ABSTRACT
Context:
Clinicians face one of the most common bacterial infections in developing countries that is urinary tract infection (UTI). Current knowledge on antimicrobial susceptibility pattern is essential for selecting appropriate therapy.
Aims:
In this study, we investigated the various bacteria causing UTI and determined the sensitivity and resistance of antibiotics pattern against most prevalent uropathogens isolated from patients at tertiary hospital, Al-Baha, Saudi Arabia.
Settings and Design:
This was a retrospective study of urine culture conducted in King Fahad Hospital at Al-Baha in Saudi Arabia.
Materials and Methods:
Laboratory reports and patient medical files of both inpatient and outpatient were collected between June 2017 and May 2018, targeting both male and female of age above 18 years of age, who had been treated for UTI.
Results:
A total of 349 patients’ urine report was studied to identify the uropathogens. Escherichia coli was the main etiologic agent in community and hospital-acquired infections. The majority of the bacteria was isolated from female (60%), whereas the remaining (40%) was from male. The most common isolates were E. coli, Klebsiella pneumoniae, Enterococcus faecalis, E. coli Extended spectrum beta-lactamases, Pseudomonas, and K. pneumoniae ESBL (these represented 37.82%, 19.20%, 10.89%, 10.32%, 6.59%, and 3.72%, of isolate, respectively). UTI due to E. coli was at a higher rate during summer than during winter. This study showed that ciprofloxacin (20.29%) and cefuroxime (16.14%) are most prescribed medications, followed by ceftriaxone (12.96%) and then tazocin (8.80%). Imipenem, meropenem, amikacin, vancomycin, tigecycline, linezolid, and colistin were highly sensitive for most types of bacteria, but gram-negative bacteria were highly resistant to ampicillin. Gram-positive bacteria showed highly resistance to cefoxitin.
Conclusion:
The microbial culture and sensitivity of the isolates from urine samples should be carried out as a routine before starting the antimicrobial therapy. Current knowledge of the antibiotic sensitivity/resistance patterns of uropathogens at a particular geographical region is a guiding factor for choosing an appropriate empirical antimicrobial treatment rather than following universal guidelines.
KEYWORDS: Al-Baha, antibiotic susceptibility, predisposing factor, Saudi Arabia, urinary tract infections, uropathogens
INTRODUCTION
Generally antibiotics are prescribed empirically for urinary tract infection (UTI) before the final urine culture laboratory results are available.[1] Choice of empirical antibiotic prescription depends on the knowledge of bacterial organisms that are prevalent locally and antibiotic sensitivities, rather than on universal guidelines.[2,3,4] In recent years, bacterial resistance to various antibiotics has raised dramatically, forcing physicians with few therapeutic choices.[5,6]
Therefore, estimating the epidemiology of UTIs prevalence, risk factors, bacterial isolates, and antibiotic sensitivity is mandatory for the hospital setup and health planners to give appropriate interventions. The aim of this study was to determine the various bacteria causing UTI and the sensitivity and resistance of antibiotic pattern of the urinary pathogens, additionally UTI ratio in gender distribution, antibiotic prescribing pattern, and concomitant disease conditions with UTI at a tertiary hospital at Al-Baha, Saudi Arabia.
SUBJECTS AND METHODS
Sample size
Retrospective study was conducted from past medical files and urine samples’ test report that were obtained from patients treated for UTI at King Fahad Hospital in Al-Baha region, Saudi Arabia, between June, 2017 and May, 2018. Urine specimens’ test report of patients of age 18 years and older, belonging to outpatient clinics and inpatient wards, suspected of having UTI were retrieved from microbiology laboratory. The total number of patients’ medical files assessed was 349.
Inclusion criteria
All adult patients infected with urinary tract infection from June 2017 to May 2018 were included in this study.
Exclusion criteria
All children (younger than 18 years) were excluded from this study.
Data collection
This was a retrospective study, conducted by collecting information from medical files such as date of sample collections, sex and age of patients, bacterial isolate, antibiotics prescribed, and concomitant diseases.
Instruments of data collection
Lab reports
The data were collected from laboratory reports, which had information about uropathogen bacterial isolate, bacterial sensitivity, and resistance to antibiotics.
Medical file
The first-line antibiotics prescribed were recorded that included both empirical antibiotics prescribed before urine laboratory test was conducted and antibiotics after the test result release. Furthermore, concomitant diseases along with UTI and their medications prescribed were recorded from patient medical files.
Urine analysis methodology previously done
Urine analysis was performed according to internal policy and procedure (IPP) conducted at the department of microbiology laboratory, King Fahad Hospital, Al-Baha, Saudi Arabia.
Statistical analysis
Data were entered into a database designed using an MS Excel spreadsheet and analyzed with Statistical Package for Social Sciences (SPSS, Chicago, Illinois) software, version 16.0. Retrospective study statistics shows uropathogen percentage and antibiotics resistance/sensitivity for particular period. The overall 1-year resistance/sensitivity rates of the most common uropathogen to the routinely tested first-line antimicrobials were calculated by dividing the number of urinary isolates resistant/sensitive to each antimicrobial agent by the number of isolates tested against an individual antimicrobial agent. Tables and graphs are presented as frequency (count) and percentages with 95% confidence interval.
