Abstract
Ca2+ influx through the L-type Ca2+ channel Cav1.2 triggers each heartbeat. The fight or flight response induces the release of the stress-response hormone norepinephrine to stimulate β adrenergic receptors, cAMP production, and protein kinase A activity to augment Ca2+ influx through Cav1.2 and consequently cardiomyocyte contractility. Emerging evidence shows that Cav1.2 is regulated by different mechanisms in cardiomyocytes compared to neurons and vascular smooth muscle cells.
The L-type Ca2+ channel Cav1.2, which is the most prevalent Ca2+ channel in the heart, mediates the initial Ca2+ influx into cardiomyocytes that subsequently induces Ca2+ release from internal stores (1). Cytoplasmic Ca2+ concentrations eventually reach a threshold for triggering cardiomyocyte contraction. The closely-related L-type channel Cav1.3 plays an important role in initiating the heart beat in the sinoatrial node, which serves as the primary pacemaker for the heart (2). Cav1.2 also mediates Ca2+ influx into neurons, which governs gene expression (3, 4) and excitability (5, 6).
The stress hormone norepinephrine increases the rate and strength of heart muscle contraction during the fight or flight response. The increase in strength is largely due to upregulation of the activity of the cardiac Cav1.2 by norepinephrine (7). Because of the central physiological role and hence the long-lasting interest in and focus on the regulation of Cav1.2, it is considered one of the holy grails of channel regulation. The timely work of Marx and colleagues delineated one major regulatory mechanism in heart muscle (8). They found that stimulation of β adrenergic receptors (β AR) by norepinephrine leads to PKA-mediated phosphorylation of the small G-protein Rad. This modification induces the release of Rad from Cav1.2. Because Rad is a potent negative regulator of the cardiac Cav1.2, its displacement from the complex results in disinhibition of channel activity (8).
The first milestone in deciphering this mechanism was the identification of Ser1928 in the C-terminus of Cav1.2 of the pore-forming α subunit of Cav1.2 as the main PKA phosphorylation site in the channel (9) (Figure 1). PKA is activated by cAMP, a second messenger that is produced upon the activation of the stimulatory trimeric Gs protein and adenylyl cyclase following stimulation of β ARs (Figure 1). PKA is the primary downstream kinase mediating norepinephrine signaling through β ARs. However, definitive evidence for a critical role of this signaling paradigm had been difficult to establish because ectopic expression of Cav1.2 in heterologous cell lines does not reliably reconstitute this regulatory pathway (10). Early evidence from studies in HEK293 cells supporting a role for Ser1928 in increasing Cav1.2 activity was limited or required complex expression system manipulations (4, 11, 12). Still, several studies demonstrating a robust increase in Ser1928 phosphorylation in heart (13) and other tissues and especially brain, where Cav1.2 plays important roles in regulating neuronal functions (14, 15) buttressed the notion that Ser1928 phosphorylation is a critical event in governing Cav1.2 activity.
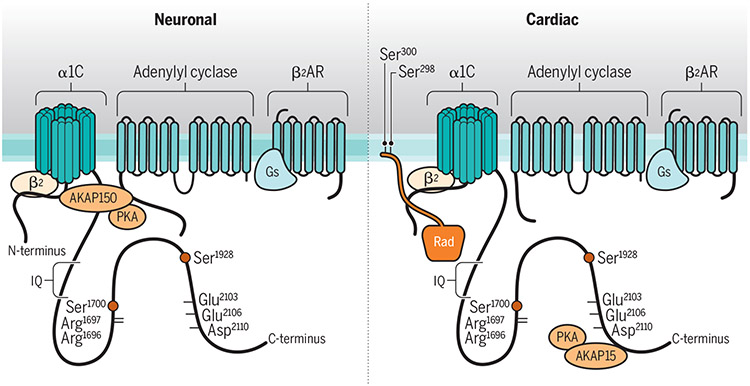
Figure 1: Differential regulation of Cav1.2 by β AR stimulation in neurons and cardiomyocytes.
