Abstract
Polyphenols represent a group of secondary metabolites of plants which have been analyzed as potent regulators of multiple biological processes, including cell proliferation, apoptosis, and autophagy, among others. These natural compounds exhibit beneficial effects and protection against inflammation, oxidative stress, and related injuries including metabolic diseases, such as cardiovascular damage, obesity and diabetes, and neurodegeneration. This review aims to summarize the mechanisms of action of polyphenols in relation to the activation of autophagy, stimulation of mitochondrial function and antioxidant defenses, attenuation of oxidative stress, and reduction in cell apoptosis, which may be responsible of the health promoting properties of these compounds.
Keywords: polyphenols, autophagy, mechanisms, oxidative stress, inflammation, disease models
1. Introduction
One of the consequences of the normal function in living organisms is the intracellular production of reactive oxygen (ROS) and nitrogen (RNS) species in significant amounts, mainly found in the cytosol, mitochondria, lysosomes, peroxisomes, and epithelial membranes. The deleterious effects of free radicals are counteracted by the antioxidant defenses present at cellular level through both enzymatic and non-enzymatic systems, leading to the concept of redox homeostasis [1]. However, when the antioxidant defenses are not able to counteract an extensive free radicals production, oxidative stress occurs by reacting with lipids, proteins, and nucleotides to produce oxidized compounds, which are the final responsible of the cell damage [2,3]. These processes are mediated by several pathways implying signal transduction such as mitochondrial kinases, protein kinase A, protein kinase B/Akt, protein kinase C (PKC), extracellular signal-regulated protein kinase, c-Jun N-terminal kinase, or p38 nitrogen activated kinase [4,5,6,7,8,9].
The main response to tissue damage caused by intense oxidative stress is cell death. The most studied type of cell death is apoptosis. This process runs through several steps, starting with the nucleus condensation and fragmentation; mitochondrial swelling and destruction of the endoplasmic reticulum (ER) structure are then observed, together with cytoplasmic vacuolization and the disappearance of the microvilli. All these changes finally cause cell contraction and death, without any immune reaction.
Moreover, in response to cellular stress, eukaryotic cells can activate several physiological degradative pathways, such as the ubiquitin (Ub)-proteasome system (UPS) and autophagy.
Autophagy is a lysosomal degradation pathway for clearing out cytoplasmic long-lived proteins and damaged organelles [10], to mitigate cellular stress, and to increase the chances of cell survival [11]. Although oxidative stress stimulates autophagy in different cellular systems, it has been shown that a prolonged oxidative stress status inhibits autophagy [12,13]. Autophagy dysfunction leads to the accumulation of protein aggregates, oxidative and ER stress, which in turn contribute to cell apoptosis and the progression of several diseases [14,15]. Although autophagy can promote cell death under certain circumstances, it is considered as a protective mechanism. Furthermore, both positive and negative regulatory loops have been described for different proteins involved in either apoptosis or autophagy processes [16,17].
As a result of an increased oxidative stress and depletion of intracellular antioxidants, additional exogenous sources of counteracting molecules such as fat-soluble vitamin E, beta-carotene, coenzyme Q, or water-soluble vitamin C, are needed. Increasing evidence demonstrates that dietary polyphenols act as antioxidants and autophagy activators, thus reducing oxidative stress [12]. Polyphenols constitute one of the main groups of secondary metabolites from plants. Their role in the vegetal cell metabolism involves the cellular defense against ultraviolet radiation or aggression by pathogens, whereas they contribute to the bitterness, astringency, color, flavor, odor, and oxidative stability of the plant.
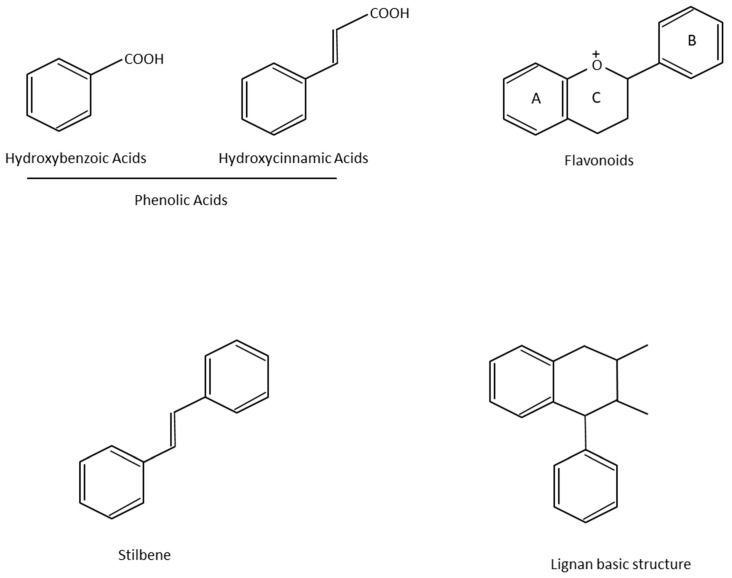
More than 8000 polyphenolic structures have been identified in plants with a wide range of molecular size depending on the number of phenolic rings and the nature and the number of the bound chemical groups, leading to simple compounds such as phenolic acids or highly polymerized molecules as tannins. The first classification system grouped natural compounds according to their chemical structure, so compounds containing at least one phenolic group could be mainly found among heterosides and tannins [18]. The biogenetic approach adopted more than 30 years ago allowed a new classification of metabolites from plants according to their main biogenetic route, leading to a division of phenolic compounds derived from shikimate (shikimic acid pathway) or acetate, with rather different biochemical and biological characteristics [18]. Thus, polyphenols derived from the shikimic acid pathway (mainly phenolic acids, coumarins, lignans, flavonoids, and anthocyanins) show prominent pharmacological activities such as antioxidant, antiinflammatory, and antiproliferative effects which led to an increased interest in the study of these compounds [19,20,21,22,23] (Table 1). Among them, flavonoids and the seven described subclasses (flavonols, flavones, flavanones, flavanonols, flavanols, anthocyanidins, and isoflavones) are the most abundant and studied group of polyphenols, although the exact mechanisms involved in the health-promoting effects of these compounds are still far from clear. Figure 1 shows the main types of polyphenols with biological interest.
Table 1.
Summarizes some of the polyphenols-mediated actions on autophagy and other important biological effects.
| Table 1. Examples of Health Benefits of Dietary Polyphenols by Autophagy Activation | |||||
|---|---|---|---|---|---|
| Class of Polyphenol | Name of Polyphenolic Compound | Molecular Formula | Molecular Weight (g/mol) | Biological Effects | References |
| Stilbenes | Resveratrol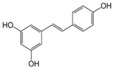
|
C14H12O3 | 228.25 | Improves mitochondrial function in vitro and in vivo | [60,149,151] |
| Autophagic activation in vitro and in vivo | [52,61,94,95,96,97,106,113,122,124,149,151,158] | ||||
| Improves insulin sensitivity in humans | [94] | ||||
| Flavonoids | Fisetin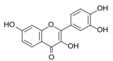
|
C15H10O6 | 286.24 | Autophagic activation in vitro | [63] |
Quercetin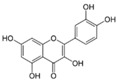
|
C15H10O7 | 302.23 | Autophagic activation in vitro and in vivo | [64,122,162,163,164,165,166,167,168] | |
Myricetin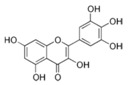
|
C15H10O8 | 318.23 | Inhibition of α-synuclein aggregation in vitro | [148] | |
Apigenin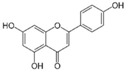
|
C15H10O5 | 270.24 | Autophagic activation in vitro | [122,169,170,171,172] | |
Luteolin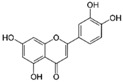
|
C15H10O6 | 286.24 | Autophagic activation in vitro and in vivo | [173,174,175,176,177,178] | |
Baicalein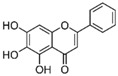
|
C15H10O5 | 270.24 | Autophagic activation in vitro and in vivo | [179,180,181,182,183] | |
| Phenolic acids | Rosmarinic acid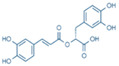
|
C18H16O8 | 360.31 | Inhibition of α-synuclein aggregation in vitro | [148] |
Nordihydro-guaiaretic acid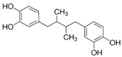
|
C18H22O4 | 302.4 | |||
Ferulic acid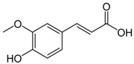
|
C10H10O4 | 194.18 | |||
| Tannins | Punicalagin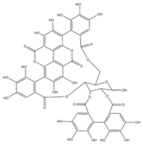
|
C48H28O30 | 1084.71 | Autophagic activation in vitro and in vivo | [68] |
| Secoiridoid | Oleuropein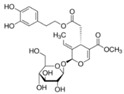
|
C25H32O13 | 540.51 | Autophagic activation in vitro and in vivo | [70,71,72,73,155,156] |
| Catechins | (-)-epigallocatechin-3-gallate 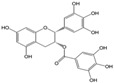
|
C22H18O10 | 442.4 | Autophagic activation in vivo | [101,102,107,121] |
| Polyphenols | Curcumin 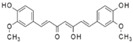
|
C21H20O6 | 368.38 | Autophagy induction in vitro and in vivo | [59,65,66,67,123] |
| Downregulation of ER stress | Reviewed in [98] | ||||
| Increase insulin sensitivity in vitro and in vivo | [99] | ||||
| Inhibition of α-synuclein aggregation in vitro | [147] | ||||
Figure 1.
Basic chemical structures of the main types of polyphenols with biological interest.
The direct antioxidant activity of polyphenols is mainly due to their redox properties which allow them to act as chelating agents of metal ions, hydrogen donators, or reducing agents.
Nevertheless, caution is needed because its total antioxidant capacity measured by in vitro tests does not reflect the real potential of substances and its kinetics does not always allow the proximity of a reductant or an antioxidant to the oxidative injury so that the redox reaction could take place [24,25].
At molecular level, polyphenols, rather than acting as merely chemical antioxidants, may induce enzymatic systems which in turn alter the steady-state levels of protective elements, such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and heme oxygenase 1 (OH1), and notably protect cells against the oxidative insult [26,27].
Other polyphenols such as hydroxycinnamic acids (i.e., caffeic and ferulic acids) prevent peroxidation mediated by UVR by inhibiting the chain reaction of lipid peroxidation and scavenging nitrogen oxides; both compounds strongly absorb UV-photons, leading to a protective effect, i.e., on human skin from UVB-induced erythema which justifies their inclusion in the formulation of several topical solutions and sunscreens [28].
More recent studies are focused in the indirect mechanisms of the antioxidant activity which are more difficult to measure but include hormesis, defined as the ability to upregulate those enzymes needed in innate detox pathways and/or the transcription of the so-called vitagenes, which in turn improve the innate mechanisms of detoxification [25]; moreover, their activity in the adaptive cellular response, genes transcription [26,29], and even in autophagic process as an important cellular mechanism underlying the beneficial effects of some polyphenols have been described [30,31].
In humans, most polyphenols are ingested from the diet (fruits—especially red berries—, vegetables, cereals, and beverages) in the form of glycosides, which are bound to one or more sugar molecules [32]. After ingestion, glycosides are hydrolyzed by intestinal hydrolases of gut microflora and the released aglycones are then absorbed by the intestinal cells and converted into their respective metabolites in both intestinal and hepatic cells. Metabolites are then transported by the blood to various cells and/or excreted by urine.
The oral bioavailability of dietary polyphenols is low due to their poor absorption, rapid excretion, and extensive biotransformation and conjugation that take place during their absorption from the intestine and at hepatic level [25,33].