RESULTS
Uropathogen percentage for complete study period
The pathogens causing UTIs are well known, Escherichia coli was the main etiologic agent in community as well as hospital-acquired infections. A total of 349 urine bacteria were isolated and identified. The majority of the bacteria was isolated from females (60%), whereas the remaining (40%) was from males. The most common isolates were E. coli, Klebsiella pneumoniae, Enterococcus faecalis, E. coli ESBL, Pseudomonas, K. pneumoniae ESBL, and Proteus (these represented 37.82%, 19.20%, 10.89%, 10.32%, 6.59%, 3.72%, and 2.29% of isolate, respectively). Percentage of uropathogens isolated between June 2017 and May 2018, is shown in Table 1 and Figure 1. Percentage of UTI occurrence during summer was higher than that during winter, which is caused by the most common uropathogen E. coli followed by E. faecalis. Percentage of various uropathogens during summer and winter season is shown in Figure 2.
Table 1.
Susceptibility pattern of Escherichia coli
| Antibiotics | Test done (td) | Resistant |
95% CI resistant | Sensitive |
95% CI sensitive | Test not done (nd) |
|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||
| Penicillin | 2 | 2 (100) | 34.2–100 | 0 (0) | 0 | 94 |
| Ampicillin | 78 | 69 (88.46) | 79.50–93.81 | 9 (11.54) | 6.19–20.50 | 51 |
| Augmentin | 33 | 14 (42.42) | 27.24–59.19 | 19 (57.58) | 40.81–72.76 | 95 |
| Carbenicillin | 0 | 0 | 0 | 0 | 0 | 115 |
| Aztreonam | 22 | 3 (13.64) | 4.75–33.33 | 19 (86.36) | 66.67–95.25 | 92 |
| Imipenem | 82 | 1 (1.22) | 0.22–6.59 | 81 (98.78) | 93.41–99.78 | 48 |
| Meropenem | 18 | 0 (0) | 0 | 18 (100 ) | 82.41–100 | 96 |
| Piperacillin | 1 | 0 (0) | 0 | 1 (100) | 20.65–100 | 128 |
| Tazocin (Zosyn) | 38 | 4 (10.53) | 4.17–24.13 | 34 (89.47) | 75.87–95.83 | 90 |
| Cephalexin | 44 | 42 (95.45) | 84.87–98.74 | 2 (4.55) | 1.26–15.13 | 84 |
| Cefoxitin | 22 | 16 (72.73) | 51.85–86.85 | 6 (27.27) | 13.15–48.15 | 125 |
| Cefuroxime | 23 | 7 (30.43) | 15.60–50.87 | 16 (69.57) | 49.13–84.40 | 56 |
| Ceftriaxone | 39 | 18 (46.15) | 31.57–61.43 | 21 (53.85) | 38.57–68.43 | 57 |
| Ceftazidime | 13 | 0 (0) | 13 (100.0) | 77.19–100 | 115 | |
| Cefepime | 26 | 8 (30.77) | 16.50–49.99 | 18 (69.23) | 50.01–83.50 | 102 |
| Amikacin | 81 | 3 (3.70) | 1.27–10.33 | 78(96.30) | 89.67–98.73 | 49 |
| Gentamicin | 29 | 4 (13.79) | 5.50–30.56 | 25 (86.21) | 69.44–94.50 | 99 |
| Ciprofloxacin | 61 | 30 (49.18) | 37.06–61.40 | 31(50.82) | 38.60–62.94 | 67 |
| Levofloxacin | 1 | 0 (0) | 0 | 1(100.00) | 20.65–100.0 | 45 |
| Norfloxacin | 53 | 28 (52.83) | 39.66–65.62 | 25 (47.17) | 34.38–60.34 | 66 |
| Erythromycin | 1 | 1 (100.00) | 20.65–100 | 0 (0) | 0 | 128 |
| Clindamycin | 1 | 1 (100.00) | 20.65–100 | 0 (0) | 0 | 128 |
| Vancomycin | 1 | 0 (0) | 0 | 1 (100.00) | 20.65–100.00 | 128 |
| Linezolid | 1 | 0 (0) | 0 | 1 (100.00) | 20.65–100.00 | 128 |
| Tigecycline | 4 | 0 (0) | 0 | 4 (100.00) | 51.01–100 | 125 |
| Colistin | 3 | 0 (0) | 0 | 3 (100.00) | 43.85–100 | 126 |
| Nitrofurantoin | 112 | 11 (9.82) | 5.57–16.73 | 101 (90.18) | 83.72-94.43 | 17 |
| TMP-SMX | 107 | 61 (57.01) | 47.55–65.99 | 46 (42.99) | 34.01–52.45 | 22 |
N = frequency, CI = confidence interval, TMP-SMX = Trimethoprim and sulfamethoxazole, td = respective antibiotic tested against E. coli, nd = respective antibiotic was not tested against E. coli
Figure 1.
