Abstract
Introduction and Objectives
As of January 2021, over 88 million people have been infected with COVID-19. Almost two million people have died of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A high SOFA score and a D-Dimer >1 µg/mL identifies patients with high risk of mortality. High lactate dehydrogenase (LDH) levels on admission are associated with severity and mortality. Different degrees of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) abnormalities have been reported in these patients, its association with a mortality risk remains controversial. The aim of this study was to explore the correlation between LDH and in-hospital mortality in Mexican patients admitted with COVID-19.
Materials & Methods
We performed a retrospective multi-centre cohort study with 377 hospitalized patients with confirmed SARS-CoV-2 in three centres in Mexico City, Mexico, who were ≥18 years old and died or were discharged between April 1 and May 31, 2020.
Results
A total of 377 patients were evaluated, 298 (79.1%) patients were discharged, and 79 (20.9%) patients died during hospitalization. Non-survivors were older, with a median age of 46.7 ± 25.7 years old, most patients were male. An ALT > 61 U/l (OR 3.45, 95% CI 1.27−9.37; p = 0.015), C-reactive protein (CRP) > 231 mg/l (OR 4.71, 95% CI 2.35−9.46; p = 0.000), LDH > 561 U/l (OR 3.03, 95% CI 1.40−6.55; p = 0.005) were associated with higher odds for in-hospital death.
Conclusions
Our results indicate that higher levels of LDH, CRP, and ALT are associated with higher in-hospital mortality risk in Mexican patients admitted with COVID-19.
Keywords: Severe acute respiratory syndrome coronavirus 2, Biochemical markers, Retrospective studies, Mortality determinants, Pandemics.
1. Introduction
In December 2019 a new human infecting betacoronavirus was described in China [1], by March 2020 coronavirus disease 2019 (COVID-19) had infected people from over a hundred countries and was deemed a pandemic [2]. As of January 2021, over 88 million people have been infected with COVID-19, and almost two million people have died of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In Mexico, over a million people have been infected, and over 131,000 deaths have been reported. In the last year, many attempts have been made to identify risk factors for mortality.
A retrospective cohort study has found that older age, a high sequential organ failure assessment (SOFA) score and a D-Dimer >1 µg/mL identifies patients with high risk of mortality [3]. Another study has found that high cytokine levels (IL-2R, IL-6, IL-10, and TNF-a), and high lactate dehydrogenase (LDH) levels on admission were associated with the severity of COVID-19 infection [4]. Neutrophilia, organ and coagulation dysfunction (as indicated by high levels of lactate dehydrogenase), have also been associated with mortality in patients with severe acute respiratory syndrome (SARS) [2].
The global prevalence of metabolic syndrome is hard to measure, however it has been estimated to be around 25% [5]. In Mexico, a recent meta-analysis reported a prevalence of 41% of metabolic syndrome (95% CI 0.34−0.47) [6], the reported prevalence of obesity (BMI ≥ 30 kg/m2) in the adult population of 2018 was 36.1% [7], the prevalence of type 2 diabetes mellitus was 10.6% and of hypertension 18.4% [8]. It has been found that SARS-CoV-2 affects older males with comorbidities [9], however an association with higher mortality risk remains controversial [3].
In a Chinese cohort, 43% of the patients with COVID-19 had different degrees of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) abnormalities [9]. A large cohort in China reported that 50% of patients who died of SARS-CoV-2 had an ALT > 40 U/l, compared with 20.1% of survivors [10]. Increased levels of serum lactate dehydrogenase indicate tissue injury, necrosis, and hypoxia [11]. High LDH levels have been found to be an important prognostic factor [11], and a predictor for mortality in patients with sepsis [12].
Risk factors for severity and mortality of COVID-19 infection have previously been reported, nevertheless, most of these studies are single-centre and based on relatively small samples. The aim of this study was to explore the correlation between serum biomarkers and in-hospital mortality in Mexican patients admitted with COVID-19.