Epidemiological data suggested that long-term consumption of a polyphenol-rich diet is associated with lower risk of developing certain cancers and coronary heart diseases, a decrease in arterial pressure and plasma concentration of lipids, as well as a protection against development of diabetes, osteoporosis, and even neurodegenerative diseases. Nonetheless, overconsumption of some types of food has been associated with the induction of oxidative stress and the consequent development of pathological transformations [27,34].
The aim of this review is to contribute to the knowledge of the main mechanisms of action of dietary polyphenols in relation to autophagy regulation and their impact on several metabolic diseases in order to allow the development of new effective drugs for the treatment of oxidative stress-related diseases.
2. Molecular Mechanisms of Autophagic Process and Its Regulation by Dietary Polyphenols
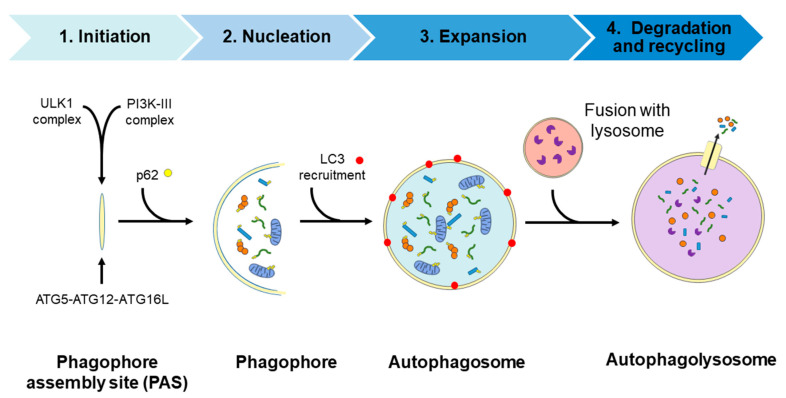
Autophagy is a physiological recycling process highly conserved in eukaryotic cells, playing an important role in maintaining cytoplasmic quality control through the elimination of cytosolic protein aggregates, damaged organelles, and invasive microbes and reducing oxidative and ER stress. This activity aims to maintain cellular homeostasis and cell survival, which contributes to enhance health and longevity [35,36,37,38]. Moreover, this catabolic process is upregulated by a wide range of extracellular and intracellular stressors such as nutrient starvation including growth factor and insulin deprivation, hypoxia, infections, ER, and oxidative stress; the resulting macromolecular constituents are then recycled. In this context, activation of autophagy allows metabolic adaptation during starvation by providing an alternative source of energy and nutrients [39] and participating in the cellular defense against infections [40,41]. The autophagic process involves the activities of proteins encoded by autophagy-related (ATG) genes and it could be considered as a nonselective degradative pathway, removing non-specific cargoes and ubiquitinated proteins (also referred as “bulk autophagy”), or highly specific, eliminating distinct cargoes including lipids (termed lipophagy), peroxisomes (pexophagy), mitochondria (mitophagy), nucleus (nucleophagy), ribosomes (ribophagy), ER (reticulophagy), and microbes (xenophagy), among others. In mammalian cells, three primary types of autophagy, depending on how the cargo is delivered to the lysosome—macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA)—have been described [42]. The present review focuses on the macroautophagy process (hereafter referred to as autophagy) which takes place through the following four steps: (1) initiation, (2) nucleation, (3) elongation, and (4) fusion and degradation [43,44,45], as described in detail in Figure 2.
Figure 2.
Diagram of the autophagic process. Steps: (1) Initiation, with the formation of the phagophore assembly site (PAS) which assemblies the Atg1/ULK1 complex and the PI3K-III complex; (2) nucleation step occurs upon the recruitment of the ubiquitin-like conjugation system (the ATG5/ATG12/ATG16L complex) to the PAS. Then, the curvature of this double-membrane (phagophore) is induced to facilitate the sequestration of the cargo. The recruitment of the ubiquitinated proteins or damaged organelles to the phagophore is carried out by the p62 protein, which links the autophagy pathway and the ubiquitin-proteasome system (UPS) by binding the ubiquitinated proteins to LC3 protein for autophagic degradation; (3) the elongation of the phagophore to form a spherical vesicle termed autophagosome; (4) finally, autophagosomes fuse with lysosomes to form autophagolysosomes/autolysosomes and the autophagic cargo are degraded by the action of resident lysosomal/vacuolar hydrolases and then are exported to the cytosol for reuse by the cell. ATG, autophagy-related; LC3, microtubule-associated protein 1A/1B-light chain 3; p62, sequestosome 1 (SQSTM1); PI3K-III, class III phosphatidylinositol 3-kinase; ULK1, unc-51-like kinase.
The autophagic process is tightly regulated depending on nutrient status by different signaling pathways that include the mammalian target of rapamycin complex 1 (mTORC1), AMP-activated kinase (AMPK), and Sirtuin-1 (SIRT1) pathways. Autophagy is negatively regulated by the mTORC1 pathway, which also regulates several important and essential processes including cell growth and protein synthesis [46]. Under nutrient-rich conditions, mTORC1 is activated and phosphorylates ULK1, thereby preventing its activation by AMP activated protein kinase (AMPK), a key activator of autophagy [47]. Moreover, mTORC1 phosphorylates and inhibits the nuclear translocation of the transcription factor TFEB, which drives the expression of genes for lysosomal biogenesis and the autophagy machinery [46].
Conversely, mTORC1 is inhibited following nutrient starvation or energy deprivation and, thus, AMPK as a key energy sensor is activated and autophagy is induced [47]. When AMPK becomes activated, it phosphorylates and activates the tuberous sclerosis complex (TSC) which is composed of the TSC1 (hamartin), TSC2 (tuberin), and TBC1D7 proteins. The TSC complex is an essential inhibitor of mTORC1 activity through the activation of its GAP (GTPase-activating protein) activity towards the small G-protein Rheb (Ras homologue enriched in brain) [48]. Inactivation of mTORC1 activity by the TSC complex occurs via lysosomal recruitment of cytosolic TSC complex, where mTORC1 is located [49].
In addition, AMPK activation stimulates the activity of the nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase SIRT1 by elevating the intracellular levels of its co-substrate, NAD+, and inducing lifespan extension [50]. Regarding post-translational modifications, the acetylation status of histones as well as cytoplasmic proteins is related with the regulation of autophagic flux [51,52]. In this sense, growing evidence indicates that both the activity of sirtuins, the deacetylation protein status, and the activation of autophagic flux decline with age [53].
Upon starvation, hypoxia, mitochondrial dysfunction, or several other circumstances in which a high intracellular levels of ROS/RSN are achieved, the c-Jun N-terminal kinase (JNK), or stress-activated protein kinase, becomes activated. JNK activation mediates the phosphorylation of B-cell lymphoma 2 (BCL2) protein to induce apoptosis. On the contrary, when JNK is not activated, dephosphorylated BCL2 interacts with beclin-1, which is a BCL2-interacting protein mediating the inhibition of beclin-1—dependent autophagy. This is only one of the main mechanisms of the complex crosstalk between autophagy and apoptosis [54].
Mitochondrial quality control mechanisms involved the activity of the phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1) and an ubiquitin E3 ligase known as Parkin. PINK1 protein acts as a molecular sensor of damaged mitochondria and promotes the recruitment of Parkin on the outer mitochondrial membrane (OMM) resulting in the ubiquitination of numerous OMM proteins, which in turn recruits other proteins to mitochondria to initiate mitophagy. It is well known that alterations of this mitophagy and/or autophagy pathways lead to the accumulation of altered proteins and damaged organelles contributing to oxidative stress.
In addition, another adaptive cellular response and a redox signaling axis that confers cellular protection against oxidative stress is the nuclear factor erythroid 2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein 1 (Keap1)-antioxidant response elements (ARE) pathway. Activated Nrf2 is translocated to the nucleus where it activates the transcription of antioxidant genes, including heme oxygenase-1 (HO-1) [55]. Of note, and regarding the crosstalk between the antioxidant effects mediated by Nrf2-Keap1-ARE activation and autophagic process, it has been described that p62/SQSTM1 protein, which is degraded by autophagic process, also represents one of the Nrf2 target. Moreover, p62/SQSTM1 protein competitively binds to the redox sensor Keap1, disrupting its interaction with Nrf2, resulting in Nrf2 activation [56]. As a consequence, this p62/SQSTM1 positive-feedback loop may have beneficial effects against oxidative stress-dependent cell death through the activation of mitophagy by increasing the degradation of defective and altered mitochondria [38]. Other mechanisms are involved in autophagy regulation by oxidative stress such as post-translational modifications of key autophagy regulators. In this sense, recent studies demonstrate that some plant polyphenols including resveratrol, curcumin, and quercetin are able to activate the Nrf2-Keap1-ARE, counteracting oxidative damage and representing a novel therapeutic approach based on the antioxidant properties of these natural compounds [29,57,58].
Moreover, there has been a growing interest in the beneficial effects of the dietary consumption of polyphenols beyond its antioxidant activity, because most of them are able to activate autophagy and, thus, play a beneficial role in the redox balance by alleviating oxidative stress. Therefore, dietary polyphenols have emerged as a promising therapeutic strategy to prevent the development of several diseases including metabolic and neurodegenerative ones [57,59].
Resveratrol (2,3,4′-trihydroxystilbene), which is a caloric restriction mimetic (CRM), stimulates in vivo SIRT1, inducing the kinase activities of liver kinase B1 (LKB1) and AMPK, and inhibiting mTORC1 signaling, leading to autophagy activation [60]. In addition, resveratrol reduces ROS production in response to D-galactose of aging cardiomyocytes, enhancing cardiac function and diminishing ischemia/reperfusion-induced cell apoptosis through the regulation of mitophagy [61]. Moreover, resveratrol could enhance the association between mTORC1 and its inhibitory protein DEPTOR (DEP-domain containing mTOR-interacting protein), thus triggering the inactivation of mTORC1 [62]. It also increases in vivo mitochondrial biogenesis and function [60].
Flavonoids such as fisetin and quercetin lead to autophagy induction by TFEB nuclear translocation via mTORC1 inhibition [63,64]. The polyphenol curcumin is able to translocate TFEB to the nucleus, leading to autophagy activation by mTORC1-independent inhibition [65]. In addition, curcumin activates AMPK, which in turn phosphorylates BCL2, and subsequently disrupts the interaction between BCL2 and BECN1, resulting in autophagy activation [66] or upregulation of the expression of BECN1 protein which reduces p62 levels [67]. More recently, it has been demonstrated that the dietary tannin punicalagin activates autophagy through the inhibition of Akt signaling pathway, resulting in the activation of the transcription factor forkhead box O3a (FOXO3a) [68]. FOXO transcription factors have been identified to promote the expression of ATG genes, including LC3b (Map1lc3b), Gabarapl1, Pi3kIII, Ulk2, Atg12l, Beclin1, Atg4b, Atg14, and Bnip3, then leading to an increase in both autophagic and mitophagic flux [69].
Oleuropein and its related compounds, which are secoiridoids present in olive oil, have also emerged as autophagic inductors because they could activate the Ca2+/CaMKKβ/AMPK signaling pathway and hence preserve TFEB in its activated state [70] or even induce the expression of proteins involved in autophagic process including BECN1 and LC3 [71,72] mainly caused by AMPK activation [73]. Besides all these specific mechanisms, both mono- and polyphenols could also activate autophagic flux by stimulating the deacetylase activity of SIRT1 and, consequently, downregulating the general acetylation status of cytosolic proteins [74].