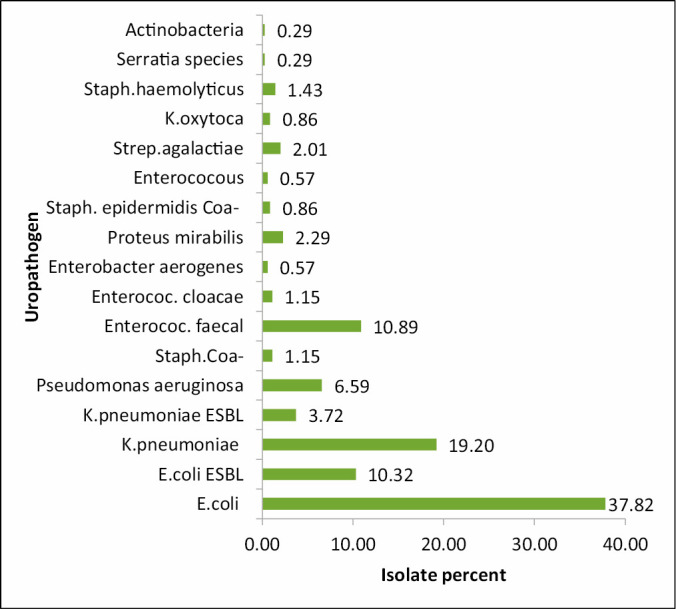
Percent of uropathogen from June 2017 to May 2018. Total 349 uropathogen isolates were identified between June 2017 and May 2018, among them four major UTI causing bacteria were identified as E. coli = 132, K. pneumoniae = 67, P. aeruginosa = 23, E. faecalis = 38
Figure 2.
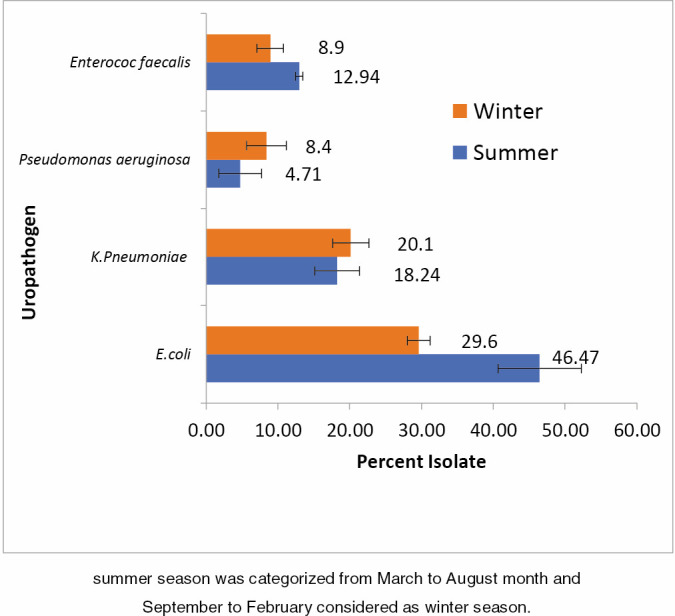
Percent of uropathogens during summer and winter. Summer season was categorized from March to August month, and September to February was considered as winter season
Antibacterial susceptibility pattern
Susceptibility pattern of E. coli was observed, it was highly sensitive toward higher and broad range of antibiotics such as meropenem, piperacillin, ceftazidime, levofloxacin, tigecycline, and colistin (100%), followed by imipenem (98.78%), amikacin (96%), nitrofurantoin (90.18%), tazocin (89.47%), aztreonam (86.36%), gentamicin (86.21%), cefuroxime (69.57%), Augmentin (57.58%), ceftriaxone (53.85%), and ciprofloxacin (50.82%). High resistance was observed for ampicillin (88.46%), cephalexin (95.45%), cefoxitin (72.73%), and trimethoprim and sulfamethoxazole (TMP-SMX) (57.01%), and norfloxacin (52.83%). Empirical treatment is based on epidemiological data and guidelines; broad spectrum is generally avoided as first-line treatment.[7] In our study, nitrofurantoin was prescribed as the first choice of antibiotics [Table 1]. Susceptibility patterns against K. pneumoniae had a trend similar to that against E. coli. It was highly sensitive to oxacillin, carbenicillin, colistin (100%), amikacin (94.59%), ceftazidime (90%), imipenem (84.78%), gentamicin (84.62%), tigecycline (83.33%), meropenem and Augmentin (75%), and nitrofurantoin (73.91%). K. pneumoniae was highly resistant against penicillin, ampicillin (100%), cephalexin (93.75%), and cefotaxime (71.43%). Augmentin and nitrofurantoin were prescribed as the first line of therapy for these types of bacteria [Table 2]. E. faecalis was highly sensitive to teicoplanin, colistin, tigecycline, linezolid and vancomycin (100%), and nitrofurantoin (83.33%). It was highly resistant against meropenem (100%), imipenem (87.50%), oxacillin (100%), gentamicin (72.73%), ciprofloxacin (66.67%), erythromycin (88.46%), and most of the beta lactam except ampicillin (53.57%). This bacterium has low susceptibility to doxycycline (50%) and TMP-SMX (52.53%) [Table 3]. P. aeruginosa was highly sensitive to tigecycline and colistin (100%) and tazocin (83.33%), imipenem (73.33%) and meropenem (71.43%), ciprofloxacin (60%), carbenicillin (66.67%), aztreonam (61.54%), ceftazidime, piperacillin, and gentamicin (50%), but highly resistant to most of the beta lactam. This bacterium was resistant against cefepime (66.66%), but it was completely resistant against levofloxacin (100%) [Table 4].
Table 2.