Models depict the hypothesized overall structural arrangements of part of the β2 AR/Cav1.2 signaling complex as might be prevalent in neurons (left) and cardiomyocytes (right). Cav1.2 is thought to mostly reside outside lipid rafts in neurons (darker membrane) and in lipid rafts in cardiomyocytes (lighter membrane) (22, 23). Rad is present predominantly in cardiomyocytes and might bind to the β subunit and the N-terminus and PCT of the α1 subunit of Cav1.2, either through multiple interactions as depicted or in multiple copies (not depicted). AKAP150 and AKAP15 seem to be the predominant AKAP in neurons and cardiomyocytes, respectively. AKAP150 links both adenylyl cyclase and PKA to Cav1.2 and has multiple interaction sites with Cav1.2 (15). AKAP15 is shorter than AKAP150 and may link only PKA to Cav1.2. How AC is linked to Cav1.2 when AKAP15 is in the Cav1.2 complex is unclear. The PCT and DCT are depicted in a ‘closed’ conformation in the left model due to salt bridges between Arg1696 and Arg1697 in the PCT and Glu2103, Glu2106 and Asp2110 in the DCT and in an ‘open’ conformation in the right model, perhaps due to differences in the environment of Cav1.2 (inside compared to outside of rafts) or associated proteins. Arg1696 and Arg1697 are part of the PKA consensus site for phosphorylation of Ser1700, which disrupts these salt bridges. Ser1928 is the main PKA phosphorylation site in α11.2. For simplicity, the linkers between the transmembrane segments of the α1 subunit of Cav1.2 are not shown.
In 2008, Hofmann and colleagues reported that a S1928A knock-in mutation of the Cav1.2 α-subunit did not affect the peripheral fight or flight response, arguing against the importance of this PKA target site in mediating β adrenergic signaling, at least in heart muscle (16). Coincidentally, Catterall and colleagues identified Ser1700 as a second PKA site in the Cav1.2 C-terminus (17) (Figure 1). By titrating the amount of the A kinase anchoring protein AKAP15/18, which recruits PKA to Cav1.2, they defined the conditions under which an increase in Cav1.2 activity and the consequent coupling of gating charge movement to pore opening could be reliably observed in HEK293 cells (17). Too little AKAP15/18 does not provide sufficient attachment sites to enable PKA association with Cav1.2 and too much may act as a sink to sequester PKA and reduce its association in Cav1.2. By titrating AKAP15/18 amounts, Fuller et al. reproducibly obtained PKA-mediated increases in Cav1.2 activity in HEK293 cells. Subsequent work in S1700A knock-in mice indicated that this phosphorylation site contributes to increasing Cav1.2 activity in heart tissue, but did not account for the full norepinephrine effect (18). Further clouding the picture were observations from expression of different Cav1.2 mutants in vivo that suggested that Ser1700 did not play a substantial role of in norepinephrine regulation of cardiac Cav1.2 activity (19). Moreover, Liu et al showed that mutating all intracellular consensus sites for PKA in Cav1.2 did not abrogate the increase in activity by β AR stimulation in the heart (8). The main factor for the regulation of the cardiac Cav1.2 had not been identified.
The next turning point was the recognition that Ser1928 is part of the attachment site for the β2 AR on Cav1.2 (20). This signaling complex also contains Gs, adenylyl cyclase, AKAPs, and PKA for highly localized regulation of Cav1.2 activity in neurons (21, 22) and cardiomyocytes (23) (Figure 1). Repetitive stimulation of the β2 AR leads to a temporary (~5 min) displacement of the β2 AR from Cav1.2 (20), an effect that was absent in S1928A knock-in mice. This mechanism creates a refractory period during which this signaling pathway cannot be re-stimulated (20), suggesting a potential negative feedback loop. However, further analysis of S1928A knock-in mice revealed that in contrast to cardiomyocytes, Ser1928 is required for upregulation of Cav1.2 by β2 AR stimulation in hippocampal neurons (24). Parallel experiments confirmed that β-adrenergic stimulation of Cav1.2 is not affected in cardiomyocytes from S1928A knock-in mice (24). At the same time upregulation of Cav1.2 activity in VSMCs upon stimulation of the Gs/PKA-coupled adenosinic P2Y11 receptor in vascular smooth muscle cells (VSMCs) also depended on Ser1928 (25, 26).
