Supplemental Digital Content is available in the text.
Keywords: Beijing fibrosis classification, hepatitis C, histology, liver fibrosis, regression, sustained virological response
Abstract
Background
Most of the studies on fibrosis regression prediction were based on noninvasive fibrosis markers and differ greatly. The ‘Beijing fibrosis classification’ can use histological results to classify fibrosis into progressive or ‘nonprogressive’ according to fibrotic septal morphology. We use this standard which served as the gold standard in order to find fibrosis regression predictors.
Aim
To study the predictors of fibrosis regression after hepatitis C virus clearance according to histological fibrosis staging by the ‘Beijing fibrosis classification’.
Materials and methods
This was a prospective cohort study. A total of 68 patients with advanced liver fibrosis or compensated cirrhosis who achieved sustained virological response were enrolled. Patients with the Ishak scores lower than 3 seemed to have fibrosis regression. The others were divided into the fibrosis progressive group and the nonprogressive group according to the ‘Beijing fibrosis classification’. Predictors of fibrosis regression were studied by logistic regression using baseline factors and the dynamic change in noninvasive fibrosis factors.
Results
Eighteen patients were assigned to the progressive group, and the others were assigned to the nonprogressive group. The baseline liver stiffness measurements (LSMs) of the progressive and nonprogressive groups were 14.35 (11.3, 27.3) kPa and 11.3 (8.3, 14.2) kPa, respectively, P = 0.02. The baseline LSM was the only predictor of fibrosis progression. With a cutoff of 11.85 kPa, the AUC was 0.71 (0.5, 0.9), and the negative predictive value was 0.92.
Conclusions
The baseline LSM was found to be the only predictor of fibrosis regression, 11.85 kPa is a possible ‘hepatic fibrosis return point’.
Introduction
The global prevalence of hepatitis C is approximately 3% and can lead to cirrhosis and liver cancer with a heavy economic burden [1,2]. Available studies have shown that, whether interferon (IFN)-based or direct oral antiviral agents (DAAs) are used, viral elimination in chronic hepatitis C has been shown to prevent progression to more advanced stages of fibrosis or cirrhosis, and the proportion is significantly lower in patients without SVR than in those with SVR [3]. While the incidence of liver cancer is higher in people whose liver fibrosis still progresses after SVR [4,5] this is a population that requires intensive monitoring and follow-up.
The gold standard for judging fibrosis regression is to compare two pathological liver fibrosis scores. There are few studies based on histological evaluation because antiviral therapy for hepatitis C does not rely on histological results, and the liver puncture is difficult for some patients with trauma. To date, only a few studies have performed paired biopsies [6,7]. Other studies have mainly analyzed the noninvasive liver fibrosis score [8,9]. Risk factors for progressive liver fibrosis after achieving sustained virological response (SVR) are currently considered to include baseline severe liver fibrosis, high BMI, diabetes, and alcohol consumption [8,10] but these are not widely recognized.
In 2017, Sun et al. [11] proposed a new pathological classification method to assess the dynamic changes of liver fibrosis properties, named the ‘Beijing fibrosis classification’. For this classification, fibrosis is the net result of dynamic changes between fibrogenesis and fibrolysis, namely, predominantly progressive (thick/broad/loose/pale septa with inflammation), predominately regressive (delicate/thin/dense/splitting septa), and indeterminate, which displayed an overall balance between progressive and regressive scarring.
The analysis of paired biopsies of patients with hepatitis B showed that this new classification was able to classify the dynamic changes in fibrosis in patients with hepatitis B after treatment and to further classify patients with the same Ishak fibrosis score or Metavir fibrosis stage to determine the fibrotic outcome. The Beijing fibrosis classification was consistent with the dynamic changes in Laennec substage, collagen percentage area, and liver stiffness. However, this criterion has not been applied to hepatitis C patients.
This study intends to assess the applicability of the Beijing fibrosis classification in patients with hepatitis C, to determine the fibrosis outcome by pathology, and to analyze the factors predicting the reversal of liver fibrosis to achieve individualized management of patients.