Susceptibility pattern of Klebsiella pneumoniae
| Antibiotics | Test Done (td) | Resistant, N (%) | 95% CI resistant | Sensitive, N (%) | 95% CI sensitive | Test not done (nd) |
|---|---|---|---|---|---|---|
| Penicillin | 2 | 2 (100) | 34.24–100 | 0(0) | 0 | 49 |
| Ampicillin | 39 | 39 (100) | 91.03–100 | 0 (0) | 0 | 28 |
| Augmentin | 16 | 4 (25) | 10.18–49.50 | 12 (75) | 50.50–89.92 | 51 |
| Oxacillin | 1 | 0 (0) | 0 | 1 (100) | 20.65–100 | 63 |
| Carbenicillin | 1 | 0 (0) | 0 | 1 (100) | 20.65–100 | 61 |
| Aztreonam | 12 | 4 (33.33) | 13.81–60.94 | 8 (66.67) | 25.38–74.62 | 46 |
| Imipenem | 46 | 7 (15.22) | 7.57–28.22 | 39 (84.78) | 71.78–92.43 | 20 |
| Meropenem | 16 | 4 (25) | 10.18–49.50 | 12 (75) | 50.50–89.82 | 48 |
| Tazocin (Zosyn) | 21 | 10 (47.61) | 28.34–67.63 | 11 (52.38) | 32.37–71.66 | 46 |
| Cephalexin | 16 | 15 (93.75) | 71.67–98.89 | 1 (6.25) | 1.11–28.33 | 51 |
| Cefoxitin | 12 | 7 (58.33) | 31.95–80.67 | 5 (41.67) | 19.33–68.05 | 55 |
| Cefuroxime | 13 | 9 (69.23) | 42.37–87.32 | 4 (30.77) | 12.68–57.63 | 37 |
| Ceftriaxone | 22 | 13 (59.09) | 38.73–76.74 | 9 (40.91) | 23.26–61.27 | 31 |
| Ceftazidime | 10 | 1 (10) | 1.79–40.42 | 9 (90) | 59.58–98.21 | 57 |
| Cefotaxime | 7 | 5 (71.43) | 35.89–91.78 | 2 (28.57) | 8.22–64.11 | 58 |
| Cefepime | 12 | 4 (33.33) | 13.81–60.94 | 8 (66.67) | 39.06–86.19 | 51 |
| Amikacin | 37 | 2 (5.41) | 1.50–17.70 | 35 (94.59) | 82.30–98.50 | 30 |
| Gentamicin | 13 | 2 (15.38) | 4.33–42.23 | 11 (84.62) | 57.77–95.67 | 50 |
| Ciprofloxacin | 43 | 20 (46.51) | 32.51–61.08 | 23 (53.49) | 38.92–67.49 | 37 |
| Levofloxacin | 3 | 2 (66.67) | 20.77–93.85 | 1 (33.33) | 6.15–79.23 | 18 |
| Norfloxacin | 28 | 10 (35.71) | 20.71–54.17 | 18 (64.29) | 33.45–64.11 | 37 |
| Clindamycin | 1 | 1 (100) | 20.65–100 | 0 (0) | 0 | 66 |
| Tigecycline | 6 | 1 (16.67) | 3.01–56.35 | 5 (83.33) | 3.55–17.79 | 61 |
| Colistin | 5 | 0 (0) | 0 | 5 (100) | 56.55–100 | 62 |
| Nitrofurantoin | 46 | 12 (26.09) | 15.60–40.26 | 34 (73.91) | 59.74–84.40 | 21 |
| TMP-SMX | 57 | 29 (50.88) | 38.26–63.38 | 28 (49.12) | 36.62–61.74 | 14 |
N = frequency, CI = confidence interval, TMP-SMX = trimethoprim and sulfamethoxazole, td = respective antibiotic tested against K. pneumoniae nd = respective antibiotic was not tested against K. pneumoniae
Table 3.
