To the Editor,
An increasing number of therapies are available to treat asthma, but inhaled corticosteroids (ICS) are still the most commonly prescribed and effective controller asthma medication1. Both environmental and genetic factors are involved in ICS response. However, few biomarkers have been associated with response to asthma treatment with ICS2. Here, we aimed to identify novel markers of ICS response combining transcriptomic and genomic data.
A full description of the methods is provided in the Supplementary Methods at the Supporting information and it is also summarized in Figure S1. In the discovery stage, RNA sequencing (RNA-seq) data from airway smooth muscle (ASM) cells isolated from four non-asthmatic donors publicly available at the Sequence Read Archive (SRA, SRP033351) (https://www.ncbi.nlm.nih.gov/sra) were analyzed3. Briefly, this study performed RNA-seq of cell lines from four non-asthmatic lung donors treated with control solution or with 1 μM dexamethasone for 18 h3. Raw sequencing data were downloaded and processed according to the procedures described in the Supplementary Methods. Differential gene expression of cells treated with glucocorticosteroids (GCs) compared to those treated with control solution was evaluated using linear models. Differentially dysregulated genes were identified after a false discovery rate (FDR) adjustment of 5% (q-value≤0.05).
Differentially expressed genes in the discovery were examined to determine if they also showed changes in peripheral blood mononuclear cells (PBMCs) obtained from ICS non-responders (n=3) and ICS responders (n=3) from the SLOVENIA study4, defined based on the presence or absence of asthma exacerbations despite ICS therapy. RNA libraries were sequenced using the BGISEQ-500 instrument (BGI Inc.) and data was analyzed following the same methodology as for the ASM cells. Gene expression levels were compared in responders to ICS and ICS non-responder patients, adopting a q-value≤0.05 to declare significance. Validation of significant differentially expressed genes found in the previous datasets was sought using three additional transcriptomic data of ASM cells after GCs exposure (GSE13168, GSE34313, and SRP098649), which were combined in a random-effects meta-analysis. Evidence of replication was considered for genes with consistent effects and q-value≤0.05.
Genetic variants located within ±100 kilobases (kb) from the genes with significant changes in expression levels across all transcriptomic datasets were examined for association with asthma exacerbations despite ICS. For that, ten studies participating in the Pharmacogenomics in Childhood of Asthma (PiCA) consortium were analyzed, including 2,681 European and 1,347 Hispanics/Latinos and African American children and youth treated with ICS with genome-wide genotyping data available (Table S1). Details about each study are described at the Supporting Information and elsewhere4.
Individuals with asthma exacerbations despite ICS treatment were considered as ICS non-responders and those without asthma exacerbations as ICS responders. Asthma exacerbations were defined by the need for emergency care, hospitalizations, administration of systemic corticosteroids, unscheduled doctor visits, and/or school absences because of asthma (Table S1). Association between imputed single nucleotide polymorphisms (SNPs) and asthma exacerbations despite ICS treatment was tested for each study through logistic regression models including age, sex, and principal components as covariates. Two separate meta-analyses were performed in Europeans and non-Europeans. A Bonferroni-like corrected threshold for significance was obtained for each population as α=0.05/total number of independent variants.
