Abstract
Purpose
The COVID-19 pandemic has led to over 92 million cases and 1.9 million deaths worldwide since its outbreak. Public health responses have focused on identifying symptomatic individuals to halt spread. However, evidence is accruing that asymptomatic individuals are infectious and contributing to this global pandemic.
Methods
Observational data of 320 index cases and their 1289 positive contacts from the National COVID-19 Database in Bahrain were used to analyze symptoms, infectivity rate and PCR Cycle threshold (Ct) values.
Results
No significant difference (p = 1.0) in proportions of symptomatic (n = 160; 50.0%) and asymptomatic index cases (n = 160; 50.0%) were seen; however, SARS-CoV-2 positive contact cases were predominantly asymptomatic (n = 1127, 87.4%). Individuals aged 0−19 years constituted a larger proportion of positive contact cases (20.8%) than index cases (4.7%; p < 0.001). A total of 22% of the positive contacts were infected by symptomatic male index cases aged between 30−39 years. The total numbers of exposed contacts (p = 0.33), infected contacts (p = 0.81) and hence infectivity rate (p = 0.72) were not different between symptomatic and asymptomatic index cases. PCR Ct values were higher in asymptomatic compared to symptomatic index cases (p < 0.001), and higher in asymptomatic compared to symptomatic positive contacts (p < 0.001). No differences between the infectivity rates of index cases with Ct values <30 and values ≥30 were observed (p = 0.13).
Conclusion
These data reveal that the high asymptomatic incidence of SARS-CoV-2 infection in Bahrain and subsequent positive contacts from an index case were more likely to be asymptomatic, showing the high “silent” risk of transmission and need for comprehensive screening for each positive infection to help halt the ongoing pandemic.
Keywords: COVID-19, Symptomatic, Asymptomatic, SARS-CoV-2, Cycle threshold, Transmission, Bahrain, Public health
Introduction
SARS-CoV-2, the virus causing Coronavirus disease 2019 (COVID-19), has infected more than 92 million people and lead to the death of more than 1.9 million people worldwide since its outbreak in December 2019 (WHO, 2020). The disease has a wide range of presentations, from asymptomatic infection to fever, cough, shortness of breath and the loss of taste and smell. Symptoms normally appear 2–14 days following exposure to the virus and may develop into mild upper respiratory tract infections or progress to severe pneumonia, which can progress to acute respiratory distress, shock, multiorgan failure and death (Huang et al., 2020, Wang et al., 2020).
The virus is thought to mainly be transmitted through person-to-person contact, with evidence that SARS-CoV-2 transmits through the inhalation of large droplets exhaled by infected individuals (WHO, 2021, Anfinrud et al., 2020). Interventions have accordingly been taken to identify, test and isolate infected people, with the aim of containing the spread of the disease. To date, international testing has mostly been carried out on symptomatic patients seeking diagnosis. However, whilst there is increased evidence of asymptomatic infections (Hu et al., 2020, Bai et al., 2020, Zhang et al., 2020, Furukawa et al., 2020, Arons et al., 2020), testing has been prioritized to the more “pressing” symptomatic individuals. This is not surprising as identification COVID-19 was extremely dependent on symptomatic diagnosis early in the pandemic, and management of symptoms is an essential part of treatment. Indeed, both the World Health Organization (WHO) and the Center for Disease Control and Prevention (CDC) have issued guidance for the identification of COVID-19 based on symptoms (Sohrabi et al., 2020). In agreement with the classical belief that viral infection normally stimulates a symptomatic response in its host, the WHO commented that transmission of COVID-19 by asymptomatic individuals is “very rare” (Anon, 2021), although this has now been retracted. The CDC estimates that 35% of COVID-19 cases are asymptomatic and 40% of transmissions occur before symptom onset (CDC, 2020). These statements were supported by reports showing that transmission between the asymptomatic index cases and contact cases mostly occurred within households or during hospital visits (Hu et al., 2020, Bai et al., 2020, Zhang et al., 2020, Furukawa et al., 2020). A recent study by Arons et al. described a COVID-19 outbreak in a Washington nursing facility: after a symptomatic healthcare worker tested positive for the virus, a facility-wide SARS-CoV-2 screen was carried out, which showed over half (56%) of workers who tested positive for SARS-CoV-2 were asymptomatic, of which 71% had viable virus by culture (Arons et al., 2020). This study demonstrates the need to take this reservoir of asymptomatic infections as a serious threat to the community spread of SARS-CoV-2.