2. Methods
We performed a retrospective multi-centre cohort study with 377 hospitalized patients with COVID-19 infection in three centres in Mexico City, Mexico (Medica Sur Clinic and Foundation, Hospital General de Mexico and Hospital Central Militar Mexico) admitted from April 1 and May 31, 2020. To confirm SARS-CoV-2 infection, throat swab samples were taken from all patients upon admission and tested using real-time reverse transcriptase-polymerase chain reaction (PCR). The general sociodemographic data including age, sex, and chronic diseases (diabetes mellitus, hypertension, history of cardiovascular disease and chronic obstructive pulmonary disease) and biochemical data, including haemoglobin, leukocytes, platelets, creatinine, total bilirubin, alkaline phosphatase, ALT, AST, C-reactive protein (CRP), international normalized ratio (INR), albumin, sodium and LDH were extracted from medical records using a standardised data collection.
We included patients with a confirmed diagnosis of SARS-CoV-2 infection who were ≥18 years old and died or were discharged between April 1 and May 31, 2020. We excluded patients with incomplete sociodemographic and biochemical data. Treatment varied from one centre to the other depending on available resources and the criteria of each physician.
2.1. Statistical analysis
Sociodemographic data are presented using numbers (percentages) or median (interquartile range [IQR]) as appropriate. Categorical variables were compared using Chi Square Test (or Fisher’s exact test when non-parametric). Continuous variables were compared using Student’s T test (or Mann Whitney’s U test when non-parametric). We subjected all variables to univariate analyses to select patients with p < 0.05 for multivariate analysis. We performed a multivariate binary logistical regression to identify factors independently associated with the primary outcome. Two-tailed p values <0.05 were considered statistically significant. Data were analysed using IBM SPSS Statistics software (version 23.0; SPSS Inc., Chicago, IL).
The present study complies with the basic principles of human research following the Helsinki Declaration of the Medical Association (Helsinki Finland 1964, last amendment at the 52nd General Assembly, in Fortaleza, Brazil, October 2013). The data collected was handled confidentially.
3. Results
A total of 377 patients were included in the final analysis, 298 (79.1%) patients were discharged, and 79 (20.9%) patients died during hospitalization (Fig. 1 ). Non-survivors were older, with a median age of 46.7 ± 25.7 years old, and most patients were male (Table 1 ). The most frequent comorbidities were diabetes mellitus and hypertension; cardiovascular disease, chronic obstructive pulmonary disease (COPD) and the need of dialysis were more frequent in the non-survivors group (Table 1).
Fig. 1.
Flowchart of study population.
Table 1.
Demographic characteristics and laboratory findings on admission.
| Variable | Survivors (n = 298) |
Non-survivors (n = 79) |
p value |
|---|---|---|---|
| Age, mean (range), years | 41.8 (±22.7) | 46.72 (±25.7) | 0.016 |
| Male, n (%) | 215 (72.1%) | 62 (78.5%) | 0.257 |
| Diabetes Mellitus, n (%) | 64 (21.5%) | 24 (30.4%) | 0.096 |
| Hypertension, n (%) | 56 (18.8%) | 20 (25.3%) | 0.199 |
| Cardiovascular disease, n (%) | 6 (2%) | 9 (11.4%) | 0.000 |
| COPD, n (%) | 8 (2.7%) | 8 (10.1%) | 0.013 |
| Dialysis, n (%) | 10 (3.4%) | 8 (10.1%) | 0.012 |
| Haemoglobin g/dl, median (IQR) | 14.8 (13.3−15.8) | 14 (12.2−15.5) | 0.022 |
| Leukocytes x103/µL, median (IQR) | 7.3 (5.4−10.8) | 9.4 (8.2−13.1) | 0.000 |
| Platelets x103/µL, median (IQR) | 219 (171.7−301.5) | 243 (180−310) | 0.454 |
| Creatinine mg/dl, median (IQR) | 0.86 (0.7−1) | 0.95 (0.7−1.7) | 0.005 |
| Total bilirubin mg/dl, median (IQR) | 0.77 (0.51−1) | 0.97 (0.6−2.2) | 0.000 |
| Alkaline phosphatase U/l, median (IQR) | 76 (58−102.5) | 102 (68−124) | 0.001 |
| ALT U/l, median (IQR) | 36 (26−56) | 46 (24−100) | 0.040 |
| AST U/l, median (IQR) | 43.5 (31−61.5) | 51 (36−142) | 0.000 |
| CRP mg/dl, median (IQR) | 121 (48−197) | 232 (126.5−321.5) | 0.000 |
| INR, median (IQR) | 1 (1−1.2) | 1 (1−1.2) | 0.888 |
| Albumin g/dl, median (IQR) | 3.5 (3−3.9) | 3.3 (3−3.7) | 0.283 |
| Sodium mmol/l, median (IQR) | 138 (136−140) | 137 (135−140) | 0.421 |
| LDH U/l, median (IQR) | 380.5 (265.7−523.2) | 536 (419−776) | 0.000 |
| MELD score, median (IQR) | 8 (6−10) | 9 (8−16) | 0.000 |
COPD (Chronic Obstructive Pulmonary Disease), ALT (alanine aminotransferase), AST (aspartate aminotransferase), CRP (C reactive protein), INR (international normalized ratio), LDH (lactate dehydrogenase), MELD (Model of End-Stage Liver Disease).