Patients and methods
Patients
The patients were infected by plasma donation in the 1990s in a town of China. They received antiviral treatment from October 2015 to December 2017. Chronic hepatitis C (CHC) patients with advanced fibrosis or compensated cirrhosis who achieved sustained viral response were included. The inclusion criteria were as follows: aged 18–70 years; hepatitis C virus (HCV) antibody positivity for at least 6 months before screening; and HCV-RNA positivity. Advanced fibrosis was diagnosed by a liver stiffness measurement (LSM) ≥ 9.5 kPa. Compensated cirrhosis was diagnosed by abdominal ultrasound, computed tomography, MRI or LSM ≥ 14 kPa. SVR was defined as HCV undetected 12 weeks after direct-acting antiviral agent (DAA) treatment or 24 weeks after PEG-Interferon ± ribavirin. The exclusion criteria were as follows: coinfection with hepatitis B or HIV; alcohol use disorders [alcohol intake > 30 g/day (men) or 20 g/day (women)]; drug abuse; other liver disease (e.g., autoimmune hepatitis); hepatocellular carcinoma or other malignant tumor; any severe heart, lung, kidney, brain, or blood diseases; severe neurological or psychological disease; and pregnancy or lactation. All patients were followed up for at least 6 months and examined every 3 months. The follow-up time was calculated from the initiation of antiviral treatment to the biopsy day. Data on demographics, the history of alcohol consumption, complications, BMI (BMI; kg/m2), and laboratory tests were collected.
Histological evaluation
Liver biopsy specimens were all formalin fixed, paraffin embedded, and sectioned using standard clinical techniques. Five-micrometer sections were stained with hematoxylin and eosin, reticulin, and Masson’s trichrome. All liver biopsy samples were evaluated by two experienced hepatopathologists (from Beijing Youan Hospital and Beijing Youyi Hospital) independently blinded to treatment assignment, biochemical response, and liver stiffness values. Discrepancies were solved by consensus reading. Necroinflammation activity and fibrosis stage were assessed by the Ishak fibrosis score and Ishak inflammation score.
The Beijing fibrosis classification, which was developed for hepatitis B, was assessed when the Ishak fibrosis score was higher than or equal to stage 3. Patients were classified into three stages, predominantly progressive, indeterminate, and predominately regressive. The Beijing standard is shown in Supplementary Table 1, Supplemental digital content 1, http://links.lww.com/WNR/A586.
Liver stiffness measurements and laboratory tests
LSMs and controlled attenuation parameters were assessed by transient elastography (FibroScan-502, Echosens, Paris, France). Transient elastography is performed on a patient lying supine, with the right arm elevated to facilitate access to the right liver lobe. The tip of the probe is contacted to the intercostal skin with coupling gel in the 9th–11th intercostal space at the level where a liver biopsy would be performed. The final result of a transient elastography session can be regarded as valid if the following criteria are fulfilled: (1) a number of valid shots of at least 10; (2) a success rate (the ratio of valid shots to the total number of shots) above 60%; and (3) an interquartile range (IQR, reflecting the variability of measurements) less than 30% of the median LSMs value (IQR/M 60.30%) [12].
The lower limit of serum HCV-RNA was 15 IU/mL (Amplicor; Roche Diagnostics, Manheim, Germany). Liver fibrosis was also evaluated by the aspartate transaminase-to-platelet ratio index (APRI) [and the fibrosis index based on the four factors (FIB-4)]. The serum markers of liver fibrosis were calculated according to the following formulas: APRI = (AST/upper limit of normal) × 100/PLT; FIB-4 = (age × AST)/(PLT × square root of ALT).
Statistical analysis
Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, Illinois, USA) and Stata 12.0 (StataCorp, College Station, Texas, USA). Categorical data are presented as numbers (percentages). Continuous variables are reported as the mean ± standard error or median (25th percentile/75th percentile). Patient characteristics were compared between progressive and nonprogressive fibrosis patients using χ2 tests for categorical variables, t-tests for variables with normal distributions, and Mann–Whitney U tests for variables with nonnormal distributions. Logistic regression analysis was used for univariate and multivariate analyses. STATA 15 was used to calculate receiver operating characteristic (ROC) curves, and the accuracy of each diagnostic criterion was evaluated according to the area under each ROC curve (AUROC). We defined the cutoff values of LSMs of liver fibrosis based on the maximum ROC curve area and a negative predictive value (NPV) of 92%.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committee of Beijing Youan Hospital. Written informed consent was provided by all patients. The study was registered with the Clinical Trials no. ChiCTR1900021376.
Results
Patient enrollment and characteristics
General cases
This group originated from the population infected with hepatitis C by apheresis blood transfusion in the 1990s in a county in Hebei province, China, with a total of 2892 patients. Among them, 550 patients had advanced liver fibrosis or cirrhosis. Seventy-three patients had liver biopsy after achieving SVR, and 68 patients were finally enrolled (Table 1; Fig. 1). There were 18 patients (26.47%) in the cirrhosis group and 50 patients (73.53%) in the advanced fibrosis group. Genotyping was dominated by types 1b and 2a, with a few classified as untyped, and the main reason for the failure of typing was low viral load.
Table 1.