Susceptibility pattern of Enterococcus faecalis
| Antibiotics | Test done (td) | Resistant, N (%) | 95% CI resistant | Sensitive, N (%) | 95% CI, sensitive | Test not done (nd) |
|---|---|---|---|---|---|---|
| Penicillin | 4 | 4 (100.00) | 51.01–100 | 0 (0) | 0 | 23 |
| Ampicillin | 28 | 13 (46.43) | 29.53–64.19 | 15 (53.57) | 35.81–70.47 | 7 |
| Augmentin | 4 | 3 (75.00) | 30.06–95.44 | 1 (25.00) | 4.56–69.94 | 34 |
| Oxacillin | 3 | 3 (100.00) | 43.85–100 | 0 (0) | 0 | 31 |
| Imipenem | 8 | 7 (87.50) | 52.91–97.76 | 1 (12.50) | 2.24–47.09 | 25 |
| Meropenem | 1 | 1 (100.00) | 20.65–100 | 0 (0) | 0 | 38 |
| Cephalexin | 4 | 4 (100.00) | 51.01–100 | 0.00 | 0 | 30 |
| Cefoxitin | 15 | 14 (93.33) | 70.18–98.81 | 1 (6.67) | 1.19–29.82 | 23 |
| Gentamicin | 22 | 16 (72.73) | 51.85–86.85 | 6 (27.27) | 13.15–48.15 | 16 |
| Ciprofloxacin | 12 | 8 (66.67) | 39.06–86.19 | 4 (33.33) | 13.81–60.94 | 26 |
| Norfloxacin | 1 | 1 (100.00) | 20.65–100 | 0 (0) | 0 | 37 |
| Erythromycin | 26 | 23 (88.46) | 71.02–96.00 | 3 (11.54) | 4.0–28.98 | 12 |
| Tetracycline | 4 | 3 (75.00) | 30.06–95.44 | 1 (25.00) | 4.56–69.94 | 27 |
| Doxycycline | 2 | 1 (50.00) | 9.45–90.55 | 1 (50.00) | 9.45–90.55 | 28 |
| Clindamycin | 15 | 14 (93.33) | 70.18–98.81 | 1 (6.67) | 1.19–29.82 | 23 |
| Vancomycin | 29 | 0 (0) | 0 | 29 (100.00) | 88.30–100 | 9 |
| Linezolid | 22 | 0 (0) | 0 | 22 (100.00) | 85.13–100 | 16 |
| Tigecycline | 4 | 0 (0) | 0 | 4 (100.00) | 51.01–100 | 40 |
| Colistin | 1 | 0 (0) | 0 | 1 (100.00) | 20.65–100 | 33 |
| Nitrofurantoin | 24 | 4 (16.67) | 6.68–35.85 | 20 (83.33) | 64.15–93.32 | 14 |
| TMP-SMX | 19 | 9 (47.37) | 27.33–68.29 | 10 (52.63) | 31.71–72.67 | 19 |
| Teicoplanin | 13 | 0 (0) | 0 | 13 (100.00) | 77.19–100 | 18 |
N = frequency, CI = confidence interval, TMP-SMX = Trimethoprim and sulfamethoxazole, td = respective antibiotic tested against E. faecalis, nd = respective antibiotic was not tested against E. faecalis
Table 4.
Susceptibility pattern of Pseudomonas aeruginosa
| Antibiotics | Test done (td) | Resistant, N (%) | 95% CI resistant | Sensitive, N (%) | 95% CI sensitive | Test not done (nd) |
|---|---|---|---|---|---|---|
| Ampicillin | 4 | 4 (100) | 51.01–100 | 0 (0) | 0 | 19 |
| Augmentin | 1 | 1 (100) | 20.65–100 | 0 (0) | 0 | 22 |
| Carbenicillin | 3 | 1 (33.33) | 6.15–79.23 | 2 (66.67) | 20.77–93.85 | 19 |
| Aztreonam | 13 | 5 (38.46) | 17.71–64.48 | 8 (61.54) | 35.52–82.29 | 9 |
| Imipenem | 15 | 4 (26.67) | 10.90–51.95 | 11 (73.33) | 48.05–89.10 | 8 |
| Meropenem | 7 | 2 (28.57) | 8.22–64.11 | 5 (71.43) | 35.89–91.78 | 15 |
| Piperacillin | 2 | 1 (50.00) | 9.45–90.55 | 1 (50.00) | 9.45–90.55 | 21 |
| Tazocin (Zosyn) | 12 | 2 (16.67) | 4.70–44.80 | 10 (83.33) | 55.20–95.30 | 11 |
| Cephalexin | 3 | 3 (100) | 43.85–100 | 0 (0) | 0 | 19 |
| Cefuroxime | 2 | 2 (100) | 34.24–100 | 0 (0) | 0 | 18 |
| Ceftriaxone | 3 | 3 (100) | 43.85–100 | 0 (0) | 0 | 18 |
| Ceftazidime | 8 | 4 (50) | 21.52–78.48 | 4 (50) | 21.52–78.48 | 16 |
| Cefepime | 6 | 4 (66.67) | 30.00–90.32 | 2 (33.33) | 9.68–70.00 | 17 |
| Amikacin | 10 | 3 (30) | 10.78–60.32 | 7 (70) | 39.68–89.22 | 13 |
| Gentamicin | 4 | 2 (50) | 15.00–85.00 | 2 (50) | 15.00–85.00 | 19 |
| Ciprofloxacin | 15 | 6 (40) | 19.83–64.25 | 9 (60) | 35.75–80.18 | 8 |
| Levofloxacin | 1 | 1 (100) | 20.65–100 | 0 (0) | 0 | 11 |
| Norfloxacin | 6 | 1 (16.67) | 3.01–56.35 | 5 (83.33) | 43.65–96.99 | 17 |
| Tigecycline | 1 | 0 (0) | 0 | 1 (100) | 20.65–100 | 22 |
| Colistin | 4 | 0 (0) | 0 | 4 (100) | 51.01–100 | 16 |
| Nitrofurantoin | 6 | 3 (50) | 18.76–81.24 | 3 (50) | 18.76–81.24 | 17 |
| TMP-SMX | 5 | 4 (80) | 37.55–96.38 | 1 (20) | 37.55–96.38 | 18 |
N = frequency, CI = confidence interval, td = respective antibiotic tested against P. aeruginosa, nd = respective antibiotic was not tested against P. aeruginosa, TMP-SMX = trimethoprim and sulfamethoxazole
Antibiotics prescribed for urinary tract infection
This study shows us that ciprofloxacin is the most prescribed medication in inpatient and outpatient departments (20.29%), followed by cefuroxime—second-generation cephalosporin (16.14%), ceftriaxone—third generation-cephalosporin (12.96%), piperacillin/tazobactam–tazocin (8.80%), nitrofurantoin (5.13%), meropenem (4.16%), imipenem and colistin (3.67%), metronidazole, and linezolid (2.69%), whereas ampicillin has shown us that it is less commonly used because of high rate of resistance [Table 5].