Although reports of these silent transmitters are limited in number, models simulating the spread of infection through China have shown that undocumented asymptomatic cases greatly contributed (Li et al., 2020, Nishiura et al., 2020). These reports reveal that undocumented cases, most of which are asymptomatic, were overlooked when evaluating the magnitude of the pandemic. This means that infectivity, and hence identification and management of these asymptomatic cases, was consequently “missed”, but appears to be vital in controlling the pandemic. However, since currently available data on asymptomatic transmission are scarce and geographically limited, there is much controversy surrounding its real impact. As a result, current public health guidelines are not adapted to asymptomatic transmission, but with predictions of an imminent second SARS-CoV-2 wave approaching there must be increased understanding of this key factor in the ongoing global pandemic.
Bahrain had recorded over 26,000 confirmed COVID-19 cases at the time this paper was written. The kingdom has been praised for its response to the pandemic, implementing gold-standard testing and contact tracing procedures to identify, test and quarantine all potential cases. As well as developing a vaccine and therapeutic cure, disease control depends on developing a better understanding of the viral infection and spread in national and international populations. This study aimed to compare the differences in characteristics between index cases and positive contacts, and to compare the public health risks between asymptomatic and symptomatic transmission of COVID-19. Therefore, it analyzed the demographics, clinical characteristics and differences in viral transmission of index cases who transmitted the virus to their contacts in Bahrain.
Methods
Study design
This was a cross-sectional observational study conducted in Bahrain between April–June 2020 in Bahrain comparing transmission between symptomatic and asymptomatic SARS-CoV-2-infected individuals. A confirmed COVID-19 case was defined as an individual who tested positive for SARS-CoV-2 Envelope (E), RNA-dependent RNA Polymerase (RdRP) and Nucleocapsid (N) genes. The test was conducted by taking a nasopharyngeal (NP) swab. The NP sample was tested for the presence of SARS-CoV-2 by polymerase chain reaction (PCR) analysis. The RT-qPCR test was conducted using Thermo Fisher Scientific (Waltham, MA) TaqPath 1-Step RT-qPCR Master Mix on the Applied Biosystems (Foster City, CA) 7500 Fast Dx RealTime PCR Instrument. The assay followed the WHO protocol and measured the viral E gene. If the E gene was detected, the sample was subsequently analyzed for the SARS-CoV-2 RdRP and N genes. When measuring the initial E gene, a Cycle threshold (Ct) value of >40 was considered negative. All confirmed cases were immediately admitted to a government isolation and treatment facility, irrespective of being symptomatic or asymptomatic, and discharged after two consecutive negative PCR tests.
“Index cases” were defined as individuals with a confirmed SARS-CoV-2 infection who had transmitted the infection to at least one close contact. Symptomatic index cases were identified on their presentation to the medical services, whilst asymptomatic index cases were identified by the program of community screening targeting close contacts, travelers and random testing in areas with outbreaks. Screening for close contacts was carried out by the contact tracing team to identify all close contacts of a positive index case and arrange testing and quarantine of the close contacts. Close contacts who tested positive were termed “positive contacts”. These two samples (index cases and all their positive contacts) were compared based on demographics (gender, age group, nationality) and clinical features (symptomatic or asymptomatic). Symptomatic individuals were those who presented with symptoms at testing, or developed symptoms in the period prior to or on admission to isolation or treatment facilities. Asymptomatic individuals were individuals who had no symptoms at testing and did not develop symptoms up to their isolation or admission to health facilities. Symptom status was not followed up after isolation of cases because these cases were no longer a public health risk.
