Abstract
Background
The impact of chronic cholestatic liver diseases such as primary biliary cholangitis (PBC) on non-alcoholic fatty liver disease (NAFLD) has yet to be described.
Objectives
To document and compare the severity and course of liver disease in patients with NAFLD/PBC versus NAFLD alone.
Methods
In this retrospective, case-control study 68 adult NAFLD/PBC patients were matched 1:2 for age and sex with 136 NAFLD alone patients. Disease activity and severity were documented by serum aminotransferases, albumin, bilirubin and international normalized ratio (INR) values and hepatic fibrosis by Fib-4 and aspartate aminotransferase/platelet ratio indices (APRI).
Results
On presentation (baseline), NAFLD/PBC patients had similar serum aminotransferase, albumin and bilirubin levels but lower INR values than NAFLD alone patients. Fib-4 and APRI levels were similar. Despite longer follow-up (favouring more advanced disease) in NAFLD/PBC patients, serum aminotransferases and bilirubin values were similar but albumin and INR levels significantly lower in NAFLD/PBC versus NAFLD alone patients at the end of follow-up. NAFLD/PBC patients also had significantly lower and less worsening of Fib-4 values at the end of follow-up. Transition from intermediate Fib-4 levels to those compatible with no or limited fibrosis was higher in NAFLD/PBC patients.
Conclusion
These findings suggest PBC does not adversely affect the severity or course of NAFLD.
Keywords: alkaline phosphatase, endotoxins, hepatocyte toxicity, non-alcoholic fatty liver disease, primary biliary cholangitis
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a common chronic liver disease that can progress to cirrhosis and/or hepatocellular carcinoma [1]. Recent data suggest progression may occur as a result of hepatic exposure to gut-derived immunogens such as endotoxins that activate hepatic innate immunity and thereby, induce a proinflammatory state [2,3].
Primary biliary cholangitis (PBC) is chronic cholestatic liver disease that predominantly affects middle-aged women [4]. Although the aetiology of PBC remains unclear, immune-mediated damage, the retention of toxic bile acids and viral pathogens have all been implicated [4].
Alkaline phosphatase (ALP) is a 78 kd protein that dephosphorylates compounds in an alkaline environment [5]. One such compound is endotoxin [6,7]. Thus, cholestatic liver diseases associated with increased hepatic ALP expression such as PBC could conceivably protect a NAFLD liver from endotoxin-mediated injury. Alternatively, because PBC tends to involve zone 1 of the liver lobule while NAFLD is predominantly a zone 3 disease, progression to decompensated liver disease may occur more rapidly in NAFLD patients with co-existing PBC [1,8].
The purpose of the present study was to determine whether patients with NAFLD and PBC have more, less or similar disease severity and progression when compared to matched patients with NAFLD alone.
Methods
Patient population
This was a single centre, retrospective, case-control analysis of adult patients seen and followed in the Liver Disease Outpatient Clinic at the Health Sciences Centre in Winnipeg, Manitoba, Canada with a diagnosis of NAFLD or NAFLD/PBC. The diagnosis of NAFLD was based on the presence of fatty infiltration of the liver on abdominal imaging (increased echogenicity relative to the right kidney, decreased visualization of portal echoes and rounding of the liver contour) and the absence of alternative causes (excess alcohol, medications, etc.). The diagnosis of PBC was established on the basis of at least two of the following three criteria being present; (1) persistently elevated serum ALP levels of greater than six months duration, (2) a positive (≥1:80 titre) antimitochondrial antibody test and (3) liver histology in keeping with PBC [1,4].
Other causes of chronic liver disease were excluded from analysis. The additional liver diseases and tests performed to exclude those conditions included chronic hepatitis B (anti-HBc) and C (anti-HCV) infections, drug-induced liver disease (introduction of a new agent within 3 months of presentation), autoimmune hepatitis (anti-nuclear antibody and anti-smooth muscle antibody positivity associated with elevated IgG levels), hereditary hemochromatosis (elevated serum ferritin and transferrin saturation), Wilson’s disease (low serum ceruloplasmin), primary sclerosing cholangitis (PSC) (diagnostic imaging or histology), IgG4 disease (elevated serum IgG4 level) and granulomatous disease of the liver (chest x-ray suggestive of sarcoidosis, tuberculosis or lymphoma). Alcohol-induced liver injury was identified on the basis of a self-reported history of weekly consumption in excess of 21 units for men and 14 units for women. Collateral histories for alcohol intake were obtained where possible if the patient’s aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio exceeded 1, there was a disproportionate increase in serum gamma-glutamyl transferase (GGT) relative to ALP levels and/or an increased mean corpuscular volume value.