Characteristics of hepatitis C patients with advanced fibrosis or cirrhosis
| Characteristics | All patients | Progressive group | Nonprogressive group | P value |
|---|---|---|---|---|
| n (%) | 68 | 14 (20.6) | 54 (79.4) | – |
| Age (years), IQR | 57.51 (55.05–63.85) | 62.52 (54.78–65.32) | 57.21 (55.06–62.92) | 0.32 |
| Male, n (%) | 22 (32.4) | 1 (7.1) | 21 (38.9) | 0.05 |
| BMI (kg/m2), mean | 26.59 ± 3.34 | 27.66 ± 2.86 | 26.29 ± 3.43 | 0.18 |
| Hypertension, n (%) | 34 (50) | 7 (50) | 27 (50) | 0.62 |
| Diabetes, n (%) | 10 (14.7%) | 3 (21.4) | 7 (13) | 0.34 |
| Hyperlipidemia, n (%) | 20 (29.4%) | 5 (35.7) | 15 (27.8) | 0.39 |
| Treatment medicine | 0.01 | |||
| PEG ± RBV | 18 | 0 | 18 | 0.07a |
| DAA | 23 | 9 | 14 | 0.00b |
| PR + DAA | 27 | 5 | 22 | 0.11c |
| Follow-up time (months), mean | 25.19 ± 8.10 | 24.14 ± 8.34 | 25.59 ± 8.09 | 0.56 |
| Platelet (×109/L), mean | 152.28 ± 52.58 | 140.14 ± 57.77 | 155.43 ± 51.26 | 0.34 |
| ALT (U/L), IQR | 54.45 (34.95–94.20) | 52.50 (20.73–1.00) | 54.45 (35.68–96.20) | 0.37 |
| AST (U/L), IQR | 50.45 (37.28–72.20) | 51.95 (33.65–80.85) | 50.45 (37.43–67.25) | 0.83 |
| ALT/AST, mean | 1.15 ± 0.37 | 0.99 ± 0.41 | 1.19 ± 0.35 | 0.07 |
| Albumin (g/L), IQR | 43.80 (41.73–46.68) | 42.30 (39.10–45.60) | 44.40 (42.50–46.70) | 0.06 |
| Bilirubin (µmol/L), IQR | 17.15 (13.68–21.80) | 17.40 (13.50–23.80) | 17.10 (13.60–21.90) | 0.76 |
| Prothrombin time (s), IQR | 11.30 (10.80–11.93) | 11.25 (10.90–11.45) | 11.50 (10.80–12.07) | 0.44 |
| INR, IQR | 1.02 (0.98–1.07) | 1.00 (0.97–1.02) | 1.03 (0.97–1.10) | 0.34 |
| HCV-RNA(log IU/ml),IQR | 3.02(0.65–7.50) | 3.28(0.63–12.23) | 3.02(0.65–6.22) | 0.76 |
| HCV genotype | 0.90 | |||
| 1b | 51 | 11 | 40 | |
| 2a | 7 | 1 | 6 | |
| Undetermined | 10 | 2 | 8 | |
| LSM (kPa), IQR | 11.70 (8.60–16.00) | 14.35 (11.33–27.33) | 11.30 (8.30–14.20) | 0.02 |
| CAP (dB/m), mean | 241.08 ± 43.31 | 259.00 ± 55.39 | 238.09 ± 41.94 | 0.12 |
| APRI, IQR | 0.92 (0.65–1.66) | 0.98 (0.74–2.42) | 0.92 (0.60–1.63) | 0.56 |
| FIB-4, IQR | 2.72 (1.97–3.89) | 3.51 (2.55–5.63) | 2.65 (1.87–3.68) | 0.08 |
ALB, albumin; ALT, alanine aminotransferase; APRI, aspartate transaminase-to-platelet ratio index; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; DAA, direct-acting antiviral agent; FIB-4, fibrosis index based on the four factors; HCV, hepatitis C virus; INR, international normalized ratio; IQR, interquartile range; LSM, liver stiffness measurement; PEG, PEG-interferon; PLT, blood platelet; RBV, ribavirin.
Comparison between the treatment group PR + DAA and group PEG ± RBV.
Comparison between the treatment group DAA and group PEG ± RBV.
Comparison between the treatment group DAA and group PR+DAA.
Fig. 1.
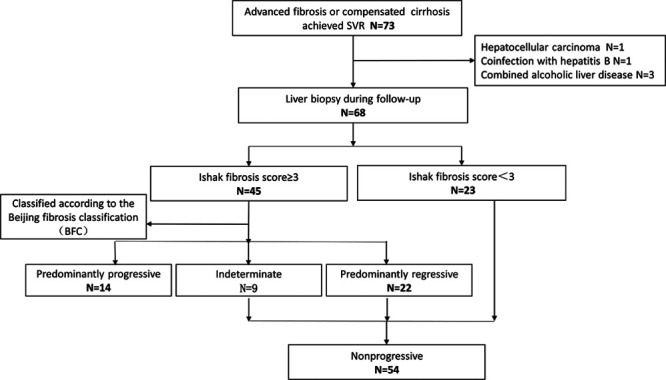
Flowchart for patient enrollment and evaluation. A total of 68 patients satisfying the inclusion criteria were enrolled in the study. Inflammation HAI score and Ishak fibrosis score were evaluated for each biopsy, and the new classification of fibrosis quality was evaluated for patients with an Ishak fibrosis score ≥3. HAI, histology activity index.