Data collection
A population of 350 index cases was obtained, all of whom were known to have documented contact tracing. After exclusion of cases with incomplete data, 320 index cases were included in the study. Demographics (gender, age group and nationality), clinical features on admission (symptomatic or asymptomatic), total number of close contacts and the number of positive contacts and their Ct values (as indicators of viral load) were extracted. Contact tracing was conducted by the Central Investigation Department and Public Health Directorate in Bahrain. All contacts of index cases were traced prior to the isolation and treatment facility admission. Demographics (gender, age group and nationality) and clinical features on admission (symptomatic or asymptomatic) were extracted for each positive contact. No positive contact cases were excluded.
Data handling and statistical analysis
The demographics (gender, age group and nationality) were compared between index cases and positive cases. Z-tests were used to compare differences in variables represented as counts and/or proportions (gender, nationality, clinical features) between samples. T-tests with Welch’s correction were used to compare means between samples, with mean values quoted as (mean ± SEM), unless otherwise stated. P-values were considered statistically significant at p < 0.05. Analyses were performed using STATA (Version 16.1) and GraphPad Prism (Version 9.0.0).
The protocol and manuscript for this study were reviewed and approved by the National COVID-19 Research Committee in Bahrain. All methods and retrospective analysis of data were approved by the National COVID-19 Research and Ethics Committee, and carried out in accordance with local guidelines and ethical guidelines of the Declaration of Helsinki 1975.
Results
Demographics
A total of 320 randomly selected index cases and their 1289 positive contacts were included in this study; their demographic characteristics are presented in Table 1 . There were significantly more males amongst both index cases (74.1%, z = 8.6, p < 0.001) and positive contacts (69.2%, z = 13.8, p < 0.001). Bahraini nationals represented a significant proportion of both the index cases sample (59.7%, z = 3.5, p < 0.001) and positive contacts sample (54.5%, z = 3.3, p = 0.0011). There was no difference in the proportion of symptomatic versus asymptomatic index cases (50%, z = 0.0, p = 1.0). However, asymptomatic presentation was significantly greater among the positive contacts (87.4%, z = 26.9, p < 0.001). The age group with the highest proportion of index cases (40.3%) and positive contacts (28.3%) was 30−39 years. The least common age group for index cases was 0−9 years (0.3%), and 60+ years for positive contacts (4.1%).
Table 1.
Demographics (gender, age group, nationality) and clinical features (symptomatic or asymptomatic) amongst the index cases and positive contacts.
| Index cases n (%) | Positive contacts n (%) | ||
|---|---|---|---|
| N | 320 | 1289 | |