3. Dietary Polyphenols and Type 2 Diabetes, Obesity, and Metabolic Syndrome
The mechanism of action of different polyphenols in the control of several metabolic diseases, including obesity and type 2 diabetes mellitus (T2DM) in relation with autophagy modulation, has been extensively studied. It is well known that polyphenols are a group of molecules with positive effects in the modulation of aging, a period in which most of the reviewed diseases show a higher prevalence. T2DM is a very complex disease and is considered as epidemic worldwide. It is the resultant from multiple factors in a progressive manner, being insulin resistance and pancreatic β cell dysfunction the most relevant. T2DM is associated with increased levels of glucose and lipids that could contribute to β-cell death and is characterized by two different phases. During the first one, which main event is insulin resistance with normal levels of glycemia, pancreatic β-cells increase their mass by hyperplasia or hypertrophy, with a concomitant insulin and amylin secretion [75]. The duration of this phase depends on the patient’s idiosyncrasy and can be extended for years. At the final stage, pancreatic β-cells fail and then hypoinsulinemia appears. In addition, amylin deposition occurs in nearly 90% of T2DM patients. During the progression to T2DM, a chronic activation of mTORC1 signaling pathway has been detected. Although this activation is necessary during the first phase of the disease, for insulin resistance compensation by pancreatic β-cells, its chronic effect is deleterious for these cells [76,77]. Importantly, this maintained activation of mTORC1 generates a blockade in autophagic flux, which is essential for a correct elimination of damaged organelles or protein aggregates. These effects generate a continuous stimulation of the unfolded protein response (UPR), which in turn upregulates ER protein chaperones in charge of promoting protein folding. However, when the ER protein folding capacity is overwhelmed, cells undergo a condition of chronic ER stress, triggering the activation of apoptosis [78,79]. Nowadays, T2DM is considered a disease affecting the folding ability of pancreatic β-cells. In fact, the expression of different endogenous ER chaperones such as the 78-kDa glucose-regulated protein (GRP78) or BiP protein and protein disulfide isomerase, or chemical chaperones, such as taurine-conjugated derivative from ursodeoxycholic acid (TUDCA) or 4-phenyl butyric acid (4-PBA), diminished β cell failure and facilitated the correct folding, avoiding protein aggregation and apoptosis [80]. In this regard, azoramide, a small molecule which modulates UPR activity, exerts a strong antidiabetic activity [81,82]. In addition to all the changes mentioned before through the progression to T2DM, mitochondrial dysfunction is also shown. In general terms, mitochondrial dysfunction occurs as the natural history of aging [83]. However, during T2DM progression a concomitant defect in the mitochondrial clearance or mitophagy occurs which promotes an accumulation of aberrant and dysfunctional mitochondria which cannot be eliminated by mitophagy [84]. In this regard, a chronic activation of mTORC1 in pancreatic β-cells is able to inhibit both general autophagy as well as mitophagy, with a higher production of oxidized mitochondrial proteins and an accumulation of mitochondria with an altered membrane potential, which are not degraded by mitophagy [85]. One of the mechanisms that could explain this failure in mitophagy is the reduction in the levels of PINK1, which is an essential component in the recognition of mitochondria with an altered membrane potential as observed in fibroblasts with mTORC1 hyperactivation [86]. In addition to that, the overexpression of human amylin in pancreatic β cells impairs both bulk autophagy as well as mitophagy [87]. More importantly, these defects have been observed in prediabetic stages as well as in T2DM patients [88].
One of the most characterized and studied polyphenols with respect to its effects in diabetes is the stilbene compound resveratrol. Apart from its protective effects in diabetes, resveratrol can be a positive treatment in diabetes-related pathologies such as diabetic cardiomyopathy [89]. In fact, positive effects of resveratrol in the reduction of oxidative stress [90] as well as an increase in the sensitivity to insulin [91] with a concomitant decrease in lipid levels have been assessed [92]. In addition to these actions, resveratrol has been proposed to modulate the expression of both pro-apoptotic and anti-apoptotic factors [31]. Furthermore, stilbenes can stimulate an important antioxidant defense such as the transcription factor called Nrf2. Nrf2 is involved in the control of the transcription of different antioxidants in response to inflammation and oxidative stress [93]. Stilbenes are also involved in the activation of autophagy, through the modulation of p62 protein levels and SIRT1 activity [94]. An essential role of SIRT1 has been proposed for the induction of autophagy in response to hypoxia in a T2DM rat model [95]. Resveratrol stimulates autophagic flux, improving diabetic cardiomyopathy in a diabetic mouse model mediated by a decrease in p62 protein levels, facilitating SIRT1 activity. Moreover, SIRT1/FOXO/Rab7 has been proposed as a potential therapeutic pathway which mediates the effects of resveratrol on autophagic flux and ameliorates dysfunctional autophagy in diabetic cardiomyopathy [96]. Resveratrol can also modulate autophagy by alternative indirect mechanisms which involve transcriptional regulation of microRNA’s (miRNA’s for short). miRNA-18a-5p is one kind of miRNA which expression is enhanced in a diabetic mouse model after treatment with resveratrol [97].
Other polyphenols with a wide protective effect in diabetes are curcumin and its analogues. These groups of molecules are able to down-regulate the chronic stimulation of ER stress, reducing apoptosis [98] and increasing insulin sensitivity [99]. There are multiple evidences indicating a pro-autophagic role of curcumin in order to moderate the negative effects of T2DM [66]. For instance, curcumin can modulate several proteins involved in autophagy including LC3, p62, and beclin-1, among others. These effects have been described in a diabetic mice model treated with curcumin, preventing podocyte apoptosis during diabetic nephropathy [67].
(−)-Epigallocatechin-3-gallate (EGCG) is a polyphenol isolated from green tea with a significant effect in autophagy modulation. EGCG is able to regulate autophagy at different levels, alleviating part of the deleterious effects observed in diabetic patients. For instance, it can avoid lipid accumulation in vascular endothelial cells, increasing the degradation of lipids by lipophagy, and facilitating the recognition between LC3B (located in the autophagosomes) and the lipid droplets for their catabolism [100]. This polyphenol has a protective effect for both mitochondrial dysfunction and aberrant autophagy described in the heart’s tissue of diabetic rats [101,102].
Punicalagin, one of the main polyphenolic components of pomegranate, is able to protect liver dysregulation in response to T2DM. In fact, this molecule has been proposed very recently as an autophagy inducer through the modulation of different protein markers such as LC3B and p62 which allow the reactivating of autophagy [68]. Furthermore, oleuropein has been related to an induction of autophagy as part of its mechanism of action [73]. Oleuropein, together with hydroxytyrosol, is involved in the induction of autophagy through the regulation of AMPK/mTORC1 signaling pathway [70,103].
Obesity, one of the main factors associated to the development of T2DM, has been implicated in autophagy dysregulation. In fact, a dysregulation in autophagic activity plays an important role in the appearance of insulin resistance [104]. Furthermore, adipocyte hypertrophy occurring in obesity contributes to insulin resistance as well, by the production of different inflammatory cytokines which lead to an increased in endoplasmic reticulum stress (ER-stress) [105]. One of the main polyphenols with protective properties in obesity and, specifically in liver steatosis, is resveratrol. In rats, resveratrol effect is similar to an energy restriction (a reduction of 15% of calories in energy intake), with a decrease in p62 protein levels and an increase in LC3B, beclin-1, and atg5 protein levels, which collectively suggests an autophagic activation [106]. Apart from resveratrol, other polyphenolic compounds with protective effects through autophagy activation in obesity have been assessed. A treatment with epigallocatechin-3-gallate (EGCG) for two weeks in a mice model of high-fat diet (HFD)-induced obesity stimulated both autophagic flux in white adipose tissue in an AMPK-dependent manner and beclin-1 activation [107]. Very interestingly, the effect of a 30 days’ supplementation with resveratrol on gene expression and adipose tissue morphology in a cohort of obese men has been reported. The authors indicated that, at the end of the study, there was a significant reduction in adipocyte size, with a downregulation of both Wnt and Notch signaling pathways. In addition, there was an upregulation in different proteins involved in the cell cycle control which suggested an increase in adipogenesis. Finally, an increase in lipophagy accompanied by a reduction in inflammation were also observed [108].
The metabolic syndrome represents several injuries including obesity, T2DM, and nonalcoholic fatty liver (NAFL); a mitochondrial dysfunction appears, thus contributing to insulin resistance. Then, mitophagy could potentially preserve mitochondrial function by the elimination of these damaged organelles [109,110]. Another important consequence of obesity is the appearance of NAFLD. It is well known that the elimination of lipid accumulation that occurs in the liver by the activation of lipophagy contributes to a decrease in NAFLD pathology [111]. In this regard, there are multiple examples assessing the protective effect of different polyphenols: pro-autophagic activity of resveratrol has been demonstrated both in vitro model of palmitic acid-induced hepatic steatosis [112] and in in vivo models of NAFLD, where it also reduces liver inflammation [113,114]. In fact, this in vivo beneficial effect of resveratrol was associated with autophagy activation, because the elimination of ULK1, which is essential in the control of autophagosome formation, impaired the protective role of resveratrol [113]. Using a cafeteria diet-induced obesity in rats, polyphenols from bergamot were able to induce lipophagy and prevent NAFLD onset. In the bergamot-treated animals, a reduction in p62 protein levels and an increase in both LC3B and beclin-1 protein levels were shown, both events being associated with an activation of the autophagic flux. All these effects were linked to a decrease in both liver lipid accumulation and inflammation [115,116]. Furthermore, an in vitro model of liver cells (HepG2) treated with different levels of fatty acids (a combination of oleic acid and palmitic acid) which are found in patients with liver steatosis, assessed the accumulation of lipids inside the cells. Very interestingly, the protective effect of different polyphenols derived from Toona sinensis A. Juss. was associated with an activation of AMPK/ULK1 signaling pathway with a concomitant increase in LC3B protein levels [117]. Similar protective results related to lipid accumulation in a mice model of NAFLD in response to blueberry polyphenols were reported [118]. Very recently, the protective effect exerted by a polyphenol extract from apple in the activation of lipophagy and restoration of lysosomal pH was related to intervention through the SIRT1/AMPK signaling [119]. In fact, polyphenols such as quercetin can stimulate lysosomal biogenesis and, hence, the clearance capacity of the cells by facilitating the nuclear translocation of TFEB [64]. Although these effects have been observed in retinal epithelium cells (RPE cells), it should be of great interest to explore the improvement of the TFEB-lysosomal axis in other pathologies such as T2DM, obesity, and metabolic syndrome. In summary, autophagy activation represents a key event in the pathophysiology of fatty liver disease and its modulation by the use of different polyphenols could contribute to prevent both the disease and related pathologies such as T2DM, obesity, and other metabolic complications [120].