Analysis of treatment methods. All patients who achieved SVR using PEG ± RBV entered the nonprogressive group, and patients who achieved SVR only with the DAA entered part of the progressive group and part of the nonprogressive group. There was a significant difference in the results of the two treatment methods P = 0.00.
This population had stable residence, the same route and time of infection, similar living environments, the initiation of treatment at the same time, and regular follow-up, with good comparability.
Ishak modified histology activity index grading and staging system
In Fig. 2, patient 1 and patient 2 are two patients with cirrhosis, and patient 3 is a patient with advanced liver fibrosis; the patients were followed up for 30, 23, and 27 months, respectively. The pathological results were the Ishak fibrosis 6/histology activity index (HAI) 6, fibrosis 4/HAI 5, and fibrosis 3/HAI 2, respectively, for patient 1, patient 2, and patient 3. Overall, 66.7% of patients with cirrhosis after SVR still remained as Ishak stage 5–6 (cirrhotic stage), and 33.3% no longer had cirrhosis (Ishak stage 0–4). Of the 50 patients with advanced liver fibrosis, 12% progressed to cirrhosis after SVR (Ishak stage 5–6), 12% had no change (Ishak stage 4), and 76% had fibrosis reversal (Ishak stage 0–3). The proportion of fibrosis reversal was lower in patients with cirrhosis than in advanced liver fibrosis patients (Table 2).
Fig. 2.
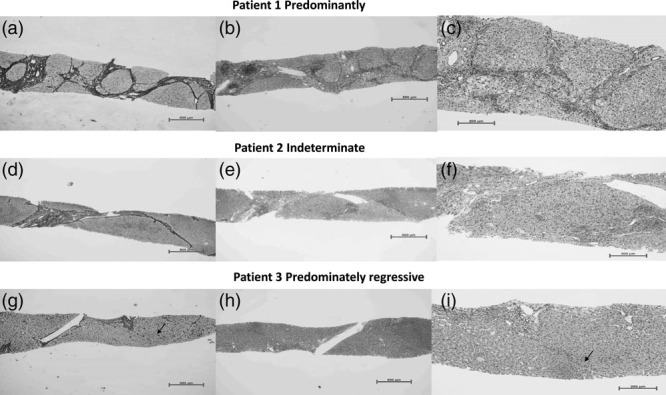
Liver biopsy samples of patients with progressive, indeterminate, and regressive septa. Patient 1: predominantly progressive, Ishak fibrosis score 6, necroinflammatory score 6. Patient 2: indeterminate, Ishak fibrosis score 4, necroinflammatory score 5. Patient 3: predominately regressive, Ishak fibrosis score 3, necroinflammatory score 2. (b and c) A moderate amount of mononuclear cell infiltration in fibrosis septa, predominantly lymphocytes, mild interfacial inflammation, partial hepatocyte edema, and balloon-like changes. (h and i) The portal area is slightly enlarged and a small amount of lymphocytes are infiltrated. (e and f) Inflammation are between b,c and h,i. (a) Fibrosis septa are wide/broad, loosely aggregated collagen fibers with inflammation; (g) Scars with thin, densely compacted stroma, septum can be fragmented and interrupted by hepatocytes (arrowhead). (d) Indeterminate.
Table 2.
The Ishak score and Beijing score of compensated cirrhosis and advanced fibrosis patients after sustained virological response
| Histological evaluation | Ishak fibrosis score | Beijing fibrosis classification | |||||
|---|---|---|---|---|---|---|---|
| 0–2 | 3 | 4 | 5–6 | Progressive | Indeterminate | Regressive | |
| All patients, N (%) | 23 (33.8) | 19 (27.9) | 8 (11.8) | 18 (26.5) | 14 (20.6) | 9 (13.2) | 45 (66.2) |
| Compensated cirrhosis, N = 18 (%) | 2 (11.1) | 2 (11.1) | 2 (11.1) | 12 (66.7) | 6 (33.3) | 4 (22.2) | 8 (44.5) |
| Advanced fibrosis, N = 50 (%) | 21 (42.0) | 17 (34.0) | 6 (12.0) | 6 (12.0) | 8 (16.0) | 5 (10.0) | 37 (74.0) |
Beijing fibrosis classification
The observation of the fibrotic septal characteristics of the above images revealed that the fibrotic septa contained wide/broad, loosely aggregated collagen fibers with inflammation, as shown in Fig. 2, patient 1. Scars were thin, densely compacted stroma, and the septa could be fragmented and interrupted by hepatocytes, as shown in Fig. 2, patient 3. The dynamic changes of liver fibrosis in patients with hepatitis C after antiviral treatment are similar to those in patients with hepatitis B.