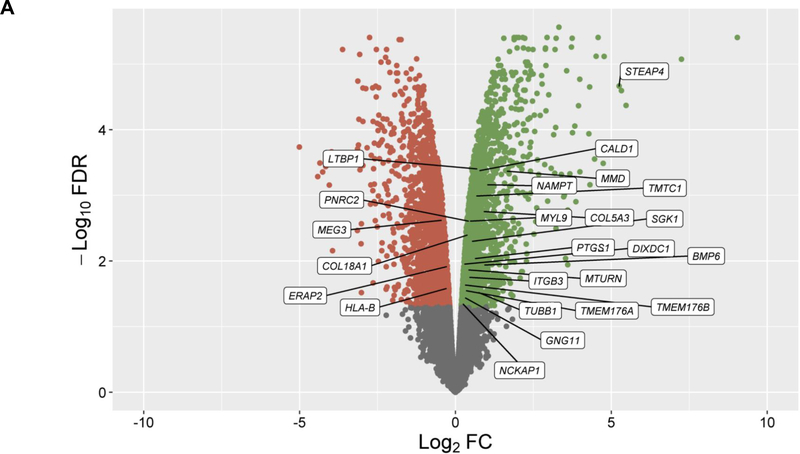
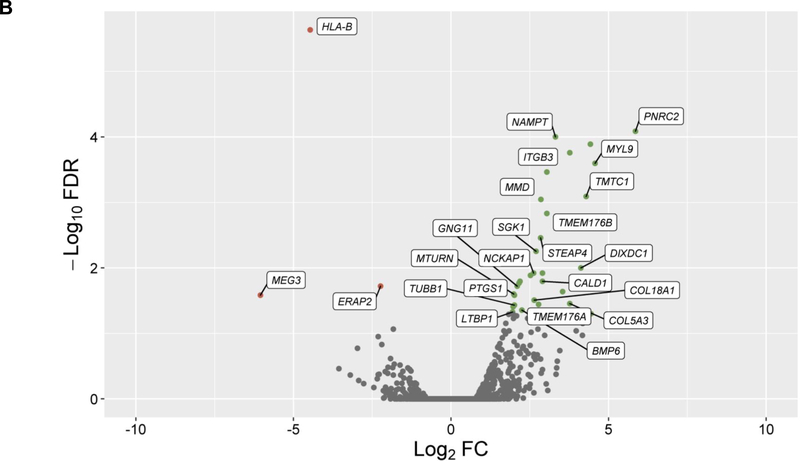
In the discovery, a total of 14,707 genes were analyzed and 4,718 of them were found to be differentially expressed in cells treated with GCs (q-value≤0.05) (Figure 1A). From these genes, 24 showed significant changes in expression levels depending on ICS response status from asthma patients in PBMCs (q-value≤0.05) with consistent alteration across both datasets based on log2 fold change (FC) values (Figure 1B, Table S2). Moreover, six out of the 24 genes were replicated with a consistent effect of up-regulation in the combined analysis of the three additional transcriptomic datasets of ASM cells treated with GCs: LTBP1 (q-value=7.46×10−4), MTURN (q-value=3.92×10−3), NAMPT (q-value=3.77×10−7), CALD1 (q-value=5.22×10−5), MMD (q-value=5.84×10−4), COL18A1 (q-value=1.93×10−3) (Table S3). The potential implication of these genes on asthma severity was assessed by evaluating their expression in PBMCs according to baseline lung function, showing no significant changes (Table S4). Additionally, only LTBP1 remained significantly differentially expressed in response to ICS treatment after accounting for baseline lung function (Table S4).
Figure 1. Results of differential expression in response to GCs in ASM cells and PBMCs.
Volcano plot of differential expression results in ASM cells after exposure to GCs compared to a control solution (panel A). Results of differential expression in PBMCs from ICS responders are shown for genes with significant changes in expression levels in ASM cells in response to GCs after adjusting with a false discovery rate (FDR) of 5% (q-value≤0.05) (panel B). Results are represented in terms of log2 fold change (log2 FC) (x-axis) and the logarithmic transformation of FDR (-log10 FDR) (x-axis). Genes significantly (q-value≤0.05) found to be up-regulated (log2 FC>0) or downregulated (log2 FC<0) are represented by means of green or red dots, respectively. Genes with consistent alteration of expression levels in ASM cells and PBMCs are labeled into white boxes.