Less than 5% of NAFLD patients were being treated with vitamin E or thiazolidinediones and greater than 90% of PBC patients were treated with ursodeoxycholic acid at a dose of 10–15 mg/kg/d. The review preceded the promotion of obeticholic acid and fibrate treatments for PBC.
All NAFLD/PBC patients fulfilling the study’s inclusion criteria with no exclusion criteria were matched 1:2 with NAFLD alone patients by age and sex.
Laboratory analyses
Liver enzyme testing included serum ALT, AST, ALP and GGT levels. Liver function tests included serum total bilirubin, albumin and international normalized ratios (INRs) of prothrombin times. A complete blood count (CBC), serum creatinine, IgA, IgG and IgM levels were also obtained at initial and follow-up visits. All laboratory testing was performed by the Health Sciences Centre’s accredited Clinical Chemistry Department using standard laboratory techniques.
Activity, severity and progression of liver disease
NAFLD disease activity was assessed by pre-treatment serum ALT and AST values, severity by liver function tests, and fibrosis by calculation of Fib-4 scores and AST/platelet ratio indices (APRI). Progression was assessed by changes in these parameters over time.
Statistics
The descriptive statistics employed included means, medians and proportions with 95% confidence intervals. The frequency and distribution of demographic variables and clinical outcomes of interest were described by proportions and compared using chi-square test or F-test when warranted. Continuous variables (age, laboratory test results, etc.) were reported as means + SDs, medians and ranges, and were analysed using Student t-test or the non-parametric Mann–Whitney U test. Activity, fibrosis and progression were assessed by paired T-test and/or Wilcoxon signed-rank test. The prevalence of activity and fibrosis stage were expressed as a proportion of cases from the totals in each of the two groups. Ordinal logistic regression models were used to assess the joint contribution of key variables to the categorical classification. Two-sided P values less than 0.05 were considered statistically significant in all analyses.
This study was approved by the University of Manitoba’s Health Research Ethics Board. Individual consents from patients in this retrospective analysis were not requested.
Results
Table 1 provides the demographic and laboratory findings in NAFLD/PBC and NAFLD alone patients on presentation to the clinic (baseline). The mean ages were 64 ± 12 and 64 ± 11 years respectively and 91% were female. CBCs and serum creatinine levels were similar in the two cohorts. As expected for PBC patients, NAFLD/PBC patients had higher serum ALP, GGT, IgG and IgM levels (P < 0.0001, respectively).
Table 1.
Baseline demographic and laboratory data in non-alcoholic fatty liver disease/primary biliary cholangitis and non-alcoholic fatty liver disease alone patients
| Variable (normal values) | NAFLD/PBC (N = 68) | NAFLD (N = 136) | P value | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Median (IQR) | Mean ± SD | Range | Median (IQR) | ||
| Age | 64 ± 12 | 41–95 | 62 (55–73) | 64 ± 11 | 41–88 | 63 (56–74) | 0.86 |
| Female (%) | 91 | 91 | |||||
| Haemoglobin (140–180 g/L) | 131 ± 13 | 94–169 | 131 (123–141) | 134 ± 19 | 90–178 | 135 (126–143) | 0.35 |
| WBC (4.5–11 × 10E9/L) | 6.5 ± 1.9 | 2.6–13.1 | 6.5 (5.1–7.6) | 6.9 ± 2.3 | 3.1–15.9 | 6.45 (5.2–8.4) | 0.16 |