Classifying the patients according to the Beijing fibrosis classification, it can be seen (Table 2) that fibrosis progressed in 33.3% and did not progress in 66.7% of patients with cirrhosis; fibrosis progressed in 16.0% and did not progress in 84.0% of patients with advanced liver fibrosis after SVR. These findings also suggest that patients with cirrhosis have a lower proportion of liver fibrosis reversal.
The Beijing fibrosis classification and clinical indicators
According to Beijing standards, all patients were divided into progressive group, indeterminate group, and regressive group (Supplementary Table 2, Supplemental digital content 1, http://links.lww.com/WNR/A586). In general, the indeterminate fibrosis group will eventually enter the regressive group; therefore, the indeterminate and regressive groups were combined into the nonprogressive group for a total of 50 patients (see Table 2). The baseline data of the indeterminate and regressive groups were significantly higher than those of the nonprogression group only in LSMs, and there was no significant difference in ALB, APRI, and FIB-4 (Table 1). Follow-up clinical data showed that albumin was significantly lower in the progression group than in the nonprogression group (42.74 ± 5.04 vs 45.02 ± 2.93 g/L, P = 0.03), LSM, APRI, and FIB-4 were all significantly higher in the nonprogression group (Table 3), suggesting that the progression group had more severe liver fibrosis and poorer liver function.
Table 3.
Histological and clinical characteristics of the progressive group and the nonprogressive group classified according to the Beijing standard
| Characteristics | Progressive | Nonprogressive | P value |
|---|---|---|---|
| PLT (per nL), mean | 156.15 ± 49.65 | 181.19 ± 57.43 | 0.15 |
| PT(S), mean | 11.90 ± 0.49 | 11.69 ± 0.65 | 0.70 |
| INR, mean | 1.06 ± 0.04 | 1.04 ± 0.06 | 0.67 |
| ALT (U/L), IQR | 17.25 (12.98–19.53) | 17.80 (13.90–24.30) | 0.59 |
| AST, IQR | 23.95 (18.73–35.33) | 21.90 (19.70–26.60) | 0.40 |
| ALT/AST | 0.67 ± 0.25 | 0.83 ± 0.28 | 0.08 |
| Bilirubin (µmol/L), IQR | 14.50 (11.35–17.85) | 15.10 (10.90–20.00) | 0.58 |
| ALB (g/L), mean | 42.74 ± 5.04 | 45.02 ± 2.93 | 0.03 |
| CAP, IQR | 264.50 (238.00–285.50) | 241.50 (208.75–282.50) | 0.09 |
| LSM, IQR | 12.00 (8.40–18.50) | 7.60 (6.05–9.00) | 0.00 |
| APRI, mean | 0.62 ± 0.44 | 0.41 ± 0.18 | 0.01 |
| FIB-4, mean | 3.01 ± 1.54 | 1.99 ± 0.75 | 0.00 |
| Inflammation HAI score, n (%) | 0.00 | ||
| 0–3 | 0 (0) | 27 (50) | |
| 4–6 | 11 (78.6) | 25 (46.3) | |
| 7–9 | 0 (0) | 2 (3.7) | |
| ≥10 | 3 (21.4) | 0 (0) | |
| Ishak fibrosis score, n (%) | 0.00 | ||
| 0–2 | 0 (0) | 23 (42.6) | |
| 3 | 3 (21.4) | 16 (29.6) | |
| 4 | 2 (14.3) | 6 (11.1) | |
| 5 | 3 (21.4) | 7 (13.0) | |
| 6 | 6 (42.9) | 2 (3.7) |
APRI, aspartate transaminase-to-platelet ratio index; CAP, controlled attenuation parameter; DAA, direct-acting antiviral agent; FIB-4, fibrosis index based on the four factors; HAI, histology activity index; HCV, hepatitis C virus; INR, international normalized ratio; IQR, interquartile range; PLT, blood platelet.
Baseline parameters predict fibrosis regression
Univariate analysis (Supplementary Table 3, Supplemental digital content 1, http://links.lww.com/WNR/A586) suggested that the baseline LSM was the only risk factor affecting fibrosis outcome. The cutoff was 11.85 kPa, and the AUROC (95% confidence interval) for predicting liver fibrosis progression was 0.708 (0.541–0.875), as shown in Fig. 3. When the baseline LSM was less than 11.85 kPa, the NPV was 92%, suggesting a 92% likelihood of the nonprogression of fibrosis after SVR in these patients (Fig. 3).