Laboratory findings on admission are presented in Table 1. In the non-survivors group, haemoglobin levels were lower, and leukocytes, creatinine, total bilirubin, alkaline phosphatase, ALT (alanine aminotransferase), AST (aspartate aminotransferase), CPR (C reactive protein) and LDH (lactate dehydrogenase) levels were higher.
In univariate analysis odds for in-hospital death were higher in patients aged >59 years old, those who needed dialysis during hospitalization, with leukocytes >11.2 × 109/L, creatinine >1.09 mg/dl, total bilirubin >1.11 mg/dl, alkaline phosphatase >111 U/l, ALT > 61 U/l, AST > 69 U/l, CRP > 231 mg/l, LDH > 561 U/l, and a MELD score > 11 on admission (Table 2 ).
Table 2.
Univariate and Multivariate Analysis.
| Variable | Univariate OR (95% CI) |
p value | Multivariate OR (95% CI) |
p value |
|---|---|---|---|---|
| Age >59 years old | 1.86 (1.08−3.19) | 0.023 | 1.47 (0.53−3.90) | 0.440 |
| Cardiovascular disease | 0.16 (0.05−0.46) | 0.000 | 0.259 (0.66−1.00) | 0.051 |
| COPD | 0.24 (0.08−0.67) | 0.013 | 0.57 (0.14−2.19) | 0.413 |
| Dialysis | 3.24 (1.23−8.52) | 0.012 | 0.61 (0.13−2.88) | 0.538 |
| Centre | 0.30 (0.15−0.61) | 0.000 | 0.455 (0.09−2.14) | 0.320 |
| Haemoglobin <15.8 g/dl | 0.67 (0.362−1.29) | 0.207 | – | – |
| Leukocytes >11.2 × 109/L | 2.00 (1.17−3.43) | 0.010 | 1.00 (0.47−2.13) | 0.986 |
| Creatinine >1.09 mg/dl | 2.96 (1.74−5.04) | 0.000 | 1.21 (0.49−2.99) | 0.679 |
| Total bilirubin >1.11 mg/dl | 2.75 (1.62−4.69) | 0.000 | 2.08 (0.84−5.14) | 0.110 |
| Alkaline phosphatase >111 U/l | 2.19 (1.28−3.75) | 0.003 | 0.86 (0.31−2.37) | 0.779 |
| ALT >61 U/l | 2.40 (1.41−4.09) | 0.001 | 3.45 (1.27−9.37) | 0.015 |
| AST >69 U/l | 2.59 (1.52−4.39) | 0.000 | 0.85 (0.32−2.30) | 0.763 |
| CRP >231 mg/l | 5.77 (3.24−10.26) | 0.000 | 4.71 (2.35−9.46) | 0.000 |
| LDH > 561U/l | 3.54 (2.08−6.00) | 0.000 | 3.03 (1.40−6.55) | 0.005 |
| MELD > 11 | 3.75 (2.11−6.64) | 0.000 | 1.79 (0.73−4.37) | 0.199 |
COPD (Chronic Obstructive Pulmonary Disease), ALT (alanine aminotransferase), AST (aspartate aminotransferase), CRP (C reactive protein), LDH (lactate dehydrogenase), MELD (Model of End-Stage Liver Disease).