| Platelets (140–440 × 10E9/L) | 261 ± 78 | 90–561 | 272 (199–304) | 242 ± 82 | 47–496 | 233.5 (189–292) | 0.11 |
| Alkaline phosphatase (30–120 U/L) | 284 ± 196 | 43 891 | 207.5 (136–399) | 120 ± 123 | 34–1448 | 103 (77–135) | <0.0001 a |
| GGT (5–38 U/L) | 324 ± 318 | 20–2154 | 263.5 (132–404) | 145 ± 205 | 13–1283 | 79 (44–165) | <0.0001 a |
| Creatinine (35–97 µmol/L) | 71 ± 18 | 43–144 | 69 (58-79) | 73 ± 24 | 41–238 | 68 (58–82) | 0.64 |
| IgA (0.7–3.8 g/L) | 3.1 ± 1.5 | 0.07–7.32 | 2.87 (2.0–3.8) | 3.2 ± 1.7 | 0.77–8.37 | 2.96 (1.9–4.1) | 0.51 |
| IgG (6.9–16.2 g/L) | 15.2 ± 4.0 | 7.6–26.8 | 13.95 (12.2–17.7) | 11.5 ± 3.2 | 0.8–22.5 | 11.4 (9.3–13.6) | 0.0001 |
| IgM (0.6–2.6 g/L) | 3.6 ± 2.0 | 0.79–9.68 | 3.43 (2.0–4.7) | 1.5 ± 1.0 | 0.25–6.69 | 1.22 (0.8–1.7) | 0.0001 |
IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cholangitis; WBC, white blood cell count.
Bold indicates significant of P values.
Non-parametric test.
The results of baseline aminotransferases and liver function tests are provided in Table 2. Serum ALT and AST values were similar in the two cohorts. Although NAFLD/PBC and NAFLD alone patients had similar serum albumin and bilirubin levels, INR levels were significantly lower in the NAFLD/PBC cohort [0.99; interquartile range (IQR) 0.9–1.0 versus 1.01; IQR 1.0–1.1, P < 0.05).
Table 2.
Results of liver enzyme and function testing at baseline in non-alcoholic fatty liver disease/primary biliary cholangitis and non-alcoholic fatty liver disease alone patients
| Test (normal range) | NAFLD/PBC (N = 68) | NAFLD (N = 136) | P value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| ALT (<30 U/L) | 71 ± 43 | 61 (40.3–94.5) | 70 ± 49 | 56 (34.3–94.8) | 0.86 |
| AST (10–32 U/L) | 61 ± 46 | 46.5 (34.3–77) | 54 ± 41 | 42 (30–59.8) | 0.34 |
| Albumin (33–45 g/L) | 39 ± 3.8 | 38 (36–41) | 40 ± 4.3 | 39 (37–420 | 0.102 |
| Total Bilirubin (3–19 µmol/L) | 15.4 ± 43.2 | 7.75 (6–11) | 10.3 ± 19.3 | 7 (5.5–10) | 0.24 |
| INR (0.9–1.1) | 0.98 ± 0.1 | 0.99 (0.9–1.0) | 1.04 ± 0.2 | 1.01 (1.0–1.1) | 0.04 a |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; IQR, interquartile range; NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cholangitis.
Bold indicates significant of P values.
Non-parametric tests.
Non-invasive determinants of liver fibrosis stages at baseline were similar in the two cohorts with Fib-4 values and percent of patients with Fib-4 values suggestive of minimal or no fibrosis (Fib-4 <1.45) and cirrhosis (Fib-4 >3.25) being similar (Table 3). APRI determinations in terms of overall mean values and percent of patients with minimal fibrosis (APRI ≤0.45) and cirrhosis (APRI ≥1.5) were in keeping with the Fib-4 findings (Table 3).
Table 3.
Parameters of minimal and advanced liver disease at baseline in non-alcoholic fatty liver disease/primary biliary cholangitis and non-alcoholic fatty liver disease alone patients
| NAFLD/PBC (N = 68) | NAFLD (N = 136) | P value | |||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| FIB-4 | 1.7 ± 1.1 | 0.4–6.6 | 1.9 ± 1.4 | 0.4–8.7 | 0.26 |
| FIB-4 <1.45 (minimal or no fibrosis) | 44% | 50% | 0.41 | ||
| FIB-4 >3.25 (cirrhosis) | 7.8% | 13% | 0.26 | ||
| APRI | 0.6 ± 0.5 | 0.09–3.3 | 0.6 ± 0.5 | 0.16–3.5 | 0.99 |
| APRI ≤0.45 (minimal fibrosis) | 45% | 42% | 0.65 | ||
| APRI ≥1.5 (cirrhosis) | 4.7% | 8.1% | 0.56 | ||
APRI, aspartate aminotransferase/platelet ratio index; Fib-4, fibrosis score; NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cholangitis.