| Gender | Males | 237 (74.1) | 892 (69.2) |
| Females | 83 (25.9) | 397 (30.8) | |
| Age group | 0−9 | 1 (0.3) | 136 (10.6) |
| 10−19 | 14 (4.4) | 131 (10.2) | |
| 0−19 | 15 (4.7) | 267 (20.8) | |
| 20−29 | 67 (20.9) | 266 (20.6) | |
| 30−39 | 129 (40.3) | 365 (28.3) | |
| 40−49 | 69 (21.6) | 222 (17.2) | |
| 50−59 | 27 (8.4) | 116 (9.0) | |
| 60+ | 13 (4.1) | 53 (4.1) | |
| Nationality | Bahraini | 191 (59.7) | 703 (54.5) |
| Non-Bahraini | 129 (40.3) | 586 (45.5) | |
| Clinical features | Symptomatic | 160 (50.0) | 162 (12.6) |
| Asymptomatic | 160 (50.0) | 1127 (87.4) | |
Age groups
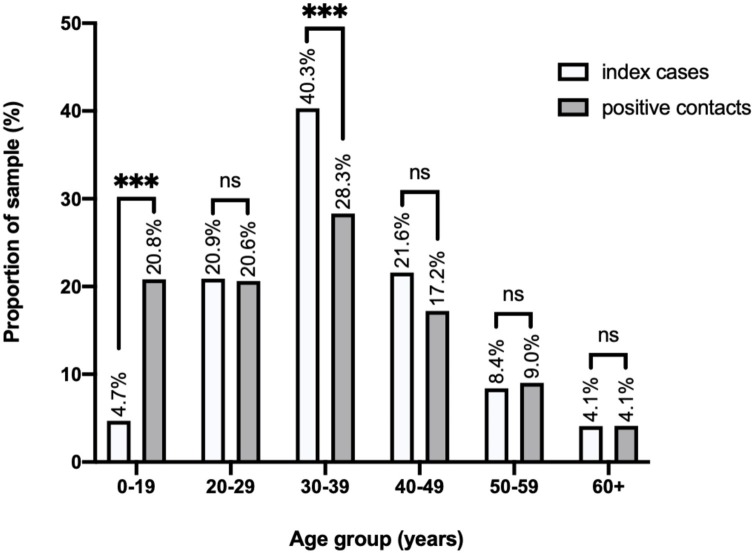
When conducting further analysis of age group distributions, the age groups 0−9 and 10−19 were merged to account for the small sample sizes. To visualize the overall infected cases and determine the transmission levels of each age group, the percentage proportions of index and positive contact cases in the age groups 0−19, 20−29, 30−39, 40−49, 50−59 and 60+ years were compared. When comparing the proportion of index cases to positive cases across all the age groups, individuals aged 0−19 years were the only group to have a significant increase (z = 6.7, p < 0.001), with 4.7% of index cases linked to 20.8% of positive cases, suggesting that the highest susceptibility was in this age group. Additionally, although the age group with the highest proportion of infected individuals (both index cases and positive contacts) was 30−39 years, this age group constituted a significantly higher proportion of index cases (40.2%) than positive contacts (28.3%) (z = 4.2, p < 0.001), suggesting higher transmission from this age group. No significant difference was observed in the other age groups (Figure 1 ).
Figure 1.
Comparison of the proportions of index cases and positive contacts by age group.
Proportions represent the total number of individuals in each sample that belong to each age group, as a proportion of the total sample size, and are plotted in percentages above each bar. The proportions were statistically compared using two-sample z-tests. Statistical significance is indicated above the error bars: p > 0.05 (ns), p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
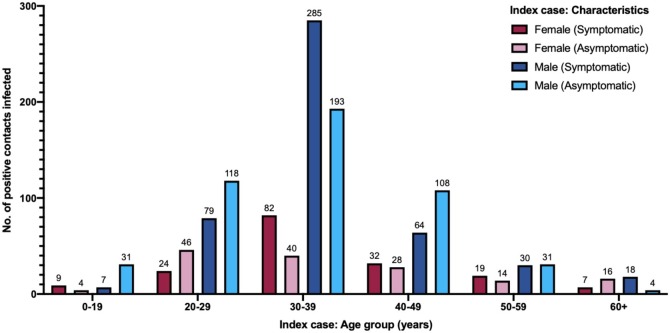
Analysis of the number of symptomatic and asymptomatic index cases of each gender was undertaken. The number of positive index cases infected was stratified according to gender, age group and symptomatic or asymptomatic clinical features. Most transmissions occurred from male index cases. The greatest number of positive contacts were infected by symptomatic male index cases aged between 30−39 years (285 cases). This corresponds to 22% of the positive contacts. This was also the category with the highest number of positive contacts for female index cases, although the number was less than the number of male cases (Figure 2 ).
Figure 2.
Number of individuals infected by index case gender, age group and clinical features.
