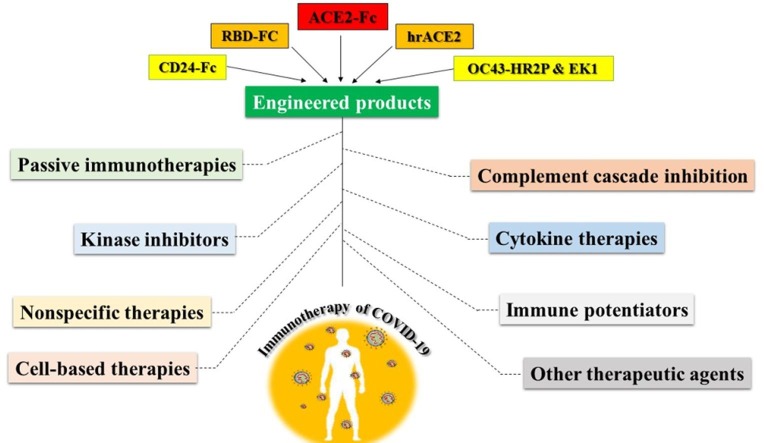
Graphical abstract
Keywords: COVID-19, Immunotherapy, SARS-CoV2, ACE2-Fc, Vaccines, Antibodies
Abstract
After the advent of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) in the late 2019, the resulting severe and pernicious syndrome (COVID-19) immediately was deployed all around the world. To date, despite relentless efforts to control the disease by drug repurposing, there is no approved specific therapy for COVID-19. Given the role of innate and acquired immune components in the control and elimination of viral infections and inflammatory mutilations during SARS-CoV2 pathogenesis, immunotherapeutic strategies appear to be beneficent. Passive immunotherapies such as convalescent plasma, which has received much attention especially in severe cases, as well as suppressing inflammatory cytokines, interferon administration, inhibition of kinases and complement cascade, virus neutralization with key engineered products, cell-based therapies, immunomodulators and anti-inflammatory drugs are among the key immunotherapeutic approaches to deal with COVID-19, which is discussed in this review. Also, details of leading COVID-19 vaccine candidates as the most potent immunotherapy have been provided. However, despite salient improvements, there is still a lack of completely assured vaccines for universal application. Therefore, adopting proper immunotherapies according to the cytokine pattern and involved immune responses, alongside engineered biologics specially ACE2-Fc to curb SARS-CoV2 infection until achieving a tailored vaccine is probably the best strategy to better manage this pandemic. Therefore, gaining knowledge about the mechanism of action, potential targets, as well as the effectiveness of immune-based approaches to confront COVID-19 in the form of a well-ordered review study is highly momentous.
1. Introduction
The widespread pandemic of COVID-19 and rising mortality rates especially in high-risk people, currently have become a major public health concern. The SARS-CoV2 is in the backroom of this pneumonia-like illness that was firstly showed up on December 2019 in Wuhan, Hubei Province, China [1]. Since then, the COVID-19 outbreak has crawled globally and incredibly crippled the humanity. SARS-CoV2, named by the International Committee on Taxonomy of Viruses (ICTV) on 11 February 2020 [2], is a new member of the Coronaviridae family, subfamily Coronavirinae and the Nidovirales order [3]. Coronaviruses (CoVs) are the largest known RNA viruses with 118–136 in diameter and 25 to 32 kb genome size [4]. Their envelope contains structural proteins that entrapping a positive-sense and single stranded RNA [5]. Triple spike (S) protein is a petal-shaped projection on the surface of CoVs that mediates attachment to angiotensin-converting enzyme 2 (ACE2) receptor and cell membrane integration [6]. ACE2 is widely expressed in diverse human tissues such as ileum, stomach, bronchus, esophagus, heart, lung, kidney, bladder, nasal mucosa [7] and even testicular tissues that make them susceptible to CoVs infection [8]. The SARS-CoV2 genome also encodes Nucleocapsid (N), Membrane (M) and Envelope (E) proteins [6] (Fig. 1 ). In addition to structural proteins, other genomic regions express specific viral enzymes involving in replication [9] and virulence of SARS-CoV2 such as papain-like protease [10] and coronavirus main protease [11]. Similar to middle east respiratory syndrome (MERS) and SARS, the SARS-CoV2 infection is cytopathic mainly to human lung epithelial and alveolar cells [12]. Most hospitalized patients have been suffered from the acute respiratory distress syndrome (ARDS), which is accompanied by numerous lymphocytes and macrophages infiltration, interstitial inflammation, hyaline membrane formation and desquamative pneumocytes. The early and most common manifestations are dry cough, fever, fatigue, headaches, myalgia and pharyngalgia [13], [14], [15]. Currently unbridled viral replication, loss of ACE2 expression via shedding or retraction, antibody dependent enhancement (ADE), imbalanced proinflammatory cytokine production and dysfunctional cellular immunity have been determined as the most prominent factors responsible for these lethal manifestations [16]. According to WHO reports over 200 COVID-19 vaccine candidates are under investigation that some of them moving toward human clinical trials [17]. However, a specific therapy or universal completely assured vaccine candidate for COVID-19 is absent. Given the widespread prevalence of COVID-19, high mortality and morbidity and the role of immunological factors in the development of lethal symptoms, immunotherapy seems to be one of the potential strategies to combat against COVID-19. So, considering the limited treatment time of infected patents, in this paper, we intend to provide evidences around the potential immunotherapeutic options for the newly discovered 2019 coronavirus, focusing on strategies that widely affecting both immune responses and viral spread. Finally, we try to render the best immune-based solutions for the prevention and recovery of high-risk and critically ill patients.
Fig. 1.
Schematic view of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) structure, pathogenicity and immunotherapeutic approaches. The novel SARS-CoV2 has four main structural proteins, including spike (S), membrane (M), nucleocapsid (N) and envelope (E) proteins and contains a positive-sense single-stranded RNA genome. SARS-CoV2 by reproducing in diverse tissues and spreading throughout the body as well as excessive inflammation, impaired coagulation activity, vascular damage and eventually hypoxia and organ failure is accompanied by devastating pathological complications. Immunotherapeutic approaches are suitable strategies to balance such disorders and limiting virus replication and spread. Vaccine candidates are being developed as promising active immunotherapies to eradicate COVID-19. Other immunotherapies including passive immunotherapy, kinase inhibitor, cytokine therapy, complement inhibition, engineered product, cell-based therapy, immune potentiator and nonspecific therapy can also be used to manage SARS-CoV2 infection and clinical manifestations.
2. Passive immunotherapies
Based on a historical look at the foretime epidemics and human dream experience with infectious viral diseases such as mumps and H1N1 influenza, passive immunotherapy has always been one of the main treatments for effective but temporary control of epidemics [18]. Generally, passive immunotherapy consists of the 1) plasma of recovered individuals from an infection (convalescent plasma (CP)) 2) purified high titers of neutralizing antibodies from pooled recovered human plasma (hyperimmune globulin (H-IG)) 3) extracted normal human immunoglobulins from pooled plasma (intravenous immunoglobulin (IVIG)) and 4) monoclonal antibodies. In relation to viral infections passive immunotherapies explicitly block viral entrance and replication in target cells and limit the viral spread via less specialized mechanisms such as opsonization and phagocytosis, antibody-dependent cellular cytotoxicity (ADCC) and complement fixation [19].
2.1. CP and H-IG
CP and H-IG are some of the most well-known passive immunotherapies that rely on the plasma of recuperated patients. As the CP or serotherapy is more accessible in a short time and does not require complex separation processes, it is considered as the first-line passive immunotherapy against infectious disease. The CP is generally composed of various organic and inorganic compounds, water and thousands of proteins including innate humoral immune factors and all pathogen-specific antibody isotypes (IgM, IgG, IgE and IgA) [19], [20]. Regarding the coronaviruses, much of the antiviral activities of the CP are provided by neutralizing antibodies, which mainly target epitopes of the nucleoprotein as well as the S1 and S2 subunits of the SARS-CoV2 S glycoprotein [21], [22]. There are also IgM and IgG non-neutralizing protective antibodies that are associated with patient recovery and improvement [18]. Importantly, the quality of neutralizing antibodies in CP samples alter during the course of the COVID-19 disease, and diverse CPs show different antiviral potentials. Therefore, it is necessary to evaluate the function and titers of neutralizing antibodies in donated CP before therapeutic use [23], [24]. Moreover, CP is most effective when used as prophylaxis or in the early stages of the disease. In general, high titers of neutralizing antibodies are required for the effectiveness of CP and dose adjustment based on the weight of the recipient is one of the challenges facing this treatment [25]. The CP has also immunomodulatory activities owing to the content of anti-inflammatory cytokines and antibodies that block complement components (e. g., C3a and C5a), inflammatory cytokines (interleukin-1-beta (IL-1β), tumor necrosis factor-alpha (TNF-α), etc.) and autoantibodies [26]. Anti-β2-glycoprotein I and anticardiolipin are among the most serious autoantibodies, which are associated with antiphospholipid syndrome-like disease and thrombotic problems [27]. Thus, the CP of COVID-19 patients critically limits the inflammatory injuries related to damaging autoantibodies, complement cascades and inflammatory cytokines. Fc receptors (FcRs) are also affected by CP administration so that the saturation of FcRn aids in diminish the lifetime of autoantibodies and immunomodulation. Also, Fcγ receptor activation following IgG attachment leads to FcγRIIB upregulation on immune cells especially B lymphocytes and downmodulate antibody production and inflammatory events [28]. Antigen presenting cells (APCs) like dendritic cells (DCs) and T lymphocytes, are also touched by CP therapy. Laboratory studies have shown that exposure of DCs to blood antibodies (CP or IVIG) repeal their (interferon (IFN)-α-mediated) maturation and induce T helper (Th)2-cytokine secretion (IL-10, IL-4, IL-13, IL-33). Downregulation of costimulatory molecules such as B7-1, B7-2, CD40 as well as MHC II were also observed at the surface of exposed DC cells [29], [30], [31]. Besides, IgG-mediated activity of Wnt-β-catenin pathway in DC cells is associated with suppression of inflammation [32]. Results showed that recovered antibodies readjust CD4+-CD8+ balance and enhanced the survival and number T regulatory (reg) cells. Cytotoxicity and proliferation of CD8+ cells are greatly suppressed in the presence of this treatment and the expanded Th17 clones and related inflammatory cytokines such as IL-17A and F, CCL20 and IL-21 were retreated [28]. Apoptosis of B lymphocytes upsurge following immunoglobulin administration, which is either contribute to the presence of anti-Fas and/or B-cell activating factor (BAFF) neutralizing antibodies. Moreover, B lymphocyte stimulation is eliminated by toll-like receptor 9 (TLR9), which is associated with nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) abrogation, diminished IL-2Rα (CD25) and CD40 expression and reduced IL-6 secretion [33], [34], [35]. M2 anti-inflammatory macrophages are also dominated by the antibody in pooled plasma, and the migration of inflammatory macrophages to certain inflammatory areas is suppressed (Fig. 2 ) (Table 1 ) [28]. On the other hand, the H-IG is prepared by apheresis from the pooled high content neutralizing antibody plasma of patients who have recovered from an infectious disease and consists mainly of highly purified specific IgG and its subclasses [36]. Unlike CP, the H-IG is applied in the second line of immunotherapy and is valued for titration and neutralizing activity during preparation [36]. Similarly, the H-IG products derived from recovered COVID-19 patients contains a set of polyclonal antibodies, targeting mainly the S protein and related receptor-binding domain (RBD) [37]. Besides, all the mentioned antibody-mediated immunomodulatory and antiviral effects of CP also apply to H-IG. However, take into consideration that the best time to obtain CP and H-IG is at least 21 days after the onset of the disease so that the titers of IgM and IgG neutralizing antibodies reach the desired level [38]. Retrospective studies have shown that both CP and H-IG treatments have been effective during the last coronavirus epidemics such as SARS and MERS and due to the similarities, these approaches seem to be beneficial in diminishing the mortality rate and hospitalization, especially in the early stages, of the COVID-19 infection [39]. Fortunately, The CP therapy has been welcomed in growing clinical trials for COVID-19 in the United States as the COVID-19 Convalescent Plasma Project [19]. A clinical trial study has been examined the effects of CP on the induction of specific immunity in COVID-19 patients (NCT04264858). Also using 200 mL of CP with at least 1: 640 titer of neutralizing antibodies in Chinese severe cases of COVID-19 led to amelioration of their signs and symptoms [40]. Anyway, compared to other passive immunization strategies, plasma donation by apheresis is preferred for many compelling reasons including no serious effects on the donors’ haemoglobin, enhancing the probable number of donations and achieving larger volumes in every session [41], [42]. Albeit, there are several limitations to CP and H-IG donation that sometimes lead to skepticism [43]: transmission of blood-borne infectious viral diseases in endemic areas, increased risk of infection by health care personnel when checking samples of infected individuals, lack of reliable and reasoned researches [44], discovering people with high titers of neutralizing antibodies against certain infections, the challenge of supportive care next to the CP administration, potential risk of ADE phenomenon in genetically predisposed patients etc. [42], [45]. Moreover, the exponential growth of COVID-19 cases especially in thickly populated areas is so high that it is likely reduce the chance of using the plasma of recovered individuals [42]. By sequencing the gene encoding the S protein of the SARS-CoV2, scientists took the opportunity to quickly access the protein in vitro. Therefore, manufacturing neutralizing antibodies could be done in laboratory animals such as mice and rabbits, although this method is not very effective against COVID-19 due to its time-consuming nature. Also, the traditional screening strategies in animal models are so lagging and novel display libraries is more ideal [46], [47]. By and large it seems that providing a cocktail of polyclonal antibodies by immunized laboratory animals or specific cell line strategies is similar to CP with more acceptable clinical outcomes and reduced risk of contamination. Also, these products make it possible to predict the dose and kinetics of antibodies and reduce the chances of escape-mutant variants by targeting different epitopes [48]. However, several evaluations should be performed to determine the neutralizing and preventive effects of such challenging antibodies against several circulating COVID-19 strains. Furthermore, employing synthetic laboratory antibodies instead of CP or H-IG will no longer have the same immunomodulatory advantages.