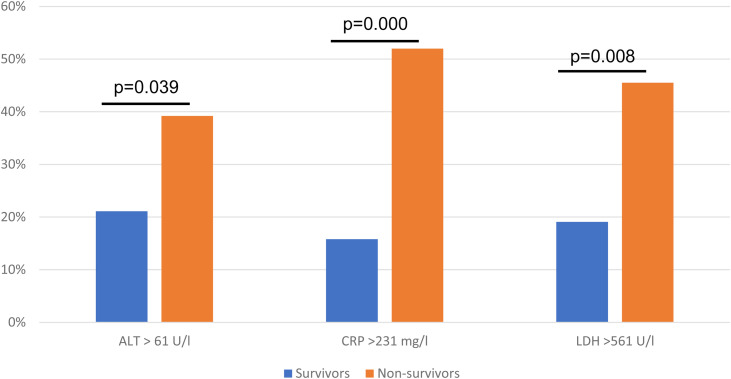
In the multivariate logistic regression model an ALT > 61 U/l (OR 3.45, 95% CI 1.27−9.37; p = 0.015), CRP > 231 mg/l (OR 4.71, 95% CI 2.35−9.46; p = 0.000), LDH > 561 U/l (OR 3.03, 95% CI 1.40−6.55; p = 0.005) were associated with higher odd for in-hospital death, the results were adjusted for study centre (Table 2, Fig. 2 ).
Fig. 2.
Difference in proportion of patients with alterations in ALT (alanine aminotransferase), CRP (C reactive protein), and LDH (lactate dehydrogenase) between survivors and non-survivors.
4. Conclusions
This retrospective cohort study of 377 patients identified several risk factors for mortality in patients hospitalized with diagnosis of COVID-19, ALT greater than 61 U/l, CRP greater than 231 mg/l, and LDH greater than 561 U/l on admission were associated with higher odds of in-hospital mortality.
In the 2003 epidemic of severe acute respiratory syndrome coronavirus (SARS-CoV), a study found that elevated LDH, as an indicator of organ damage, was elevated in 58% of the patients at admission [13]. Elevated neutrophil count, D-Dimer, BUN, creatinine and LDH have been found to be a common occurrence during hospitalization in non-survivor patients with SARS-CoV-2 [14].
LDH is an ubiquitous enzyme in the human tissue that serves as the last step of aerobic glycolysis by catalysing the conversion of pyruvate to lactate reversibly [12]. In septic shock, inadequate whole-body oxygen delivery results in a reduced ability of tissues to extract oxygen to less than 50% so that lactic acid formation increases, the endothelial inflammatory response also results in microcirculatory dysfunction that results in microregional oxygen delivery not being sufficient to match demand [15]. Higher LDH levels have been previously found in non-survivor patients with sepsis [11], with an area under the curve for prediction for mortality of 0.783 [12], and for severity of 0.799 [16].
In murine models with acute liver failure, it has been found that increased LDH concentrations in nuclear fractions in the liver are associated with increased histone H3 hyper-acetylation, which induces the expression of genes related to damage response [17]. It has also been found that the production of LDH by hepatocytes is markedly increase at the acute phase of acute liver failure [18].
Our findings agree with other studies where a LDH higher than 245 U/l (OR 45.5, 95% CI 6.10–338.44; p = 0.0002) was associated with higher in-hospital death [3].
Whether COVID-19 directly causes liver injury, or the changes are secondary to systemic inflammation remains to be demonstrated [19], the most frequent abnormal liver biochemical marker is reported to be albumin (39.8%) [20]. Concentrations of ALT have been found to be higher in non-survivors [21], a recent meta-analysis found that ALT is the most commonly elevated liver enzyme among these patients [20], elevations of ALT have been found to be mild (less than 5 times normal) in 2-5% of patients [22]. Nevertheless, the predictive value of abnormal ALT is not completely elucidated, some studies have found an association with progression to severe illness [23] and mortality [20,24,25], while others have not found an association [26,27]. In our study, higher levels of ALT were associated with higher odds for in-hospital mortality.
