan between low vs. high SBP groups (p-interaction=0.80). Symptomatic hypotension was also more common in patients who required inotropes during the index admission (23.5% vs. 13.0%; p=0.02), but the risk was consistent in patients treated with sacubitril/valsartan vs. enalapril.
Finally, none of the pre-selected high-risk subgroups had a significantly increased risk of hyperkalemia (Supplemental Table 1), and the rates of hyperkalemia were consistent with sacubitril/valsartan vs. enalapril in all subgroups (Supplemental Table 3).
Discussion
Initiating evidence-based therapies for HFrEF can improve outcomes in the high-risk transition period following HF hospitalization. However, questions remain about the efficacy and safety of in-hospital initiation of sacubitril/valsartan in patients at the highest risk for poor outcomes, including therapy-related complications. In this analysis, we found that there was a robust treatment effect and no evidence of diminished tolerability of sacubitril/valsartan, as compared with enalapril, among patients enrolled in PIONEER-HF at potentially higher risk of complications. Therefore, consistent with the overall trial result, these data support the in-hospital initiation of sacubitril/valsartan, including in higher-risk patients with HFrEF who are stabilized during hospitalization for ADHF.
Efficacy of sacubitril/valsartan in the post-hospitalization period
Similar to prior studies, the present analysis confirms the prognostic association of NT-proBNP concentration and frequency of prior hospitalizations for HF as important markers of clinical risk in patients admitted for ADHF.7 In addition, these results add to other work highlighting the prognostic significance of severe clinical congestion in both ambulatory and hospitalized HFrEF patients.6, 8 Since patients with these clinical risk factors are at particularly high risk for cardiovascular death or rehospitalization for HF in the early post-hospitalization period, strategies for mitigating this risk are especially important. Reassuringly, the results from this analysis demonstrate that the risk of cardiovascular death or rehospitalization for HF in these subgroups is modifiable with in-hospital initiation of sacubitril/valsartan. These data therefore reinforce the notion that in-hospital initiation of sacubitril/valsartan can improve outcomes, including in higher-risk patients with HFrEF.
Safety endpoints in patients with acute decompensated heart failure
The results of this analysis also confirm that the risks of treatment-related adverse effects are not uniform across all patients admitted with ADHF. Specifically, patients with lower SBP, lower eGFR, and higher NT-proBNP concentrations, and those who are admitted to the ICU or who require inotropes during the index hospitalization, appear to be more susceptible to certain adverse events, which has important implications for how these patients are monitored both during hospitalization and following discharge. Notably, these data also indicate that inpatient initiation of sacubitril/valsartan, as compared with enalapril, does not increase the risk of worsening renal function, symptomatic hypotension, or hyperkalemia, and thus provide reassurance that sacubitril/valsartan may be safely initiated even in these higher risk patients.
Limitations
Several limitations of these data deserve mention. First, statistical tests of heterogeneity may be underpowered when subgroups are small, which may have limited the ability to identify clinically meaningful differences in the efficacy and safety outcomes between subgroups. For this reason, most of the subgroups were categorized to optimize the symmetry of subgroup size; however, this was not possible for certain subgroups of interest (e.g., ICU admission, inotrope use during the index hospitalization). Second, these results were obtained from a clinical trial population and may not be generalizable to all patients admitted for ADHF. They are therefore most directly relevant to patients who meet the trial inclusion criteria.
Conclusions
Among HFrEF patients stabilized during hospitalization for ADHF who are at potentially higher risk for poor outcomes, including therapy-related complications, there was a robust treatment effect and no evidence of lack of tolerability of sacubitril/valsartan, as compared with enalapril. Therefore, consistent with the overall trial result, these data support the in-hospital initiation of sacubitril/valsartan, including in higher-risk patients with HFrEF who are stabilized during hospitalization for ADHF.
Supplementary Material
What is New?
In the PIONEER-HF trial, patients with higher baseline NT-proBNP concentrations, more severe clinical congestion during the index admission, and those with at least one prior hospitalization for heart failure during the preceding year had an increased risk of cardiovascular death or rehospitalization for heart failure during the transition period following heart failure hospitalization.
There was a robust treatment effect and no evidence of diminished tolerability of sacubitril/valsartan, as compared with enalapril, among patients at potentially higher risk of complications in PIONEER-HF.
What are the Clinical Implications?