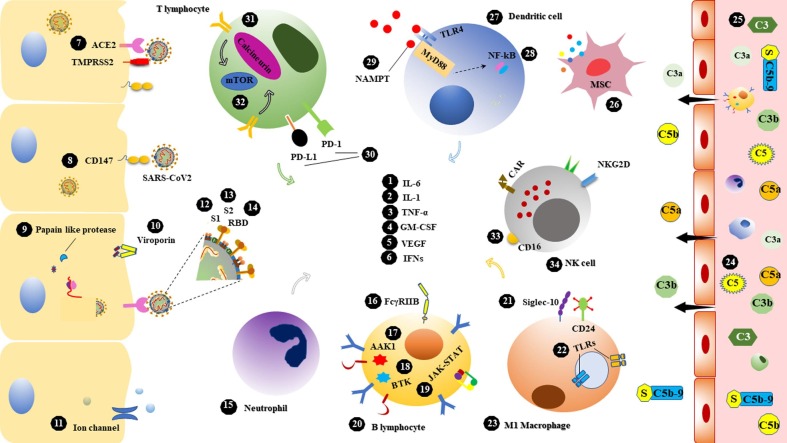
Fig. 2.
Potential targets in the immunotherapy of SARS-CoV2 infection. 34 molecular and cellular targets associated with host immune responses as well as factors involved in SARS-CoV2 pathogenesis have been conceived. 1) interleukin (IL)-6, prominent driver of hyperinflammatory syndromes which participates in lung pathology 2) IL-1, one of the main pillars of cytokine storm 3) tumor necrosis factor-alpha (TNF-α), a proinflammatory cytokine that involves in alveolar and epithelial injury 4) granulocyte–macrophage colony-stimulating factor (GM-CSF), involved in both the pathogenesis of COVID-19-like syndromes and the physiology of the lungs 5) vascular endothelial growth factor (VEGF), increases vascular permeability and is associated with hypoxia, edema, and lung damage 6) interferons (IFNs), induce antiviral defense in various cells 7) angiotensin-converting enzyme 2 (ACE2), main receptor for SARS-CoV2 entrance via S protein 8) CD147, mediates SARS-CoV2 invasion to host cells 9) Papain like protease, essential coronavirus protease to produce replicase complex 10) Viroporin, inducing inflammasome and related to viral life cycle 11) Ion channel, targets of dewetting monoclonal antibodies to block water flow and viral spread 12) S1, The major subunit of the spike protein and mediates ACE2 binding 13) S2, up to 88% sequence homology with SARS-CoV and contains conserved motifs for cross-neutralizing antibodies 14) receptor binding domain (RBD), the main part of the S1 subunit and the target of many neutralizing antibodies 15) neutrophil, the causative agent of excessive inflammation related to poor prognosis 16) FcγRIIB, an immunosuppressant receptor constrains antibody-dependent enhancement (ADE) by some antibodies 17) AP2-associated protein kinase 1 (AAK1), contributor of receptor-mediated viral endocytosis 18) Bruton's tyrosine kinase (BTK), activate in macrophage polarization, humoral immunity and hyperinflammatory outcomes 19) Janus kinase (JAK)-signal transducer and activator of transcription (STAT), the major arbitrators in proinflammatory cytokines and chemokines signaling 20) B lymphocyte, reservoir of antibody production and involvement in inflammatory responses 21) Sialic acid-binding Ig-like lectin 10 (Siglec-10), mediates B cell tolerance and dendritic cell suppression following CD24 interaction 22) endosomal and membrane-bound toll like receptors (TLRs) ligands, inducing antiviral responses, 23) M1 macrophage, cause of inflammation and tissue damage 24) C5 & 25) C3, role in hyper inflammatory syndromes and thrombotic microangiopathy 26) mesenchymal stem cell (MSC), anti-inflammatory and tissue repairing potentials 27) Dendritic cell, the main APC at the onset of inflammatory responses 28) nuclear factor-κB (NF-κB) driver of lethal inflammation 29) Nicotinamide phosphoribosyl transferase (NAMPT), released following physical stress and mediated hyper inflammation 30) programmed death-ligand 1 (PD-L1) & programmed cell death (PD-1), immune checkpoint inhibitors cause T cell exhaustion 31) Calcineurin, role in cytokine storm and early T cell activation 32) mammalian target of rapamycin (mTOR), related to the complications of obesity and inflammation 33) CD16, role in antibody mediated cytotoxicity and viral clearance 34) natural killer (NK) cell, immune homeostasis and eradicating viral infections.
Table 1.
Summary of key immunotherapeutic approaches against COVID-19, potential target and mechanism of action.
 |
S, spike; ↓, decline; ↑, enhance; tDC, tolerogenic DC; EM, extracellular matrix; ROS, reactive oxygen species; WBC, white blood cell.
2.2. IVIG therapy
IVIG is part of the plasma of thousands of healthy donors that has been used to treat immune deficiencies, improve inflammatory conditions and resolute several infectious diseases. Moreover, the IVIG could provide passive protection against multiple pathogens. Several anti-inflammatory and immunomodulatory activities of IVIG have been reported that possibly dampen the hyper inflammatory response in COVID-19 infection. These effects are largely due to autoreactive antibodies against inflammatory cytokines and chemokines, autoantibodies, complement component, idiotypic determinants of antibodies and IgG dimers that block FcγR downstream signaling on phagocytic cells [49]. In a case series study around 3 severe SARS-CoV2 infected Chinese patients showed that IVIG injection ameliorate respiratory symptoms during five days of treatment. Also, previous reports revealed that the SARS patients have benefited from the therapeutic effects of IVIG. Experiments in life-threatening cases of COVID-19 showed that IVIG therapy suppressed Th1 and Th17 inflammatory lymphocytes and recovered the decreased Treg cells number in peripheral blood [50], [51]. Monitoring of 58 patients with severe COVID-19 who received IVIG in the hospital showed that 2 days after treatment, the patients’ symptoms improved significantly and the hospitalization rate as well as the need for respiratory supportive treatments decreased [52]. Thus, it is clear that IVIG therapy can be beneficial in patients with COVID-19, especially in people with underlying disease at least to improve the inflammatory and pulmonary symptoms (Fig. 2) (Table 1).
2.3. Monoclonal antibodies
Among the methods that limit the entry of the virus into host cells, monoclonal antibodies are of great importance mainly owing to lower risk of transmitting blood-borne infections, functional specificity and high purity [53]. However, producing the right amount of these antibodies that can meet the needs of a large number of patients in populous countries is costly, time consuming and very arduous. Therefore, we should think about designing systems that can produce a large volume of different proteins at a reasonable cost in a short time [53]. To date, the most common target of neutralizing antibodies has been the epitopes on the S protein of SARS-CoV2 virions to prevent the infection of host cells. Despite the multitudinous similarities between SARS-CoV and SARS-CoV2, almost the majority of previous anti-S protein neutralizing antibodies are unable to bind and inhibit SARS-CoV2 viral particles [54]. Recent in silico studies have demonstrated the ability of CR3022 neutralizing antibody to bind the RBD domain of the SARS-CoV2 S protein, but its neutralizing effects have not yet been confirmed [55], [56]. However, some studies have claimed that the CR3022 neutralized the virus through epitopes other than RBD. The m396 antibody outwardly composed electrostatic interactions and salt bridges with the conserved residues of RBD in SARS-CoV and SARS-CoV2 [38]. Contrariwise, the F26G19 anti-RBD monoclonal antibody through dissimilar interactions, neutralized SARS-CoV2 better than SARS-CoV [57]. Besides, the cross-neutralizing potential of anti-HR2 monoclonal antibodies such as 1G10, 1A9, 2B2 and 4B12 against the SARS-CoV2 have been established [58]. The S309 monoclonal antibody from SARS-CoV recovered plasma also showed promising neutralizing activities via targeting domains other than RBD in SARS-CoV2 [59]. Nicotinamide phosphoribosyl transferase (NAmPRTase or NAMPT) is an alert molecule with enhanced expression following physical stress in the lung and mediates inflammatory turmoil by TLR4 activation [60]. Therefore, inhibition of the NAMPT with monoclonal antibodies may be useful in COVID-19 patients. Just like the influenza infection, dewetting monoclonal antibodies which block the water flow through ion channels maybe operative in damping SARS-CoV2 infection. Viroporins are tiny virally encoded hydrophobic ion channel proteins that participate in different parts of the virus infectivity cycle. In this regard navigating dewetting antibodies towards SARS-CoV2 viroporins most likely hinder virus binding and spread [61]. Blocking of ACE2 receptors on the surface of COVID-19 involving organs with specific monoclonal antibodies is also helpful. This method, like a double-edged sword, both prevents the viral entry and may disrupt the physiological function of normal tissues. Therefore, the application of this method requires more in-depth studies. CD147 or Basigin is a hyper glycosylated transmembrane molecule which acts as an extracellular matrix metalloproteinase inducer (EMMPRIN) [62]. Cell culture experiments showed that the SARS-CoV2 virions exploited CD147 molecule as an accessory receptor to attack human cells [63]. Wang et al. recently confirmed the spike protein/CD147 associations on SARS-CoV2 infecting cells by ELISA, immunoprecipitation and electron microscopy approaches [62]. Woefully, CD147 is also expressed by undifferentiated and tissue specific stem cells which makes them susceptible to direct infection by SARS-CoV2 [64]. Then not only tissue-specific cells but the regenerative potential of the engaging tissues is destroyed by depleting stem cell sources. On the other hand, in patients with COVID-19, excessive differentiation of stem cells into myofibroblasts is observed, which is associated with mass deposition of extracellular matrix components mainly fibrous filaments [65], [66]. This is probably why fibrosis, especially pulmonary type, occurs in people with COVID-19. Also, enhanced level of CD147 expression in macrophages and type II pneumocytes around the fibrous area in SARS-CoV2 infected lung has been discovered [67]. In vivo results showed that in response to TGF-β1, crafted CD147-overexpressed lung fibroblasts revealed rising proliferation and tendency to myofibroblast phenotype [67]. Experiments in mic model indicated that the angiotensin II was one the main basis for development of fibrosis [68]. On the other hand, the ACE2 receptor usually decomposes angiotensin II and blocks fibrotic formation. So, dysfunction of ACE2 as a result of SARS-CoV2 infection likely leads to pulmonary fibrosis. Therefore, administering monoclonal antibodies against CD147 such as meplazumab not only block the entry of the SARS-CoV2 but also avoid tissue fibrosis by reducing fibroblast foci formation [63]. Though, it is too early to conclude, since the results of several clinical trials on CD147 inhibition in patients with COVID-19 is underway. CCR5, CD16, TLR3, G-CSF, MCP-1, IL-4, IL-10 and immunoreceptor tyrosine-based activation motif (ITAM) are the other potential targets of monoclonal antibodies that can be considered in SARS-CoV2 infection studies (Fig. 2) (Table 1).
3. Kinase inhibitors
Although, the ACE2 is the main receptor for SARS-CoV2 in order to invading host cells but the new coronavirus also employ endocytosis trafficking mechanisms for penetration. AP2-associated protein kinase 1 (AAK1) and Janus-associated kinase (JAK) are the known key regulators of endocytosis that can be considered as potential therapeutic options in controlling SARS-CoV2 entry [69]. So far, many JAK inhibitors have been identified, including tofacitinib, baricitinib, ruxolitinib, upadacitinib and fedratinib, which have been used primarily to treat myelofibrosis and multiple inflammatory diseases. Lymphocytopenia and cytokine storm are the prominent features of COVID-19 infection that possibly resolute by JAK inhibitors. However, JAK inhibitors likely delay viral clearance by widespread immunosuppression influencing IFN-α production and other antiviral responses [70]. The JAK inhibitors either restrain the activity of all JAK molecules (including JAK1, JAK2, JAK3 and TYK2) or selectively target desirable ones [71]. Fedratinib is a JAK2 inhibitor that showed beneficial effects in ameliorating cytokine storm by reducing IL-22, IL-17 and granulocyte–macrophage colony-stimulating factor (GM-CSF) production via Th17 suppression. Also, the JAK2 inhibitor had transient and reversible effects on B cell function with negligible perturbation on innate immune responses [55]. Baricitinib (a JAK1/JAK2 inhibitor) mainly targets cyclin G-related kinases as the regulator of endocytosis [72]. Richardson et al. hypothesized that baricitinib could significantly diminish the infection of pulmonary cells with SARS-CoV2 via AP2-associated protein kinase 1 (AAK1) and G-related kinases inhibition [73], [74]. Besides the immunomodulatory effects of baricitinib can be beneficial to hyperinflammatory status and immune mediated pulmonary failure in severe COVID-19 patients. Compared to other inhibitors the baricitinib is better tolerated because of lower side effects, higher potential pharmacokinetics and minimal interference with drug transporters and vital enzymes. However, the baricitinib should be prescribed with more caution due to the reactivation of latent viral infections such as varicella zoster and lymphocytopenia development [75], [76]. Ruxolitinib is the other JAK1/2 inhibitor that improved lung and kidney functions and helped maintain hemodynamic balance in a 11-year-old haemophagocytic lymphohistiocytosis (HLH) patient [77]. The study of primary and secondary HLH (sHLH) animal models also showed signs of reduced tissue inflammation mainly through blocking STAT1-dependent CD8+ T cell proliferation by ruxolitinib [78]. Therefore, based on the similarities between sHLH and severe COVID-19, ruxolitinib may be helpful to improve hyperinflammation status of COVID-19 patients. Unluckily, the other JAK inhibitors, filgotinib and upadacitinib also perturbated antiviral responses via IFNs and revitalized latent infections [79]. Therefore, only tofacitinib, baricitinib and ruxolitinib have succeeded in entering the clinical trial studies for the treatment of COVID-19. Currently multiple controlled trial experiments have been evaluating the healing potential of baricitinib and ruxolitinib in lung injury of COVID-19 suffering individuals [80]. Bruton tyrosine kinase (BTK) inhibitors are the other strategy that was firstly approved for treatment of patients with mantle cell lymphoma. Ibrutinib, acalabrutinib and zanubrutinib are such inhibitors that block proliferation and cytokine release from B cells [81]. A pharmaceutical company recently has studied the effects of acalabrutinib on suppressing cytokine storm and inflammation in COVID-19 patients [48]. Other kinase inhibitors that are mainly used to treat cancers including sunitinib, a receptor tyrosine kinase (RTK) inhibitor, and erlotinib, an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase, were shown to debar viral entry [75], [76], [82]. Sorafenib is also against many protein kinases such as vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) that its application in the treatment of COVID-19 may be effective (Fig. 2) (Table 1) [82].