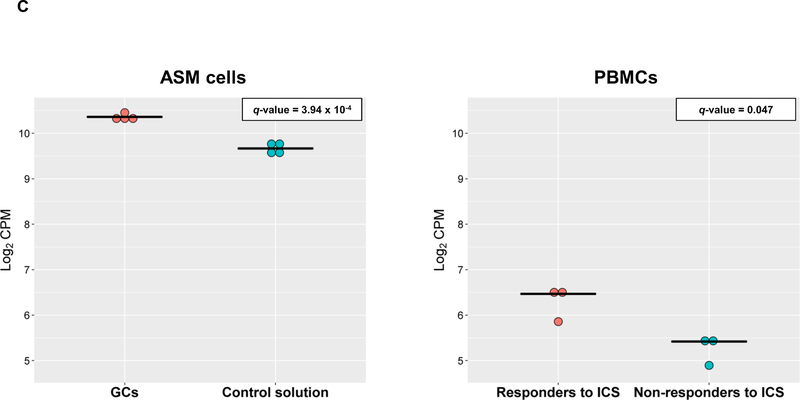
Dots plots of differential expression for LTBP1 in ASM cells and PBMCs (panel C). Gene expression levels are represented in terms of log2 counts per million (CPM) in the y-axis as dots for cases (red) and control (blue) samples. The median expression level is represented for each sample group by a black horizontal line. P-values adjusted by false discovery rate are shown (q-value).
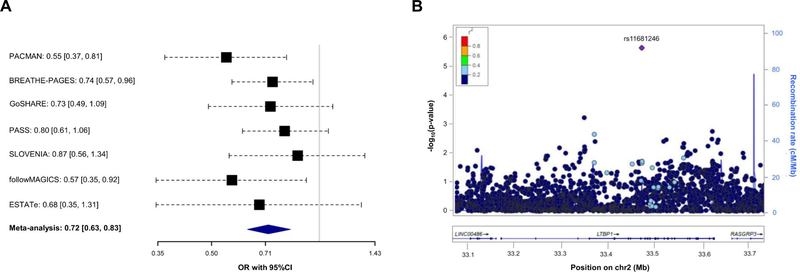
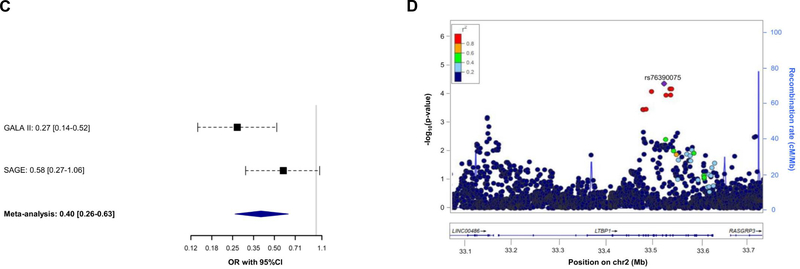
Association of 7,042 SNPs with asthma exacerbations despite ICS use within ±100 kilobases from the six genes validated was tested in Europeans. After applying a Bonferroni-like correction (p≤4.96×10−5 for 1,007 independent variants tested), the SNP rs11681246 located within LTBP1 (Figure 1C) was significantly associated with asthma exacerbations despite ICS use in Europeans (OR for G allele: 0.72, 95% CI: 0.63, 0.83, p=3.28×10−6) (Figure 2A–2B). The effect of this association was similar after adjusting by treatment step, as a proxy of disease severity, in a subset of 2,282 patients with complete information about asthma medication (OR for G allele: 0.74, 95% CI: 0.63, 0.86, p=1.13×10−4). However, this SNP was not replicated in non-Europeans. Nonetheless, we assessed the association of alternative polymorphisms in non-Europeans, revealing the association of six an intronic LTBP1 variants in high linkage disequilibrium (r2>0.95), all dependent from the association of rs76390075 (OR for C allele: 0.40, 95% CI: 0.26, 0.63, p=6.76×10−5), after adjustment for 234 independent variants tested (p≤2.14×10−4) (Figure 2C–2D). Moreover, this association was robust to the adjustment by medication step, OR for C allele: 0.41, 95% CI: 0.26, 0.65, p=1.12×10-4. However, the association signals detected in European and admixed populations are not in LD (r2<0.01). A description of the potential functional implications of these variants is available at the Supporting Information.