The work by Marx and his colleagues is based on a proximity assay in which Cav1.2 was tagged with ascorbate peroxidase, which biotinylates proteins within a 20 nm region around the channel (8). Biotinylation of Rad was decreased upon β AR stimulation, which was the strongest effect for any of the biotinylated proteins and which suggested that β AR stimulation robustly displaces Rad from the Cav1.2 complex. Rad belongs to the RGK family of small G proteins, which also includes Rem, Rem2 and Gem/Kir. These G proteins inhibit Cav1.2 activity (27-29). The authors found that co-expression of Rad reduced Cav1.2 activity in HEK293 cells. Stimulation of adenylyl cyclase augmented Cav1.2 activity in the presence of Rad, but had no further effect on the already fairly high activity of Cav1.2 in the absence of Rad. Mutating the putative PKA sites Ser272 and Ser300 at the extreme C-terminus of Rad, a region that binds to negatively charged phospholipids such as PIP2, abrogated the regulation by cAMP. This was consistent with the importance of the interaction of the C-terminus of Rem with the membrane for Cav1.2 inhibition (30, 31). Liu et al. showed that PKA-mediated phosphorylation displaced Rad from the auxiliary β subunit in the Cav1.2 complex, thereby disinhibiting the channel activity (8). This was coherent with an essential role of β subunits in upregulation of Cav1.2 activity by β adrenergic signaling in the heart (32).
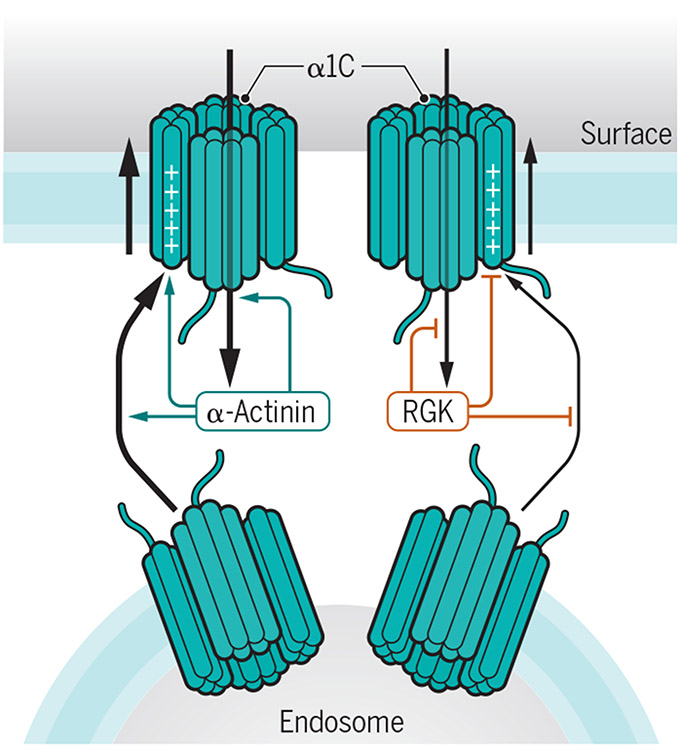
The mechanism of Cav1.2 inhibition by Rad is most likely as complex as that shown for the closely related Rem. Rem impairs the functional availability of Cav1.2 in cardiomyocytes in three ways (Figure 2) (31). Firstly, Rem reduces surface abundance, possibly by binding to auxiliary β subunits in Cav1.2, which otherwise augment trafficking of Cav1.2 through the secretory pathway (31). Secondly, Rem reduces the open probability (Po) of Cav1.2 channels. Thirdly, Rem impairs the movement of the voltage sensors in Cav1.2. The effects of Rem and Rad on surface localization and Po depend on their binding to the β subunit whereas those on gating charge movement do not (33). Rem can directly bind to the N- and C-terminus of the central pore forming α11.2 subunit of Cav1.2 (30, 33) (Figure 1). How Rad impairs Cav1.2 functions is currently unclear. However, it is tempting to speculate that it could affect α-actinin binding to the IQ motif in the membrane-proximal portion of the C-terminus (PCT) of α11.2 because this interaction increases the same three parameters, namely surface abundance and localization (34, 35), gating charge movement, and its coupling to pore opening (with the latter two determining Po) (36). In fact, Cav1.2 has low activity in the absence of α-actinin binding – the Po of α-actinin binding-deficient Cav1.2 mutants is ~15-20% of wild type Cav1.2 - but becomes primed for activation when at its designated location at the cell surface by α-actinin, an effect that minimizes unintended Ca2+ flux activity at inappropriate locations such as the secretory pathway (36). Binding of RGK proteins instead of α-actinin to Cav1.2 could contribute to keeping the channel inactive in the secretory pathway. In turn, α-actinin binding to the PCT could impair binding of Rad to the α1 or β subunit and thereby augment Po. An antagonistic effect of RGK proteins on α-actinin binding would also explain the opposite effects of these two proteins on the surface abundance and Po of Cav1.2 (31, 36).