4. Cytokine therapies
Due to the inflammatory nature of COVID-19 infection and immense volume of proinflammatory cytokines and chemokines production specially in late-stage patients, using immunomodulators seems to be beneficial. The levels of IL-1β, IL-2, IL-6, IL-7, IL-10, GM-CSF, interferon gamma-induced protein (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1-alpha (MIP-1-α), TNF-α and also IFNs are imbalanced in SARS-CoV2 infection and subsequent cytokine storm symptoms were observed (Fig. 2) (Table 1) [13], [83].
4.1. IL-6 inhibition
IL-6 is a prominent driver of inflammation that mediates multiple immune and physiological functions [84]. It plays a crucial role in the defense against viral respiratory infections and deemed to be as a predictor of respiratory failure [85]. The concentration of IL-6 in COVID-19-borne patients increases dynamically with enhancing the disease severity and deteriorates lung pathology [13]. Besides, the number of CD14+/CD16+ inflammatory monocytes that considered as a main source of IL-6 substantially elevated in severe COVID-19 patients. It is assumed that GM-CSF and IFNγ that were produced by hyperactive Th1 lymphocytes lead to this elevation [86]. Several IL-6 (siltuximab, sirukumab and clazakizumab) and IL-6 receptor (tocilizumab and sarilumab) targeting antibodies have investigated in COVID-19 patients [87]. Tocilizumab, a humanized monoclonal antibody against both soluble and membrane bound isoforms of IL-6 receptor [88], [89] repressing proinflammatory downstream JAK-STAT signaling pathway following IL-6 binding [90]. In a systematic review, despite the reduction in mortality rate of severe COVID-19 patients upon using tocilizumab compared to the control group, but the difference was not statistically significant and the same intensive care unit (ICU) admission were observed [91]. Although this insignificant result may be due to the basic characteristics of the tocilizumab-receiving group, but this treatment apparently does not help improve the severe COVID-19 cases. A current Chinese study exhibited that treating critically ill patients of COVID-19 by tocilizumab led to the improvement of clinical symptoms as well as laboratory findings in a few days [92]. Paradoxically, diminishing cytokine levels by inhibiting the innate and acquired arms of the immune system can be associated with unrestrained viral replication, delayed patient recovery, and impaired antiviral responses. That's why the IL-6 inhibition may not be the ultimate solution, and conversely will help the viral spread.
4.2. IL-1 inhibition
IL-1 is also one of the constituents of cytokine storm that was produced by the NLRP3 inflammasome complex through various stimuli. It is supposed that the IL-1 is the main culprits in the pathogenesis of COVID-19, because its presence has been discovered in the lung tissue of infected individuals by several laboratory techniques [93]. Some small case series studies reported the benefit of IL-1 inhibition in COVID-19 patients [93], [94]. Filocamo et al. showed that using anakinra, an IL-1 receptor antagonist, led to effective treatment of a critical COVID-19 case [95]. Also, in a cohort study in patients with severe COVID-19, anakinra administration significantly reduced the need for mechanical ventilation in the patient group. Defined safety index, prompt dissociation, long therapeutic window and the ease of subcutaneous or intravenous administration has made anakinra popular with COVID-19 patients [93]. A phase II/III clinical trial study is exploring the therapeutic efficiency of simultaneous IL-1 (anakinra) and IFNγ (emapalumab) inhibition in order to ameliorating inflammatory and pulmonary problems (NCT04324021). Although there are certainly patients with uncontrolled levels of extravagant inflammation for whom the use of IL-1 and IL-6 inhibitory therapies can be beneficial, but no method has yet been found that can reliably identify these patients.
4.3. TNF-α inhibition
TNF-α is a pro-inflammatory cytokine that play destructive roles in the pathogenesis of many inflammatory and autoimmune diseases as well as SARS-CoV infection [96]. As the COVID-19 also has an inflammatory basis, it is supposed that TNF-α blocking agents maybe ameliorates cytokine storm and terminates subsequent alveolar and epithelial injury. Etanercept is an anti TNF-α monoclonal antibody that currently hypothesized to modulate immune responses in SARS-CoV2 infection [97]. However, it should be noted that due to the conclusive role of TNF-α in maintaining the structure of granulomas, there is a possibility of re-eruption of latent mycobacterium infections if TNF-α is inhibited.
4.4. GM-CSF inhibition
GM-CSF is essentially a proinflammatory cytokine that induced by various stimuli such as bacterial endotoxin and downstream of other inflammatory cytokines [98]. Various cell types including monocyte, macrophage, epithelial and endothelial cells, fibroblast and T lymphocyte are potential sources of GM-CSF in innate and acquired immune responses [98], [99]. Moreover, this cytokine plays dynamic roles in lung physiology. Intranasal utilization of GM-CSF in animal model of respiratory infections propitiated the symptoms and enhanced the alveolar macrophage (AM) expansion [100], [101]. Conversely multiple clinical studies have been detected the systemic and localized upregulated level of GM-CSF in the involved tissue of several inflammatory and autoimmune disorders as well as severe COVID-19-like syndromes such as ARDS and cytokine release syndrome (CRS) [102]. Patients with ARDS have high levels of GM-CSF in the bronchoalveolar fluid [103], [104], which contributes to the small blood vessels damage in the lungs by increasing neutrophil survival [105], [106]. Besides, elevated serum levels of GM-CS have been recently detected in patients affected by COVID-19 [13]. A Chinese online paper reported that in the lung of COVID-19 suffering particularly shut-in patients, massive gathering of GM-CSF and IL-6 by pathogenic Th1 and CD14+/CD16+ monocytes led to hyperinflammatory deleterious events [86]. Also, in pathological pulmonary lesions and bronchial lymphatic structures of coronavirus-related diseases including COVID-19, substantial infiltration of inflammatory monocyte, macrophage and DCs has been reported [107]. As the cytokine is a key growth factor of myeloid cells and promote proinflammatory features, most likely GM-CSF is a main exciter of immunopathological outcomes in COVID-19 patients. So, blocking GM-CSF receptor or soluble GM-CSF possibly quenching the exaggerated inflammation and confined the subsequent lung injury in SARS-CoV2 infected patients. Preliminary studies exhibited that COVID-19 patients who once received mavrilimumab, a blocking anti-GM-CSF receptor IgG4 monoclonal antibody, had upgraded blood oxygen level and lowered hospitalization. Also, certain randomized case control studies having started and in progress and some anti-GM-CSF neutralizing monoclonal antibodies including TJ003234 and gimsilumab will be explored in prospective trials. Lenzilumab, is the only anti-GM-CSF monoclonal antibody which has received FDA approval for expanded access in COVID-19 infection [107]. Inspired by the desirable achievement following intranasal administration of GM-CSF in mouse experiments, the alternative strategy is to apply human recombinant GM-CSF (hrGM-CSF) in such pulmonary failures. Sargramostim is a recombinant GM-CSF that daily intravenous administration was examined in patients suffering from lung injury. Findings showed no significant difference in the improvement of pulmonary symptoms and patient mortality rates [108]. Also, prescribing low dose of the other hrGM-CSF, molgramostim, as an antibiotic therapy in patients with generalized sepsis and pulmonary deficiency in a placebo-controlled trial restored pulmonary gas exchange and AM functionality with no change in a month mortality [109]. It is supposed that GM-CSF protects from viral-mediated injury and facilitates SARS-CoV2 clearance especially in the early stages while its remarkable elevation during disease progression as a part of cytokine storm made damaging sequels. So, in a clinical trial study the beneficial effects of sargramostim inhalation to ameliorating COVID-19-mediated lung injury is under consideration (NCT04326920). Nevertheless, it is necessary to remember that GM-CSF inherently accelerate microbial scavenging and pulmonary repair [110]. Therefore, further research is required to determine whether an increase in GM-CSF level is involved in the pathology of COVID-19 or as a compensatory response to the severity of the disease.
4.5. Vascular endothelial growth factor (VEGF) inhibition
VEGF is a growth factor that increases vascular permeability by releasing vasodilators such as nitric oxide (NO). Recent reports revealed that the elevated level of VEGF in the sera of patients with COVID-19 were associated with hypoxia, edema, and lung damage. Moreover, the role of VEGF in the pathogenesis of acute lung injury and ARDS has been well established [111]. Since acute pneumonia and respiratory distress occur in many COVID-19 patients, inhibition of VEGF can help improve pulmonary symptoms and lung function. Bevacizumab is a humanized monoclonal antibody against VEGF that was firstly approved for treatment of several cancers and eye diseases [112]. Accordingly, in a pilot study the efficacy and therapeutic potential of bevacizumab in severe COVID-19 patients is under investigation (NCT04275414). Therefore, with further studies and ensuring their safety, VEGF inhibitors can be licensed to improve respiratory symptoms in patients with COVID-19.
4.6. IFNs prescription
Coronaviruses commonly interrupt the antiviral responses of type I IFNs in target cells with multiple strategies. Therefore, inducing noticeable type I IFN responses has attracted the attention of scientists to combat SARS-CoV2 pandemics [113]. Since the type I IFNs activate the antiviral defense shield and revealed immunomodulatory activities, so its administration as prophylaxis in healthy people and also patients in the early stages of COVID-19 maybe beneficial. Promising results obtained from a phase II trial showed that subcutaneous injection of IFN-β-1b with antiretroviral drugs in mild and moderate cases of COVID-19 infection alleviated clinical symptoms, reduced hospital stay and rapid viral resolution with no specific side effects [114]. Nevertheless, lack of knowledge about the timing and appropriate dosing of type I IFNs and increasing the chances of immunopathology by further stimulating proinflammatory signals are the most important barriers to this therapy [115], [116]. So, type III IFNs is proposed as an alternative strategy that does not have the restrictions of type I IFNs such as direct activation of NK cells, immunopathology and cellular toxicity [117]. Pegylated IFN-λ, a type III IFN, is a potential therapeutic candidate that is being investigated in patients suffering from mild COVID-19 (NCT04331899). IFN-λ indirectly activate NK cells through IL-12 induction in macrophages and reduced tissue mediated injury by neutrophils in mucosal surface [118], [119]. However, finding implies that IFN-λ leads to delayed tissue repair and possibly elevates the risk of secondary infection in COVID-19 patients [120]. Therefore, considering the increase in mRNA level of type I and III IFNs along with inflammatory cytokines in the lower part of the lung in severe COVID-19, interferon or other cytokine therapies should be done with extreme caution at the appropriate time.
5. Complement cascade inhibition
The complement system as one of the humoral components of innate immunity participates in microbe and damaged cell clearance with the help of antibodies and phagocytic cells [121]. Uncontrolled complement activity may be associated with multiple microvascular injuries and inflammatory or immunothrombosis disorders in several organs. Since the COVID-19 is a hyperinflammatory syndrome along with diffused microvascular thrombosis, complement inhibitory therapies have attracted the attention of many researchers [122], [123], [124]. Elevated C5a and soluble C5b-9 (sC5b-9) membrane attack complex levels has been reported in the vascular bed of multiple organs especially the lungs of individuals with COVID-19 [125], [126], [127]. The C3 mRNA expression level also increased in the lung epithelial cell and nasopharyngeal swab samples of COVID-19 suffering patients [128], [129]. Notably the level of surface C3a receptor (R) and CD46, as a complement regulatory and cofactor protein, on various epithelial, endothelial, myeloid and lymphoid cells is closely related to the vulnerability to COVID-19 infection [130]. Studies have shown that the amplified signaling of C3aR, participating in inflammatory events of COVID-19 patients [131]. Probably enervated C3aR signaling, downsizing C3a-related opsonization of vascular and alveolar cells and subsequent tissue injury are the main involved reasons. In cohort studies around the C5 and C3 blocking in COVID-19 patients by eculizumab monoclonal antibody and AMY-101 cyclic peptide respectively, results showed that complement inhibition ameliorated hyperinflammation that exposed as intense decline in serum IL-6 and c-reactive protein (CRP), decreased neutrophil count and neutrophil extracellular traps (NETs) formation as well as evident lung performance correction and lymphocyte recovery [132]. Deviant NETosis containing tissue factor (TF) by COVID-19 neutrophils in proximity of intact C3 milling, leading to hyper coagulopathy disorders [124]. Moreover, the NET structure provides a trellis to reinforce the complement cascade activation. So, the AMY-101 therapy restored the lymphopenia by reducing the IL-6 inflammatory burden on peripheral lymphocytes and restraining C3 and C5 convertase activities. Also, the COVID-19-mediated thrombocytopenia was largely compensated by AMY-101 therapy. Unlike the AMY-101, patients treating with eculizumab showed persistent and high volume of C3a that in turn promoted neutrophil, monocyte and other inflammatory cells recruitment to the lungs and deteriorated coagulation problems. The blood level of factor B in eculizumab-treated COVID-19 patients was constantly diminished, indicating the unremitting activity of alternative complement pathway by SARS-CoV2 infection. Interestingly, the effects of C3 blocking were more pronounced since patients with COVID-19 that receiving AMY-101 drug experienced lower C3a and C5b-9 complex formation. Also, the rapid and early reduction of LDH in the presence of C3 convertase inhibitor indicated the greater importance of this treatment in improving lung damage and vascularization along with microvascular injuries [132]. The C3 cleavage is the intersection of different complement pathways, so its blocking appears to donate more significant therapeutic effects. Overall, it seems that C3 blocking likely more beneficial for COVID-19 patients because it both inhibits inflammation and cytokine storm and prevents thrombotic disorders and aberrant NET formation (Fig. 2) (Table 1).