Figure 2. Association results with asthma exacerbations despite ICS treatment for LTBP1.
Forest plot of association effects of the SNPs rs11681246 (panel A) across the European studies and rs76390075 (panel C) in non-European studies. The effects are shown in terms of OR for the effect alleles (G and C, respectively) for each study and after performing a meta-analysis of the results by black boxes and a blue diamond, respectively. The 95% CI are represented by black dash lines. Results are not provided for BREATHE since this SNP did not pass quality control checks in this study.
Regional plot of association results with asthma exacerbations despite ICS use among Europeans (panel B) and non-Europeans (panel D). The y-axis represents the logarithmic transformation of the association results (-log10 p-value) by chromosome position (x-axis) for each SNP as a dot. The diamond corresponds to the most significant variant after Bonferroni-like correction; the remaining SNPs are color-coded based on pairwise linkage disequilibrium (r2 values) with that SNP for Europeans (panel B) or Admixed American populations (panel D) from 1KGP (GRCh37/hg19 build).
This study describes the results of transcriptomic analyses of several datasets revealing LTBP1 as a gene involved in ICS response among asthma patients. GCs were found to increase LTBP1 expression levels in four datasets of ASM cells experimentally exposed to different types of GCs. Similar effects were detected in PBMCs obtained from asthma patients that were ICS-responders, but not in non-responders. This suggests that ICS treatment may not induce LTBP1 expression in PBMCs from non-responders. Additionally, the association of two variants within this gene with asthma exacerbations despite ICS use was found in diverse populations.
LTBP1 encodes a member of the family of latent-transforming growth factor-beta (TGF-β) binding proteins. LTBP1 is involved in the regulation of the TGF-β1 activity, including its activation from a precursor form, folding, secretion out from the cell, and deposition at the extracellular matrix through interactions with fibrillin molecules5. Interestingly, TGF-β1 has been proposed to play a key role in cell growth and differentiation, immune response, and airway remodeling6. Specifically, increased levels of the active form of TGF-β1 have been detected in asthma patients, which has been suggested to recruit myofibroblasts triggering an increased collagen deposition and the development of subepithelial fibrosis in asthma7. LTBP1 has also been proposed to be involved in allergic diseases and idiopathic pulmonary fibrosis (IPF), where LTBP1 interacts with fibulin 1c (FBLN1), modulating lung remodeling and fibrosis through the regulation of TGF-β1 activation8. In fact, the inhibition of FBLN1 binding to LTBP1 has been proposed as a therapeutic strategy to reduce fibrotic processes8. Interestingly, genetic variants near or within LTBP1 are also associated with lung function measurements among participants from the UKBiobank9.
We acknowledge the limited sample size of the transcriptomic datasets and the lack of functional experiments as major limitations of our study. Therefore, validation of the association in independent populations analyzing larger sample sizes and functional studies are needed. These will provide insights about the biological mechanisms implicating LTBP1 in ICS response and to test its clinical relevance predicting the treatment response. Additionally, a different gene expression profile would be obtained if the analyses would have been restricted only to ASM cells, as indicated by Kan et al10.
In summary, our study revealed LTBP1 as a potential novel locus for ICS response in asthma patients. These results indicate that combining publicly available data from different omic sources could be a powerful approach to provide novel insights about the mechanisms involved in the ICS treatment response.
Supplementary Material
Acknowledgments
The authors would like to thank the patients, families, recruiters, health care providers, and community clinics for their participation in all the studies included in the PiCA consortium (http://pica-consortium.org). The authors thank the contribution of Teide High-Performance Computing facilities (http://teidehpc.iter.es) provided by the Instituto Tecnológico y de Energías Renovables (ITER, S.A.) to the results of this research and also to the Centro Nacional de Genotipado-Plataforma de Recursos Biomoleculares-Instituto de Salud Carlos III (CeGen-PRB3-ISCIII; www.cegen.org) for the genotyping services provided.