As shown in Table 4, the duration of follow-up was longer in NAFLD/PBC than NAFLD alone patients (7.0; IQR 3.6–8.8 versus 3.0; IQR 1.7–5.0). At last follow-up visit, serum AST values were similar and ALT lower in NAFLD/PBC versus NAFLD alone patients. In terms of liver function, total bilirubin levels were similar while albumin and INR levels were lower in NAFLD/PBC patients.
Table 4.
Results of liver enzyme and function testing at the end of follow-up in non-alcoholic fatty liver disease/primary biliary cholangitis and primary biliary cholangitis alone patients
| Test (normal range) | NAFLD/PBC (N = 68) | NAFLD (N = 136) | P value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Follow-up (years) | 6.6 ± 15.2 | 7.0 (3.6–8.8) | 3.6 ± 2.6 | 3.0 (1.7–5.0) | 0.001 a |
| ALT (<30 U/L) | 34 ± 33 | 27 (19–40) | 45 ± 37 | 35 (23–58) | 0.008 a |
| AST (10–32 U/L) | 33 ± 51 | 29 (22–40) | 43 ± 51 | 32 (23–47) | 0.13a |
| Albumin (33–45 g/L) | 36 ± 4.4 | 37 (34–39) | 38 ± 4.4 | 39 (36–41) | 0.006 |
| Total Bilirubin (3–19 µmol/L) | 14.5 ± 45 | 7 (5.1–9) | 8.6 ± 5.3 | 7 (6–9) | 0.79a |
| INR (0.9–1.1) | 0.99 ± 0.1 | 0.99; 0.9–1.0 | 1.11 ± 0.4 | 1.1 (0.93–1.1) | 0.02 a |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; IQR, interquartile range; NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cholangitis.
Bold indicates significant of P values.
Non-parametric tests.
Patients with NAFLD/PBC had significantly lower Fib-4 levels at last follow-up visit when compared to NAFLD alone patients (1.23; IQR 0.92–1.83 versus 1.51; IQR 0.98–2.47, P < 0.05) (Table 5). The overall but not annual change in Fib-4 levels during follow-up was also less in NAFLD/PBC patients. These findings were not supported by APRI determinations where the last follow-up visit and overall change in APRI values were also lower in NAFLD/PBC patients but the differences did not reach statistical significance (P = 0.08 and 0.09, respectively).
Table 5.
Changes in Fib-4 and aspartate aminotransferase/platelet ratio index values during follow-up in non-alcoholic fatty liver disease/primary biliary cholangitis and primary biliary cholangitis alone patients
| NAFLD/PBC (N = 68) | NAFLD (N = 136) | P valuea | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| FIB-4 baseline | 1.7 ± 1.1 | 1.47 (0.93 to 1.74) | 1.9 ± 1.4 | 1.45 (0.98 to 2.25) | 0.26 |
| FIB-4 last follow-up | 1.6 ± 1.2 | 1.23 (0.92 to 1.83) | 2.2 ± 2.4 | 1.51 (0.98 to 2.47) | 0.04 |
| FIB-4 change (overall) | −0.06 ± 1.3 | −0.08 (−0.42 to 0.33) | 0.33 ± 1.8 | 0.06 (−0.29 to 0.50) | 0.05 |
| FIB-4 change per year | −0.003 ± 0.6 | −0.01 (−0.13 to 0.06) | 0.10 ± 0.7 | 0.02 (−0.11 to 0.13) | 0.3 |
| APRI baseline | 0.6 ± 0.5 | 0.51 (0.33 to 0.77) | 0.6 ± 0.5 | 0.50 (0.28 to 0.75) | 0.99 |
| APRI follow-up | 0.4 ± 0.3 | 0.32 (0.21 to 0.46) | 0.6 ± 0.8 | 0.39 (0.23 to 0.68) | 0.08 |
| APRI change (overall) | −0.23 ± 0.5 | −0.13 (−0.03 to 0.01) | −0.06 ± 0.8 | −0.05 (−0.27 to 0.08) | 0.09 |
| APRI change per year | −0.07 ± 0.3 | −0.03 (−0.10 to 0.003) | −0.03 ± 0.3 | −0.02 (-0.07 to 0.02) | 0.41 |
APRI, aspartate aminotransferase/platelet ratio index; Fib-4, fibrosis score; IQR, interquartile range; NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cholangitis.