6. Engineered products
6.1. CD24-Fc
CD24 is expressed at higher levels in metabolically active and progenitor cells with ambiguous functions, while plays crucial roles in innate and acquired immunities [133]. Surprisingly, it has been demonstrated that the CD24 is associated with several damage-associated molecular patterns (DAMPs) such as nucleolins, heat shock proteins (HSPs) and high mobility group box 1 (HMGB1) [134]. Siglecs are co-receptors that belongs to immunoglobulin superfamily and express on various immune cells [135]. Siglec-10 in humans by recognizing self-ligands induce B cell tolerance and prevent lethal inflammatory response by DCs to damaged tissues. Researches indicated that following CD24 interaction with Siglec10, it suppressed the pathological inflammatory responses to DAMPs (Fig. 2) (Table 1) [136], [137], [138], [139]. The CD24-Fc is a chimeric molecule consists of nonpolymorphic parts of the CD24 and Fc region of human IgG1 which can act as an immunomodulator [140]. Using CD24-Fc in phase I/II clinical trials in addition to exhibiting safety and suppressing several inflammatory cytokines in healthy volunteers, it mitigated the severity of graft versus host disease (GvHD) in hematopoietic stem cell (HSC) transplanted recipients (NCT02663622). Besides, CD24-Fc in preclinical study of HIV/SIV infected models recovered T lymphocytes number and functionality and confined multiple organ leukocyte infiltration. It is noteworthy that occurrence of pneumonia in SIV-infected monkeys decreased by 50% after the use of CD24-Fc [140]. Subsequently, this chimeric biological immunomodulator possibly considered as a nonspecific immune modifier for ameliorating severe COVID-19. Accordingly, a phase III clinical trial involving 270 randomized COVID-19 infected patients is evaluating therapeutic efficacy of CD24-Fc treatment and the results will be forthcoming soon (NCT04317040).
6.2. ACE2 blocking agents
As the SARS-CoV2 constantly mutates its cell surface ligands and subsequently escape from the neutralizing antibodies, one strategy is applying therapeutic agents that block the target of such ligands on host cells like ACE2 (Fig. 2). These have two main benefits, the first is that unlike the virions the possibility of any changes in the structure of the human ACE2 receptor is near to zero and secondly the viral pathogens don’t have the opportunity to exploit alternative receptor for infecting receptor-bearing cells [141]. Same as the SARS-CoV virions the S protein of the SARS-CoV2 chiefly its RBD key domain binds to ACE2 receptor and enter the host cells. In relation to the SARS, cell culture experiments showed that administering the RBD domain successfully harnessed the viral infection. Given the similarities, it can be assumed that this therapy helps to better handling the SARS-CoV2 infection. Due to the small size of RBD protein (193 amino acids), it penetrates effectively into biological tissues and nowadays is widely manufactured in bacterial hosts by Chinese research organizations [142]. Also, in order to enhancing the circulatory half-life of the engineered RBD molecules scientist have made RBD-Fc conjugates that effectively block viral infection in MERS mice models [141]. Besides the RBD-Fc fusion induced specific antiviral responses against the strange peptide. But it is noteworthy that attaching the FcRs to the Fc domain of the related conjugates lead to cytotoxicity even in normal cells, which is alleviated by creating dysfunctional Fc regions through mutations [143]. An alternative strategy is applying monoclonal antibodies that target ACE2 receptors in human cells that showed its blocking potentials in SARS researches. However, there is no published sequences for ACE2 antibodies but the related hybridoma cell line is accessible and clones during a few days [141]. Like the RBD-Fc fusion protein, the functional Fc domains of the related monoclonal antibodies should be changed or removed to extinguishing the flare of the subsequent inflammation. As the proper functionality of the Fc domain in human depends critically on the complex glycosylation process, so such therapies require the lagging, costly and mincing mammalian cell systems which considered as “after death the doctor” for several COVID-19 infected patients [141]. Nevertheless, Fc free structures such as single chain variable fragment, nanobodies and camelids-derived VHH domains are the other potential ACE2 targeting agents that due to their tiny size have considerable biological activities (Table 1) [144], [145]. But the main demerit is their short half-life in the absence of Fc domain. In general, there are many drawbacks to these treatments. Regarding the variability of the ACE2 expression profile in certain organs the required dose of RBD in order to blocking cell attachment throughout the body and the saturation percent of the ACE2 receptors is not known [146]. Besides, the turnover level of the ACE2 receptors on the surface of diverse cell types influence the need for reminder dose of the ACE2 blocking therapies. To compensate for this, especially in susceptible areas such as the lungs increased dose of the therapy should be administered locally [141]. Also, blocking the ACE2 receptor can disrupt the normal physiological functions of multiple organs and conversely, worsen the patient’s condition. In a murine model of SARS infection administering RBD-Fc conjugates worsen the lung edema [147]. Therefore, it seems that the best time for ACE2 blocking therapies is at the beginning of the disease or as prophylaxis.
6.3. S protein blocking agents
The S glycoprotein on the surface of coronaviruses consists of S1 and S2 subunits. The S1 part indicates the cellular specificity of virion and contains the receptor-binding domain (RBD), while the S2 part composed of the heptad repeats 1 (HR1) and heptad repeats 2 (HR2) consecutive domains that facilitate viral fusion process (Fig. 2) [148]. Therefore, by determining the protected peptide sequences in the subunits of S protein and targeting them in different ways, we can be armed against the spread of new coronaviruses. In this regard OC43-HR2P peptide derived from the HR2 domain of the human coronavirus OC43 (HCoV-OC43) extensively inhibited the integration of several human coronaviruses into target cell membrane. EK1 is the optimized structure of the OC43-HR2P peptide which made unchanging six-helix conformations with HR1 domain and effectively hampered fusion entry [149]. The HR1 domain is almost identical in SARS-CoV and SARS-CoV2 viruses, except for the difference of 7 amino acids in the center of the fusion region. This shift does not appear to affect the binding between EK1 and the HR1 domain, and hopefully has the potential to be considered as the potent inhibitor of SARS-CoV2 infection [148]. In general, mutations are more common in the S1, and the S2 subunit is more conserved [150]. So, targeting the S1 domain including the RBD epitopes by a mixture of monoclonal antibodies critically diminishes the chance of resistance to treatment by amino acid substitution [151]. According to the possible problems following anti-ACE2 therapy, a better alternative is to blocking viral infection through soluble forms of the receptor. By using the soluble ACE2 in cell culture experiments, it has been determined that the number of SARS infected cells diminished effectively [152]. Besides, the soluble receptor was able to bind to its target on the surface of the SARS virions with a comparable affinity similar to monoclonal antibodies [153]. So, it is supposed that the soluble ACE2 likely binds to the S protein of SARS-CoV2 virion with the similar affinity and was considered as a promising strategy to fight against COVID-19. Efforts to improve the biological activities of this treatment led to the design of a recombinant ACE2-Fc fusion protein. In this way purportedly scientists killed two birds with one stone because they both neutralized the viral particles and stimulated the phagocytic and NK immune cells through FcRs. The Fc domain from all IgG subclasses can be used, however previous findings showed that the IgG1 Fc domain has the highest ability to induce antimicrobial responses. In a mouse model of SARS infection, the Fc domain of the ACE2-Fc conjugate played a vital role in triggering antiviral responses and eliminating SARS infection [154]. As the in vitro results regarding the SARS neutralization by the recombinant molecule fusing the extracellular domain of ACE2 and the human IgG1 Fc domain (ACE2-NN-Ig) with the IC50 of 2 nM, reliable evidence has been obtained to strengthen the inhibitory potential of this fusion protein against the SARS-CoV2 infection [153]. A common complication associated with SARS and COVID-19 infections is a pneumonia-like syndrome which is accompanied by ARDS and lung failure. The main cause of such disturbing manifestations is the ACE2 removal or a decrease in its expression at least to some extent by the viral S protein [147]. Since the ACE2 is one of the inhibitors of the renin-angiotensin system, recombinant ACE2 administration mitigates the acute lung injury via diminishing angiotensin II production [155]. Also, the occurrence of ARDS significantly decreased following ACE2 consumption in H5N1 influenza [156] and RSV [157] infected patients. Accordingly, the hrACE2 was designed and entered to clinical trials with the hope of improving ARDS. The hrACE2 showed some tolerable on-target efficacies [158] like the Ang1-8 peptide reduction but same as the ACE2-Fc has no proven efficacy in ameliorating the ARDS in COVID-19 infection (Table 1) [159]. Findings showed that the region containing the amino acids 18 to 615 of the ACE2 is adequate for covering the SARS S protein [151]. Although, so far there is no precise information about the SARS-CoV2 S protein and its binding characteristics but given the similarities, it can be assumed that the same sequence of the ACE2 also binds to the SARS-CoV2 S protein. Though it should be considered that likely the SARS-CoV2 lowers its binding affinity to ACE2 molecules via mutation and escape neutralization like the re-emergent SARS infection in 2003 [160]. The other limitation is the unknown effects of the elevated level of extracellular ACE2 which requires further investigation as the cells normally secrete a small amount of this receptor [161]. Generally, the ACE2-Fc therapy is well-tolerated but if still concerning the enzymatic activity of the ACE2 could be incapacitate via targeted mutation while the binding domain remains intact. However, some researchers suggest that maintaining the peptidase activity of the ACE2 is important for improving lung injury [141]. Also, there are some concerns about the unsought transmission of the virions via the CD16 to competent immune cells as it was proved in ex vivo experiments around the MERS neutralizing antibodies [162].
7. Cell-based therapies
7.1. Natural killer (NK) cell supply
NK cells are the key innate immune responders to multiple human viral infections and play decisive roles in immunosurveillance and immunomodulation. Clinical studies showed that the NK cell numbers significantly reduced in COVID-19 patients and most expressing the CD markers related to exhausted phenotype. Considering this, exhaustion and reduced number of peripheral NK cells may be the reasons of unbridled progression of COVID-19 infection [120]. Furthermore, the traces of NK cells in the immunopathology of many viral infections such as RSV [163] and influenza A [164] have been determined. Zheng et al. initially revealed an inverse and significant relationship between the severity of COVID-19 infection and the amount of NK cells in the peripheral blood [165]. Besides the NK cell responsivity had decreased markedly and appeared as upregulated NKG2A inhibitory receptor and diminished IL-2, IFNγ, TNF-α and granzyme B secretion. Corresponding to the reduction of disease severity the number of NK cells with lowered NKG2A in the peripheral blood of COVID-19 patients also increased [165]. Besides, patients with mild COVID-19 had more NKG2A+/CD94+ NK cell infiltrate in the fluid from pulmonary lavage than in severe disease [166]. Other similar studies showed reduced mature peripheral NK cells in COVID-19 patients with ARDS and pneumonia-like manifestations along with elevated CD39, NKG2A and programmed cell death protein 1 (PD-1) expression levels [167]. In a case-control study of 7 patients with COVID-19, it was found that both CD56bright and CD56dim NK cell subpopulations vanished in severe cases, while the CD56dim cells were the only NK subtypes in milder forms of COVID-19. Also, the level of lymphocyte-activation gene 3 (LAG-3) and t-cell immunoglobulin and mucin-domain containing-3 (TIM-3) exhaustion markers were elevated on the surface of COVID-19 NK cells [168]. Although the exact mechanism of SARS-CoV2-mediated NK depletion is not yet known, but its reduction in peripheral blood is apparently due to the entry into extravascular affected areas [120]. Moreover, Inhibitory factors especially TGF-β appears to be involved in petrifying NK cells. In this regard Huang et al. revealed that the patients with SARS infection had higher serum level of TGF-β compare to the control group and significantly correlated with recovery of patients [169]. In return, findings corroborated the role of NK cell-mediated cytokine storm and subsequent hyperinflammatory pulmonary failure. Accordingly, although the initial presence of NK cells is critical to fighting against infection, paradoxically, extreme and constant stimulation of these cells lead to systemic inflammation, cellular exhaustion, and tissue damage [120]. Therefore, therapeutic approaches that rehabilitate the attenuated functionality and homeostasis of NK cells possibly are respected for COVID-19 patients. Since NK-based therapies were substantially proposed to handle cancer patients, employing similar strategies and fuel mechanisms provide anti-COVID-19 policies. The CYNK-001 product is the only NK-based remedy which has been approved by FDA for clinical analysis in COVID-19 patients [170]. A phase I/II clinical trial is evaluating the therapeutic potential as well as unwanted side effects of CYNK-001 therapy in outpatients of COVID-19 (NCT04365101). Also genetically manipulated NK cells and engineered chimeric antigen receptor (CAR) NK cells recognizing arbitrary targets are the other possible options on the table [170], [171]. However, because CRS is more likely to occur after the CAR-related products administration, their evaluation in patients with severe COVID-19 should be performed with extreme caution [172]. Another phase I/II study on newly COVID-19 infected individuals which is supposed to examine the effects of NKG2D, ACE2 and NKG2D-ACE2 CAR-NK cell constructs compare to normal and IL-15-secreting NK cells is underway [173]. As the previous reports showed elevated NKG2D ligand expression on the cell surface following viral infection, NKG2D containing CAR-NK cell therapies are more promising [174]. The alternative strategy with unknown outcomes is deliberate ACE2 expression on NK cells to facilitate SARS-CoV2 clearance. However, it is not yet clear whether ACE2-NK cells operate as moving traps to prevent the viral spread or later become a viral repository [175]. In the case of IL-15-producing NK cells, it should be noted that IL-15, as a proinflammatory cytokine, may deteriorate the patient’s state. According this, in some cases of persistent inflammatory lung ailments, elevated level of IL-15 has been observed [176], [177]. On the other hand, based on experiments performed on juvenile and aged monkey models of SARS-CoV infection, it was found that the level of this cytokine was high in young monkeys, while aged monkeys showed lower levels [178]. For this reason, this type of NK cells seems to better tolerated by aged people with COVID-19, although it has not yet been confirmed. In general, due to the limitations of using external sources of NK cells and the need to spend a lot of time and money to produce engineered NK cells, so it seems that manipulating the patient’s own NK cells will bring more benefits. Blocking NKG2A by monoclonal antibodies restored the attenuated NK cell cytotoxicity [179] while its therapeutic effects in COVID-19 patients have not yet been studied. Bystander activation of NK cells via inducing innate immune cells such as macrophage and plasmacytoid dendritic cells (pDCs) possibly effective in coronavirus clearance and dampening COVID-19 severity [120]. Administering ascorbic acid or vitamin C also revitalized NK cell cytotoxicity and enhanced the IFNγ production by NK cells. Moreover, the CD25, NKp46 and CD69 stimulatory receptors upregulated following vitamin C consumption on NK cells of influenza bearing patients [180]. Another therapeutic option are recombinant cytokines to modulating NK and the other immune cell functions including IL-2, IL-15:IL-15R heterodimers or IL-15 “super agonists” (Fig. 2) (Table 1) [175].