Fig. 3.
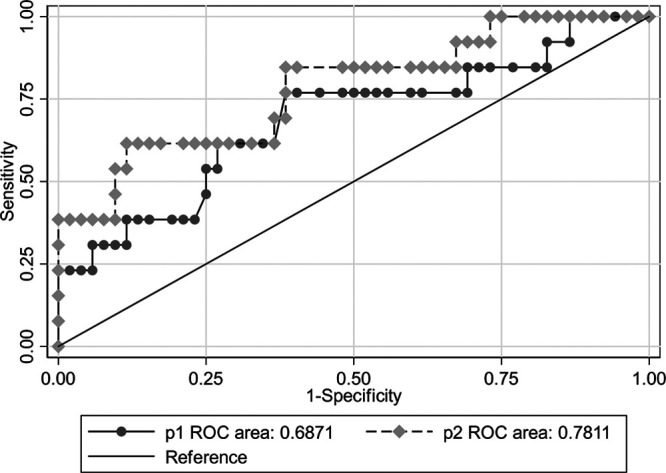
Receiver operating characteristic curve of LSM and ΔLSM predicting histological fibrosis regression. P1: the LSM indicator alone; P2: the combination of ΔLSM and LSM. Cutoff values of liver stiffness measurements obtained the Sensitivity >90%, maximizing Youden’s index, Specificity >90%. AUROC, the area under the receiver operating characteristic; CI, confidence interval; LSM, liver stiffness measurement; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
Dynamic changes in indicators predict fibrosis regression
After antiviral treatment, most patients’ LSMs rapidly decreased in both the progressive and nonprogressive groups, as shown in (Fig. 4). APRI, FIB-4, and other indicators showed the same decreasing trend (data not shown), and the degree and proportion of the decrease in LSM, FIB-4, and APRI did not predict the outcome of liver fibrosis (Supplementary Table 4, Supplemental digital content 1, http://links.lww.com/WNR/A586). The combination of ΔLSM and LSM was used to predict liver fibrosis, and the prediction model was Y = 3.45–0.19 × LSM + 3.04 × ΔLSM, with an AUROC of 0.781 (0.633–0.929) (Fig. 3). Compared to the LSM indicator alone, Z = 1.23, P = 0.27, the results demonstrate that the prediction model is not better than the baseline LSM alone.
Fig. 4.
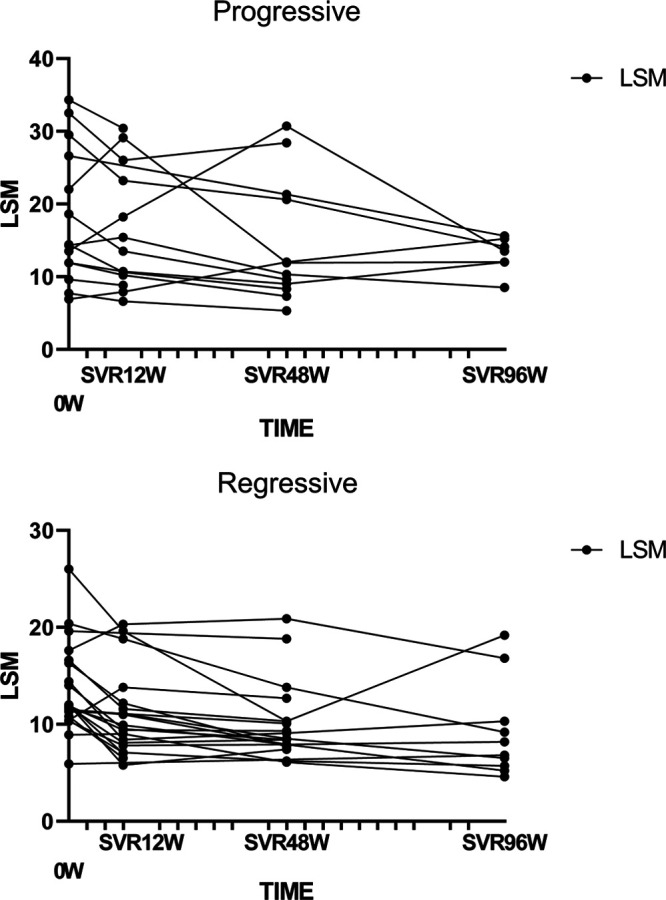
Dynamic changes in LSM after SVR. LSM, liver stiffness measurement; SVR, sustained virological response.
Discussion
This study, based on histology, found that the baseline LSM was the only predictor of fibrosis regression, and a baseline LSM less than 11.85 kPa could be used as a predictive factor for fibrosis regression, which can also be called the liver fibrosis returnable point. This finding is particularly important from a clinical point of view, given that it can have a potential impact in the follow-up strategies after SVR.