4. Effect of Polyphenols on Cardiovascular Diseases
Cardiovascular diseases are a direct consequence of aging. During the aging process, there is a progressive deterioration of mitochondrial function and a decline in cardiac efficiency. Using a cellular model of senescent-like cardiomyocytes, treatment with resveratrol improved cardiac function by the activation of SIRT1 signaling pathway. In addition, the effect of resveratrol in the correct function of two essential proteins in mitophagy namely PINK1 and PARKIN was also assessed [61]. A polyphenol mixture from green tea (catechin > 90% and EGCG > 70%) exerted a protective role in the reduction of atherogenesis in an ApoE-knock-out mice model through the activation of autophagy. In this model, there was an increased production of p62 protein levels linked to an increased autophagosome formation flux, with an increase in LC3B and beclin-1 protein levels in the polyphenol-treated group [121]. Oleuropein aglycone has been studied in a cellular model with an increased oxidative stress in cardiomyocytes. In these cells, there was an induction of autophagy, detected by an increase in LC3B and beclin-1 protein levels. However, very importantly, there was an improvement of TFEB translocation to the nucleus which suggested an improved autophagy induction [72]. In this regard, different polyphenols have been involved in the protection against cardiovascular disease and vascular inflammation, including EGCG, quercetin, resveratrol, apigenin, and curcumin, through autophagy modulation among other mechanisms [122,123]. Recent reports indicate that resveratrol, by autophagy activation could be useful for the treatment of the ischemic process found in diabetic myocardium. The beneficial effect of resveratrol was associated with an increase in beclin-1 and LC3B protein levels and a reduction in inflammation, by diminishing two inflammatory cytokines such as TNF-alpha and IL-6 [124]. In general terms, mTOR signaling pathway has been proposed as a target for treating atherosclerosis, cardiac hypertrophy, and heart failure. Then, every natural compound with proven activity towards the inhibition of mTORC1, could represent an interesting approach to reduce both the above cited diseases and the number of deaths caused by cardiovascular disease worldwide [125].
5. Dietary Polyphenols and Neurodegeneration
Dysfunction of autophagy contributes to protein misfolding and aggregation which underly the pathogenesis of human proteinopathies such as Alzheimer (AD), Parkinson (PD), and Huntington diseases [126]. Protein homeostasis is essential for the cell function but it is especially relevant in postmitotic neurons, which have a limited regenerative potential and thus, mechanisms involved in the elimination of damaged organelles or protein aggregates are crucial for preserving cell survival [127]. Although the mechanisms that control the above cited diseases are different, they share a common feature which is the accumulation of different protein aggregates in neurons, leading to cell toxicity and neurodegeneration. As mentioned for diabetes, neurodegenerative diseases prevalence increases during aging. It is well known that all the systems involved in protein quality control including UPS and autophagy-lysosome ones fail with aging, thus promoting the accumulation of aggregates which are not present in younger organisms.
In the case of Alzheimer’s patients, there is an accumulation of amyloid β (Aβ) and an intracellular accumulation of an hyperphosphorylated form of Tau protein which leads to a structure called neurofibrillary tangles. Although a small percentage of patients (1%) with Alzheimer’s disease shows a mutation in one of the genes involved in the disease, including presenilins 1 and 2 or amyloid precursor protein (APP), most patients (around 90%) are called sporadic ones and depend on both genetic and environmental determinants [128]. Aβ oligomers and the hyperphosphorylated form of tau produce several alterations in neurons including inhibition of proteasome and autophagy-lysosome degradation systems, which contribute to a higher accumulation of these proteins [129]. In addition, it has been shown that Aβ is able to accumulate lysosomes inside itself, thus altering their membrane permeability [130]. Tau protein is involved in both microtubule assembly and stability, depending on its phosphorylation degree, which is the result of the activity of kinases as well as phosphatases. Microtubule stability is associated with an unphosphorylated form of tau protein [131,132]. In contrast, hyperphosphorylation of tau provokes a microtubule instability which generates an alteration in the polymerization of microtubules, altering the correct transport and movement of different organelles, which are in turn essential processes in the regulation of synapsis [133].
With respect to PD, another protein known as α-synuclein is involved. This protein can assemble into aggregates and form new deposits known as Lewy bodies (LB) mainly in dopaminergic neurons [134]. In the same line with AD, different mutations related to the increased capacity of this protein to aggregate have been described for PD. This aggregation alters normal cellular physiology and organelles, including ER as well as mitochondria, which finally contribute to cell death [135,136]. Multiple genes exist associated with the disease such as LRRK2, SNCA, LAMP3, and ATP13A2. Although SNCA, which encodes α-synuclein, is the major genetic alteration related with the pathology, other genetic mutations are also involved in the mechanisms related to the control of the mitochondrial quality, such as PINK1 and protein deglycase (DJ-1). However, other different mechanisms have been found to be altered as a consequence of α-synuclein aggregation, including vesicular trafficking between compartments such as ER–Golgi transport and Golgi apparatus fragmentation [137,138]. In addition, several posttranslational modifications such as phosphorylation, ubiquitination, and acetylation have been described to alter the structure of α-synuclein, its correct function, and its aggregation capacity [139]. Very interestingly, an hyperphosphorylation state of α-synuclein has been related with a change in its subcellular localization, leading to an interaction with histones and the inhibition of their activity inducing neurotoxicity and cellular apoptosis [140,141,142]. This phosphorylation could be mediated by different kinases in cells. Although the exact mechanism remains unknown, polo-like kinases (PLK) could be responsible for regulating this phosphorylation event in the serine 129 [143]. Apart from phosphorylation, lysine acetylation status of α-synuclein has been involved in the pathogenesis of the disease as well [144]. In addition, this protein modification impairs mitophagy in fibroblasts, as shown for PD patients [145].
Autophagy modulation is a key avenue for the treatment of neurodegenerative diseases as it has been very recently reviewed [146]. In this regard, different polyphenols have been proposed as protective in different neurodegenerative diseases such as AD and PD. Curcumin is able to modulate autophagic machinery in order to restore the defects observed in the neurons of patients affected with these diseases [59], and it is also able to directly interact with α-synuclein, then avoiding its aggregation and hence its toxicity [147]. This effect on aggregation which reduces synaptic toxicity in response has been described not only for curcumin, but also for other phenolic compounds such as myricetin, rosmarinic acid, norhydroguaiaretic acid, and ferulic acid [148]. A synthetic form of curcumin, called C1, is able to activate autophagy by inducing TFEB, a master transcription factor for lysosomal biogenesis as well as autophagy machinery [65]. Using a fly model of PD associated with a loss of function of an essential protein involved in mitophagy, a grape skin extracts exerted a protective effect by promoting autophagy and preserving mitochondrial function, provoking a rescue in mitochondrial structural defects by the activation of mitophagy [149]. In this regard, alpha-arbutin obtained from different species of the Ericaceae family, protects from rotenone-induced mitochondrial dysfunction in a neuroblastoma cell line (SH-SY5Y). The protective effect of this polyphenol is due to its ability to induce both AMPK and autophagy [150]. Similarly, resveratrol protects mitochondrial function, by enhancing autophagic flux, in a mitogen-activated protein kinase (MEK)-dependent manner, indicating that this signaling pathway is essential in the actions of resveratrol, at least in the modulation of mitochondrial dynamics [151]. Very recently, a polyphenol-enriched extract from lychee seed has demonstrated a neuroprotective effect through the activation of autophagy by increasing beclin-1 and LC3B protein levels, mainly by AMPK activation [152].
Another mode of action of polyphenols is by acting on stem cells. In general terms, neuroprotection by polyphenols and stimulation of neurogenesis [153] occurs at different levels, including modulation of antiapoptotic proteins, stimulation of different signaling pathways inside the cells, inhibition of pro-oxidant enzymes, and alteration of mitochondrial function. Furthermore, polyphenols exert an active role in quenching free radical species and chelating metal ions, with the ability to regulate the major degradative pathways of proteins [154]. Oleuropein is involved in alleviating oxidative stress as well as inducing autophagic flux [155]. In this regard, it is also involved in the profound reduction of β-amyloid deposits, with a concomitant increase in autophagy activation. In parallel, there is a great reduction in mTORC1 signaling pathway in response to oleuropein [71]. Oleuropein aglycone induces autophagy and favors the elimination of protein aggregates, decreasing the cognitive decline [156]. Using a mice model of AD, the treatment with hydroxytyrosol increases autophagic flux mainly by activation of MAPK signaling pathway this resulting in neuroprotection [157]. Resveratrol and other compounds called sirtuin activating compounds or STACS have been assessed as strongly inducing SIRT1 [158]. SIRT1 inhibits c-Jun by deacetylation, decreasing the transcriptional activity of the activator protein-1 (AP1) involved in the expression of several inflammatory and stress genes [159,160]. The essential role of SIRT1 as autophagy regulator derives from the fact that SIRT1 is able to bind to an ATG protein, regulating its activity by deacetylation and, hence, autophagy [161].
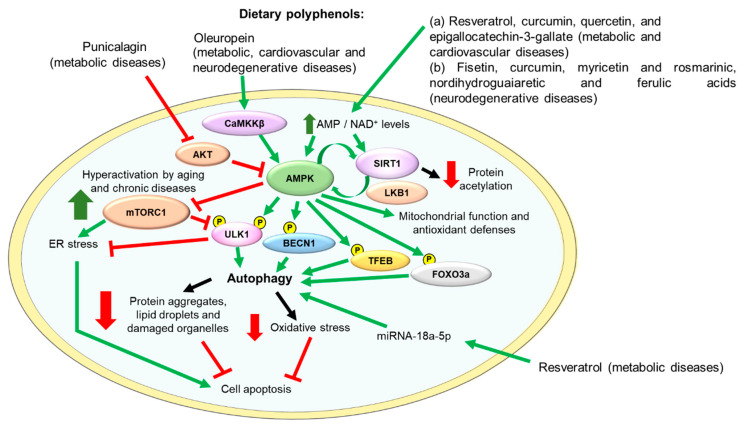
Figure 3 depicts the main molecular targets of some polyphenols and their beneficial effects on metabolic, cardiovascular, and neurodegenerative diseases.
Figure 3.
Main molecular mechanisms involved in the antioxidant activity and the beneficial effects of dietary polyphenols in metabolic, cardiovascular, and neurodegenerative diseases. The dietary polyphenols mentioned in this figure have been demonstrated to have a beneficial impact on the prevention of these diseases by different mechanisms including increasing the intracellular levels of AMP and NAD+, activating CaMKKβ or inhibiting AKT. The regulation of these molecular pathways results in the activation of both AMPK and SIRT1 activities. AMPK activates SIRT1, and SIRT1 activates AMPK by inducing LKB1 activity. Activated AMPK diminishes mTORC1 pathway activation, reducing ER stress, activates autophagy, and stimulates mitochondrial function and antioxidant defenses, and, consequently, reduces oxidative stress and cell apoptosis. Green arrows indicate activation and red lines indicate inhibition of the activity of the target protein. AMPK, adenosine monophosphate (AMP)-activated protein kinase; AKT, protein kinase B; BECN1, beclin-1; CaMKKβ, calcium/calmodulin-dependent protein kinase kinase β; ER, endoplasmic reticulum; FOXO3a, forkhead box O3a; LKB1, liver kinase B1; miRNA, microRNA; mTORC1, mammalian/mechanistic target of rapamycin complex 1; NAD+, nicotinamide adenine dinucleotide; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; SIRT1, sirtuin-1; TFEB, transcription factor EB; ULK1, unc-51-like kinase 1.
6. Conclusions
Natural products with dietary polyphenols among them have emerged as a promising group of potential agents in health promotion, supported by their structure and results from molecular studies. Polyphenols may act on multiple molecular targets in relation to autophagic flux activation with potential beneficial effects on metabolic and neurodegenerative diseases. In this review, different protein targets and signaling pathways modulated by these natural products have been discussed.