The GALA II study collaborators include Shannon Thyne, UCSF; Harold J. Farber, Texas Children’s Hospital; Denise Serebrisky, Jacobi Medical Center; Rajesh Kumar, Lurie Children’s Hospital of Chicago; Emerita Brigino-Buenaventura, Kaiser Permanente; Michael A. LeNoir, Bay Area Pediatrics; Kelley Meade, UCSF Benioff Children’s Hospital, Oakland; William Rodriguez-Cintron, VA Hospital, Puerto Rico; Pedro C. Avila, Northwestern University; Jose R. Rodriguez-Santana, Centro de Neumologia Pediatrica; Luisa N. Borrell, City University of New York; Adam Davis, UCSF Benioff Children’s Hospital, Oakland; Saunak Sen, University of Tennessee and Fred Lurmann, Sonoma Technologies, Inc.
The authors acknowledge the families and patients for their participation and thank the numerous health care providers and community clinics for their support and participation in GALA II. In particular, the authors thank study coordinator Sandra Salazar; the recruiters who obtained the data: Duanny Alva, MD, Gaby Ayala-Rodriguez, Lisa Caine, Elizabeth Castellanos, Jaime Colon, Denise DeJesus, Blanca Lopez, Brenda Lopez, MD, Louis Martos, Vivian Medina, Juana Olivo, Mario Peralta, Esther Pomares, MD, Jihan Quraishi, Johanna Rodriguez, Shahdad Saeedi, Dean Soto, Ana Taveras; and the lab researcher Celeste Eng who processed the biospecimens.
The SAGE study collaborators include Harold J. Farber, Texas Children’s Hospital; Emerita Brigino-Buenaventura, Kaiser Permanente; Michael A. LeNoir, Bay Area Pediatrics; Kelley Meade, UCSF Benioff Children’s Hospital, Oakland; Luisa N. Borrell, City University of New York; Adam Davis, UCSF Benioff Children’s Hospital, Oakland and Fred Lurmann, Sonoma Technologies, Inc.
The authors acknowledge the families and patients for their participation and thank the numerous health care providers and community clinics for their support and participation in SAGE. In particular, the authors thank study coordinator Sandra Salazar; the recruiters who obtained the data: Lisa Caine, Elizabeth Castellanos, Brenda Lopez, MD, Shahdad Saeedi; and the lab researcher Celeste Eng who processed the biospecimens.
Funding
This study was supported by the award (AC15/00015) funded by the Instituto de Salud Carlos III (ISCIII) through Strategic Action for Health Research (AES) and European Community (EC) within the Active and Assisted Living (AAL) Programme framework (MP-Y), and the SysPharmPedia grant from the ERACoSysMed 1st Joint Transnational Call from the European Union under the Horizon 2020. This study was also funded by the Spanish Ministry of Economy, Industry, and Competitiveness, the State Research Agency and the European Regional Development Funds from the European Union (MINECO/AEI/FEDER, UE, SAF2017–83417R). N.H-P was supported by a fellowship (FI16/00136) from Instituto de Salud Carlos III (ISCIII) and co-funded by the European Social Funds from the European Union (ESF) “ESF invests in your future”. MP-Y was funded by the Ramón y Cajal Program (RYC-2015–17205) by the Spanish Ministry of Economy, Industry, and Competitiveness. The PACMAN study was funded by a strategic alliance between GlaxoSmithKline and Utrecht Institute for Pharmaceutical Sciences. The SLOVENIA study was financially supported by the Slovenian Research Agency (research core funding No. P3–0067) and from the SysPharmPedia grant, co-financed by the Ministry of Education, Science and Sport Slovenia (MIZS) (contract number C3330–16-500106). GALA II was supported by the National Heart, Lung, and Blood Institute of the National Institute of Health (NIH) grants R01HL117004 and X01HL134589; study enrolment supported by the Sandler Family Foundation, the American Asthma Foundation, the RWJF Amos Medical Faculty Development Program, Harry Wm. and Diana V. Hind Distinguished Professor in Pharmaceutical Sciences II and the National Institute of Environmental Health Sciences grant R01ES015794. SAGE was funded by the National Heart, Lung, and Blood Institute of the National Institute of Health (NIH) grants R01HL117004 and X01HL134589; study enrolment supported by the Sandler Family Foundation, the American Asthma Foundation, the RWJF Amos Medical Faculty Development Program, Harry Wm. and Diana V. Hind Distinguished Professor in Pharmaceutical Sciences II. The SHARE Bioresource (GoSHARE) and SHARE have ongoing funding from NHS Research Scotland and established by funding from The Wellcome Trust Biomedical Resource [Grant No. 099177/Z/12/Z]. ESTATe was funded by an independent research grant by ZonMw project (113201006). The PASS study was funded by the NHS Chair of Pharmacogenetics via the UK Department of Health. MP is Emeritus NIHR Senior Investigator. Genotyping of samples from BREATHE-PAGES, GoSHARE, and SCSGES was carried out at CeGen-PRB3-ISCIII; supported by ISCIII and European Regional Development Fund (ERDF) (PT17/0019).