Table 5.
Percentage of antibiotics prescribed between June 2017 and May 2018
| Antibiotics | Total* prescribed | Prescribed percentage | 95% CI |
|---|---|---|---|
| Ampicillin | 1 | 0.24 | 0.04–1.37 |
| Amoxicillin | 6 | 1.47 | 0.67–3.16 |
| Augmentin | 10 | 2.44 | 1.33–4.44 |
| Imipenem | 15 | 3.67 | 2.23–5.96 |
| Meropenem | 17 | 4.16 | 2.61–6.56 |
| Colistin | 15 | 3.67 | 2.23–5.96 |
| Tazocin | 36 | 8.80 | 6.43–11.95 |
| Cefepime | 1 | 0.24 | 0.04–1.37 |
| Ceftriaxone | 53 | 12.96 | 10.05–16.56 |
| Cefuroxime | 66 | 16.14 | 12.89–20.01 |
| Cefazolin | 7 | 1.71 | 0.83–3.49 |
| Cephalexin | 1 | 0.24 | 0.04–1.37 |
| Ceftazidime | 8 | 1.96 | 0.99–3.81 |
| Levofloxacin | 7 | 1.71 | 0.83–3.49 |
| Metronidazole | 11 | 2.69 | 1.51–4.75 |
| Nitrofurantoin | 21 | 5.13 | 3.38–7.72 |
| Linezolid | 11 | 2.69 | 1.51–4.75 |
| Azithromycin | 10 | 2.44 | 1.33–4.44 |
| Doxycycline | 3 | 0.73 | 0.25–2.13 |
| Ciprofloxacin | 83 | 20.29 | 16.68–24.46 |
| Co-trimoxazole | 3 | 0.73 | 0.25–2.13 |
| Amikacin | 5 | 1.22 | 0.52–2.83 |
| Gentamicin | 1 | 0.24 | 0.04–1.37 |
| Tigecycline | 8 | 1.96 | 0.99–3.81 |
| Vancomycin | 3 | 0.73 | 0.25–2.13 |
| Clindamycin | 2 | 0.49 | 0.13–1.77 |
| Total | 409 |
*Total antibiotics prescribed during June 2017 to May 2018, for all UTI cases
Concomitant disease with urinary tract infection
The patients with UTI during study period had following concomitant diseases: diabetes mellitus was the greatest risk of UTIs (21.11%), then hypertension (20.49%), urinary tract disorders (9.55%), chronic kidney disease (6.47%), and pregnancy (3.85%) [Figure 3].
Figure 3.
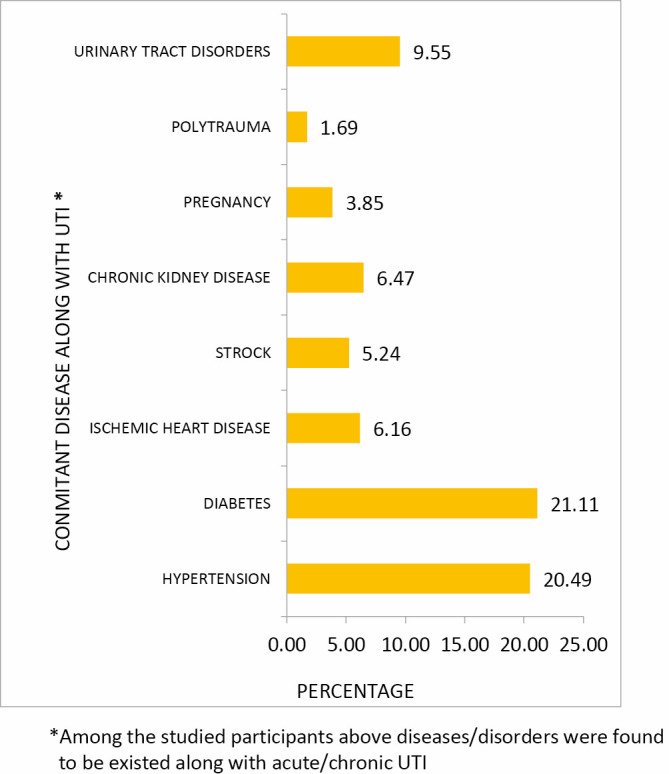
Concomitant diseases with urinary tract infection.