Since years, the positive effects of a variety of plant extracts and dietary polyphenols including resveratrol, curcumin, epigallocatechin-3-gallate, punicalagin, oleuropein, myricetin and rosmarinic, norhydroguaiaretic, and ferulic acids have been reported. These compounds are CRM and anti-aging molecules which may activate AMPK and SIRT1 activities by increasing intracellular levels of AMP and NAD+, leading to mitochondrial function improvement and autophagy stimulation. Activation of autophagy removes protein aggregates, lipid droplets, and damaged organelles, stimulates antioxidant defenses, and alleviates both ER and oxidative stress mediated by mTORC1 hyperactivation, resulting in an enhancement of cell survival.
In conclusion, in view of their potential beneficial effects, polyphenols represent a promising chemical group for which further studies, including clinical trials, should be conducted for their in-depth knowledge which would likely be helpful for translational outcomes.
Abbreviations
Aβ: Amyloid beta; AD, Alzheimer’s disease; AMPK, adenosine monophosphate (AMP)-activated protein kinase; APP, amyloid precursor protein; Atg, autophagy related; CMA, chaperone-mediated autophagy; ER, endoplasmic reticulum; LC3, Microtubule-associated protein 1A/1B-light chain 3; LB, Lewy bodies; miRNA, microRNA; mTORC1, mammalian/mechanistic target of rapamycin complex 1; NAFLD, non-alcoholic fatty liver disease; PD, Parkinson’s disease; T2DM, type 2 diabetes; TFEB, transcription factor EB; TSC, tuberous sclerosis complex; UPR, unfolded protein response; UPS, ubiquitin-proteasome system; Ub, ubiquitin.
Author Contributions
A.G.-A., O.P., M.B., and C.G. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants SAF 2017-82133-R from Ministerio de Ciencia e Innovación, and Spanish Diabetes and Associated Metabolic Research Network (CIBERDEM) to M. Benito, Instituto de Salud Carlos III, Spain.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jia M., Qin D., Zhao C., Chai L., Yu Z., Wang W., Tong L., Lv L., Wang Y., Rehwinkel J., et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat. Immunol. 2020;21:727–735. doi: 10.1038/s41590-020-0699-0. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012;70:257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. Free radical involvement in aging. Pathophysiology and therapeutic implications. Drugs Aging. 1993;3:60–80. doi: 10.2165/00002512-199303010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 5.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radi E., Formichi P., Battisti C., Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014;3:S125–S152. doi: 10.3233/JAD-132738. [DOI] [PubMed] [Google Scholar]
- 7.Moldogazieva N.T., Mokhosoev I.M., Feldman N.B., Lutsenko S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018;52:507–543. doi: 10.1080/10715762.2018.1457217. [DOI] [PubMed] [Google Scholar]
- 8.Ou H.C., Pandey S., Hung M.Y., Huang S.H., Hsu P.T., Day C.H., Pai P., Viswanadha V.P., Kuo W.W., Huang C.Y. Luteolin: A Natural Flavonoid Enhances the Survival of HUVECs against Oxidative Stress by Modulating AMPK/PKC Pathway. Am. J. Chin. Med. 2019;47:541–557. doi: 10.1142/S0192415X19500289. [DOI] [PubMed] [Google Scholar]
- 9.Checa J., Aran J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020;13:1057–1073. doi: 10.2147/JIR.S275595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine B., Klionsky D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 11.Ding W.X., Ni H.M., Gao W., Yoshimori T., Stolz D.B., Ron D., Yin X.M. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am. J. Pathol. 2007;171:513–524. doi: 10.2353/ajpath.2007.070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limanaqi F., Biagioni F., Mastroiacovo F., Polzella M., Lazzeri G., Fornai F. Merging the Multi-Target Effects of Phytochemicals in Neurodegeneration: From Oxidative Stress to Protein Aggregation and Inflammation. Antioxidants. 2020;9:1022. doi: 10.3390/antiox9101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janda E., Lascala A., Carresi C., Parafati M., Aprigliano S., Russo V., Savoia C., Ziviani E., Musolino V., Morani F., et al. Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2: Implications for neuroprotection. Autophagy. 2015;11:1063–1080. doi: 10.1080/15548627.2015.1058683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Settembre C., Fraldi A., Rubinsztein D.C., Ballabio A. Lysosomal storage diseases as disorders of autophagy. Autophagy. 2008;4:113–114. doi: 10.4161/auto.5227. [DOI] [PubMed] [Google Scholar]
- 15.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukhopadhyay S., Panda P.K., Sinha N., Das D.N., Bhutia S.K. Autophagy and apoptosis: Where do they meet? Apoptosis. 2014;19:555–566. doi: 10.1007/s10495-014-0967-2. [DOI] [PubMed] [Google Scholar]
- 17.Fairlie W.D., Tran S., Lee E.F. Crosstalk between apoptosis and autophagy signaling pathways. Int. Rev. Cell Mol. Biol. 2020;352:115–158. doi: 10.1016/bs.ircmb.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Singla R.K., Dubey A.K., Garg A., Sharma R.K., Fiorino M., Ameen S.M., Haddad M.A., Al-Hiary M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019;102:1397–1400. doi: 10.1093/jaoac/102.5.1397. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson L.R. Role of plant polyphenols in genomic stability. Mutat. Res. 2001;475:89–111. doi: 10.1016/S0027-5107(01)00073-2. [DOI] [PubMed] [Google Scholar]
- 20.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun A.Y., Wang Q., Simonyi A., Sun G.Y. Botanical phenolics and brain health. Neuromol. Med. 2008;10:259–274. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka T., Kojima T., Kawamori T., Wang A., Suzui M., Okamoto K., Mori H. Inhibition of 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis by the naturally occurring plant phenolics caffeic, ellagic, chlorogenic and ferulic acids. Carcinogenesis. 1993;14:1321–1325. doi: 10.1093/carcin/14.7.1321. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T., Kojima T., Kawamori T., Yoshimi N., Mori H. Chemoprevention of diethylnitrosamine-induced hepatocarcinogenesis by a simple phenolic acid protocatechuic acid in rats. Cancer Res. 1993;53:2775–2779. [PubMed] [Google Scholar]
- 24.Han R.M., Zhang J.P., Skibsted L.H. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules. 2012;17:2140–2160. doi: 10.3390/molecules17022140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco R., Navarro G., Martinez-Pinilla E. Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals. Antioxidants. 2019;8:373. doi: 10.3390/antiox8090373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewlings S.J., Kalman D.S. Curcumin: A Review of Its Effects on Human Health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saija A., Tomaino A., Trombetta D., De Pasquale A., Uccella N., Barbuzzi T., Paolino D., Bonina F. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 2000;199:39–47. doi: 10.1016/S0378-5173(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 29.Suraweera T.L., Rupasinghe H.P.V., Dellaire G., Xu Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants. 2020;9:973. doi: 10.3390/antiox9100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pallauf K., Rimbach G. Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 2013;12:237–252. doi: 10.1016/j.arr.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Yessenkyzy A., Saliev T., Zhanaliyeva M., Masoud A.R., Umbayev B., Sergazy S., Krivykh E., Gulyayev A., Nurgozhin T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients. 2020;12:1344. doi: 10.3390/nu12051344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 33.Jung U.J., Kim S.R. Beneficial Effects of Flavonoids Against Parkinson’s Disease. J. Med. Food. 2018;21:421–432. doi: 10.1089/jmf.2017.4078. [DOI] [PubMed] [Google Scholar]
- 34.Cherkas A., Holota S., Mdzinarashvili T., Gabbianelli R., Zarkovic N. Glucose as a Major Antioxidant: When, What for and Why It Fails? Antioxidants. 2020;9:140. doi: 10.3390/antiox9020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung H.S., Chung K.W., Won Kim J., Kim J., Komatsu M., Tanaka K., Nguyen Y.H., Kang T.M., Yoon K.H., Kim J.W., et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Ebato C., Uchida T., Arakawa M., Komatsu M., Ueno T., Komiya K., Azuma K., Hirose T., Tanaka K., Kominami E., et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Bartolome A., Guillen C., Benito M. Autophagy plays a protective role in endoplasmic reticulum stress-mediated pancreatic beta cell death. Autophagy. 2012;8:1757–1768. doi: 10.4161/auto.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pena-Oyarzun D., Bravo-Sagua R., Diaz-Vega A., Aleman L., Chiong M., Garcia L., Bambs C., Troncoso R., Cifuentes M., Morselli E., et al. Autophagy and oxidative stress in non-communicable diseases: A matter of the inflammatory state? Free Radic. Biol. Med. 2018;124:61–78. doi: 10.1016/j.freeradbiomed.2018.05.084. [DOI] [PubMed] [Google Scholar]
- 39.Singh R., Cuervo A.M. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deretic V., Saitoh T., Akira S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eskelinen E.L., Saftig P. Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochim. Biophys. Acta. 2009;1793:664–673. doi: 10.1016/j.bbamcr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Parzych K.R., Klionsky D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox. Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y., He D., Yao Z., Klionsky D.J. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y.K., Lee J.A. Role of the mammalian ATG8/LC3 family in autophagy: Differential and compensatory roles in the spatiotemporal regulation of autophagy. BMB Rep. 2016;49:424–430. doi: 10.5483/BMBRep.2016.49.8.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martens S., Fracchiolla D. Activation and targeting of ATG8 protein lipidation. Cell Discov. 2020;6:23. doi: 10.1038/s41421-020-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang J., Manning B.D. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menon S., Dibble C.C., Talbott G., Hoxhaj G., Valvezan A.J., Takahashi H., Cantley L.C., Manning B.D. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canto C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banreti A., Sass M., Graba Y. The emerging role of acetylation in the regulation of autophagy. Autophagy. 2013;9:819–829. doi: 10.4161/auto.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Aguilar A., Guillen C., Nellist M., Bartolome A., Benito M. TSC2 N-terminal lysine acetylation status affects to its stability modulating mTORC1 signaling and autophagy. Biochim. Biophys. Acta. 2016;1863:2658–2667. doi: 10.1016/j.bbamcr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 53.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menon M.B., Dhamija S. Beclin 1 Phosphorylation-at the Center of Autophagy Regulation. Front. Cell Dev. Biol. 2018;6:137. doi: 10.3389/fcell.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katsuragi Y., Ichimura Y., Komatsu M. Regulation of the Keap1–Nrf2 pathway by p62/SQSTM1. Curr. Opin. Toxicol. 2016;1:54–61. doi: 10.1016/j.cotox.2016.09.005. [DOI] [Google Scholar]