Bold indicates significant of P values.
Non-parametric tests.
In 57% of NAFLD/PBC patients, Fib-4 levels were stable, 13% increased and 30% decreased during follow-up while in NAFLD alone patients the percentages were 75, 15 and 9.6%, respectively (Table 6). The differences in percent of stable and decreased Fib-4 levels between the two cohorts were statistically significant (P = 0.01 and 0.0002, respectively). According to APRI values, stable, increased and decreased percentages were similar in the two cohorts.
Table 6.
Trends in Fib-4 and aspartate aminotransferase/platelet ratio index values from baseline to end of follow-up in non-alcoholic fatty liver disease/primary biliary cholangitis and non-alcoholic fatty liver disease alone patients
| Score | NAFLD/PBC (N = 63) | NAFLD (N = 136) | P value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| FIB-4 change | |||||
| Stable | 36 | 57.1 | 102 | 75.0 | 0.01 |
| Increase | 8 | 12.7 | 21 | 15.4 | 0.61 |
| Decrease | 19 | 30.2 | 13 | 9.6 | 0.0002 |
| APRI change | |||||
| Stable | 37 | 58.7 | 92 | 67.0 | 0.22 |
| Increase | 4 | 6.3 | 9 | 6.6 | 0.94 |
| Decrease | 22 | 34.9 | 35 | 25.7 | 0.18 |
Bold indicates significant of P values.
Finally, aside from more NAFLD/PBC patients with baseline intermediate Fib-4 levels (>1.45 <3.25) transitioning to minimal or no fibrosis levels (<1.45) than those with NAFLD alone (53% versus 16%, respectively, P < 0.0005) there were no significant differences in improvement or regression of liver disease stages in the two cohorts during follow-up (Table 7 and Fig. 1).
Table 7.
Transition of patients with minimal, moderate or advanced fibrosis from baseline to end of follow-up in non-alcoholic fatty liver disease/primary biliary cholangitis and non-alcoholic fatty liver disease alone patients
| NAFLD/PBC | NAFLD | P value | |||||
|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | ||
| Low FIB-4 score (<1.45) | |||||||
| Baseline | 64 | 28 | 44 | 136 | 68 | 50 | 0.41 |
| End of follow-up | 66 | 39 | 59 | 136 | 64 | 47 | 0.11 |
| Changes in low FIB-4 group by the end of follow-up period | |||||||
| Stable (remains <1.45) | 28 | 21 | 75 | 68 | 55 | 80.8 | 0.52 |
| Moderate increase (>1.45 <3.25) | 28 | 6 | 21.4 | 68 | 12 | 17.7 | 0.66 |
| Cirrhosis (>3.25) | 28 | 1 | 3.6 | 68 | 1 | 1.5 | 0.51 |
| High FIB-4 score (>3.25) | |||||||