The total number of individuals infected represent the positive contacts, with the total count indicated by the number above the bar. The key representing the categories of data plotted is indicated on the top-right corner.
Infectivity rates
Of all 1289 positive contacts, 656 (50.9%) were contacts of symptomatic index cases and 633 (49.1%) were contacts of asymptomatic index cases and the difference between the number of people infected by a symptomatic or asymptomatic index case did not differ (z = 0.6, p = 0.52). The mean number of close contacts from symptomatic index cases was 12.4 ± 1.2 contacts, which did not differ to the mean number of close contacts from asymptomatic index cases, which was 14.0 ± 1.1 contacts (p = 0.33). The majority of positive contacts were asymptomatic (87.4%).
To evaluate the effect of the presence or absence of symptoms upon rate of infection, this study compared the mean number of positive contacts infected by either symptomatic cases (4.1 ± 0.5 infected/case) or asymptomatic index cases (4.0 ± 0.3 infected/case). No association was observed between clinical symptoms and the number of cases infected (t = 0.2, p = 0.81). Second, infectivity rates were calculated to adjust for number of exposed contacts. Infectivity rate for each index case was calculated using total positive contacts as a proportion of all exposed contacts.
The mean infectivity rate for symptomatic index cases was 39.3% ± 2.0% that did not differ to asymptomatic index cases was 38.3% + 2.0% (p = 0.72).
Ct-PCR value analysis
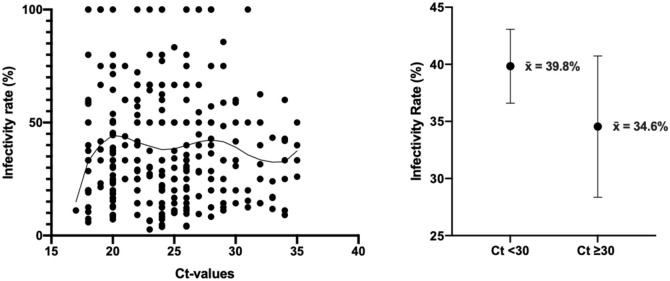
Ct values were available for 282 individuals within the index cases sample: 145 were symptomatic index cases and 137 were asymptomatic index cases, with values ranging between 17–34 and 18–35, respectively. The mean Ct value was significantly higher in asymptomatic (25.5 ± 0.4), compared to symptomatic (23.3 ± 0.3) index cases (t = 4.2, p < 0.001). The analysis of Ct values was expanded to test for an association with infectivity rate. The spread of data is shown in Figure 3 a, with infectivity rates that steeply increased with increasing Ct values up to Ct values of 20, after which the curve plateaued. Interestingly, infectivity rate peaked at Ct values of 20 and 28, and dipped at 24 and 33. Index cases were categorized by Ct value (<30 or ≥30), and infectivity rates compared between these two groups. The average infectivity rate observed for Ct values <30 was 39.8% ± 1.6% (n = 243) that did not differ compared to Ct values ≥30 was 34.6% ± 3.1% (n = 39) (p = 0.13) (Figure 3b).
Figure 3.
Analysis of infectivity rates by PCR Ct values. (a) PCR Ct values and corresponding infectivity rates for 282 index cases, with a fitted Lowess curve showing the association between these variables; (b) Average infectivity rates for index cases with Ct values <30 and ≥30, plotted as mean±95% confidence limits.
Ct values were available for 1017 individuals within the positive contacts sample, of those 93 were symptomatic and 924 were asymptomatic, with Ct values ranging between 17–35 and 15–36, respectively. The mean Ct value was significantly higher amongst asymptomatic (27.1 ± 0.1) than symptomatic (25.2 ± 0.4) positive contacts (p < 0.001).