It must be highlighted that some of the characteristics of COVID-19 related liver injury may be shared with drug-induced liver injury (DILI) and herb-induced liver injury (HILI). However, in these patients where polypharmacy is often present, along with various factors that can cause liver injury, it can become difficult to determine the cause. It has been suggested that the updated 2016 Roussel Uclaf Causality Assessment Method (RUCAM) [28] can be a valuable tool to help associate DILI or HILI as a cause of liver injury in these population [29].
CRP is a pattern recognition molecule, its synthesis by hepatocytes rapidly increases after tissue injury or infection, suggesting a contribution in the host defence [30], it has been suggested to be an indicator of disease progression [31]. A CRP greater than 41.8 mg/l has been found to predict disease severity and severe complications with an area under the curve of 0.858 [32]. CRP levels have also been found to correlate with the diameter of lung lesions (correlation coefficient = 0.873, p < 0.001) and sever presentation (correlation coefficient = 0.734, p < 0.001) [33]. Another study found that high CRP and LDH levels correlate with PaO2/FiO2 values, suggesting a relationship between tissue damage and infective status [34]. A positive relationship between CRP, LDH and AST with both APACHE II and SOFA scores has also been reported [35].
Non-survivors had higher levels of CRP, LDH and ALT, this could probably be a reflection of acute inflammatory injury, reflecting hypoxia and tissue injury due to a strong inflammatory response to infection. Thus, patients with abnormal levels upon arrival should be closely monitored for a prompt intervention and outcome improvement.
In spite of the fact that some studies have found that the presence of comorbidities such as cardiovascular disease and diabetes mellitus are associated with higher odds for mortality [2], the association has been inconsistent [3], and was not found in our study.
One of the main strengths of our study is that it includes patients form three different centres, a private hospital, a public hospital and a military hospital. Another strength is that the sample size is one of the largest published in Mexico.
Limitations of the study are those inherent to retrospective studies. Second, although this was a multicentre study, the cohort consisted only of Mexicans treated in Mexico City, so results cannot be generalized to other populations. Third, only hospitalized patients were included, which may be a selection bias for more severely ill patients. Fourth, we had insufficient data to calculate the risk of severity, intensive care unit (ICU) admission or need of mechanical ventilation.
Abbreviation
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SOFA
sequential organ failure assessment
- SARS
severe acute respiratory syndrome
- BMI
body mass index
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- LDH
lactate dehydrogenase
- PCR
real-time reverse transcriptase-polymerase chain reaction
- CRP
C-reactive protein
- INR
international normalized ratio
- MELD
Model of End-Stage Liver Disease
- COPD
Chronic Obstructive Pulmonary Disease
- SARS-CoV
severe acute respiratory syndrome coronavirus
- APACHE II
acute physiology and chronic health disease classification system II
- ICU
intensive care unit.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet [Internet] 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet [Internet]. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. https://linkinghub.elsevier.com/retrieve/pii/S0140673620305663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y. 2020. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saklayen M.G. The global epidemic of the metabolic syndrome. Curr Hypertens Rep [Internet] 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. http://link.springer.com/10.1007/s11906-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutiérrez-Solis A.L., Datta Banik S., Méndez-González R.M. Prevalence of metabolic syndrome in Mexico: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2018;16(8):395–405. doi: 10.1089/met.2017.0157. [DOI] [PubMed] [Google Scholar]
- 7.Barquera S., Rivera J.A. Obesity in Mexico: rapid epidemiological transition and food industry interference in health policies. Lancet Diabetes Endocrinol. 2020;8(9):746–747. doi: 10.1016/S2213-8587(20)30269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamah-Levy T., Rivera-Dommarco J. Encuesta Nacional de Salud y Nu-trición 2018. Resultados en localidades con menos de 100 000 habitantes. Salud Publica Mex. 2019;61(6):707–708. doi: 10.21149/11013. [DOI] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet [Internet]. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duman A., Akoz A., Kapci M., Ture M., Orun S., Karaman K., et al. Prognostic value of neglected biomarker in sepsis patients with the old and new criteria: predictive role of lactate dehydrogenase. Am J Emerg Med. 2016;34(11):2167–2171. doi: 10.1016/j.ajem.