| Baseline | 64 | 5 | 7.8 | 136 | 18 | 13.2 | 0.38 |
| End of follow-up | 66 | 4 | 6.1 | 136 | 21 | 15.4 | 0.057 |
| Changes in high FIB-4 group by the end of follow-up period | |||||||
| Stable (remains cirrhotic >3.25) | 5 | 2 | 40 | 18 | 13 | 72 | 0.18 |
| Decreased to moderate (>1.45 <3.25) | 5 | 3 | 60 | 18 | 4 | 22 | |
| Decreased to low (<1.45) | 5 | 0 | 18 | 1 | 6 | ||
| Intermediate FIB-4 score (>1.45 <3.25) | |||||||
| Baseline | 64 | 31 | 48 | 136 | 50 | 36.8 | 0.12 |
| End of follow-up | 66 | 22 | 33 | 136 | 51 | 37.5 | 0.71 |
| Changes in intermediate FIB-4 group by the end of follow-up period | |||||||
| Stable (remains moderate) | 30 | 13 | 43.3 | 50 | 35 | 70 | 0.018 |
| Increased to cirrhosis (>3.25) | 30 | 1 | 3.3 | 50 | 7 | 14 | 0.12 |
| Decreased to low (<1.45) | 30 | 16 | 53.3 | 50 | 8 | 16 | 0.0004 |
| Low APRI (≤0.45) | |||||||
| Baseline | 64 | 29 | 45 | 136 | 57 | 42 | 0.65 |
| End of follow-up | 66 | 49 | 74.2 | 136 | 81 | 59.6 | 0.05 |
| Changes in low APRI group by the end of follow-up period | |||||||
| Stable (remains ≤0.45) | 29 | 27 | 93 | 57 | 50 | 88 | 0.44 |
| Moderate increase (≥0.45 ≤1.5) | 29 | 2 | 7 | 57 | 7 | 12 | |
| Cirrhosis (≥1.5) | 29 | 0 | 57 | 0 | |||
| High APRI score (>1.5) | |||||||
| Baseline | 64 | 3 | 4.7 | 136 | 11 | 8.1 | 0.56 |
| End of follow-up | 66 | 1 | 1.5 | 136 | 8 | 5.9 | 0.29 |
| Changes in high APRI group by the end of follow-up period | |||||||
| Stable (remains cirrhotic ≥1.5) | 3 | 0 | 11 | 5 | 45.4 | ||
| Decreased to moderate (≥0.5 ≤1.5) | 3 | 2 | 66.6 | 11 | 4 | 36.4 | 0.54 |
| Decreased to low (≤0.5) | 3 | 1 | 33.3 | 11 | 2 | 18.2 | 0.82 |
| Intermediate APRI score (>0.45 <1.5) | |||||||
| Baseline | 64 | 31 | 48 | 136 | 68 | 50 | 0.88 |
| End of follow-up | 66 | 16 | 24 | 136 | 47 | 35 | 0.15 |
| Changes in intermediate APRI group by the end of follow-up period | |||||||
| Stable (remains moderate) | 31 | 11 | 35.5 | 68 | 36 | 53 | 0.13 |
| Increased to cirrhosis (≥1.5) | 31 | 1 | 3.2 | 68 | 3 | 4 | |
| Decreased to low (≤0.5) | 31 | 19 | 61.3 | 68 | 29 | 43 | 0.085 |
Fig. 1.
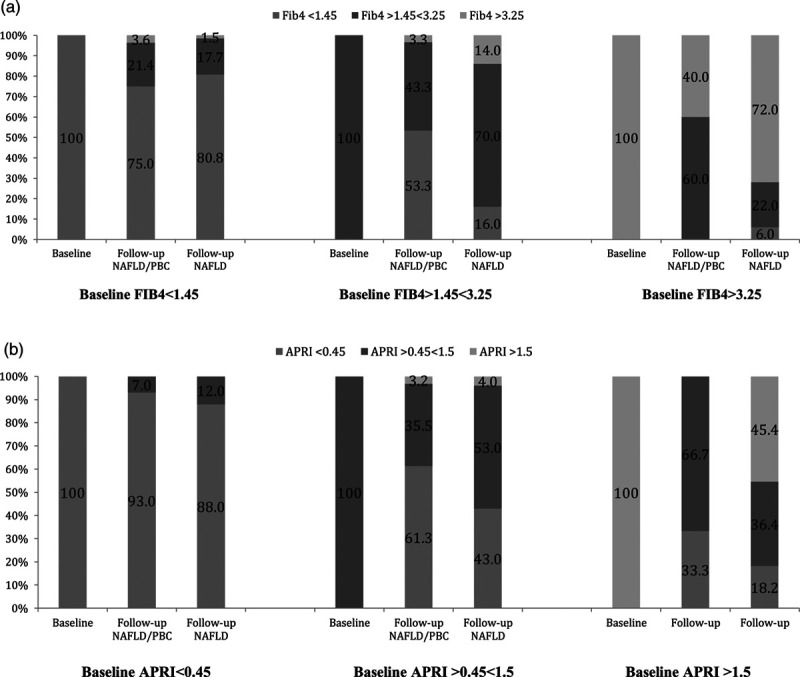
Transition of patients with minimal, moderate or advanced fibrosis from baseline to end of follow-up in NAFLD/PBC and NAFLD alone patients (as proportions). (a) Transition of FIB-4. (b) Transition of APRI. NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cholangitis.