Discussion
These data showed that 320 index cases transmitted the infection to 1289 positive contacts and, on average, each positive contact infected four individuals, showing the high infectivity and reflecting the SARS-C0V-2 pandemic that has resulted. This study has shown that the risk of transmission by asymptomatic individuals may be higher than previously expected. This study selected a random sample of 320 index cases who had documented links to exposed contacts who tested positive. It was found that asymptomatic individuals constituted a larger proportion of index cases than expected (50.0%). This finding was even greater amongst positive contacts (87.4%). This may be due to early diagnosis of close contacts. In Bahrain and during the pandemic all close contacts were identified, tested and quarantined within 48 h. This led to detection of a significant number of positive contacts early during their infection course. Bahrain has been globally praised for the excellent response and infrastructure setup early during the pandemic to identify, test and isolate infected individuals. Contact tracing was rapidly and strictly implemented to control spread. In addition, random community testing enabled identification of cases before they escalated and, hence, limited transmission. These measures have led to increased diagnosis of cases of asymptomatic infection and may explain these findings.
Moreover, several studies have suggested that children are less likely to develop severe COVID-19 and more likely to be asymptomatic (Swann et al., 2020, Han et al., 2020, Sola et al., 2020). The significantly higher rates of children that the current study observed amongst positive contacts compared to index cases may also be a contributor to this increased asymptomatic presentation amongst positive contacts. These data are higher than those that have previously been reported, where 30–40% of all COVID-19 infections were suggested to be asymptomatic (Oran and Topol, 2020), with higher estimates being suggested (Nishiura et al., 2020), whilst in a cruise ship outbreak it was estimated that 81% of COVID-19 were asymptomatic (Mizumoto et al., 2020); however, the data are in accord with previous data on international arrivals into Bahrain, where asymptomatic patients were greater than those who were symptomatic (Al-Qahtani et al., 2021). In addition, a recent study using a model to assess SARS-CoV-2 transmission showed that asymptomatic transmissions may account for at least 50% of all SARS-CoV-2 infections. The current results support these projections, in that 50% of index cases (i.e. transmitters) in this sample were asymptomatic (Johansson et al., 2021).
The current data also showed a higher proportion of positive contacts aged 0−19 years than index cases. This is consistent with reports from several studies stating that transmission from children is less common than from adults (Cai et al., 2020, Danis et al., 2020). In addition, a recent prospective cohort study also showed lower rates in ages 0–17 years amongst index cases compared to positive contacts (Luo et al., 2020). With current public health measures in Bahrain comprising the suspension of face-to-face teaching at schools and closures of youth socializing spaces, restaurants, cinemas and arcades, young people are more likely to remain at home and less likely to transmit in the community. This may clarify why this age group is over-represented in positive contacts compared to index cases; however, this study did not quantify transmission or infectivity by age group and the results are exclusively epidemiological.
The most significant category of index cases and, hence, transmitters of COVID-19 in the studied sample were male index cases aged 30−39 years. This was not unexpected, as this constitutes the working age group in Bahrain and there is a higher proportion of working males in the country (LMRA, 2021). This group generated the greatest number of contact cases, who were usually household contacts and included children, explaining the higher prevalence of infected children in the positive contacts found. Interestingly, the segment of the sample aged 60+ years constituted a steady proportion of 4.1% of both index cases and positive contacts. This is relatively comparable to the proportion of the total population of individuals aged 60+ years in Bahrain (5.4%) (iGA, 2019). In conjunction, these results suggest that the behavior elicited by this age group, and perhaps individuals around them, may be of increased awareness of risk to this age group and hence cautiousness in their interactions. Additionally, interactions of this age group may comprise small social circles of similarly aged individuals, with similar lifestyles and levels of cautiousness. Luo et al. also reported similar proportions of index cases and positive contacts for individuals aged 60+ years at 27.9% and 26.8%, respectively (Luo et al., 2020); however, it is believed that these results were most likely due to behavioral tendencies, as susceptibility of this age group to infection is extensively documented (Davies et al., 2020, Channappanavar and Perlman, 2020, Crimmins, 2020).