- 57.Costa L.G., Garrick J.M., Roque P.J., Pellacani C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell Longev. 2016;2016:2986796. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janda E., Isidoro C., Carresi C., Mollace V. Defective autophagy in Parkinson’s disease: Role of oxidative stress. Mol. Neurobiol. 2012;46:639–661. doi: 10.1007/s12035-012-8318-1. [DOI] [PubMed] [Google Scholar]
- 59.Perrone L., Squillaro T., Napolitano F., Terracciano C., Sampaolo S., Melone M.A.B. The Autophagy Signaling Pathway: A Potential Multifunctional Therapeutic Target of Curcumin in Neurological and Neuromuscular Diseases. Nutrients. 2019;11:1881. doi: 10.3390/nu11081881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price N.L., Gomes A.P., Ling A.J., Duarte F.V., Martin-Montalvo A., North B.J., Agarwal B., Ye L., Ramadori G., Teodoro J.S., et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren X., Chen L., Xie J., Zhang Z., Dong G., Liang J., Liu L., Zhou H., Luo P. Resveratrol Ameliorates Mitochondrial Elongation via Drp1/Parkin/PINK1 Signaling in Senescent-Like Cardiomyocytes. Oxid. Med. Cell Longev. 2017;2017:4175353. doi: 10.1155/2017/4175353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu M., Wilk S.A., Wang A., Zhou L., Wang R.H., Ogawa W., Deng C., Dong L.Q., Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J. Biol. Chem. 2010;285:36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim S., Choi K.J., Cho S.J., Yun S.M., Jeon J.P., Koh Y.H., Song J., Johnson G.V., Jo C. Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors. Sci. Rep. 2016;6:24933. doi: 10.1038/srep24933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang Y., Chen Y., Shaw A.M., Goldfine H., Tian J., Cai J. Enhancing TFEB-Mediated Cellular Degradation Pathways by the mTORC1 Inhibitor Quercetin. Oxid. Med. Cell Longev. 2018;2018:5073420. doi: 10.1155/2018/5073420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song J.X., Sun Y.R., Peluso I., Zeng Y., Yu X., Lu J.H., Xu Z., Wang M.Z., Liu L.F., Huang Y.Y., et al. A novel curcumin analog binds to and activates TFEB in vitro and in vivo independent of MTOR inhibition. Autophagy. 2016;12:1372–1389. doi: 10.1080/15548627.2016.1179404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao Q., Ke Z.Q., Guo S., Yang X.S., Zhang F.X., Liu X.F., Chen X., Chen H.G., Ke H.Y., Liu C. Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J. Mol. Cell Cardiol. 2018;124:26–34. doi: 10.1016/j.yjmcc.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Zhang P., Fang J., Zhang J., Ding S., Gan D. Curcumin Inhibited Podocyte Cell Apoptosis and Accelerated Cell Autophagy in Diabetic Nephropathy via Regulating Beclin1/UVRAG/Bcl2. Diabetes Metab. Syndr. Obes. 2020;13:641–652. doi: 10.2147/DMSO.S237451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y., Cao Y., Chen J., Qin H., Yang L. A New Possible Mechanism by Which Punicalagin Protects against Liver Injury Induced by Type 2 Diabetes Mellitus: Upregulation of Autophagy via the Akt/FoxO3a Signaling Pathway. J. Agric. Food Chem. 2019;67:13948–13959. doi: 10.1021/acs.jafc.9b05910. [DOI] [PubMed] [Google Scholar]
- 69.Webb A.E., Brunet A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014;39:159–169. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rigacci S., Miceli C., Nediani C., Berti A., Cascella R., Pantano D., Nardiello P., Luccarini I., Casamenti F., Stefani M. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget. 2015;6:35344–35357. doi: 10.18632/oncotarget.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grossi C., Rigacci S., Ambrosini S., Ed Dami T., Luccarini I., Traini C., Failli P., Berti A., Casamenti F., Stefani M. The polyphenol oleuropein aglycone protects TgCRND8 mice against Ass plaque pathology. PLoS ONE. 2013;8:e71702. doi: 10.1371/journal.pone.0071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miceli C., Santin Y., Manzella N., Coppini R., Berti A., Stefani M., Parini A., Mialet-Perez J., Nediani C. Oleuropein Aglycone Protects against MAO-A-Induced Autophagy Impairment and Cardiomyocyte Death through Activation of TFEB. Oxid. Med. Cell Longev. 2018;2018:8067592. doi: 10.1155/2018/8067592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nediani C., Ruzzolini J., Romani A., Calorini L. Oleuropein, a Bioactive Compound from Olea europaea L.; as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants. 2019;8:578. doi: 10.3390/antiox8120578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pietrocola F., Marino G., Lissa D., Vacchelli E., Malik S.A., Niso-Santano M., Zamzami N., Galluzzi L., Maiuri M.C., Kroemer G. Pro-autophagic polyphenols reduce the acetylation of cytoplasmic proteins. Cell Cycle. 2012;11:3851–3860. doi: 10.4161/cc.22027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prentki M., Nolan C.J. Islet beta cell failure in type 2 diabetes. J. Clin. Investig. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guillen C., Benito M. mTORC1 Overactivation as a Key Aging Factor in the Progression to Type 2 Diabetes Mellitus. Front. Endocrinol. 2018;9:621. doi: 10.3389/fendo.2018.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ardestani A., Lupse B., Kido Y., Leibowitz G., Maedler K. mTORC1 Signaling: A Double-Edged Sword in Diabetic beta Cells. Cell Metab. 2018;27:314–331. doi: 10.1016/j.cmet.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Fonseca S.G., Burcin M., Gromada J., Urano F. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr. Opin. Pharmacol. 2009;9:763–770. doi: 10.1016/j.coph.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fonseca S.G., Urano F., Burcin M., Gromada J. Stress hypERactivation in the beta-cell. Islets. 2010;2:1–9. doi: 10.4161/isl.2.1.10456. [DOI] [PubMed] [Google Scholar]
- 80.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R.O., Gorgun C.Z., Hotamisligil G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu S., Yalcin A., Lee G.Y., Li P., Fan J., Arruda A.P., Pers B.M., Yilmaz M., Eguchi K., Hotamisligil G.S. Phenotypic assays identify azoramide as a small-molecule modulator of the unfolded protein response with antidiabetic activity. Sci. Transl. Med. 2015;7:292ra98. doi: 10.1126/scitranslmed.aaa9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guillen C. Azoramide: A new drug for the treatment of type 2 diabetes? Ann. Transl. Med. 2016;4:S45. doi: 10.21037/atm.2016.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Natarajan V., Chawla R., Mah T., Vivekanandan R., Tan S.Y., Sato P.Y. Mallilankaraman, K. Mitochondrial Dysfunction in Age-Related Metabolic Disorders. Proteomics. 2020;20:e1800404. doi: 10.1002/pmic.201800404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee Y.H., Kim J., Park K., Lee M.S. beta-cell autophagy: Mechanism and role in beta-cell dysfunction. Mol. Metab. 2019;27S:S92–S103. doi: 10.1016/j.molmet.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bartolome A., Kimura-Koyanagi M., Asahara S., Guillen C., Inoue H., Teruyama K., Shimizu S., Kanno A., Garcia-Aguilar A., Koike M., et al. Pancreatic beta-cell failure mediated by mTORC1 hyperactivity and autophagic impairment. Diabetes. 2014;63:2996–3008. doi: 10.2337/db13-0970. [DOI] [PubMed] [Google Scholar]
- 86.Bartolome A., Garcia-Aguilar A., Asahara S.I., Kido Y., Guillen C., Pajvani U.B., Benito M. MTORC1 Regulates both General Autophagy and Mitophagy Induction after Oxidative Phosphorylation Uncoupling. Mol. Cell Biol. 2017;37:e00441-17. doi: 10.1128/MCB.00441-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hernandez M.G., Aguilar A.G., Burillo J., Oca R.G., Manca M.A., Novials A., Alcarraz-Vizan G., Guillen C., Benito M. Pancreatic beta cells overexpressing hIAPP impaired mitophagy and unbalanced mitochondrial dynamics. Cell Death. Dis. 2018;9:481. doi: 10.1038/s41419-018-0533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhansali S., Bhansali A., Walia R., Saikia U.N., Dhawan V. Alterations in Mitochondrial Oxidative Stress and Mitophagy in Subjects with Prediabetes and Type 2 Diabetes Mellitus. Front. Endocrinol. 2017;8:347. doi: 10.3389/fendo.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhagani H., Nasser S.A., Dakroub A., El-Yazbi A.F., Eid A.A., Kobeissy F., Pintus G., Eid A.H. The Mitochondria: A Target of Polyphenols in the Treatment of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2020;21:4962. doi: 10.3390/ijms21144962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sabu M.C., Smitha K., Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol. 2002;83:109–116. doi: 10.1016/s0378-8741(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 91.Brasnyo P., Molnar G.A., Mohas M., Marko L., Laczy B., Cseh J., Mikolas E., Szijarto I.A., Merei A., Halmai R., et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 92.Zang M., Xu S., Maitland-Toolan K.A., Zuccollo A., Hou X., Jiang B., Wierzbicki M., Verbeuren T.J., Cohen R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 93.Li S., Eguchi N., Lau H., Ichii H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020;21:6973. doi: 10.3390/ijms21186973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reinisalo M., Karlund A., Koskela A., Kaarniranta K., Karjalainen R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid. Med. Cell Longev. 2015;2015:340520. doi: 10.1155/2015/340520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma L., Fu R., Duan Z., Lu J., Gao J., Tian L., Lv Z., Chen Z., Han J., Jia L., et al. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol. Res. Pract. 2016;212:310–318. doi: 10.1016/j.prp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 96.Wang B., Yang Q., Sun Y.Y., Xing Y.F., Wang Y.B., Lu X.T., Bai W.W., Liu X.Q., Zhao Y.X. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J. Cell Mol. Med. 2014;18:1599–1611. doi: 10.1111/jcmm.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu X.H., Ding D.F., Yong H.J., Dong C.L., You N., Ye X.L., Pan M.L., Ma J.H., You Q., Lu Y.B. Resveratrol transcriptionally regulates miRNA-18a-5p expression ameliorating diabetic nephropathy via increasing autophagy. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4952–4965. [PubMed] [Google Scholar]
- 98.Shakeri A., Zirak M.R., Wallace Hayes A., Reiter R., Karimi G. Curcumin and its analogues protect from endoplasmic reticulum stress: Mechanisms and pathways. Pharmacol. Res. 2019;146:104335. doi: 10.1016/j.phrs.2019.104335. [DOI] [PubMed] [Google Scholar]
- 99.Jin T., Song Z., Weng J., Fantus I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. Endocrinol. Metab. 2018;314:E201–E205. doi: 10.1152/ajpendo.00285.2017. [DOI] [PubMed] [Google Scholar]