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Lu J., Wei Z., Jiang H., Cheng L., Chen Q., Chen M., et al. Lactate dehydrogenase is associated with 28-day mortality in patients with sepsis: a retrospective observational study. J Surg Res [Internet]. 2018;228(250):314–321. doi: 10.1016/j.jss.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Liu C.L., Lu Y.T., Peng M.J., Chen P.J., Lin R.L., Wu C.L., et al. Clinical and laboratory features of severe acute respiratory syndrome vis-à-vis onset of fever. Chest [Internet]. 2004;126(2):509–517. doi: 10.1378/chest.126.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suetrong B., Walley K.R. Lactic acidosis in sepsis: It’s Not All anaerobic: Implications for diagnosis and management. Chest [Internet]. 2016;149(1):252–261. doi: 10.1378/chest.15-1703. [DOI] [PubMed] [Google Scholar]
- 16.Hu J., Zhou J., Dong F., Tan J., Wang S., Li Z., et al. Combination of serum lactate dehydrogenase and sex is predictive of severe disease in patients with COVID-19. Bull Sch Med Univ Md. 2020;99(42) doi: 10.1097/MD.0000000000022774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferriero R., Nusco E., De Cegli R., Carissimo A., Manco G., et al. Pyruvate dehydrogenase complex and lactate dehydrogenase are targets for therapy of acute liver failure. J Hepatol [Internet]. 2018;69(2):325–335. doi: 10.1016/j.jhep.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotoh K., Kato M., Kohjima M., Tanaka M., Miyazaki M., Nakamura K., et al. Lactate dehydrogenase production in hepatocytes is increased at an early stage of acute liver failure. Exp Ther Med. 2011;2(2):195–199. doi: 10.3892/etm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Méndez-Sánchez N., Valencia-Rodríguez A., Qi X., Yoshida E.M., Romero-Gómez M., George J., et al. What has the COVID-19 pandemic taught us so far? Addressing the problem from a hepatologist’s perspective. J Clin Transl Hepatol. 2020;8(2):109–112. doi: 10.14218/JCTH.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y., Li H., Guo X., Yoshida E.M., Mendez-Sanchez N., Levi Sandri G.B., et al. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14(5):621–637. doi: 10.1007/s12072-020-10074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368(March):1–14. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertolini A., van de Peppel I.P., Bodewes F.A.J.A., Moshage H., Fantin A., et al. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology. 2020;72(5):1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai Q., Huang D., Yu H., Zhu Z., Xia Z., Su Y., et al. COVID-19: abnormal liver function tests. J Hepatol [Internet]. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phipps M.M., Barraza L.H., LaSota E.D., Sobieszczyk M.E., Pereira M.R., Zheng E.X., et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. Cohort. Hepatology. 2020;72(3):807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei F., Liu Y.M., Zhou F., Qin J.J., Zhang P., Zhu L., et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72(2):389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vespa E., Pugliese N., Piovani D., Capogreco A., Danese S., Aghemo A. Liver tests abnormalities in COVID-19: trick or treat? J Hepatol [Internet]. 2020;73(5):1275–1276. doi: 10.1016/j.jhep.2020.05.033. https://linkinghub.elsevier.com/retrieve/pii/S0168827820303524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Zheng L., Liu L., Zhao M., Xiao J., Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40(9):2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 28.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17(1):1–33. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Silva L.A., Simonato L.E., Ramos R.R. Phylogeny and pathogenesis of SARS-CoV-2: a systematic study. J Mod Med Chem [Internet]. 2020;8(1):49–55. https://synergypublishers.com/downloads/jmmcv8a6/ [Google Scholar]
- 30.Black S., Kushner I., Samols D. C-reactive protein. J Biol Chem. 2004;279(47):48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J., et al. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis [Internet]. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu F., Li L., Da Xu M., Wu J., Luo D., Zhu Y.S., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol [Internet]. 2020;127(April):104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect [Internet] 2020;50(4):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poggiali E., Zaino D., Immovilli P., Rovero L., Losi G., Dacrema A., et al. 2020. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier connect, the company’ s public news and information. [Google Scholar]
- 35.Han Y., Zhang H., Mu S., Wei W., Jin C., Tong C., et al. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging (Albany NY). 2020;12(12):11245–11258. doi: 10.18632/aging.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]