This study found no difference in numbers of symptomatic and asymptomatic index cases and their respective infectivity rates. It is believed that this shows that asymptomatic individuals play a larger role in the spread of the disease, and therefore impose a higher public health risk than currently believed. In addition, no significant difference in the numbers of contacts infected by symptomatic and asymptomatic index cases were found. One hypothesis is that symptomatic individuals are more cautious and aware of the probability of being COVID-19 positive, and therefore take precautions to reduce the transmission from what it would normally be for a symptomatic index case (Pollock and Lancaster, 2020). Simultaneously, asymptomatic individuals would be unaware of asymptomatic transmission, and therefore normally interact with others and spread the disease. However, if this were the case and similarities in findings were entirely behavioral, a larger average number of exposed contacts for asymptomatic index cases than symptomatic would have been expected; however, this was not seen and no difference in average exposed contacts or positive contact between symptomatic and asymptomatic index cases was observed. This study suggests that globally asymptomatic transmission should be urgently addressed in addition to that of symptomatic transmission.
It has been widely adopted that viral load can be quantified as an inverse relation to PCR Ct values (Zou et al., 2020, Zhou et al., 2020) and this hypothesis was applied in this study. Index cases with high infectivity rates were found to have lower Ct values and higher viral loads. When compared by symptoms, asymptomatic index cases showed a higher Ct value (i.e. lower viral load), with a mean of 25.5. La Scola et al. reported that patients with Ct values <33–34 are considered infectious, and since the average Ct values for asymptomatic individuals in this study were well below this cut-off, this adds evidence that asymptomatic cases are infectious (La Scola et al., 2020). However, the current data showed that cases with Ct values up to 35 remained infectious, regardless of symptoms. It is possible that these individuals were at a later stage of their infection, and hence a delay in detection of these cases may actually mean that infection previously happened at lower Ct values. This may also explain the inconclusive result for the association between Ct value and infectivity rate; therefore, these results must be interpreted with caution. Furthermore, it is important to consider the selection of patients in this study: they were all index cases who transmitted the virus and hence this would have biased the Ct value association with infectivity.
The current identification measures of positive contacts are prioritized by symptomatic rather than asymptomatic patients because until the SARS-CoV-2 outbreak, asymptomatic infection and transmission were believed to be unlikely (Anon, 2021). However, the current results show that symptomatic presentation of COVID-19 may not be the only contributor to its spread. Indeed, temperature and symptom-based detection are not effective at surveilling asymptomatic individuals for COVID-19. The magnitude of asymptomatic transmission revealed in this study may explain the difficulty experienced worldwide in controlling the pandemic.
A strength of this study was that all individuals were admitted to health facilities on confirmation of infection and their symptoms were determined, so those who were symptomatic or asymptomatic could be distinguished.
This study also had a number of limitations, including that the index cases were collected from the contact tracing databases and only index cases who transmitted the virus were included. This is an important consideration, as this may have influenced the results related to infectivity rates and transmission. In addition, in order to study infectivity as accurately as possible, the index cases who were selected had identifiable contacts, resulting in a decrease in sample size. Larger samples are required to confirm associations and give clearer indications for trends in cases, and better advice for public health policies is necessary to control this pandemic. Finally, the Ct value analysis conducted in this study should be interpreted with caution, as there was no inclusion of the number of days after exposure to the virus that the value corresponded to. This was not possible due to difficulty in identifying the point of exposure for the index cases, especially for the asymptomatic cases.
In conclusion, these data show that the high asymptomatic incidence of SARS-CoV-2 infection in Bahrain and subsequent positive contacts from an index case are more likely to be asymptomatic, showing the high “silent” risk of transmission and the need for comprehensive screening for each positive infection to help halt the ongoing pandemic.
Funding
None.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
Approval granted after review from National COVID-19 Research and Ethics Committee.
Consent to participate
Not applicable.
Consent for publication
All authors approved publishing of this data.