- 100.Kim H.S., Montana V., Jang H.J., Parpura V., Kim J.A. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: A potential role for reducing lipid accumulation. J. Biol. Chem. 2013;288:22693–22705. doi: 10.1074/jbc.M113.477505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J., Tang Y., Feng Z., Liu J., Liu J., Long J. (−)-Epigallocatechin-3-gallate attenuated myocardial mitochondrial dysfunction and autophagy in diabetic Goto-Kakizaki rats. Free Radic. Res. 2014;48:898–906. doi: 10.3109/10715762.2014.920955. [DOI] [PubMed] [Google Scholar]
- 102.Yan J., Feng Z., Liu J., Shen W., Wang Y., Wertz K., Weber P., Long J., Liu J. Enhanced autophagy plays a cardinal role in mitochondrial dysfunction in type 2 diabetic Goto-Kakizaki (GK) rats: Ameliorating effects of (−)-epigallocatechin-3-gallate. J. Nutr. Biochem. 2012;23:716–724. doi: 10.1016/j.jnutbio.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 103.de Pablos R.M., Espinosa-Oliva A.M., Hornedo-Ortega R., Cano M., Arguelles S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019;143:58–72. doi: 10.1016/j.phrs.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 104.Tao T., Xu H. Autophagy and Obesity and Diabetes. Adv. Exp. Med. Biol. 2020;1207:445–461. doi: 10.1007/978-981-15-4272-5_32. [DOI] [PubMed] [Google Scholar]
- 105.Garcia-Barrado M.J., Iglesias-Osma M.C., Perez-Garcia E., Carrero S., Blanco E.J., Carretero-Hernandez M., Carretero J. Role of Flavonoids in The Interactions among Obesity, Inflammation, and Autophagy. Pharmaceuticals. 2020;13:342. doi: 10.3390/ph13110342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Milton-Laskibar I., Aguirre L., Etxeberria U., Milagro F.I., Martinez J.A., Portillo M.P. Involvement of autophagy in the beneficial effects of resveratrol in hepatic steatosis treatment. A comparison with energy restriction. Food Funct. 2018;9:4207–4215. doi: 10.1039/C8FO00930A. [DOI] [PubMed] [Google Scholar]
- 107.Choi C., Song H.D., Son Y., Cho Y.K., Ahn S.Y., Jung Y.S., Yoon Y.C., Kwon S.W., Lee Y.H. Epigallocatechin-3-Gallate Reduces Visceral Adiposity Partly through the Regulation of Beclin1-Dependent Autophagy in White Adipose Tissues. Nutrients. 2020;12:3072. doi: 10.3390/nu12103072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Konings E., Timmers S., Boekschoten M.V., Goossens G.H., Jocken J.W., Afman L.A., Muller M., Schrauwen P., Mariman E.C., Blaak E.E. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 2014;38:470–473. doi: 10.1038/ijo.2013.155. [DOI] [PubMed] [Google Scholar]
- 109.Su Z., Nie Y., Huang X., Zhu Y., Feng B., Tang L., Zheng G. Mitophagy in Hepatic Insulin Resistance: Therapeutic Potential and Concerns. Front. Pharmacol. 2019;10:1193. doi: 10.3389/fphar.2019.01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma X., McKeen T., Zhang J., Ding W.X. Role and Mechanisms of Mitophagy in Liver Diseases. Cells. 2020;9:837. doi: 10.3390/cells9040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ueno T., Komatsu M. Autophagy in the liver: Functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2017;14:170–184. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y., Chen M.L., Zhou Y., Yi L., Gao Y.X., Ran L., Chen S.H., Zhang T., Zhou X., Zou D., et al. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol. Nutr. Food Res. 2015;59:1443–1457. doi: 10.1002/mnfr.201500016. [DOI] [PubMed] [Google Scholar]
- 113.Li L., Hai J., Li Z., Zhang Y., Peng H., Li K., Weng X. Resveratrol modulates autophagy and NF-kappaB activity in a murine model for treating non-alcoholic fatty liver disease. Food Chem. Toxicol. 2014;63:166–173. doi: 10.1016/j.fct.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 114.Ding S., Jiang J., Zhang G., Bu Y., Zhang G., Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE. 2017;12:e0183541. doi: 10.1371/journal.pone.0183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parafati M., Lascala A., Morittu V.M., Trimboli F., Rizzuto A., Brunelli E., Coscarelli F., Costa N., Britti D., Ehrlich J., et al. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J. Nutr. Biochem. 2015;26:938–948. doi: 10.1016/j.jnutbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 116.Lascala A., Martino C., Parafati M., Salerno R., Oliverio M., Pellegrino D., Mollace V., Janda E. Analysis of proautophagic activities of Citrus flavonoids in liver cells reveals the superiority of a natural polyphenol mixture over pure flavones. J. Nutr. Biochem. 2018;58:119–130. doi: 10.1016/j.jnutbio.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 117.Chen Y.C., Chen H.J., Huang B.M., Chen Y.C., Chang C.F. Polyphenol-Rich Extracts from Toona sinensis Bark and Fruit Ameliorate Free Fatty Acid-Induced Lipogenesis through AMPK and LC3 Pathways. J. Clin. Med. 2019;8:1664. doi: 10.3390/jcm8101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhuge Q., Zhang Y., Liu B., Wu M. Blueberry polyphenols play a preventive effect on alcoholic fatty liver disease C57BL/6 J mice by promoting autophagy to accelerate lipolysis to eliminate excessive TG accumulation in hepatocytes. Ann. Palliat. Med. 2020;9:1045–1054. doi: 10.21037/apm.2020.03.38. [DOI] [PubMed] [Google Scholar]
- 119.Li D., Cui Y., Wang X., Liu F., Li X. Apple polyphenol extract alleviates lipid accumulation in free-fatty-acid-exposed HepG2 cells via activating autophagy mediated by SIRT1/AMPK signaling. Phytother. Res. 2020:1–16. doi: 10.1002/ptr.6902. [DOI] [PubMed] [Google Scholar]
- 120.Martinez-Lopez N., Singh R. Autophagy and Lipid Droplets in the Liver. Annu Rev. Nutr. 2015;35:215–237. doi: 10.1146/annurev-nutr-071813-105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ding S., Jiang J., Yu P., Zhang G., Zhang G., Liu X. Green tea polyphenol treatment attenuates atherosclerosis in high-fat diet-fed apolipoprotein E-knockout mice via alleviating dyslipidemia and up-regulating autophagy. PLoS ONE. 2017;12:e0181666. doi: 10.1371/journal.pone.0181666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serino A., Salazar G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients. 2018;11:53. doi: 10.3390/nu11010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu W., Wu J., Cai F., Xiang J., Zha W., Fan D., Guo S., Ming Z., Liu C. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS ONE. 2012;7:e52013. doi: 10.1371/journal.pone.0052013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qu X., Chen X., Shi Q., Wang X., Wang D., Yang L. Resveratrol alleviates ischemia/reperfusion injury of diabetic myocardium via inducing autophagy. Exp. Ther. Med. 2019;18:2719–2725. doi: 10.3892/etm.2019.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanches-Silva A., Testai L., Nabavi S.F., Battino M., Pandima Devi K., Tejada S., Sureda A., Xu S., Yousefi B., Majidinia M., et al. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharmacol. Res. 2020;152:104626. doi: 10.1016/j.phrs.2019.104626. [DOI] [PubMed] [Google Scholar]
- 126.Menzies F.M., Fleming A., Rubinsztein D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015;16:345–357. doi: 10.1038/nrn3961. [DOI] [PubMed] [Google Scholar]
- 127.Sonninen T.M., Goldsteins G., Laham-Karam N., Koistinaho J., Lehtonen S. Proteostasis Disturbances and Inflammation in Neurodegenerative Diseases. Cells. 2020;9:2183. doi: 10.3390/cells9102183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ruz C., Alcantud J.L., Vives Montero F., Duran R., Bandres-Ciga S. Proteotoxicity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020;21:5646. doi: 10.3390/ijms21165646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harris L.D., Jasem S., Licchesi J.D.F. The Ubiquitin System in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2020;1233:195–221. doi: 10.1007/978-3-030-38266-7_8. [DOI] [PubMed] [Google Scholar]
- 130.Nixon R.A. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: Inseparable partners in a multifactorial disease. FASEB J. 2017;31:2729–2743. doi: 10.1096/fj.201700359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dixit R., Ross J.L., Goldman Y.E., Holzbaur E.L. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alonso A., Zaidi T., Novak M., Grundke-Iqbal I., Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ittner A., Ittner L.M. Dendritic Tau in Alzheimer’s Disease. Neuron. 2018;99:13–27. doi: 10.1016/j.neuron.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 134.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 135.Hsu L.J., Sagara Y., Arroyo A., Rockenstein E., Sisk A., Mallory M., Wong J., Takenouchi T., Hashimoto M., Masliah E. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 2000;157:401–410. doi: 10.1016/S0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Colla E. Linking the Endoplasmic Reticulum to Parkinson’s Disease and Alpha-Synucleinopathy. Front. Neurosci. 2019;13:560. doi: 10.3389/fnins.2019.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Colla E., Coune P., Liu Y., Pletnikova O., Troncoso J.C., Iwatsubo T., Schneider B.L., Lee M.K. Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. J. Neurosci. 2012;32:3306–3320. doi: 10.1523/JNEUROSCI.5367-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ganguly U., Chakrabarti S.S., Kaur U., Mukherjee A., Chakrabarti S. Alpha-synuclein, Proteotoxicity and Parkinson’s Disease: Search for Neuroprotective Therapy. Curr. Neuropharmacol. 2018;16:1086–1097. doi: 10.2174/1570159X15666171129100944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schaffert L.N., Carter W.G. Do Post-Translational Modifications Influence Protein Aggregation in Neurodegenerative Diseases: A Systematic Review. Brain Sci. 2020;10:232. doi: 10.3390/brainsci10040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mbefo M.K., Fares M.B., Paleologou K., Oueslati A., Yin G., Tenreiro S., Pinto M., Outeiro T., Zweckstetter M., Masliah E., et al. Parkinson disease mutant E46K enhances alpha-synuclein phosphorylation in mammalian cell lines, in yeast, and in vivo. J. Biol. Chem. 2015;290:9412–9427. doi: 10.1074/jbc.M114.610774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kontopoulos E., Parvin J.D., Feany M.B. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 2006;15:3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- 142.Goers J., Manning-Bog A.B., McCormack A.L., Millett I.S., Doniach S., Di Monte D.A., Uversky V.N., Fink A.L. Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry. 2003;42:8465–8471. doi: 10.1021/bi0341152. [DOI] [PubMed] [Google Scholar]
- 143.Mbefo M.K., Paleologou K.E., Boucharaba A., Oueslati A., Schell H., Fournier M., Olschewski D., Yin G., Zweckstetter M., Masliah E., et al. Phosphorylation of synucleins by members of the Polo-like kinase family. J. Biol. Chem. 2010;285:2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang R., Sun H., Wang G., Ren H. Imbalance of Lysine Acetylation Contributes to the Pathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2020;21:7182. doi: 10.3390/ijms21197182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yakhine-Diop S.M.S., Niso-Santano M., Rodriguez-Arribas M., Gomez-Sanchez R., Martinez-Chacon G., Uribe-Carretero E., Navarro-Garcia J.A., Ruiz-Hurtado G., Aiastui A., Cooper J.M., et al. Impaired Mitophagy and Protein Acetylation Levels in Fibroblasts from Parkinson’s Disease Patients. Mol. Neurobiol. 2019;56:2466–2481. doi: 10.1007/s12035-018-1206-6. [DOI] [PubMed] [Google Scholar]