DISCUSSION
UTI is reported as one of the most common microorganism infections despite widely accessible diagnostic aids, antibiotics, and increased knowledge about its pathogenesis. The first line of defense in the lower urinary tract will, in most cases, succeed in preventing bacterial attachment and establishment. However, sometimes, and more often in certain risk groups, namely the elderly and the pregnant women, patients with diabetes, with preexisting urinary tract structural abnormalities/obstruction, and transplanted kidney, bacteria will establish themselves in the urinary tract to cause infection.[7] Less commonly, bacteria spread to the upper urinary tract causing pyelonephritis. The patients with recurrent UTI are often had been prescribed antibiotics repeatedly or as prophylaxis for longer period. The problem of bacterial resistance to antibiotics used in UTI is increasing at a high speed, and new treatment strategies are therefore needed. To be able to therapeutically intervene in the host–pathogen interaction in the urinary tract, better understanding of the antibiotic sensitivity and resistance is needed.[8]
In our study, high prevalence of UTI in females than that in males was observed, which correlates with the findings of Akhtar et al.,[9] which revealed that the frequency of UTI is greater in females as compared to males. In another short period study from March to June in Qassim province by Alzohairy and Khadri,[5] it was found that females had more UTI than males, principally due to anatomical and physical factors. The most common uropathogens associated with uncomplicated UTI are E. coli, Staphylococcus saprophyticus, Enterococcus species, K. pneumoniae, Proteus mirabilis; complicated UTI is caused by E. coli, P. aeruginosa, Acinetobacter baumannii, Enterococcus species, and Staphylococcus spp. Catheter-associated urinary tract infection is caused by P. mirabilis, Morganella morganii, Providencia stuartii, Corynebacterium urealyticum, Candida species; and recurrent UTI is caused by P. mirabilis, K. pneumoniae, Enterobacter species, antibiotic-resistant E. coli, Enterococcus species, and Staphylococcus species.[10] The predominant isolates in our study were E. coli, followed by Klebsiella species. These findings are in conformity with the reports by other researchers.[9,11] According to Alzohairy and Khadri[9] and Patil and Jain,[1]Pseudomonas species was the third most common uropathogen, but this is contrary to our study, which showed that E. faecalis is the third one.
The resistance to antibiotic drugs has been observed since its use and is an increasing worldwide problem. In this study, high rate of E. coli and K. pneumoniae isolates were resistant to ampicillin, this result is same as that of another study in Ethiopia[4] and central Riyadh hospital in Saudi Arabia.[3] In agreement with El-Kersh et al.,[12] our susceptibility data indicated that all tested gram-negative bacteria showed high resistance to ampicillin. Moreover, E. coli, K. pneumoniae, and other gram-negative bacteria showed susceptibility against meropenem, amikacin, and gentamicin. Also as similar to a study by El-Kersh et al.,[12] we observed that most tested bacteria were far more susceptible to amikacin than that toward gentamicin.
In another Saudi study conducted in a major tertiary hospital, the King Saud University Medical City (formerly known as King Khalid University Hospital) Riyadh,[13]E. coli showed highest resistance to ampicillin, followed by TMP-SMX and ciprofloxacin, whereas K. pneumoniae showed highest resistance to ampicillin, TMP-SMX, followed by cefuroxime. Compared to our results, E. coli showed high resistance to ampicillin followed by cephalexin, then TMP-SMX and norfloxacin, whereas K. pneumoniae showed high resistance against penicillin ˃ ampicillin ˃ cephalexin ˃ cefotaxime. Similar results were that ESBL producing E. coli were resistant to third-generation cephalosporin and fluoroquinolone, but highly susceptible to carbapenems, amikacin, and piperacillin/tazobactam. Hence, nitrofurantoin and TMP-SMX can be used as first line of treatment.
Previous study in Riyadh had shown that the drugs commonly used to treat P. aeruginosa infection was limited, which includes ciprofloxacin, amikacin, gentamicin, ceftazidime, piperacillin, tazocin, and imipenem.[14] This report was parallel with our data for these antibiotics; additionally, last but not the least, colistin and tigecycline can be used. It is recommended to use nitrofurantoin as first line because it has good susceptibility to most microorganisms. This is consistent with the findings by Biswas et al.[15] and Shaifali et al.[2] We suggest that nitrofurantoin may be considered as a viable alternative to fluoroquinolones.
When comparing our result of uropathogen sensitivity and resistance treated with co-trimoxazole (TMP-SMX) versus the result of a study by Biswas et al.[15] conducted in Bangladesh, an increasing pattern of resistance of E. coli and P. aeruginosa was observed in our study. However, this drug shows good susceptibility similar to that observed in the study by Biswas et al.[15] study for E. coli ESBL and Klebsiella, but low sensitivity, particularly, to E. coli in Al-Baha region, Saudi Arabia. Additionally, Al-Mijalli, in Central Riyadh Hospital, has revealed high resistance when compared to our study. Their result stated that trimethoprim was more resistant to E. coli and K. pneumoniae.[15]
Also Balkhi et al.[13] stated that the gram-positive organism Enterococcus faecium showed a high resistance to ampicillin and ciprofloxacin. This was similar to our study in resistance against ciprofloxacin except ampicillin. Staphylococcus Coa negative (gram positive) showed high susceptible to gentamicin and ciprofloxacin, on the contrary, erythromycin and Augmentin were fully resistant. Okopi et al.,[16] following sensitivity pattern, observed ciprofloxacin ˃ gentamicin ˃ erythromycin but no resistance was found for these antibiotics. Generally, the drug of choice as short-term therapy with nitrofurantoin was successful in most of the cases. Co-trimoxazole, fluoroquinolone, or cephalosporin is not considered as the first choice of treatment.