Figure 2: Antagonistic effects of Rem and α-actinin on Cav1.2.
Schematic depiction of α-actinin promoting (green arrows) and Rem antagonizing (red lines) three major parameters that affect Cav1.2 functions: surface abundance, movement of the voltage sensing S4 transmembrane helices, which carry the gating charges, and coupling of gating movements to pore opening. Impaired coupling has been directly determined for loss of α-actinin binding to Cav1.2 (36) and is suggested by analogy for Rem binding (31, 33). For simplicity, the linkers between the transmembrane segments of the α1 subunit of Cav1.2 are not shown.
Why is phosphorylation of Ser1928 critical in neurons but not cardiomyocytes? One key difference is that upregulation of Cav1.2 activity by β adrenergic signaling is exclusively mediated by the β2 AR in neurons (24) and mostly by the β1 AR in cardiomyocytes (37, 38). However, the β2 AR can contribute to upregulation of Cav1.2 activity under certain conditions in the heart (23, 39), especially under pathological conditions such as heart failure (37, 38). But why would the β1 AR and the β2 AR differentially couple to Cav1.2 regulation in heart and brain? The answer might lie in differences in the microenvironment, which could affect Cav1.2 conformation and thereby its regulation. In heart but not in brain, most of Cav1.2 appears to be located within membrane rafts or caveolae (22, 23). In fact, pharmacological disruption of rafts as well as caveolin-3 knockdown impairs the limited regulation of Cav1.2 by the β2 AR in heart that occurs upon pertussis toxin blockade of the inhibitory trimeric Gi protein (23, 39). The requirement of the Gi block to detect this β2 AR regulation is likely due to the ability of the β2 AR to switch from Gs coupling to Gi coupling (40). That such a Gi block is not necessary in hippocampal neurons, where signaling is exclusively mediated by the β2 AR and not the β1 AR and apparently outside of membrane rafts, fits with the notion that the microenvironment could affect Cav1.2 conformation and regulation. An additional reason could be the association of Cav1.2 with different sets of auxiliary subunits or their isoforms. For instance, there are four different genes encoding β subunits, each exhibiting multiple splice isoforms (10), which could impart different properties and conformations to the Cav1.2 complexes. In fact, emerging evidence indicates that alterations in the binding site for β subunit between domains I and II affect regulation of Cav1.2 activity by PKA (41).
How could the microenvironment influence Cav1.2 regulation? The ~660 residue long C-terminus of α11.2 is functionally divided into roughly two ~ 300 residue long portions, the PCT and the membrane-distal one (DCT). These two domains interact through salt bridges between Arg1696/Arg1697 and Glu2103/Glu2106/Asp2110 (42) (Figure 1). Deletion of DCT results in increased Cav1.2 activity (42, 43), an effect that can be reversed by expression of the DCT as a separate polypeptide (42). Arg1696 and Arg1697 form a consensus sequence for the phosphorylation of the nearby Ser1700 by PKA, which augments Cav1.2 activity (17). Collectively, these findings suggest that Ser1700 phosphorylation disrupts the PCT-DCT interaction (42) and thereby augments Cav1.2 activity. Similarly, Ser1928 phosphorylation could increase Cav1.2 activity (20) by disrupting the PCT-DCT interaction (Figure 1). The degree to which the DCT impairs the channel activity could depend on the microenvironment – perhaps the interaction between the PCT and DCT is prominent outside (neurons) but not inside membrane rafts (cardiomyocytes). Such a cell type dependence on membrane rafts would predict that phosphorylation of Ser1700 or Ser1928 results in disinhibition of Cav1.2 in neurons where this interaction curbs the channel activity but not in cardiomyocytes where this interaction might be less prevalent (Figure 1). Ser1700 phosphorylation appears to be less important than Ser1928 phosphorylation in upregulating Cav1.2 activity in neurons. A form of long-lasting synaptic potentiation that is induced by coincident prolonged synaptic activation at the 5-8 Hz theta rhythm and β adrenergic stimulation and involves Cav1.2 requires phosphorylation of Ser1928 but not that of Ser1700 (20, 24).