The available evidence shows that HCV eradication with both IFN-based and IFN-free therapies can improve fibrosis and portal hypertension [13–16]. However, few studies have used paired biopsies to evaluate the influencing factors of liver fibrosis regression. Poynard et al. [7] collected data on 3010 patients with paired biopsies before and after interferon-based therapy, with a mean follow-up of 20 months and an SVR rate of 36.3%. The factors influencing recovery from severe liver fibrosis after treatment were baseline liver fibrosis stage, SVR, age, BMI, mild baseline inflammation, and low viral load. Mauro et al. [6] performed a second liver puncture after antiviral therapy in 112 liver transplant patients with hepatitis C. The results indicated that 67% of patients had fibrosis reversal: seen in 43% of patients with cirrhosis and 72–85% of patients with other stages of liver fibrosis (P = 0.002), with pretreatment hepatic venous pressure gradient (HVPG) and LSM being the main predictive factors. The LSM irreversible point was 25.3 kPa. Pan et al. [15] performed paired biopsies in 15 patients with advanced liver fibrosis and cirrhosis, and 13 patients improved. The post-SVR liver biopsies of only four patients showed F1–F2, while 11 patients showed F3–F4, but this study did not analyze the influencing factors of liver fibrosis regression.
As mentioned earlier, both the morphological characteristics of fibrotic septa and the consistency of clinically noninvasive liver fibrosis indicators suggest that the Beijing fibrosis classification is suitable for assessing the dynamic changes in fibrosis after SVR for hepatitis C. The classic histological classification of liver fibrosis, such as Ishak staging, focuses on the severity of fibrosis rather than the dynamic changes of fibrosis. Some patients with cirrhosis in this cohort still had Ishak stages 5–6 after SVR, but it was shown that the fibrous tissue was degrading, as indicated by thin, densely compacted stroma, etc., suggesting that fibrosis was recovering and regressing. These patients are likely to have Ishak downstaging and cirrhosis reversal in the future. The Beijing fibrosis classification makes up for the short duration of clinical follow-up. Liver fibrosis was regressive but had not reached downstaging, suggesting that the Beijing fibrosis classification is a useful addition to Ishak staging.
Our results suggest a low proportion of liver fibrosis reversal in patients with cirrhosis and a high LSM at baseline, similar to other findings. Hedenstierna et al. [8] analyzed a total of 269 patients with advanced fibrosis or cirrhosis, as determined by Fibroscan, who were followed up for a mean of 7.7 years (range, 0–20 years) after treatment with interferon for hepatitis C. Twenty-one percent of the patients with cirrhosis who were followed for more than 10 years still had advanced fibrosis. Risk factors for persistent fibrosis were pretreatment cirrhosis, old age, and BMI. In the study of Lledó et al. [9], a total of 260 HCV patients were treated with DAAs and 246 patients achieved SVR, 57.2% of whom had advanced fibrosis or cirrhosis. At SVR12, 40% of patients had significant fibrosis regression. Multivariate analysis showed that only the baseline LSM was associated with liver fibrosis reversal. Mauro et al. [6] also found the baseline differences in LSM between patients with cirrhosis with (n = 14) and without fibrosis regression (n = 20) were also statistically significant: 17.1 kPa (13.0–21.6) vs 26.6 kPa (25.3–35.6; P = 0.003), respectively. They have proposed that in patients with compensated cirrhosis and HVPG > 10 mmHg and LSM > 21 kPa are predictive points at which liver fibrosis cannot be reversed. Our study showed that LSM was the only factor that predicted the reversal of liver fibrosis and that liver fibrosis did not progress in 92% of patients when LSM < 11.85 kPa.
LSM often shows a rapid and significant decrease after DAA treatment, but this does not represent a reversal of liver fibrosis [17]. Although, in our study, the type of treatment (INF vs INF-free) was significantly different in the progressive group and the nonprogressive group, this association was no longer significant in the univariate and multivariate analysis. And in the progressive group, the histological inflammation and fibrosis score was significantly higher than in the nonprogressive group. Enomoto et al. [18] also found significant histological inflammation of unknown cause in some patients. Additionally, improvement in liver fibrosis was not evident in the short term after achieving a sustained virologic response to direct-acting antiviral treatment. The reasons are unknown, maybe the persistent inflammation after SVR cause fibrosis progression and the amount of specimens can be expanded to further clarify.
Our results also showed that neither the degree nor the proportion of LSM reduction was effective in predicting liver fibrosis reversal. In a study by Mauro et al. [6], the median reduction in LSM was 47 and 30% in the progressive and nonprogressive groups of liver fibrosis, respectively. The percentage decrease in LSM predicted liver fibrosis regression with an AUC of 0.653; a 50% decrease predicted liver fibrosis recovery with a positive predictive value of 77.8% and an NPV of 44% and was able to correctly distinguish only 55% of patients. Therefore, LSM dynamic changes are not good indicators of fibrosis regression.