We found that UTIs are common among patients with type 2 diabetes mellitus due to an impaired immune status and increased glucose content of the urine. This is in consistence with the finding of a study that was carried out as systematic review on observational studies by evaluating 37 articles.[17] All kidney and urinary tract related diseases also had UTI, which was prominent in our study.
Recommendations
Broad-spectrum antibiotics should be avoided as empirical treatment.
E. coli > K. pneumoniae > E. faecalis seems to be common causative uropathogen.
Strong evidence of resistance have been noted for penicillin, ampicillin, cephalexin and cefoxitin against E.coli induced UTI.
Fluoroquinolones, Augmentin, cephalosporin, and nitrofurantoin are good choice of drugs as empirical treatment.
Fluoroquinolone-resistant cases can be treated with nitrofurantoin.
Broad-spectrum antibiotics, namely carbapenam, piperacillin, vancomycin, linezolid, tigecycline, and colistin should be reserved for special cases of complicated UTI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Patil T, Jain MA. Retrospective analysis of prevalence of uropathogens and antibiotic sensitivity pattern in patients of urinary tract infection in a tertiary care teaching hospital. Int Archiv Biomed Clin Res. 2016;2:14. [Google Scholar]
- 2.Shaifali I, Gupta U, Mahmood SE, Ahmed J. Antibiotic susceptibility patterns of urinary pathogens in female outpatients. N Am J Med Sci. 2012;4:163–9. doi: 10.4103/1947-2714.94940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mijalli SHS. Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in Riyadh hospital, Saudi Arabia. Cell Mol Med. 2017;3:1. [Google Scholar]
- 4.Beyene G, Tsegaye W. Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in Jimma University Specialized Hospital, southwest Ethiopia. Ethiop J Health Sci. 2011;21:141–6. doi: 10.4314/ejhs.v21i2.69055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzohairy M, Khadri H. Frequency and antibiotic susceptibility pattern of uro-pathogens isolated from community and hospital-acquired infections in Saudi Arabia—a prospective case study. Br J Med Med Res. 2010;2:45–56. [Google Scholar]
- 6.Fasugba O, Mitchell BG, Mnatzaganian G, Das A, Collignon P, Gardner A. Five-year antimicrobial resistance patterns of urinary Escherichia coli at an Australian tertiary hospital: time series analyses of prevalence data. PLoS One. 2016;11:e0164306. doi: 10.1371/journal.pone.0164306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.EAU Guidelines Office. Arnhem, The Netherlands: [Last accessed on 2020 June 03]. Available from: http://uroweb.org/guidelines/compilations-of-all-guidelines/ [Google Scholar]
- 8.Kang CI, Kim J, Park DW, Kim BN, Ha US, Lee SJ, et al. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother. 2018;50:67–100. doi: 10.3947/ic.2018.50.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhtar N, Rahman R, Shahin Sultana S, Rezwanur Rahman R. Antimicrobial sensitivity pattern of bacterial pathogens associated with urinary tract infection. Delta Med Coll J. 2017;5:57–62. [Google Scholar]
- 10.Sobel JD, Kaye D. Urinary tract infections. In: Mandell GL, Bennett JE, editors. Principles and practice of infectious diseases. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014. pp. 886–913. [Google Scholar]
- 11.Behzadi P, Behzadi E, Yazdanbod H, Aghapour R, Akbari Cheshmeh M, Salehian Omran D. A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica (Buchar) 2010;5:111–5. [PMC free article] [PubMed] [Google Scholar]
- 12.El-Kersh T, Marie M, Al-Sheikh Y, Al-Kahtani S. Prevalence and risk factors of community-acquired urinary tract infections due to ESBL-producing Gram negative bacteria in an Armed Forces Hospital in Sothern Saudi Arabia. Global Adv Res J Med Med Sci. 2015;4:321–30. [Google Scholar]
- 13.Balkhi B, Mansy W, AlGhadeer S, Alnuaim A, Alshehri A, Somily A. Antimicrobial susceptibility of microorganisms causing urinary tract infections in Saudi Arabia. J Infect Dev Ctries. 2018;12:220–7. doi: 10.3855/jidc.9517. [DOI] [PubMed] [Google Scholar]
- 14.Hossain MA, Mohal S, Islam MS, Yusuf M. Prevalence of ciprofloxacin resistance among gram-negative bacilli isolated from urinary tract infection specimens at a specialist hospital in Riyadh, Saudi Arabia. J Sci Foundation. 2013;11:11–6. [Google Scholar]
- 15.Biswas R, Rabbani R, Ahmed H, Sarker M, Zafrin N, Rahman M. Antibiotic sensitivity pattern of urinary tract infection at a tertiary care hospital. Bangladesh Crit Care J. 2014;2:21–4. [Google Scholar]
- 16.Okopi J, Okojokwu O, Ramyil S, Bakwet P, Okechalu J, Agada G, et al. Bacterial and antibiotic susceptibility pattern of urinary tract infection isolated from asymptomatic and symptomatic diabetic patients attending tertiary hospital in Jos, Nigeria. Trends Med. 2017;17:1–5. [Google Scholar]
- 17.Alrwithey F, Alahmadi A, Alshehri A, Abalhassan I, Alhamad F, Khedher Y, et al. Urinary tract infection in patients with diabetes mellitus. Egypt J Hospital Med. 2017;69:2133–6. [Google Scholar]