The dependence of β adrenergic upregulation of Cav1.2 in neurons but not in cardiomyocytes on Ser1928 phosphorylation could theoretically be due to differential regulation of this phosphorylation event by β2 AR compared to β1 AR signaling, leading to variant target phosphorylation in neurons compared to cardiomyocytes. This possibility would be consistent with the β2 AR but not the β1 AR being part of the Cav1.2/Gs/adenylyl cyclase/PKA signaling complex (22, 23). However, β AR stimulation leads to a robust increase in Ser1928 phosphorylation not only in neurons (20) but also in cardiomyocytes (13), arguing that this possibility is not the main explanation for the tissue-specific differential functions of Ser1928 .
Another interesting twist is the observation that the β2 AR locally restricts signaling by the β1 AR in cardiomyocytes to Cav1.2 (44). This restriction prevents β1 AR-induced phosphorylation of the juxtaposed ryanodine receptor, which mediates Ca2+-induced Ca2+ release (CICR) after Ca2+ influx through Cav1.2. Upregulation of CICR from the sarcoplasmic reticulum but not of Cav1.2 currents upon stimulation of the β1 AR with the non-selective β AR agonist isoproterenol is impaired only by strong activation of the β2 AR by co-application of the β2 AR selective agonist salbutamol but not by the modest activation induced by isoproterenol alone (44). This restriction of β1 AR to the nanospace around Cav1.2 by the β2 AR appears to depend on the PDE4 family of phosphodiesterases (PDEs) and the C-terminus of the β1 AR, because this effect is prevented by the PDE4 selective inhibitor rolipram or injection of a peptide corresponding to the C-terminal 36 residues of the β1 AR, which includes its binding site for PDZ-domain containing scaffolding proteins. This restraint of β1 AR signaling is also abrogated by injection of β-arrestin-1 (but not β-arrestin-2) and an inhibitor of the G protein coupled receptor kinase GRK2. Further experiments showed that activation of the β2 AR but not β1 AR caused GRK2 translocation to Caveolin-3 containing membrane sites, consistent with the recruitment to membrane rafts where Cav1.2 resides in cardiomyocytes. In knock-in mice expressing a form of the β1 AR in which the three serine residues near the extreme C-terminus that are phosphorylated by GRKs are replaced with alanine residues, which would be expected to abolish arrestin binding, the spatial restriction of β1 AR signaling by β2 AR signaling is lost (44). Collectively, these results suggest that β2 AR activation results in β1 AR phosphorylation by GRKs, and that binding of β-arrestin-1 to the β1 AR compartmentalizes cAMP signaling by the β1 AR to the immediate Cav1.2 environment. In the presence of β2 AR signaling, β1 AR signaling downstream of cAMP/PKA does not upregulate CICR by the juxtaposed ryanodine receptor, which is only a few nanometers away and otherwise effectively regulated by β1 AR signaling (1). β2 ARs can exist as monomers and dimers, which become differentially phosphorylated upon isoproterenol stimulation at Ser365/Ser366 only in monomers by GRKs or at Ser261/Ser262 only in dimers by PKA. The monomers but not dimers undergo agonist-induced endocytosis (45). Given that Ser261/Ser262 phosphorylation is required for upregulation of Cav1.2 by β2 AR signaling (45) it appears likely that the dimeric form of the β2 AR associates with and regulates Cav1.2. These observations support the notion that the β2 AR adopts different conformations without ligand stimulation, thus allowing its selective interaction with downstream targets and thereby potentially creating defined microenvironments.
Could Rad or another RGK also regulate Cav1.2 in neurons? Rad, Rem, and Gem are all poorly expressed in brain (46). However, there is a subset of neurons in which Gem2 is abundant (47). Thus, it seems likely that in most neurons, RGK proteins are simply not present in sufficient amounts to interact with and inhibit Cav1.2 activity. In neurons in which Gem2 is abundant, β2 AR signaling-mediated upregulation of Cav1.2 may require phosphorylation of both Ser1928 as well as Rem2.