These data suggest that in-hospital initiation of sacubitril/valsartan can improve clinical outcomes, with an acceptable safety profile, in higher-risk patients with heart failure with reduced ejection fraction who are stabilized during hospitalization for acute decompensated heart failure.
Acknowledgments
Sources of Funding:
The PIONEER-HF study was supported by Novartis Pharmaceuticals Corp.
Disclosures:
Dr. Berg is supported by Harvard Catalyst KL2/CMeRIT (NIH/NCATS UL 1TR002541) and reports grant support to his institution from AstraZeneca. Dr. Samsky has nothing to disclose. Dr. Velazquez reports grants from Novartis, Amgen, Phillips, and NHLBI/NIH. Dr. Duffy is an employee of Novartis Pharmaceuticals Corp. Dr. Gurmu received grant support from Novartis. For the work under consideration, Dr. Braunwald reports grant support to his institution from Novartis for the conduct of the PIONEER-HF Trial, for serving on the Executive Committee of the PARADISE trial and the Steering Committee of the PARAGLIDE trial, and for participation in an Advisory Board Meeting. For outside the submitted work, Dr. Braunwald reports grants to his institution from Astra Zeneca, Daiichi Sankyo, and Merck; personal fees for consultancies with Amgen, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Morrow reports grants from Abbott Laboratories, Amgen, AstraZeneca, Eisai, GlaxoSmithKline, Medicines Company, Merck, Novartis, Pfizer, Roche Diagnostics, and Takeda. He has received personal fees from Abbott Laboratories, Aralez, AstraZeneca, Bayer Pharma, GlaxoSmithKline, InCarda, Merck, Peloton, and Roche Diagnostics. Dr. DeVore has received research funding from AstraZeneca, Amgen, the American Heart Association, Bayer, Luitpold Pharmaceuticals, Medtronic, the National Heart, Lung, and Blood Institute, PCORI, and Novartis; and has served as a consultant for AstraZeneca, LivaNova, Mardil Medical, Novartis, and Procyrion.
Non-standard Abbreviations and Acronyms
- ADHF
(acute decompensated heart failure)
- BNP
(B-type natriuretic peptide)
- HF
(heart failure)
- HFrEF
(heart failure with reduced ejection fraction)
- ICU
(intensive care unit)
- LVEF
(left ventricular ejection fraction)
- NT-proBNP
(N-terminal pro-B type natriuretic peptide)
- SBP
(systolic blood pressure)
References
- 1.Hollenberg SM, Warner Stevenson L, Ahmad T, Amin VJ, Bozkurt B, Butler J, Davis LL, Drazner MH, Kirkpatrick JN, Peterson PN, Reed BN, Roy CL and Storrow AB. 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966–2011. [DOI] [PubMed] [Google Scholar]
- 2.Heart Failure Society of A, Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR and Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E and Investigators P-H. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 4.Morrow DA, Velazquez EJ, DeVore AD, Desai AS, Duffy CI, Ambrosy AP, Gurmu Y, McCague K, Rocha R and Braunwald E. Clinical Outcomes in Patients With Acute Decompensated Heart Failure Randomly Assigned to Sacubitril/Valsartan or Enalapril in the PIONEER-HF Trial. Circulation. 2019;139:2285–2288. [DOI] [PubMed] [Google Scholar]
- 5.Berg DD, Braunwald E, DeVore AD, Lala A, Pinney SP, Duffy CI, Gurmu Y, Velazquez EJ and Morrow DA. Efficacy and Safety of Sacubitril/Valsartan by Dose Level Achieved in the PIONEER-HF Trial. JACC Heart Fail. 2020;8:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr., Grinfeld L, Udelson JE, Zannad F, Gheorghiade M and Investigators ET. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43. [DOI] [PubMed] [Google Scholar]
- 7.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA, Candesartan in Heart failure: Assessment of Reduction in M and morbidity I. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. [DOI] [PubMed] [Google Scholar]
- 8.Selvaraj S, Claggett B, Pozzi A, McMurray JJV, Jhund PS, Packer M, Desai AS, Lewis EF, Vaduganathan M, Lefkowitz MP, Rouleau JL, Shi VC, Zile MR, Swedberg K and Solomon SD. Prognostic Implications of Congestion on Physical Examination Among Contemporary Patients With Heart Failure and Reduced Ejection Fraction: PARADIGM-HF. Circulation. 2019;140:1369–1379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.