7.2. Mesenchymal stem cell (MSC) adoption
MSCs are heterogeneous cluster of stromal cells that reside in diverse tissues, including bone marrow, umbilical cord and adipose tissue [181]. Among their characteristics, high replication, self-renewal, differentiation into various cell types, low immunogenicity, tissue repair as well as immunomodulation are more prominent. The immunomodulatory and suppressive activities of MSCs impress both innate and acquired immunities (Fig. 2) [182]. Several lymphocyte subpopulations, professional APCs, and many soluble inflammatory factors, such as IFNγ, TNF-α and IL-1, are affected by MSCs therapy [183]. In response to inflammatory stimuli, MSCs produce large volumes of immunosuppressive agents, including indoleamine-pyrrole 2, 3-dioxygenase (IDO), prostaglandin E2 (PGE2) and IL-10. For example, PGE2 lonely suppresses the function of macrophages, NK cells and T cell subsets and severely reduces T cell proliferation along with the IDO [184]. Moreover, these stem cells participate in tissue repair by interacting with multiple immune and non-immune cells [185]. MSCs are valuable sources of chemokines and growth factors including CCL2, CXCL9, CXCL10, CXCL11, VEGF, fibroblast growth factor (FGF) FGF, PDGF and EGF and improve the restoration of virus mediated lung injury through altering the tissue microenvironment as well as the polarity of immune responses [186]. In vivo experiments showed that MSC injection to animal models of H5N1 influenza impressively ameliorates lung lesions [183]. Moreover, epigenetic changes caused by MSC through Chaf1 and Sumo2 molecules led to inhibition of multiple viruses (Table 1) [187]. To date, several safety studies have confirmed MSC therapy, while many restrictions such as large-scale production, maintenance, providing the appropriate dose and effective injection route have remarkably limited its application [80]. Nevertheless, multiple ongoing clinical trials have been exploring the virtue of MSC therapy in SARS-CoV2 infections [188], [189]. In a recent clinical study in COVID-19 bearing patients intravenous administration of MSCs surprisingly improved lung function and the patient’s clinical symptoms. Moreover, peripheral blood analysis indicated roll back recovery of lymphocyte count, reducing CRP and TNF-α intensity and depleting hyperinflammatory immune cells. Similarly, a group of tolerogenic DC subsets substantially magnified following MSC therapy [190]. Leung et al. showed that a single (106 cells/kilograms of body weight) injection of MSCs improved the pulmonary gas exchange and reduced inflammatory cytokines production. Conversely, the level of serum IL-10, peripheral Treg cells and CD14+/CD11c+/CD11bmid DC cells with immunomodulatory effects were significantly increased [191]. In addition to MSCs, the endogenous lung progenitor cells are the other sources of stem cells for replacement of injured lung cells. Nevertheless, as yet there is little evidence regarding their efficiency in lung tissue repair.
8. Immune potentiators
Viral sepsis following an exorbitance inflammatory response in the lungs of COVID-19 suffering patients is a common finding that eventually leads to death via septic shock and ARDS. Several physiological processes including innate and acquired immune responses, are disrupted by viral sepsis. Findings showed that nonspecific anti-inflammatory agents were unable to ameliorate hyperinflammatory syndrome by viruses. The purpose of immune potentiator therapeutic strategies is to stimulate innate and acquired immune responses via multiple mechanisms in order to eliminating viral infections. These are including antimicrobial peptides, immune checkpoint inhibitors, pattern recognition receptor (PRR) ligands and signaling compartments. One of the signs of immunosuppression during viral sepsis is the increased expression of inhibitory checkpoint molecules such as PD-1 and PD-L1 on the surface of T cells which leads to lymphocytopenia [192]. Also, elevated PD-1 modulates NK cell activity and its increased expression in COVID-19 patients has been demonstrated in previous studies [193]. Various clinical and preclinical studies have shown that blocking such inhibitory molecules recovered the weakened immune response and diminished the viral resistance [194]. So, devitalization of PD-1 and/or PD-L1 possibly considered as a beneficial anti-sepsis therapeutic candidate in COVID-19 infecting patients. The efficacy of neutralizing antibodies against PD-1 alone or in combination with thymosin, as a thymus-derived hormone, are being investigated in SARS-CoV2 cases (NCT04268537). This treatment is especially useful in obese people who have high levels of serum IL-6 as well as overexpression of PD-1 on the surface of their T cells. Also, blood analysis of patients with SARS-CoV2 infection indicated that the number of TIM-3+ CD8+ T cells increased with the disease progression [195]. So, as shown in influenza infection and cancer research using immune checkpoint inhibitors that target PD-1 and TIM-3 on exhausted T cells possibly reestablish the paralyzed cellular immunity. The other solution is to hindering the secretion of cytokines that are involved in the COVID-19 pathogenesis. In addition to elevated levels of inflammatory cytokines, anti-inflammatory agents may also be involved in lung damages. TGF-β1 is one of the anti-inflammatory cytokines that has been linked to pulmonary fibrosis and decreased apoptosis of virus-infected cells in people with SARS infection [196]. Similarly, increased levels of this cytokine have been detected in SARS-CoV2 infection, so its blocking likely inhibit pulmonary complications. Pirfenidone is a potential therapeutic option that reduces cytokine-induced fibrosis and extracellular matrix formation by TGF-β1 and PDGF induction. Also, significant anti-inflammatory and antioxidant effects of this drug have been proven [197]. Therefore, a clinical trial is studying the therapeutic potentials of pirfenidone in the recovery of COVID-19 patients (NCT04282902). Further researches showed that unlike the TGF-β1 the expression level of IFN-α/β is downregulated in MERS-CoV and SARS-bearing individuals [198], [199]. Moreover, it is specified that the IFN-α/β signaling pathway was not activated upon SARS infection [200]. Type I IFNs are the main mechanism of innate humoral immune defense against viral infections and play an important role in restraining viral replication and dissemination. Therefore, many studies are investigating the therapeutic effects of various type of IFNs in COVID-19 patients. But the alternative strategy is using viral dsRNA and ssRNA as the TLR3 and TLR7 ligands respectively. These ligands not only produce IFN-α/β and inflammatory cytokines but also lead to faster viral eradication [201]. So, a current clinical trial is trying to explore the antiviral efficacy of an dsRNA analogous in COVID-19 infecting patients. Besides, demethylated CpG islands is the most well-known ligands of TLR9 that showed hopeful protective results in SARS-CoV infection [202]. Two clinical trials are examining the preventative effect of the PUL-042 aerosol drug containing TLR 2/6 and TLR9 agonist on SARS-CoV2 infection (NCT04313023, NCT04312997). Another treatment option based on the innate immune system components are defensins. These natural antibiotic peptides seem to be useful against SARS-CoV2 due to their proven antimicrobial, antifungal and especially antiviral potentials [203]. Previous results showed that defensin administration in SARS mouse models only succeeded in altering the cytokine milieu of the lung parenchyma without any change in viral replication and lung injury. Although its prophylactic application intranasally in mouse models significantly protected them against SARS infection [204]. IL-7 is a multipotent growth factor that differentiates bone marrow hematopoietic stem cells toward lymphoid progenitor cells and stimulates the proliferation of all lymphoid lineages including NK, B and T lymphocytes. Besides, it promotes NK and T cell survival, B cell maturation and participates in development and immune hemostasis (Fig. 2) (Table 1)[205]. Nowadays the IL-7 applies as an immunotherapy to recover lowered lymphocyte counts and reinforce the lost immune responses in sepsis and JC virus-mediated leukoencephalopathy [206], [207]. Therefore, IL-7 should be considered as a potential treatment option in COVID-19 patients. Surprisingly, unlike IL-6 inhibition in patients with sepsis involving cytokine storm disaster similar to that of SARS-CoV2 infection, administering IL-7 or PD-1 blocking agent (nivolumab) left beneficial effects without worsening the inflammation [208].
9. Nonspecific therapies
9.1. Calcineurin inhibitors
Calcineurin inhibitors including tacrolimus and cyclosporine are immunosuppressive agents that was mainly prescribed in solid organ transplantation. Recently, their ability to subtract hyperinflammation in patients with moderate to severe COVID-19 has been considered. Findings showed that tacrolimus by blocking calcineurin restrained initial activation and inflammatory cytokine production in T cells (Table 1). Thus, tacrolimus could possibly suppress the induced cytokine storm in COVID-19 patients. The cell experiments exhibited that the tacrolimus effectively blocked SARS-COV replication in target cell line. Also, a case study of two kidney transplanted patients with MERS-CoV infection showed that administration of tacrolimus as part of the transplant process greatly improve clinical symptoms. However, there is no evidence that cyclosporine is effective in ameliorating COVID-19 infection [209], [210].
9.2. Corticosteroids
Corticosteroids are basically divided into two main classes including mineralocorticoids and glucocorticoids. Last reports have shown that corticosteroids are widely used to alleviate pulmonary inflammation and prevent subsequent lung injury by viral infections such as SARS-CoV and MERS-CoV. Therefore, corticosteroids specially glucocorticoids also appear to be useful in relieving hyperinflammation and lung damage in COVID-19 patients. Glucocorticoids and their derivatives have been used for many years to maintain the condition of patients with diverse autoimmune diseases mainly through immunosuppressive activities (Table 1). As mentioned, ARDS is a hyperinflammatory syndrome that benefits from glucocorticoid therapies. In coronavirus infections ARDS, severe pneumonia and alveolar injury-mediated inflammation are the common manifestations. So, glucocorticoids are considered appropriate treatment options to reduce inflammation and lung damage in SARS-CoV2 involving patients. A published clinical trial study in June 2020 found that dexamethasone reduced the mortality rate of under ventilated COVID-19 inpatients by one-third compared to the control group. Thenceforth, several clinical trials have been designing to explore the healing effects of multiplicity of steroid and non-steroid anti-inflammatory agents to abolish lung injury in COVID-19 patients [211]. Ciclesonide is the only glucocorticoid with known anti-COVID-19 antiviral activities that is being tested in ongoing trials in adult patients with mild COVID-19 (NCT04330586). Although, it is noteworthy that the administration of corticosteroids, as seen in some patients with MERS-CoV, can impair antiviral defense and delayed the clearance of the virus [212]. For this reason, as well as the risk of ARDS, corticosteroids should be more cautiously prescribed to COVID-19 patients and should be discontinued to ensure the results of the present clinical trials.
9.3. Non-steroidal anti-inflammatory drugs (NSAIDs)
NSAIDs are a group of over-the-counter medications including indomethacin, ibuprofen, naproxen etc. that are commonly prescribed to relieve pain, fever, and inflammation [120]. Evidences suggested that indomethacin blocks SARS-CoV reproduction in vero cell line and naproxen had antiviral potentials against influenza virus [213]. Accordingly, a clinical trial is being evaluated the therapeutic benefits of naproxen on sever COVID-19 patients (NCT04325633). Oppositely findings showed that ibuprofen interfere with natural immune responses including antibody production and many of NSAIDs suppress cytokine production by NK cells (Table 1) [214]. Moreover, the ACE2 expression levels increased significantly in the presence of ibuprofen and facilitates SARS-CoV2 entry [215]. Therefore, the effects of each of the NSAIDs should be evaluated before administration to COVID-19 patients.
9.4. mTOR inhibitors
ADE or immune enhancement is a phenomenon by which the entry of the viral pathogens into host cells is mediated by the induction of cross-reactive and sub optimal antibodies. Thus, the virions escape from the immune system arms and continues to untroubled proliferation. Previous results demonstrated that mammalian target of rapamycin (mTOR) inhibitors possibly by banning the memory B cell activities prevented the ADE in viral infections (Fig. 2) (Table 1). As the mTOR inhibitors could effectively restrain the MERS-CoV replication in laboratory studies [216], so the rapamycin and sirolimus were supposed to take over the COVID-19 pandemic. Sirolimus have been shown to block viral dissemination and replication in patients with severe respiratory failure [217]. Moreover, multiple in silico analysis inaugurate the therapeutic potential of sirolimus in SASR-CoV-2 infection [218]. In this regard, a recent clinical ongoing study has been evaluating sirolimus in COVID-19 suffering patients.