Rad and Rem can also suppress the activity of the L-type Ca2+ channel Cav1.3 and the N-type Ca2+ channel Cav2.2 and cAMP signaling can disinhibit their channel activities (8). The regulation of Cav1.3 by Rad is relevant for cardiac function, because Cav1.3 is a major contributor to the pacemaker activity of the sinoatrial node and thereby our heart rate (2). Accordingly, Rad-dependent regulation of Cav1.3 likely contributes to the increase in heart rate during the fight or flight response.
By defining the key phosphorylation sites in the Cav1.2 complexes for norepinephrine signaling, the journey to understand norepinephrine-mediated regulation of Cav1.2 activity has reached a prominent milestone. Yet, we have only arrived at the next course of questions now awaiting answers. Perhaps the most crucial questions at this point are: How does Ser1928 phosphorylation translate into increased Cav1.2 activity, and why does this phosphorylation lead to increased channel activity in neurons but not cardiomyocytes? The next level of mechanistic insight into the fascinating and remarkable complexity of Cav1.2 regulation is now ripe for further exploration.
Acknowledgments
Funding: Research in the author’s laboratory was supported by NIH grants R01 NS-078792, R01 MH097887, and R01 AG 055357.
Footnotes
Competing Interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Bers DM, Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Mangoni ME et al. , Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proceedings of the National Academy of Sciences of the United States of America 100, 5543–5548 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheeler DG et al. , Cav1 and Cav2 Channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell 149, 1112–1124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy JG et al. , AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell reports 7, 1577–1588 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkefeld H et al. , BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science (New York, N.Y 314, 615–620 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Marrion NV, Tavalin ST, Selective activation of Ca2+-activated K+ channels by co-localized Ca2+channels in hippocampal neurons. Nature 395, 900–905 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Reuter H, Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature 301, 569–574 (1983). [DOI] [PubMed] [Google Scholar]
- 8.Liu G et al. , Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. Nature 577, 695–700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jongh KS et al. , Specific phosphorylation of a site in the full length form of the a1 subunit of the cardiac L-type calcium channel by adenosine 3',5'-cyclic monophosphate-dependent protein kinase. Biochem. 35, 10392–10402 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Dai S, Hall DD, Hell JW, Supramolecular Assemblies and Localized Regulation of Voltage-gated Ion Channels. Physiological reviews 89, 411–452 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao T et al. , cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron 19, 185–196 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Oliveria SF, Dell'acqua ML, Sather WA, AKAP79/150 Anchoring of Calcineurin Controls Neuronal L-Type Ca(2+) Channel Activity and Nuclear Signaling. Neuron 55, 261–275 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA, Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation. Proceedings of the National Academy of Sciences of the United States of America 103, 16574–16579 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davare MA, Hell JW, Increased phosphorylation of the neuronal L-type Ca(2+) channel Ca(v)1.2 during aging. Proceedings of the National Academy of Sciences of the United States of America 100, 16018–16023 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall DD et al. , Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry 46, 1635–1646 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Lemke T et al. , Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1.2 phosphorylation site S1928A mutant mice. The Journal of biological chemistry 283, 34738–34744 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA, Molecular Mechanism of Calcium Channel Regulation in the Fight-or-Flight Response. Sci Signal 3, ra70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, Westenbroek RE, Scheuer T, Catterall WA, Basal and beta-adrenergic regulation of the cardiac calcium channel CaV1.2 requires phosphorylation of serine 1700. Proceedings of the National Academy of Sciences of the United States of America 111, 16598–16603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L et al. , beta-adrenergic regulation of the L-type Ca2+ channel does not require phosphorylation of alpha1C Ser1700. Circulation research 113, 871–880 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patriarchi T et al. , Phosphorylation of Cav1.2 on S1928 Uncouples the L-type Ca2+ Channel from the β2 Adrenergic Receptor. The EMBO journal 35, 1330–1345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davare MA, Dong F, Rubin CS, Hell JW, The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C L-type calcium channels in neurons. J. Biol. Chem 274, 30280–30287 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Davare MA et