Availability of data and material
Available on reasonable request.
Code availability
All data were entered in Microsoft excel and analyzed with STATA and GraphPad Prism.
Authors' contributions
MAA, AA, SAS, DAS were involved in data collection, analysis of the data and initial manuscript preparation. NJS and SLA reviewed the manuscript. MAQ, MAA, AA, NJS developed the idea and final drafting of the manuscript.
References
- Al-Qahtani M., AlAli S., AbdulRahman A., Salman Alsayyad A., Otoom S., Atkin S.L. The prevalence of asymptomatic and symptomatic COVID-19 in a cohort of quarantined subjects. Int J Infect Dis. 2021;102:285–288. doi: 10.1016/j.ijid.2020.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfinrud P., Stadnytskyi V., Bax C.E., Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering. N Engl J Med. 2020;382(21):2061–2063. doi: 10.1056/NEJMc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Virtual Press Conference: 8 June 2020 [press release].
- Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Xu J., Lin D., Yang Z., Xu L., Qu Z. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2020. Pandemic planning scenarios.https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html [23 June 2020]. Available from: [Google Scholar]
- Channappanavar R., Perlman S. Age-related susceptibility to coronavirus infections: role of impaired and dysregulated host immunity. J Clin Invest. 2020;130(12):6204–6213. doi: 10.1172/JCI144115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E.M. Age-Related vulnerability to coronavirus disease 2019 (COVID-19): biological, contextual, and policy-related factors. Public Policy Aging Rep. 2020;30(4):142–146. doi: 10.1093/ppar/praa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis K., Epaulard O., Bénet T., Gaymard A., Campoy S., Botelho-Nevers E. Cluster of coronavirus disease 2019 (COVID-19) in the French Alps, February 2020. Clin Infect Dis. 2020;71(15):825–832. doi: 10.1093/cid/ciaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Eggo R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- Furukawa N.W., Brooks J.T., Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.S., Choi E.H., Chang S.H., Jin B.L., Lee E.J., Kim B.N. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- iGA . 2019. Information and eGovernment authority - Bahrain open data portal: population. [Google Scholar]
- Johansson M.A., Quandelacy T.M., Kada S., Prasad P.V., Steele M., Brooks J.T. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scola B., Le Bideau M., Andreani J., Hoang V.T., Grimaldier C., Colson P. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39(6):1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LMRA. Labour market regulatory authority - Bahrain labour market indicators [5 August 2020]. Available from: http://blmi.lmra.bh/2019/06/mi_dashboard.xml.
- Luo L., Liu D., Liao X., Wu X., Jing Q., Zheng J. Contact settings and risk for transmission in 3410 close contacts of patients with COVID-19 in Guangzhou, China : a prospective cohort study. Ann Intern Med. 2020 doi: 10.7326/M20-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S.M., Hayashi K. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) IJID. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020 doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock A.M., Lancaster J. Asymptomatic transmission of covid-19. BMJ. 2020;371:m4851. [Google Scholar]
- Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola A.M., David A.P., Rosbe K.W., Baba A., Ramirez-Avila L., Chan D.K. Prevalence of SARS-CoV-2 infection in children without symptoms of coronavirus disease 2019. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann O.V., Holden K.A., Turtle L., Pollock L., Fairfield C.J., Drake T.M. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int [15 January 2021]. Available from: [Google Scholar]
- WHO. Coronavirus disease (COVID-19) advice for the public [23 June 2020]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public.
- Zhang J., Tian S., Lou J., Chen Y. Familial cluster of COVID-19 infection from an asymptomatic. Crit Care. 2020;24(1):119. doi: 10.1186/s13054-020-2817-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Li F., Chen F., Liu H., Zheng J., Lei C. Viral dynamics in asymptomatic patients with COVID-19. Int J Infect Dis. 2020;96:288–290. doi: 10.1016/j.ijid.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in Upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on reasonable request.