9.5. Metabolic enzyme blockers
These are a broad spectrum of medicinal compounds that interfere with metabolic pathways and they are usually prescribed in treating leukemias and various solid tumors. In addition, these metabolic toxins are able to curtail transplant rejection by immune suppression via disturbance in the proliferation of T lymphocyte progenitors. Papain-like protease is a vital enzyme in processing coronavirus polyproteins and required for viral replication and spread (Fig. 2). Previous results indicated that some antimetabolite drugs including 6-mercaptopurine, mycophenolate mofetil and 6-thioguanine counteracted with papain-like proteases in MERS-CoV and SARS-CoV virions. Therefore, antimetabolite drugs may be useful in suppressing inflammatory responses and inhibiting the proliferation of SARS-CoV2, however no preclinical and clinical confirmations of their efficacy in COVID-19 patients have not yet been reported (Table 1) [97], [219].
10. COVID-19 vaccine-a high priority need
To date, several therapeutics including immune and non-immune based approaches have been introduced to inhibit SARS-CoV2 replication and strengthen or reprogram the immune system. Although, there is a lack of definitive or specific remedy to control this infectious disease. With the previous history of combating similar viral prevalence, almost all researchers worldwide have convinced that designing an effective and safe vaccine is the only (probably most effective) way to protect especially vulnerable populations and expunge the COVID-19 from the community. Our knowledge around the molecular and immunogenic structures of SARS-CoV2 is very limited, but scientists have employed existing similarities (SARS-CoV) and relied on previous knowledge to rationally develop antigen candidates for COVID-19 vaccine development. Several studies have shown that the N and S structural proteins are the most pivotal targets in the design of the COVID-19 vaccine [220], [221]. The N protein with high immunogenicity is vastly expressed during viral infection, while the S protein is the key to the virus entering the host cells and its epitopes are the main targets of many neutralizing antibodies [147], [222]. Moreover, the dominant and long-lived cellular immune responses against N, S and M viral proteins have been detected in sundry COVID-19 patients [223]. Therefore, using the gained knowledge and experience near other scientific findings, as of February 5, 2021, 63 COVID-19 vaccine candidates are passing through the phase Ι to ΙΙΙ clinical trials in humans and 20 of which have reached the final large-scale efficacy analysis. Another 175 candidate vaccines are completing the preclinical evaluations in animal models [17]. Whole viruses (inactivated (killed) and live attenuated), protein subunits, virus like particles, recombinant DNA or RNA, replicating and non-replicating vectors along with artificial APCs constitute the forerunner platforms for COVID-19 vaccine scenario. Live attenuated viral vaccines elicit long-lasting antibody and T cell responses without the need for adjuvants and considered as suitable candidates to achieve herd immunity. Multiple passages in culture medium or modern codon deoptimization technology is commonly used to create attenuated viral strains. However, there are reports of pathogenic conversion and safety concerns in live attenuated vaccines. In contrast, killed vaccines do not chase the same safety concerns, but rely on adjuvants and booster doses to induce effective immune responses. Protein subunits along with recombinant DNA and RNA-based vaccines are the potent stimulants of CD4+ and CD8+ T cell responses, although their fickle stability in the in vivo environment poses challenges to the design of such vaccines. In addition, vaccines with nucleic acid platform, especially mRNA-based vaccines, should be addressed with more caution in human experiments due to the lack of solid evidence concerning disturbance in natural cellular mechanisms and to shove the cell towards malignant valley. Viral vectors, both replication-incompetent and replication-competent ones, also have been used extensively in COVID-19 vaccine researches and showed promising results. However, it should be noted that the presence of pre-formed immune responses against human viral vectors such as type 5 and 26 adenoviruses most likely reduce their effectiveness. Therefore, using animal viral vectors (e. g., chimpanzee adenoviral (ChAd) vector) or different priming and boosting vectors can solve this problem. Viral-like particles also provide the intact structure of viral antigens and stimulate the immune responses like the natural infection. Though, attributable to the lack of genetic material and inability to reproduction in the host body, they need a reminder dose and adjuvant [192], [224]. Out of 12 leading vaccine candidates against COVID-19, BBIBP-CorV (inactivated), BNT162b2 and mRNA-1273 (mRNA-based) were emergency approved for full use and another 8 vaccines were licensed for limited or initial practice in some countries. The two other vaccines including Ad26.COV2.S by Johnson & Johnson and NVX-CoV2373 by Novavax are going through the phase ΙΙΙ clinical trials but have not yet received any approval for clinical application [225]. Further information on pioneering vaccine candidates is shown in Table 2 .
Table 2.
Major characteristics of pioneering COVID-19 vaccine candidates.
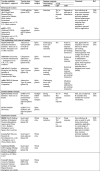 |
aComirnaty and tozinameran; bSputnik V; cCovishield; dConvidecia; ePiCoVacc; fBBV152 A, B, C; rAd5, recombinant human serotype 5 adenovirus; rAd26, recombinant human serotype 26 adenovirus; ChAd, chimpanzee adenovirus; SARS- CoV-2, severe acute respiratory syndrome coronavirus 2; LNP, lipid nanoparticle; Th, T helper; S, spike; rPA, recombinant peptide antigen; U.S, United States; E.U, European Union; U.A.E, United Arab Emirates; NIAID, National Institute of Allergy and Infectious Diseases.
11. Bird’s-eye view immunotherapy challenges
Like many other remedies, immunotherapeutic approaches along with all the potential benefits, often come with tangible weaknesses and limitations. Such limitations are especially thoughtful in relation to vaccines as to be administered to a wide range of people with different health status. Since the pandemic stretches and COVID-19 vaccines are being produced and engaged in local quantities, the mentality is shared that the early endpoint of this vaccination most likely is to prevent disease rather than infection prevention. That is to say, although these vaccines can prevent infections leading to more serious illnesses and hospitalization and subsequently reduce the burden on patients in hospitals, but without the evidences, it seems that current vaccines have little effect on avoiding and the rate of COVID-19 infection in society (as a major indicator of social and political concern). Considering this, even if a large percentage of the population is vaccinated, the infection will still diffuse and herd immunity will be essentially ineffective and will become a fallacy. Another issue is unwanted side effects such as documented severe allergic reactions, which is sometimes problematic. Life-threatening anaphylactic reactions often occur in response to vaccine contents and eliminated by epinephrine injection. However, in large populations the occurrence of such complications in a very small proportion of vaccinated individuals is not uncommon and is inevitable. Similarly, other immunotherapeutic options have more general constraints beyond their own fluctuations, especially in populous countries, which challenge their application. In such communities, population heterogeneity and genetic diversity created by evolutionary selection have given rise to a wide range of susceptibility and immunity to novel pandemics such as COVID-19 [228]. This heterogeneity can provide different responses to similar immunotherapies and make it difficult to decide on the effectiveness of treatment. On the other hand, the high rate of disease transmission as well as the large number of COVID-19 patients often succeeds in confronting immunotherapeutic agents, so that the manufactured immune-based therapies will not meet the needs of patients in waiting line. Studies have shown that the use of immunotherapies such as CP and monoclonal antibodies in the best case will be mainly useful for hospitalized patients and in general will not have much effect on changing the disease status in the population [229]. Environmental factors for example the risk of local chance of exposure to COVID-19 and limited access to support services, infusion units and intensive cares can also affect the efficacy of immunotherapies in densely populated countries. In this regard, setting out the clinical trials of COVID-19 immunotherapies in overcrowded areas is also challenging and requires special attention. Unlike recent studies, small case series cannot reflect the predominant population response to immunotherapies and mostly randomized, multi‐center controlled trials are needed [230]. However, the large population and uninterrupted transmission of infection in the current situation is problematic and greatly slows down the research process. Also, follow-up patients before and after treatment is a daunting task in the population overload and often owing to non-intelligent and worn recording systems, mainly limited evidences are available from under-treatment individuals. Therefore, due to the unintentional mismatch of the study groups, reliable results may not be achieved and may be associated with errors or false results. Patients admitted to the clinical trials also need constant follow-up, which is far-fetched by busy healthcare providers in houseful communities with large numbers of COVID-19 patients [230]. In any case, the set of limitations are key aspects that should be carefully considered in the design of immunotherapy protocols and clinical trial studies, especially in densely populated countries, in order to provide accurate and correct results and open up the door toward the possible hope for the COVID-19 tragedy.
12. Discussion and future perspectives
Despite the growth of the knowledge of scientific societies about the new SARS-CoV2, as the causative agent of COVID-19, no effective or specific therapy has been found so far. Therefore, the solution is to find a treatment option that can both alleviate patients’ symptoms and prevent death. This could be provided through immunotherapeutic approaches, since traces of immune components have been observed at different stages of COVID-19 immunopathology. Modulation of immune responses by intervention methods should be collimated based on the stages of the COVID-19 disease and it is usually done through vaccines or other immunotherapies including CP and IVIG, monoclonal antibodies, cytokine therapies, kinase and complement inhibitors, cell-based therapies and nonspecific anti-inflammatory and immunomodulatory drugs. Vaccines are the most impressive step toward preventing and control infectious pandemics such as COVID-19 in pre-disease stage. However, manufacturing and fair distribution of effective vaccines is costly and not possible in a short time especially in low-income countries. Out of 12 pioneering vaccine candidates for COVID-19, only BNT162b2, mRNA-1273 and BBIBP-CorV have received emergency approval for clinical application in some countries, and the highest hope is for their success [225]. BNT162b2 and mRNA-1273 showed significant safety with 95% and 94.5% efficacy respectively against SARS-CoV2 [231], while the Sinopharm stated that the other potential candidate, BBIBP-CorV, is 79.34% effective [225]. Other leading vaccine candidates have shown promising results and licensed for emergency or limited use but have not yet received approval. Nevertheless, lack of solid documentation regarding the sustainable efficacy of existing vaccines, clarification of their precise mechanisms of action and future safety concerns, have faced their general approval with restriction and caution. So, we have a long way to go to bring a safe and effective vaccine to market. With considering this, stimulation of immune responses using other immunotherapeutic approaches and compensating for the lost antiviral immune responses by SARS-CoV2 is the most important measure for patients to recover in the early stages of the disease. Among the mentioned approaches and therapeutic agents, it seems that ACE2-Fc recombinant product has a greater chance of successful harnessing the SARS-CoV2 infection. Because, in addition to supplying ACE2 deficiency in the lungs and improving pulmonary damage, neutralizes the SARS-CoV2 and also stimulates immune responses to remove viral particles. Besides, the ACE2-Fc can be used as prophylaxis in high-risk personals such as clinical providers and people who may have been exposed recently. Unlike CP and H-IG preparation the ACE2-Fc fusion protein construction is independent to infected individuals and could be scaled straightforwardly. Also emerging the scape viral mutants against ACE2-Fc, unlike monoclonal antibodies, is rare, because mutations in S protein will subsequently reduce the pathogenicity of SARS-CoV2. The coding sequence of the ACE2-Fc biologic is available and simply could be placed inside desired protein expression vectors. Besides, the ACE2-Fc is produced in a short time and thanks to the presence of the IgG Fc domain is easily purified with the help of staphylococcal protein A. Reciprocally, erecting an effectual vaccine is challenging because providing effective immunogenicity, inducing the appropriate immune response, determining the correct adjuvant, etc. are very complex, and the vaccine will not provide protection for people who have recently become infected with the viral agent. Therefore, maybe the ACE2-Fc in the role of a neutralizing antibody ceasing the spread of this pandemic and be the treatment that many researchers are still looking for. Also, the RBD-Fc construct is the alternative that in addition to block viral adhesion likely induce protective antibody response against RBD. However, this therapy has limitations regarding interference with ACE2 natural activities as well as attracting immune responses toward self-tissues. In the next step the soluble RBD and hrACE2 could be considered as therapeutic option because their half-life is shorter than immunoadhesins and owing to the variable expression of ACE2 in different tissues, it is difficult to achieve the appropriate therapeutic concentration. The other spike protein blocking agents like the recombinant OC43-HR2P and EK1 peptides, greatly avoid the viral spreading and multiplying in other tissues, although this group of treatments, similar to monoclonal antibodies, have little chance of success because of mutations in their molecular targets. The other non-specific chemicals and therapeutic strategies that are commonly used to handle infectious and inflammatory syndromes or even immunosuppressive drugs, may help to alleviate cytokine storm intensity and ameliorate inflammatory and coagulopathy manifestations in critically ill patients, but it is unlikely to have much effects on viral spread and eradication. Anyhow, as reaching a confident vaccine or definitive strategy to pause SARS-CoV2 propagation is time- consuming and given the potential of immunotherapeutic strategies in similar coronavirus infections alongside the results of recent trials in COVID-19 patients, it seems that the effectiveness of such therapies in improving the condition of COVID-19 patients is encouraging.
13. Conclusion
Overall, it seems that universal vaccination is possibly the most successful way to eradicate COVID-19 infection. So far, significant progress has been made in the manufacturing of COVID-19 vaccine candidates, but regarding various unknown aspects of SARS-CoV2 pathogenicity and safety concerns around the vaccine contents, it seems that we are still at the beginning of the road and attaining effective vaccines will take a long time. Therefore, given the decisive role of the immune system in the course of COVID-19 infection, modulation of immune responses using corresponding immunotherapies based on the stage of the disease along with virus-neutralizing engineered products particularly ACE2-Fc is a sensible strategy. Also, the novel RBD-Fc or hrACE2 are the other alternatives that could also be placed on the tracking command. Therefore, immunotherapies have a considerable capacity to somewhat unravel the COVID-19 enigma and can probably be used at least as adjunctive therapy. However, their widespread prescription requires further researches in the phases of clinical trials.