Many studies have suggested that old age, obesity, diabetes, and other factors are associated with persistent liver inflammation and fibrosis after SVR for hepatitis C [8,19]. There were no similar findings in our study, which may be related to the small sample size.
There are certain shortcomings in this study. For example, the sample size was small, and the follow-up period was only 25 months. A longer follow-up and larger sample sizes are needed to further clarify this result in the future.
Acknowledgements
Supported by the Scientific Research Project of Beijing Youan Hospital, Capital Medical University, 2018, No. YNKTTS20180105. Beijing Municipal Administration of Hospitals Incubating Program, 2018, No. PX2018058.
This study was approved by the Institutional Review Board of Beijing Youan Hospital of the Capital University of Medical Science, Beijing, China.
All patients were informed in writing of the use of their data for clinical research purposes and accepted.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.eurojgh.com
References
- 1.Blach S, Zeuzem S, Manns M, Altraif I, Duberg AS, Muljono DH, Abaalkhail F. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017; 2:161–176. [DOI] [PubMed] [Google Scholar]
- 2.Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995; 21:650–655. [PubMed] [Google Scholar]
- 3.Poynard T, Moussalli J, Munteanu M, Thabut D, Lebray P, Rudler M, et al. ; FibroFrance-GHPS Group. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol. 2013; 59:675–683. [DOI] [PubMed] [Google Scholar]
- 4.Tachi Y, Hirai T, Miyata A, Ohara K, Iida T, Ishizu Y, et al. Progressive fibrosis significantly correlates with hepatocellular carcinoma in patients with a sustained virological response. Hepatol Res. 2015; 45:238–246. [DOI] [PubMed] [Google Scholar]
- 5.Huang CF, Yeh ML, Huang CI, Liang PC, Lin YH, Lin ZY, et al. Post-treatment fibrotic modifications overwhelm pretreatment liver fibrosis in predicting HCC in CHC patients with curative antivirals. Hepatol Int. 2018; 12:544–551. [DOI] [PubMed] [Google Scholar]
- 6.Mauro E, Crespo G, Montironi C, Londoño MC, Hernández-Gea V, Ruiz P, et al. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology. 2018; 67:1683–1694. [DOI] [PubMed] [Google Scholar]
- 7.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002; 122:1303–1313. [DOI] [PubMed] [Google Scholar]
- 8.Hedenstierna M, Nangarhari A, El-Sabini A, Weiland O, Aleman S. Cirrhosis, high age and high body mass index are risk factors for persisting advanced fibrosis after sustained virological response in chronic hepatitis C. J Viral Hepat. 2018; 25:802–810. [DOI] [PubMed] [Google Scholar]
- 9.Lledó GM, Carrasco I, Benítez-Gutiérrez LM, Arias A, Royuela A, Requena S, et al. Regression of liver fibrosis after curing chronic hepatitis C with oral antivirals in patients with and without HIV coinfection. AIDS. 2018; 32:2347–2352. [DOI] [PubMed] [Google Scholar]
- 10.van der Meer AJ, Berenguer M. Reversion of disease manifestations after HCV eradication. J Hepatol. 2016; 65:S95–S108. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Zhou J, Wang L, Wu X, Chen Y, Piao H, et al. New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment. Hepatology. 2017; 65:1438–1450. [DOI] [PubMed] [Google Scholar]
- 12.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008; 48:835–847. [DOI] [PubMed] [Google Scholar]
- 13.D’Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012; 56:532–543. [DOI] [PubMed] [Google Scholar]
- 14.Rockey DC. Fibrosis reversal after hepatitis C virus elimination. Curr Opin Gastroenterol. 2019; 35:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan JJ, Bao F, Du E, Skillin C, Frenette CT, Waalen J, et al. Morphometry confirms fibrosis regression from sustained virologic response to direct-acting antivirals for hepatitis C. Hepatol Commun. 2018; 2:1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei L, Huang YH. Long-term outcomes in patients with chronic hepatitis C in the current era of direct-acting antiviral agents. Expert Rev Anti Infect Ther. 2019; 17:311–325. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J hepatol. 2015; 63:237–264. [DOI] [PubMed] [Google Scholar]
- 18.Enomoto M, Ikura Y, Tamori A, Kozuka R, Motoyama H, Kawamura E, et al. Short-term histological evaluations after achieving a sustained virologic response to direct-acting antiviral treatment for chronic hepatitis C. United Eur Gastroenterol J. 2018; 6:1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desbois AC, Cacoub P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: a contemporary review. World J Gastroenterol. 2017; 23:1697–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