Discussion
The results of this study are somewhat counter-intuitive. The co-existence of two chronic liver diseases, particularly those that predominantly affect different regions of the liver lobule, would be expected to result in more severe and progressive liver disease than when only one disorder is present. Yet, in an appreciable number of matched patients followed for periods of time that favour more advanced disease developing in NAFLD/PBC patients, the severity and progression of liver disease in this cohort of patients was either similar or less progressive than in patients with NAFLD alone.
Although previous studies have documented the impact of NAFLD on other liver conditions [9–11], there are no published reports describing the impact of other liver diseases on NAFLD. Thus, comparisons with previous reports could not be undertaken.
If the results of the present study are subsequently confirmed, the question arises as to how might the presence of PBC not adversely affect the severity and course of NAFLD. One consideration is whether treatment of PBC with ursodiol (90% of PBC patients were receiving this agent) would favourably impact on NAFLD. However, the results of previous therapeutic trials of ursodiol in NAFLD were largely negative [12]. It is also important to note that ursodiol merely halts or delays the progression of PBC. Thus, the NAFLD component of NAFLD/PBC patients could presumably have progressed unabated in these patients despite the use of ursodiol. Also to be considered is whether PBC decreases the prevalence or severity of the principal risk factors associated with NAFLD progression such as weight gain and the various components of the metabolic syndrome [1,13]. However, data to date suggest that PBC either has no effect or is not associated with a decreased risk of developing these conditions. Indeed, dyslipidemia is more common in PBC patients [4]. Finally, the ALP property of inactivating endotoxin and the significantly higher ALP levels in NAFLD/PBC patients compared to those with NAFLD alone, raises the possibility that PBC and perhaps other cholestatic liver diseases, attenuate the severity and course of NAFLD by eliminating a variable (endotoxin) thought to play an important role in the pathogenesis of NAFLD [6,7]. Clearly, additional experimentation is required to address this possibility.
Certain discrepancies in the results of this study require discussion. For example, although baseline and last follow-up APRI results were in keeping with Fib-4 findings, unlike Fib-4, the APRI results did not reach statistical significance. This discrepancy may reflect the relatively lower accuracy of APRI determinations when compared to Fib-4 values in NAFLD patients [14]. Also to be reconciled are the lower serum albumin (suggesting more impaired liver function) and INR (suggesting more intact liver function) levels in NAFLD/PBC compared to NAFLD alone patients. Here, it should be noted that although differences existed, the values of both tests remained within the normal ranges. Moreover, whether vitamin K deficiency and/or supplementation was present to a greater or lesser extent in one cohort compared to the other, was not captured, nor were non-hepatic causes of hypoalbuminemia excluded.
Again, if confirmed, the results of this study raise the intriguing possibility of treating NAFLD patients with ALP. Of note, systemic administration of ALP has been previously employed in animal models and patients with acute on chronic liver disease, sepsis and renal failure with promising results [15–17]. In addition, previous data indicate systemically administered ALP is distributed to the liver where its effect on endotoxin is required [18]. Whether long-term, oral ALP formulations would also be effective has yet to be determined. None-the-less, the high cost of such treatment and recent advances in NAFLD therapy render ALP treatment of NAFLD of academic interest at this time.
There are a number of limitations to this study that warrant emphasis. First, the study design involved a single centre and was retrospective. Second, body weights and clinical evidence of hepatic decompensation (ascites, encephalopathy, etc.) were not captured in the database. Thus, other parameters of disease severity such as the NAFLD fibrosis score, BARD score for NAFLD fibrosis and Child-Pugh class could not be calculated. Third, liver histology was not available and therefore, histologic evidence of disease activity and fibrosis (as well as tissue endotoxin levels) could not be ascertained. Finally, due to their low prevalences, other chronic cholestatic liver conditions such as PSC were not included in the analysis.