Authors’ contribution
Maryam Bayat, Mohammad Reza Mohammadi, Mahsa Sanaei, Mozhdeh Namvarpour, Reyhaneh Eftekhari and Yahya Asemani were involved in data collection, classification of information and writing the manuscript. Interpretation of the data and revision of the manuscript was done by Yahya Asemani and Maryam Bayat.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
References
- 1.Zhu N., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya A.E., et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses–a statement of the Coronavirus Study Group. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal M., et al. Severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2): An update. Cureus. 2020;12(3) doi: 10.7759/cureus.7423. e7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne S. Family Coronaviridae. Viruses. 2017:149–158. [Google Scholar]
- 5.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis, Advances in virus research. Elsevier. 2011:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King A.M., et al. Elsevier; 2011. Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. [Google Scholar]
- 7.Zou X., et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan C., et al. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection. Front. Med. 2020;2(12) doi: 10.3389/fmed.2020.563893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui J., et al. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Báez-Santos Y.M., et al. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziebuhr J., et al. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81(4):853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 12.Razzaque M.S., Taguchi T. Pulmonary fibrosis: cellular and molecular events. Pathol. Int. 2003;53(3):133–145. doi: 10.1046/j.1440-1827.2003.01446.x. [DOI] [PubMed] [Google Scholar]
- 13.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baig A.M., et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 15.Yang X., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y., et al. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.W.H. Organization, COVID-19 - Landscape of novel coronavirus candidate vaccine development worldwide 2021. https://www.who.int/docs/default-source/coronaviruse/novel-coronavirus_landscape_covid-1942359771-d8bb-4f89-8b72-5b02b0d69acf.xlsx?sfvrsn=617c8d90_6&download=true.
- 18.Bloch E.M., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Investig. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morabito C.J., Gangadharan B. Active Therapy with Passive Immunotherapy May Be Effective in the Fight against Covid-19. Clin. Transl. Sci. 2020;13(5):835–837. doi: 10.1111/cts.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin R.J., McLaughlin L.S. Plasma components: properties, differences, and uses. Transfusion. 2012;52:9S–19S. doi: 10.1111/j.1537-2995.2012.03622.x. [DOI] [PubMed] [Google Scholar]
- 21.Du L., et al. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsueh P.-R., et al. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin. Microbiol. Infect. 2004;10(12):1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiberghien P., et al. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how? Vox Sang. 2020;115(6):488–494. doi: 10.1111/vox.12926. [DOI] [PubMed] [Google Scholar]
- 24.Cao W.C., et al. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl. J. Med. 2007;357(11):1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 25.Abraham J. Passive antibody therapy in COVID-19. Nat. Rev. Immunol. 2020;20(7):401–403. doi: 10.1038/s41577-020-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lünemann J.D., et al. Intravenous immunoglobulin in neurology—mode of action and clinical efficacy. Nat. Rev. Neurol. 2015;11(2):80. doi: 10.1038/nrneurol.2014.253. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojas M., et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102554. 102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma M., et al. Intravenous immunoglobulin-induced IL-33 is insufficient to mediate basophil expansion in autoimmune patients. Sci. Rep. 2014;4(1):1–6. doi: 10.1038/srep05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tjon A.S., et al. Intravenous immunoglobulin treatment in humans suppresses dendritic cell function via stimulation of IL-4 and IL-13 production. J. Immunol. 2014;192(12):5625–5634. doi: 10.4049/jimmunol.1301260. [DOI] [PubMed] [Google Scholar]
- 31.Bayry J., et al. Intravenous immunoglobulin abrogates dendritic cell differentiation induced by interferon-α present in serum from patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48(12):3497–3502. doi: 10.1002/art.11346. [DOI] [PubMed] [Google Scholar]
- 32.Karnam A., et al. Therapeutic normal IgG intravenous immunoglobulin activates Wnt-β-catenin pathway in dendritic cells. Commun. Biol. 2020;3(1):1–13. doi: 10.1038/s42003-020-0825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Séité J.-F., et al. TLR9 responses of B cells are repressed by intravenous immunoglobulin through the recruitment of phosphatase. J. Autoimmun. 2011;37(3):190–197. doi: 10.1016/j.jaut.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Bendaoud B., et al. BAFF, a new target for intravenous immunoglobulin in autoimmunity and cancer. J. Clin. Immunol. 2007;27(3):257–265. doi: 10.1007/s10875-007-9082-2. [DOI] [PubMed] [Google Scholar]
- 35.Prasad N.K., et al. Therapeutic preparations of normal polyspecific IgG (IVIg) induce apoptosis in human lymphocytes and monocytes: a novel mechanism of action of IVIg involving the Fas apoptotic pathway. J. Immunol. 1998;161(7):3781–3790. [PubMed] [Google Scholar]
- 36.Perricone C., et al. Intravenous Immunoglobulins at the Crossroad of Autoimmunity and Viral Infections. Microorganisms. 2021;9(1):121. doi: 10.3390/microorganisms9010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elshabrawy H.A., et al. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS-CoV spike protein are more broadly neutralizing. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0050366. e50366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long Q.X., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 39.Lai S. Treatment of severe acute respiratory syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24(9):583–591. doi: 10.1007/s10096-005-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan K., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dodd R.Y. Emerging pathogens and their implications for the blood supply and transfusion transmitted infections. Br. J. Haematol. 2012;159(2):135–142. doi: 10.1111/bjh.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marano G., et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colebunders R.L., Cannon R.O. Large-scale convalescent blood and plasma transfusion therapy for Ebola virus disease. J. Infect. Dis. 2015;211(8):1208–1210. doi: 10.1093/infdis/jiv043. [DOI] [PubMed] [Google Scholar]
- 44.Katz L.M., Tobian A.A. Ebola virus disease, transmission risk to laboratory personnel, and pretransfusion testing. Transfusion. 2014;54(12):3247–3251. doi: 10.1111/trf.12913. [DOI] [PubMed] [Google Scholar]
- 45.Fleming A.B., Raabe V. Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of antibody-dependent enhancement. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104388. 104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin Y.W., et al. Selection of vaccinia virus-neutralizing antibody from a phage-display human-antibody library. J. Microbiol. Biotechnol. 2019;29(4):651–657. doi: 10.4014/jmb.1812.12024. [DOI] [PubMed] [Google Scholar]
- 47.Keck Z.-Y., et al. Springer; Hepatitis C Virus Protocols: 2019. Isolation of HCV neutralizing antibodies by yeast display; pp. 395–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owji H., et al. Immunotherapeutic approaches to curtail COVID-19. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106924. 106924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwab I., Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat. Rev. Immunol. 2013;13(3):176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 50.Bonam S.R., et al. Adjunct immunotherapies for the management of severely ill COVID-19 patients. Cell Rep. Med. 2020;1(2) doi: 10.1016/j.xcrm.2020.100016. 100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.AminJafari A., Ghasemi S. The possible of immunotherapy for COVID-19: A systematic review. Int. Immunopharmacol. 2020;83 doi: 10.1016/j.intimp.2020.106455. 106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie Y., et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shanmugaraj B., et al. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020;38(1):10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 54.Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020;16(10):1718. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian X., et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes. Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu X., et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park T., et al. Spike protein binding prediction with neutralizing antibodies of SARS-CoV-2. BioRxiv. 2020 [Google Scholar]
- 58.Zheng Z., et al. Monoclonal antibodies for the S2 subunit of spike of SARS-CoV cross-react with the newly-emerged SARS-CoV-2. Eurosurveillance. 2020;25(28):2000291. doi: 10.2807/1560-7917.ES.2020.25.28.2000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinto D., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 60.Camp S.M., et al. Unique toll-like receptor 4 activation by NAMPT/PBEF induces NFκB signaling and inflammatory lung injury. Sci. Rep. 2015;5:13135. doi: 10.1038/srep13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.A. Tozzi, Three Options Against SARS-CoV-2: Pediatric Sera, Mithridatization, Dewetting Antibodies, Biochemistry 1 1-8.
- 62.Wang K., et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv. 2020;3(14):1–10. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev. 2020;16(3):434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ling T.-Y., et al. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc. Natl. Acad. Sci. 2006;103(25):9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Agha E., et al. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell. 2017;21(2):166–177. doi: 10.1016/j.stem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Wang J., et al. Advances in the research of mechanism of pulmonary fibrosis induced by Corona Virus Disease 2019 and the corresponding therapeutic measures. Zhonghua Shao Shang Za Zhi. 2020;36 doi: 10.3760/cma.j.cn501120-20200307-00132. E006 E6. [DOI] [PubMed] [Google Scholar]
- 67.Guillot S., et al. Increased extracellular matrix metalloproteinase inducer (EMMPRIN) expression in pulmonary fibrosis. Exp. Lung Res. 2006;32(3–4):81–97. doi: 10.1080/01902140600710512. [DOI] [PubMed] [Google Scholar]
- 68.Li X., et al. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295(1):178–185. doi: 10.1152/ajplung.00009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bekerman E., et al. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J. Clin. Investig. 2017;127(4):1338–1352. doi: 10.1172/JCI89857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. 108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fragoulis G.E., et al. JAK-inhibitors New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology. 2019;58(1):i43–i54. doi: 10.1093/rheumatology/key276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorrell F.J., et al. Family-wide structural analysis of human numb-associated protein kinases. Structure. 2016;24(3):401–411. doi: 10.1016/j.str.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richardson P., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223) doi: 10.1016/S0140-6736(20)30304-4. e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segler M.H., et al. Planning chemical syntheses with deep neural networks and symbolic AI. Nature. 2018;555(7698):604–610. doi: 10.1038/nature25978. [DOI] [PubMed] [Google Scholar]
- 75.Pu S.-Y., et al. Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antiviral Res. 2018;155:67–75. doi: 10.1016/j.antiviral.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stebbing J., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Broglie L., et al. Ruxolitinib for treatment of refractory hemophagocytic lymphohistiocytosis. Blood Adv. 2017;1(19):1533. doi: 10.1182/bloodadvances.2017007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Das R., et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood. 2016;127(13):1666–1675. doi: 10.1182/blood-2015-12-684399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Favalli E.G., et al. Baricitinib for COVID-19: a suitable treatment? Lancet Infect. Dis. 2020;20(9):1012–1013. doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhong J., et al. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2(7):E428–E436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Owen C., et al. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr. Oncol. 2019;26(2) doi: 10.3747/co.26.4345. e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.S. Khaerunnisa, et al., Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study, Preprint 20944 (2020) 1-14.