al. , A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. [see comments]. [erratum appears in Science 2001 Aug 3;293(5531):804]. Science (New York, N.Y 293, 98–101 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ, From the Cover: Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for beta2-adrenergic regulation. Proceedings of the National Academy of Sciences of the United States of America 103, 7500–7505 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian H et al. , Phosphorylation of Ser1928 mediates the enhanced activity of the L-type Ca2+ channel Cav1.2 by the beta2-adrenergic receptor in neurons. Sci Signal 10, eaaf9659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nystoriak MA et al. , Ser1928 phosphorylation by PKA stimulates the L-type Ca2+ channel CaV1.2 and vasoconstriction during acute hyperglycemia and diabetes. Sci Signal 10, eaaf9647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prada MP et al. , A Gs-coupled purinergic receptor boosts Ca(2+) influx and vascular contractility during diabetic hyperglycemia. eLife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beguin P et al. , Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature 411, 701–706 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Finlin BS, Crump SM, Satin J, Andres DA, Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proceedings of the National Academy of Sciences of the United States of America 100, 14469–14474 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finlin BS et al. , Analysis of the complex between Ca2+ channel beta-subunit and the Rem GTPase. The Journal of biological chemistry 281, 23557–23566 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Pang C et al. , Rem GTPase interacts with the proximal CaV1.2 C-terminus and modulates calcium-dependent channel inactivation. Channels (Austin) 4, 192–202 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang T, Xu X, Kernan T, Wu V, Colecraft HM, Rem, a member of the RGK GTPases, inhibits recombinant CaV1.2 channels using multiple mechanisms that require distinct conformations of the GTPase. The Journal of physiology 588, 1665–1681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L et al. , Cardiac CaV1.2 channels require beta subunits for beta-adrenergic-mediated modulation but not trafficking. J Clin Invest 129, 647–658 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang T, Puckerin A, Colecraft HM, Distinct RGK GTPases differentially use alpha1- and auxiliary beta-binding-dependent mechanisms to inhibit CaV1.2/CaV2.2 channels. PLoS One 7, e37079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall DD et al. , Competition Between α-Actinin and Ca2+-Calmodulin Controls Surface Retention of the L-type Ca2+ Channel CaV1.2. Neuron 78, 483–497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tseng PY et al. , alpha-Actinin Promotes Surface Localization and Current Density of the Ca2+ Channel CaV1.2 by Binding to the IQ Region of the alpha1 Subunit. Biochemistry 56, 3669–3681 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner M et al. , alpha-Actinin-1 promotes activity of the L-type Ca(2+) channel Cav 1.2. The EMBO journal, e102622 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg SF, The molecular basis for distinct beta-adrenergic receptor subtype actions in cardiomyocytes. Circulation research 85, 1101–1111 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Kamp TJ, Hell JW, Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circulation research 87, 1095–1102 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Chen-Izu Y et al. , G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca(2+) channels. Biophys J 79, 2547–2556 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daaka Y, Luttrell LM, Lefkowitz RJ, Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390, 88–91 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Papa A et al. , Adrenergic CaV1.2 Activation via Rad Phosphorylation Converges at alpha1C I-II Loop. Circulation research, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA, Autoinhibitory Control of the CaV1.2 Channel by its Proteolytically Processed Distal C-terminal Domain. The Journal of physiology 576, 87–102 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei X et al. , Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac alpha 1 subunit. Journal of Biological Chemistry 269, 1635–1640 (1994). [PubMed] [Google Scholar]
- 44.Yang HQ et al. , beta2-Adrenergic Stimulation Compartmentalizes beta1 Signaling Into Nanoscale Local Domains by Targeting the C-Terminus of beta1-Adrenoceptors. Circulation research 124, 1350–1359 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen A et al. , Functionally distinct and selectively phosphorylated GPCR subpopulations co-exist in a single cell. Nature communications 9, 1050 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correll RN, Pang C, Niedowicz DM, Finlin BS, Andres DA, The RGK family of GTP-binding proteins: regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cellular signalling 20, 292–300 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liput DJ, Lu VB, Davis MI, Puhl HL, Ikeda SR, Rem2, a member of the RGK family of small GTPases, is enriched in nuclei of the basal ganglia. Scientific reports 6, 25137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]