In conclusion, patients with coexisting NAFLD and PBC do not have biochemical or non-invasive fibrosis marker evidence of more severe or progressive liver disease than age- and sex-matched patients with NAFLD alone. Additional research is required to provide an explanation for this counter-intuitive finding.
| APRI | FIB-4 | |
|---|---|---|
| Definitions | ||
| Low | ≤0.45 | <1.45 |
| Intermediate | >0.45<1.5 | >1.45<3.25 |
| High | ≥1.5 | >3.25 |
APRI, aspartate aminotransferase/platelet ratio index; Fib-4, fibrosis score; NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cholangitis.
Acknowledgements
We wish to thank Ms. R. Vizniak for her prompt and accurate typing of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017; 377:2063–2072. [DOI] [PubMed] [Google Scholar]
- 2.Ganz M, Szabo G. Immune and inflammatory pathways in NASH. Hepatol Int. 2013; 7(Suppl 2):S771–S781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010; 103:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019; 69:394–419. [DOI] [PubMed] [Google Scholar]
- 5.Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem. 2014; 29:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poelstra K, Bakker WW, Klok PA, Kamps JA, Hardonk MJ, Meijer DK. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am J Pathol. 1997; 151:1163–1169. [PMC free article] [PubMed] [Google Scholar]
- 7.Poelstra K, Bakker WW, Klok PA, Hardonk MJ, Meijer DK. A physiologic function for alkaline phosphatase: endotoxin detoxification. Lab Invest. 1997; 76:319–327. [PubMed] [Google Scholar]
- 8.Springer J, Cauch-Dudek K, O’Rourke K, Wanless IR, Heathcote EJ. Asymptomatic primary biliary cirrhosis: a study of its natural history and prognosis. Am J Gastroenterol. 1999; 94:47–53. [DOI] [PubMed] [Google Scholar]
- 9.Shi JP, Fan JG, Wu R, Gao XQ, Zhang L, Wang H, Farrell GC. Prevalence and risk factors of hepatic steatosis and its impact on liver injury in Chinese patients with chronic hepatitis B infection. J Gastroenterol Hepatol. 2008; 23:1419–1425. [DOI] [PubMed] [Google Scholar]
- 10.Ye Q, Qian BX, Yin WL, Wang FM, Han T. Association between the HFE C282Y, H63D polymorphisms and the risks of non-alcoholic fatty liver disease, liver cirrhosis and hepatocellular carcinoma: an updated systematic review and meta-analysis of 5,758 cases and 14,741 controls. PLoS One. 2016; 11:e0163423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minuk GY, Iliant V, Zhou N, Kaita KD, Wong SG, Peretz D, Uhanova J. Concomitant nonalcoholic fatty liver disease does not alter the activity, severity or course of primary biliary cholangitis. Liver Int. 2018; 38:1110–1116. [DOI] [PubMed] [Google Scholar]
- 12.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778. [DOI] [PubMed] [Google Scholar]
- 13.Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016; 17:E774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna WN, Jr, Harrison SA. Noninvasive imaging methods to determine severity of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2016; 64:2234–2243. [DOI] [PubMed] [Google Scholar]
- 15.Su F, Brands R, Wang Z, Verdant C, Bruhn A, Cai Y, et al. Beneficial effects of alkaline phosphatase in septic shock. Crit Care Med. 2006; 34:2182–2187. [DOI] [PubMed] [Google Scholar]
- 16.Pickkers P, Heemskerk S, Schouten J, Laterre PF, Vincent JL, Beishuizen A, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care. 2012; 16:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelmann C, Adebayo D, Oria M, et al. Recombinant Alkaline Phosphatase is a Novel Therapy for the Prevention of Acute on Chronic Liver Failure but not Acute Liver Failure. San Francisco: Abstract 0113, AASLD Meeting; 2018. [Google Scholar]
- 18.Alvaro D, Benedetti A, Marucci L, Delle Monache M, Monterubbianesi R, Di Cosimo E, et al. The function of alkaline phosphatase in the liver: regulation of intrahepatic biliary epithelium secretory activities in the rat. Hepatology. 2000; 32:174–184. [DOI] [PubMed] [Google Scholar]