Conflict of Interest Statement
NH-P declares funding from Instituto de Salud Carlos III (ISCIII) and the European Social Funds. SM reports funding from The Gannochy Trust, Perth and Kinross City Council, and Scottish Enterprises Tayside. MP declares funding from the MRC Clinical Pharmacology Training Scheme, EPSRC, Astra Zeneca, and Bristol Myers Squibb. KV reports funding from ZonMw, Yamanouchi, Pfizer/Boehringer Ingelheim, Novartis, and GSK. MK declares funding from the European Union, German Ministry of Education, Research, German Research Foundation, and other sources. EGB reports funding from the National Institute of Health, National Institute of Health and Environmental Health Sciences, National Institute on Minority Health and Health Disparities, National Institute of General Medical Sciences, National Human Genome Research Institute, Sandler Family Foundation, American Asthma Foundation, Robert Wood Johnson Foundation. A-HM declares funding from GlaxoSmithKline, Boehringer Ingelheim, and Astra Zeneca. UP reports funding from the Slovenian Research Agency and the Ministry of Education, Science, and Sport of Slovenia. MP-Y declares funding from ISCIII and Spanish Ministry of Economy, Industry, and Competitiveness.
REFERENCES
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention 2019. http://ginasthmaorg/.
- 2.Keskin O, Farzan N, Birben E, et al. Genetic associations of the response to inhaled corticosteroids in asthma: a systematic review. Clin Transl Allergy 2019;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Himes BE, Jiang X, Wagner P, et al. RNA-Seq transcriptome profiling identifies CRISPLD2 as a glucocorticoid responsive gene that modulates cytokine function in airway smooth muscle cells. PLoS One 2014;9:e99625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Pacheco N, Farzan N, Francis B, et al. Genome-wide association study of inhaled corticosteroid response in admixed children with asthma. Clin Exp Allergy 2019;49:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci 2004;41:233–264. [DOI] [PubMed] [Google Scholar]
- 6.Peng X, Mathai SK, Murray LA, et al. Local apoptosis promotes collagen production by monocyte-derived cells in transforming growth factor beta1-induced lung fibrosis. Fibrogenesis Tissue Repair 2011;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingram JL, Huggins MJ, Church TD, et al. Airway fibroblasts in asthma manifest an invasive phenotype. Am J Respir Crit Care Med 2011;183:1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu G, Cooley MA, Jarnicki AG, et al. Fibulin-1c regulates transforming growth factor-beta activation in pulmonary tissue fibrosis. JCI Insight 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kichaev G, Bhatia G, Loh PR, et al. Leveraging Polygenic Functional Enrichment to Improve GWAS Power. Am J Hum Genet. 2019;104(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kan M, Koziol-White C, Shumyatcher M, et al. Airway Smooth Muscle-Specific Transcriptomic Signatures of Glucocorticoid Exposure. Am J Respir Cell Mol Biol 2019;61:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.