- 83.Liu L., et al. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4) doi: 10.1172/jci.insight.123158. e123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calabrese L.H., Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat. Rev. Rheumatol. 2014;10(12):720. doi: 10.1038/nrrheum.2014.127. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka T., et al. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 86.Zhou Y., et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+ CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. BioRxiv. 2020 [Google Scholar]
- 87.Calabrese L.H. Cytokine storm and the prospects for immunotherapy with COVID-19. Clevel. Clin. J. Med. 2020;87(7):389–393. doi: 10.3949/ccjm.87a.ccc008. [DOI] [PubMed] [Google Scholar]
- 88.In Ah C., et al. Effects of tocilizumab therapy on serum interleukin-33 and interleukin-6 levels in patients with rheumatoid arthritis. Arch. Rheumatol. 2018;33(4):389. doi: 10.5606/ArchRheumatol.2018.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riegler L.L., et al. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther. Clin. Risk Manag. 2019;15:323. doi: 10.2147/TCRM.S150524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimabukuro-Vornhagen A., et al. Cytokine release syndrome. J. ImmunoTher. Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malgie J., et al. Decreased Mortality in Coronavirus Disease 2019 Patients Treated With Tocilizumab: A Rapid Systematic Review and Meta-analysis of Observational Studies. Clin. Infect. Dis. 2020;2(2):1–8. doi: 10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao Q., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fawley N., Abdelmalak B. Procedural sedation in the COVID-19 era. Clevel. Clin. J. Med. 2020;7(1):28. doi: 10.3949/ccjm.87a.ccc043. [DOI] [PubMed] [Google Scholar]
- 94.Huet T., et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(10):586–587. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filocamo G., et al. Use of anakinra in severe COVID-19: A case report. Int. J. Infect. Dis. 2020;96:607–609. doi: 10.1016/j.ijid.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tobinick E. TNF-alpha inhibition for potential therapeutic modulation of SARS coronavirus infection. Curr. Med. Res. Opin. 2004;20(1):39–40. doi: 10.1185/030079903125002757. [DOI] [PubMed] [Google Scholar]
- 97.Russell B., et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1022. e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shiomi A., Usui T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/568543. 568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griffin J.D., et al. The biology of GM-CSF: regulation of production and interaction with its receptor. Int. J. Cell. Cloning. 1990;8(1):35–44. doi: 10.1002/stem.5530080705. [DOI] [PubMed] [Google Scholar]
- 100.Huang F.F., et al. GM-CSF in the lung protects against lethal influenza infection. Am. J. Respir. Crit. Care Med. 2011;184(2):259–268. doi: 10.1164/rccm.201012-2036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Subramaniam R., et al. Delivery of GM-CSF to Protect against Influenza Pneumonia. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0124593. e0124593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sterner R.M., et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133(7):697–709. doi: 10.1182/blood-2018-10-881722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matute-Bello G., et al. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit. Care Med. 2000;28(1):1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Aggarwal A., et al. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur. Respir. J. 2000;15(5):895–901. doi: 10.1034/j.1399-3003.2000.15e14.x. [DOI] [PubMed] [Google Scholar]
- 105.Rebetz J., et al. The Pathogenic Involvement of Neutrophils in Acute Respiratory Distress Syndrome and Transfusion-Related Acute Lung Injury. Transfus. Med. Hemother. 2018;45(5):290–298. doi: 10.1159/000492950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bonaventura A., et al. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc. Res. 2019;115(8):1266–1285. doi: 10.1093/cvr/cvz084. [DOI] [PubMed] [Google Scholar]
- 107.Bonaventura A., et al. Targeting GM-CSF in COVID-19 pneumonia: rationale and strategies. Front. Immunol. 2020;11:1625. doi: 10.3389/fimmu.2020.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paine R., 3rd, et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit. Care Med. 2012;40(1):90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Presneill J.J., et al. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am. J. Respir. Crit. Care Med. 2002;166(2):138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 110.Rösler B., Herold S. Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia—a new therapeutic strategy? Mol. Cell. Pediatr. 2016;3(1):29. doi: 10.1186/s40348-016-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Medford A., Millar A. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61(7):621–626. doi: 10.1136/thx.2005.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferrara N., et al. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005;333(2):328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 113.Channappanavar R., et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hung I.F.-N., et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Teijaro J.R. Type I interferons in viral control and immune regulation. Curr. Opin. Virol. 2016;16:31–40. doi: 10.1016/j.coviro.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Major J., et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369(6504):712–717. doi: 10.1126/science.abc2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lazear H.M., et al. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50(4):907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davidson S., et al. IFN λ is a potent anti-influenza therapeutic without the inflammatory side effects of IFN α treatment. EMBO Mol. Med. 2016;8(9):1099–1112. doi: 10.15252/emmm.201606413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Groen R.A., et al. IFN-λ-mediated IL-12 production in macrophages induces IFN-γ production in human NK cells. Eur. J. Immunol. 2015;45(1):250–259. doi: 10.1002/eji.201444903. [DOI] [PubMed] [Google Scholar]
- 120.Market M., et al. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front. Immunol. 2020;11:1512. doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Janeway C.A., Jr, et al. 5th edition, Garland Science; 2001. The complement system and innate immunity, Immunobiology: The Immune System in Health and Disease. [Google Scholar]
- 122.Risitano A.M., et al. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Java A., et al. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5(15) doi: 10.1172/jci.insight.140711. e140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Skendros P., et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020;130(11):6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carvelli J., et al. Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature. 2020;6:1–5. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gao T., et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv. 2020;29(3) [Google Scholar]
- 127.Cugno M., et al. Complement activation in patients with COVID-19: a novel therapeutic target. J. Allergy Clin. Immunol. 2020;146(1):215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ramlall V., et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020;26(10):1609–1615. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blanco-Melo D., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan B., et al. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyper-activation. Research Square. 2020;1:1–22. [Google Scholar]
- 131.Ackermann M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mastellos D.C., et al. Complement C3 vs C5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin. Immunol. 2020;220 doi: 10.1016/j.clim.2020.108598. 108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fang X., et al. CD24: from A to Z. Cell. Mol. Immunol. 2010;7(2):100–103. doi: 10.1038/cmi.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen G.Y., et al. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323(5922):1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paulson J.C., et al. Siglecs as sensors of self in innate and adaptive immune responses. Ann. N. Y. Acad. Sci. 2012;1253(1):37. doi: 10.1111/j.1749-6632.2011.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol. Rev. 2009;230(1):128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 137.Duong B.H., et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J. Exp. Med. 2010;207(1):173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen G.-Y., et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat. Biotechnol. 2011;29(5):428–435. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Y., et al. CD24-Siglec G/10 discriminates danger-from pathogen-associated molecular patterns. Trends Immunol. 2009;30(12):557–561. doi: 10.1016/j.it.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tian R.R., et al. CD24 and Fc fusion protein protects SIVmac239-infected Chinese rhesus macaque against progression to AIDS. Antiviral Res. 2018;157:9–17. doi: 10.1016/j.antiviral.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 141.R.L. Kruse, Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China, F1000research 9(72) (2020) 1-24. [DOI] [PMC free article] [PubMed]
- 142.Wong S.K., et al. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279(5):3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang X., et al. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9(1):63–73. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Desmyter A., et al. Viral infection modulation and neutralization by camelid nanobodies. Proc. Natl. Acad. Sci. U.S.A. 2013;110(15):E1371–E1379. doi: 10.1073/pnas.1301336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Koch K., et al. Selection of nanobodies with broad neutralizing potential against primary HIV-1 strains using soluble subtype C gp140 envelope trimers. Sci. Rep. 2017;7(1):8390. doi: 10.1038/s41598-017-08273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170(4):1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kuba K., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu F., et al. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan. China, Microbes Infect. 2020;22(2):74–79. doi: 10.1016/j.micinf.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xia S., et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019;5(4) doi: 10.1126/sciadv.aav4580. eaav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Buchholz U.J., et al. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. 2004;101(26):9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bakker A.B., et al. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J. Virol. 2005;79(14):9062–9068. doi: 10.1128/JVI.79.14.9062-9068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li W., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sui J., et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. 2004;101(8):2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yasui F., et al. Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus. Virology. 2014;454–455:157–168. doi: 10.1016/j.virol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Imai Y., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zou Z., et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gu H., et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016;6:19840. doi: 10.1038/srep19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haschke M., et al. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013;52(9):783–792. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- 159.Khan A., et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21(1):234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li W., et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24(8):1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shao Z., et al. Increasing serum soluble angiotensin-converting enzyme 2 activity after intensive medical therapy is associated with better prognosis in acute decompensated heart failure. J. Card. Fail. 2013;19(9):605–610. doi: 10.1016/j.cardfail.2013.06.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wan Y., et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020;94(5):e02015–e2019. doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li F., et al. Natural killer cells are involved in acute lung immune injury caused by respiratory syncytial virus infection. J. Virol. 2012;86(4):2251–2258. doi: 10.1128/JVI.06209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abdul-Careem M.F., et al. Critical role of natural killer cells in lung immunopathology during influenza infection in mice. J. Infect. Dis. 2012;206(2):167–177. doi: 10.1093/infdis/jis340. [DOI] [PubMed] [Google Scholar]
- 165.Zheng M., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liao M., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 167.Carvelli J., et al. Identification of immune checkpoints in COVID-19. Nature. 2020;1(1) [Google Scholar]
- 168.Wilk A.J., et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;26(7):1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang K.J., et al. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Han J., et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci. Rep. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu E., et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang W., et al. CAR-NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer Lett. 2020;472:175–180. doi: 10.1016/j.canlet.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 173.Parihar R., et al. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol. Res. 2019;7(3):363–375. doi: 10.1158/2326-6066.CIR-18-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hammer Q., et al. Natural killer cell specificity for viral infections. Nat. Immunol. 2018;19(8):800–808. doi: 10.1038/s41590-018-0163-6. [DOI] [PubMed] [Google Scholar]
- 175.Seay K., et al. In vivo activation of human NK cells by treatment with an interleukin-15 superagonist potently inhibits acute in vivo HIV-1 infection in humanized mice. J. Virol. 2015;89(12):6264–6274. doi: 10.1128/JVI.00563-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Muro S., et al. Expression of IL-15 in inflammatory pulmonary diseases. J. Allergy Clin. Immunol. 2001;108(6):970–975. doi: 10.1067/mai.2001.119556. [DOI] [PubMed] [Google Scholar]
- 177.Noroozi R., et al. Altered cytokine levels and immune responses in patients with SARS-CoV-2 infection and related conditions. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155143. 155143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Clay C.C., et al. Severe acute respiratory syndrome-coronavirus infection in aged nonhuman primates is associated with modulated pulmonary and systemic immune responses. Immun. Ageing. 2014;11(1):4. doi: 10.1186/1742-4933-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li F., et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144(2):392–401. doi: 10.1053/j.gastro.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 180.Kim H., et al. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J. Pharm. Pharmacol. 2016;68(3):406–420. doi: 10.1111/jphp.12529. [DOI] [PubMed] [Google Scholar]
- 181.Du J., et al. Autophagy induces G0/G1 arrest and apoptosis in menstrual blood-derived endometrial stem cells via GSK3-β/β-catenin pathway. Stem Cell Res. Ther. 2018;9(1):330. doi: 10.1186/s13287-018-1073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fu Y., et al. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng. Part B. 2017;23(6):515–528. doi: 10.1089/ten.TEB.2016.0365. [DOI] [PubMed] [Google Scholar]
- 183.Chan M.C., et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. 2016;113(13):3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Spaggiari G.M., et al. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2, 3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 185.Lee D.E., et al. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016;7(1):1–8. doi: 10.1186/s13287-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shi Y., et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20(5):510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 187.Yang B.X., et al. Systematic identification of factors for provirus silencing in embryonic stem cells. Cell. 2015;163(1):230–245. doi: 10.1016/j.cell.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Simonson O.E., et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl. Med. 2015;4(10):1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zheng G., et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir. Res. 2014;15(1):39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Atluri S., et al. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician. 2020;23(2):E71–E83. [PubMed] [Google Scholar]
- 191.Leng Z., et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Florindo H.F., et al. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15(8):630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hsu J., et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018;128(10):4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Patil N.K., et al. Targeting immune cell checkpoints during sepsis. Int. J. Mol. Sci. 2017;18(11):2413. doi: 10.3390/ijms18112413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Diao B., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhao X., et al. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-β signaling. J. Biol. Chem. 2008;283(6):3272–3280. doi: 10.1074/jbc.M708033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stahnke T., et al. Suppression of TGF-β pathway by pirfenidone decreases extracellular matrix deposition in ocular fibroblasts in vitro. PLoS ONE. 2017;12(2) doi: 10.1371/journal.pone.0172592. e0172592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hu W., et al. SARS-CoV regulates immune function-related gene expression in human monocytic cells. Viral Immunol. 2012;25(4):277–288. doi: 10.1089/vim.2011.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prompetchara E., et al. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 200.Thevarajan I., et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26(4):453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Wit E., et al. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bode C., et al. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines. 2011;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.White M.R., et al. Impact of neutrophils on antiviral activity of human bronchoalveolar lavage fluid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293(5):L1293–L1299. doi: 10.1152/ajplung.00266.2007. [DOI] [PubMed] [Google Scholar]
- 204.Wohlford-Lenane C.L., et al. Rhesus theta-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J. Virol. 2009;83(21):11385–11390. doi: 10.1128/JVI.01363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dias S., et al. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J. Exp. Med. 2005;201(6):971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Levy Y., et al. Enhanced T cell recovery in HIV-1–infected adults through IL-7 treatment. J. Clin. Investig. 2009;119(4):997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu H., et al. Interleukin-7 improves reconstitution of antiviral CD4 T cells. Clin. Immunol. 2005;114(1):30–41. doi: 10.1016/j.clim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 208.Gardiner D., et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0063818. e63818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.AlGhamdi M., et al. MERS CoV infection in two renal transplant recipients: case report. Am. J. Transplant. 2015;15(4):1101–1104. doi: 10.1111/ajt.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Carbajo-Lozoya J., et al. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165(1):112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Singh A.K., et al. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes. Metab. Syndr. 2020;14(5):971–978. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Arabi Y.M., et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 213.Amici C., et al. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir. Ther. 2006;11(8):1021. [PubMed] [Google Scholar]
- 214.Bancos S., et al. Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells. Cell. Immunol. 2009;258(1):18–28. doi: 10.1016/j.cellimm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fang L., et al. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zheng Y., et al. Immunoregulation with mTOR Inhibitors to Prevent COVID-19 Severity: A Novel Intervention Strategy beyond Vaccines and Specific Antiviral Medicines. Med. Virol. 2020;92(9):1495–1500. doi: 10.1002/jmv.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang C.-H., et al. Adjuvant treatment with a mammalian target of rapamycin inhibitor, sirolimus, and steroids improves outcomes in patients with severe H1N1 pneumonia and acute respiratory failure. Crit. Care Med. 2014;42(2):313–321. doi: 10.1097/CCM.0b013e3182a2727d. [DOI] [PubMed] [Google Scholar]
- 218.Zhou Y., et al. Network-based drug repurposing for human coronavirus. Cell Discov. 2020;6(14):1–18. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chen X., et al. Thiopurine analogue inhibitors of severe acute respiratory syndrome-coronavirus papain-like protease, a deubiquitinating and deISGylating enzyme. Antivir. Chem. Chemother. 2009;19(4):151–156. doi: 10.1177/095632020901900402. [DOI] [PubMed] [Google Scholar]
- 220.Jiang S., et al. SARS vaccine development. Emerg. Infect. Dis. 2005;11(7):1016. doi: 10.3201/eid1107.050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ahmed S.F., et al. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3):254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Leung D.T.M., et al. Antibody response of patients with severe acute respiratory syndrome (SARS) targets the viral nucleocapsid. J. Infect. Dis. 2004;190(2):379–386. doi: 10.1086/422040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Grifoni A., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Haque A., Pant A.B. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines. 2020;8(4):739. doi: 10.3390/vaccines8040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zimmer C., et al. Coronavirus Vaccine Tracker. 2021 [Google Scholar]
- 226.Jeyanathan M., et al. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Khuroo M.S., et al. COVID-19 vaccines: A race against time in the middle of death and devastation! J. Clin. Exp. Hepatol. 2020;10(6):610–621. doi: 10.1016/j.jceh.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sambaturu N., et al. Role of genetic heterogeneity in determining the epidemiological severity of H1N1 influenza. PLoS Comput. Biol. 2018;14(3) doi: 10.1371/journal.pcbi.1006069. e1006069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tokumaru Y., et al. Current status and limitations of immunotherapy for breast cancer. Surgery. 2020;167(3):628–630. doi: 10.1016/j.surg.2019.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Datta S.S., Basu S. Randomization amid a pandemic–a critical appraisal regarding convalescent plasma therapy clinical trials for COVID-19 patients. ISBT. Sci. Ser. 2020;15(4):417–418. [Google Scholar]
- 231.Polack F.P., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]