- 146.Rahman M.A., Rahman M.R., Zaman T., Uddin M.S., Islam R., Abdel-Daim M.M., Rhim H. Emerging Potential of Naturally Occurring Autophagy Modulators Against Neurodegeneration. Curr. Pharm. Des. 2020;26:772–779. doi: 10.2174/1381612826666200107142541. [DOI] [PubMed] [Google Scholar]
- 147.Jha N.N., Ghosh D., Das S., Anoop A., Jacob R.S., Singh P.K., Ayyagari N., Namboothiri I.N., Maji S.K. Effect of curcumin analogs onalpha-synuclein aggregation and cytotoxicity. Sci. Rep. 2016;6:28511. doi: 10.1038/srep28511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takahashi R., Ono K., Takamura Y., Mizuguchi M., Ikeda T., Nishijo H., Yamada M. Phenolic compounds prevent the oligomerization of alpha-synuclein and reduce synaptic toxicity. J. Neurochem. 2015;134:943–955. doi: 10.1111/jnc.13180. [DOI] [PubMed] [Google Scholar]
- 149.Wu Z., Wu A., Dong J., Sigears A., Lu B. Grape skin extract improves muscle function and extends lifespan of a Drosophila model of Parkinson’s disease through activation of mitophagy. Exp. Gerontol. 2018;113:10–17. doi: 10.1016/j.exger.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 150.Ding Y., Kong D., Zhou T., Yang N.D., Xin C., Xu J., Wang Q., Zhang H., Wu Q., Lu X., et al. Alpha-Arbutin Protects Against Parkinson’s Disease-Associated Mitochondrial Dysfunction In Vitro and In Vivo. Neuromol. Med. 2020;22:56–67. doi: 10.1007/s12017-019-08562-6. [DOI] [PubMed] [Google Scholar]
- 151.Lin K.L., Lin K.J., Wang P.W., Chuang J.H., Lin H.Y., Chen S.D., Chuang Y.C., Huang S.T., Tiao M.M., Chen J.B., et al. Resveratrol provides neuroprotective effects through modulation of mitochondrial dynamics and ERK1/2 regulated autophagy. Free Radic. Res. 2018;52:1371–1386. doi: 10.1080/10715762.2018.1489128. [DOI] [PubMed] [Google Scholar]
- 152.Qiu W.Q., Pan R., Tang Y., Zhou X.G., Wu J.M., Yu L., Law B.Y., Ai W., Yu C.L., Qin D.L., et al. Lychee seed polyphenol inhibits Abeta-induced activation of NLRP3 inflammasome via the LRP1/AMPK mediated autophagy induction. Biomed. Pharmacother. 2020;130:110575. doi: 10.1016/j.biopha.2020.110575. [DOI] [PubMed] [Google Scholar]
- 153.Tandon A., Singh S.J., Chaturvedi R.K. Stem Cells as Potential Targets of Polyphenols in Multiple Sclerosis and Alzheimer’s Disease. Biomed. Res. Int. 2018;2018:1483791. doi: 10.1155/2018/1483791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nabavi S.F., Sureda A., Dehpour A.R., Shirooie S., Silva A.S., Devi K.P., Ahmed T., Ishaq N., Hashim R., Sobarzo-Sanchez E., et al. Regulation of autophagy by polyphenols: Paving the road for treatment of neurodegeneration. Biotechnol. Adv. 2018;36:1768–1778. doi: 10.1016/j.biotechadv.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 155.Achour I., Arel-Dubeau A.M., Renaud J., Legrand M., Attard E., Germain M., Martinoli M.G. Oleuropein Prevents Neuronal Death, Mitigates Mitochondrial Superoxide Production and Modulates Autophagy in a Dopaminergic Cellular Model. Int. J. Mol. Sci. 2016;17:1293. doi: 10.3390/ijms17081293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cordero J.G., Garcia-Escudero R., Avila J., Gargini R., Garcia-Escudero V. Benefit of Oleuropein Aglycone for Alzheimer’s Disease by Promoting Autophagy. Oxid. Med. Cell Longev. 2018;2018:5010741. doi: 10.1155/2018/5010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nardiello P., Pantano D., Lapucci A., Stefani M., Casamenti F. Diet Supplementation with Hydroxytyrosol Ameliorates Brain Pathology and Restores Cognitive Functions in a Mouse Model of Amyloid-beta Deposition. J. Alzheimers Dis. 2018;63:1161–1172. doi: 10.3233/JAD-171124. [DOI] [PubMed] [Google Scholar]
- 158.Albani D., Polito L., Signorini A., Forloni G. Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors. 2010;36:370–376. doi: 10.1002/biof.118. [DOI] [PubMed] [Google Scholar]
- 159.Gao Z., Ye J. Inhibition of transcriptional activity of c-JUN by SIRT1. Biochem. Biophys. Res. Commun. 2008;376:793–796. doi: 10.1016/j.bbrc.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Csiszar A., Labinskyy N., Jimenez R., Pinto J.T., Ballabh P., Losonczy G., Pearson K.J., de Cabo R., Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mech. Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee I.H., Cao L., Mostoslavsky R., Lombard D.B., Liu J., Bruns N.E., Tsokos M., Alt F.W., Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad Sci. USA. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang K., Liu R., Li J., Mao J., Lei Y., Wu J., Zeng J., Zhang T., Wu H., Chen L., et al. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR- and hypoxia-induced factor 1alpha-mediated signaling. Autophagy. 2011;7:966–978. doi: 10.4161/auto.7.9.15863. [DOI] [PubMed] [Google Scholar]
- 163.Lai L.L., Lu H.Q., Li W.N., Huang H.P., Zhou H.Y., Leng E.N., Zhang Y.Y. Protective effects of quercetin and crocin in the kidneys and liver of obese Sprague-Dawley rats with Type 2 diabetes: Effects of quercetin and crocin on T2DM rats. Hum. Exp. Toxicol. 2020:960327120954521. doi: 10.1177/0960327120954521. [DOI] [PubMed] [Google Scholar]
- 164.Lou M., Zhang L.N., Ji P.G., Feng F.Q., Liu J.H., Yang C., Li B.F., Wang L. Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Biomed. Pharmacother. 2016;84:1–9. doi: 10.1016/j.biopha.2016.08.055. [DOI] [PubMed] [Google Scholar]
- 165.Lee M.S., Chyau C.C., Wang C.P., Wang T.H., Chen J.H., Lin H.H. Flavonoids Identification and Pancreatic Beta-Cell Protective Effect of Lotus Seedpod. Antioxidants. 2020;9:685. doi: 10.3390/antiox9080658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qu L., Liang X., Gu B., Liu W. Quercetin alleviates high glucose-induced Schwann cell damage by autophagy. Neural Regen Res. 2014;9:1195–1203. doi: 10.4103/1673-5374.135328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang Z.X., Ma J., Li X.Y., Wu Y., Shi H., Chen Y., Lu G., Shen H.M., Lu G.D., Zhou J. Quercetin Induces p53-independent Cancer Cell Death via TFEB-mediated Lysosome Activation and ROS-dependent Ferroptosis. Br. J. Pharmacol. 2020 doi: 10.1111/bph.15350. [DOI] [PubMed] [Google Scholar]
- 168.Zhu X., Xiong T., Liu P., Guo X., Xiao L., Zhou F., Tang Y., Yao P. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem. Toxicol. 2018;114:52–60. doi: 10.1016/j.fct.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 169.Sung B., Chung H.Y., Kim N.D. Role of Apigenin in Cancer Prevention via the Induction of Apoptosis and Autophagy. J. Cancer Prev. 2016;21:216–226. doi: 10.15430/JCP.2016.21.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yammine A., Zarrouk A., Nury T., Vejux A., Latruffe N., Vervandier-Fasseur D., Samadi M., Mackrill J.J., Greige-Gerges H., Auezova L., et al. Prevention by Dietary Polyphenols (Resveratrol, Quercetin, Apigenin) Against 7-Ketocholesterol-Induced Oxiapoptophagy in Neuronal N2a Cells: Potential Interest for the Treatment of Neurodegenerative and Age-Related Diseases. Cells. 2020;9:2346. doi: 10.3390/cells9112346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Adham A.N., Hegazy M.E.F., Naqishbandi A.M., Efferth T. Induction of Apoptosis, Autophagy and Ferroptosis by Thymus vulgaris and Arctium lappa Extract in Leukemia and Multiple Myeloma Cell Lines. Molecules. 2020;25:5016. doi: 10.3390/molecules25215016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu J., Meng Z., Chen Y., Yu L., Gao B., Zheng Y., Guan S. Apigenin induced autophagy and stimulated autophagic lipid degradation. Food Funct. 2020;11:9208–9215. doi: 10.1039/D0FO00949K. [DOI] [PubMed] [Google Scholar]
- 173.Yao H., Zhou L., Tang L., Guan Y., Chen S., Zhang Y., Han X. Protective effects of luteolin-7-O-glucoside against starvation-induced injury through upregulation of autophagy in H9c2 Cells. Biosci. Trends. 2017;11:557–564. doi: 10.5582/bst.2017.01111. [DOI] [PubMed] [Google Scholar]
- 174.Siracusa R., Paterniti I., Impellizzeri D., Cordaro M., Crupi R., Navarra M., Cuzzocrea S., Esposito E. The Association of Palmitoylethanolamide with Luteolin Decreases Neuroinflammation and Stimulates Autophagy in Parkinson’s Disease Model. CNS Neurol. Disord. Drug Targets. 2015;14:1350–1365. doi: 10.2174/1871527314666150821102823. [DOI] [PubMed] [Google Scholar]
- 175.Liu S., Su Y., Sun B., Hao R., Pan S., Gao X., Dong X., Ismail A.M., Han B. Luteolin Protects Against CIRI, Potentially via Regulation of the SIRT3/AMPK/mTOR Signaling Pathway. Neurochem. Res. 2020;45:2499–2515. doi: 10.1007/s11064-020-03108-w. [DOI] [PubMed] [Google Scholar]
- 176.Tan X., Yang Y., Xu J., Zhang P., Deng R., Mao Y., He J., Chen Y., Zhang Y., Ding J., et al. Luteolin Exerts Neuroprotection via Modulation of the p62/Keap1/Nrf2 Pathway in Intracerebral Hemorrhage. Front. Pharmacol. 2019;10:1551. doi: 10.3389/fphar.2019.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu H., Yu W., Sun S., Li C., Zhang Y., Ren J. Luteolin Attenuates Doxorubicin-Induced Cardiotoxicity Through Promoting Mitochondrial Autophagy. Front. Physiol. 2020;11:113. doi: 10.3389/fphys.2020.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liao Y., Xu Y., Cao M., Huan Y., Zhu L., Jiang Y., Shen W., Zhu G. Luteolin Induces Apoptosis and Autophagy in Mouse Macrophage ANA-1 Cells via the Bcl-2 Pathway. J. Immunol. Res. 2018;2018:4623919. doi: 10.1155/2018/4623919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Y.F., Tang Z.H., Li T., Xu X.H., Chen X., Wang Y., Wang Y.T., Lu J.J. Baicalein protects tertbutyl hydroperoxideinduced hepatotoxicity dependent of reactive oxygen species removal. Mol. Med. Rep. 2017;16:8392–8398. doi: 10.3892/mmr.2017.7592. [DOI] [PubMed] [Google Scholar]
- 180.Kuang L., Cao X., Lu Z. Baicalein Protects against Rotenone-Induced Neurotoxicity through Induction of Autophagy. Biol. Pharm. Bull. 2017;40:1537–1543. doi: 10.1248/bpb.b17-00392. [DOI] [PubMed] [Google Scholar]
- 181.Hung K.C., Huang H.J., Wang Y.T., Lin A.M. Baicalein attenuates alpha-synuclein aggregation, inflammasome activation and autophagy in the MPP(+)-treated nigrostriatal dopaminergic system in vivo. J. Ethnopharmacol. 2016;194:522–529. doi: 10.1016/j.jep.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 182.Wu C., Xu H., Li J., Hu X., Wang X., Huang Y., Li Y., Sheng S., Wang Y., Xu H., et al. Baicalein Attenuates Pyroptosis and Endoplasmic Reticulum Stress Following Spinal Cord Ischemia-Reperfusion Injury via Autophagy Enhancement. Front. Pharmacol. 2020;11:1076. doi: 10.3389/fphar.2020.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu B.Y., Li L., Liu G.L., Ding W., Chang W.G., Xu T., Ji X.Y., Zheng X.X., Zhang J., Wang J.X. Baicalein attenuates cardiac hypertrophy in mice via suppressing oxidative stress and activating autophagy in cardiomyocytes. Acta Pharmacol. Sin. 2020 doi: 10.1038/s41401-020-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]