ABSTRACT
Background and Purpose:
There has been a long-standing belief that generic drugs are of lower value in comparison to their branded name counterparts. They are in particular under scrutiny due to their low market price. Even though the reduction in costs is largely based on skipping expensive preclinical studies and clinical trials for generic drugs, the purity and quality of the raw materials in the production of generic drugs is debatable. Thus, the objective of the study was to analyze and assess the quality comparability of generic furosemide 40 mg (FSD) tablets to branded product available in the market.
Materials and Methods:
Quality control tests, in vitro drug release assessments, and thermal analysis investigations for both analog products of FSD were performed. Various physical parameters related to the tablet quality, such as hardness, weight variation, and friability tests, were examined. In vitro drug release behavior evaluations were conducted according to United States Pharmacopeia (USP) specifications and guidelines, whereas thermal analysis was carried out using thermal gravimetric analysis (TGA), and tablets were further evaluated by Fourier transform infrared (FTIR) spectroscopy.
Results:
The results indicated a significant variation between the two products in terms of hardness, weight variation, and friability. This could be correlated to variation appeared in thermal and spectroscopic spectra between the two products using TGA and FTIR. Drug release of FSD was slightly different between both products following incubation in different pH media (1.2, 3.0, and 6.5; 120 min), however, this was in accordance with USP dissolution requirements as < 80% of drug release was obtained within the first 30 min from each product.
Conclusion:
This study is a useful example for the independent investigations using thermal and spectroscopic analysis to confirm potential hidden variations between generic and branded products that could not be obtained by the bioequivalence studies.
KEYWORDS: Dissolution test, Fourier transform infrared spectroscopy, furosemide, quality control test, thermal gravimetric analysis, thermal analysis
INTRODUCTION
It is well known that brand and generic drugs are the two major categories of medicines in pharmaceutical market. Both are similar in terms of drug content but possibly different in their inert ingredients and formulation features, such as color, shape, weight, method of preparations, and manufacturing process.[1,2] In many countries, there is a positive attitude toward generic medicines. Public, pharmacists, and physicians consider generic medicines as a cheaper, safe equivalent alternative compared to expensive branded medicines.[3,4,5,6] To approve the generic drugs for marketing, it should be interchangeable to the branded drug based on the principle known as “essential similarity.” Generic drugs should ensure that they contain the exact amount of similar active ingredient, follow a similar route of administration, and show equivalent therapeutic effectiveness as that of brand drugs. In addition, all pharmacokinetic parameters should be similar to some acceptable extent according to Food and Drug Administration (FDA) requirements, and this can be confirmed by running bioequivalence comparison studies for the active ingredients between brand and generic drugs.[7,8,9,10] Lately, it has been reported that the number of prescriptions for generic over branded drugs has been increased remarkably in clinical practice.[11] At present, nearly 63% of US prescribed drugs are generic drugs due to their lower selling price as compared to brand drugs. Tens of billions of dollars every year could be saved for consumers and purchasers by choosing generic drugs.[12,13,14] Similarly, prescriptions of generic drugs in Europe have been noticeably increased for economic reasons.[15,16] Therefore, most of the healthcare providers and health insurance companies prefer prescribing generic drugs to their clients, although a controversial discussion still remains between patients regarding which one is better to use—the generics or the brands.[2]
Many healthcare providers all over the world have taken a plan to minimize drug costs.[12] However, some studies showed that alteration of excipients in the generic drug formulations may trigger unwanted adverse events or allergic medication reactions.[9] Substitution of some excipients in generic drug contents has been reported to show some side effects or contraindications in certain cases that are not shown with similar branded drug.[16,17] Some excipients might be of high concern with some patients when used as a substitution.[18] For example, croscarmellose sodium in furosemide tablet has been reported to show allergic reaction in some patients.[16] Nevertheless, croscarmellose is considered as an inert pharmaceutical excipient used in tablets as either a glidant, binder, or anti-adherent.[19] Patients with lactose intolerance may experience gastrointestinal problems on taking generic drug with lactose in the formulation.[20] This can affect overall absorption of the drug in gut, hence affecting the systemic drug levels.[21] Other studies showed recurrence of some symptoms with patients on taking generic anti-arrhythmic drugs.[15] All of the aforementioned cases were associated with generic drugs that were bioequivalent to the branded drug, hence this may indicate that bioequivalence studies are merely useful for the determination of active ingredients only.[13,22] As bioavailability and bioequivalence (BABE) studies largely focus on pharmacokinetics, they do not necessarily refer to the quality of formulation.[19] Several factors, such as composition, purity, or alteration in the preparation steps can make changes in the quality of some generic drug formulations.[23] Heritage capsules (250 and 500 mg), the generic antibiotic tetracycline HCL, for example, was recently withdrawn from the US market because it has failed FDA dissolution specification even though it was passing the BABE studies earlier.[24] Similar dissolution failure was also reported for the generic drug Wellbutrin (bupropion hydrochloride) which resulted in their withdrawal from the US market through the FDA.[25]
Thermal analysis is commonly used to define the identity, quality, and purity of excipients. In particular, it is a powerful method to analyze drug–excipient interaction and stability.[26] Alterations in the drug degradation temperature are a sign for potential interactions among the components. Furthermore, thermal analysis can help to select the optimal drug carrier ratio in the formulation.[27] For example, differential scanning calorimetry (DSC) measurements revealed interactions of the drug naproxen with the polymer matrix.[28] Thermal analysis can also be used to investigate the compatibility between drugs and excipients as shown for glibenclamide[29] and ralidoxime chloride.[30] In general, the development of other types of analytical methods such as high-performance liquid chromatography (HPLC) or liquid chromatography mass spectrometry (LC/MS) have been proven to be a challenging and time-consuming method.[31,32,33] Therefore, thermal analysis techniques such as thermal gravimetric analysis (TGA) and DSC as well as Fourier transform infrared (FTIR) spectroscopy can be used as an easy and immediate method to scan the inactive ingredient contents in both drug types.[2,34,35]
The rational of this study was not only to compare the active ingredient but also to include the impact of the excipients of the generic drug knowing that their type, amounts, and purity can have an influences on physicochemical properties of the final drug formulation at different extent.[36] Alterations in stability, for example, might have an impact on the solubility and permeability behavior of the drug.[37] Dissolution failure with generic drug formulations of tetracycline HCL and Wellbutrin XL was reported.[24,25]
In this work, an investigation of in vitro dissolution, quality control, and thermal analysis tests on furosemide 40 mg tablets (brand vs. generic) was assessed. As expected, pH and temperature values tested were similar to the standard process applied in BABE studies, which confirmed that the proposed generic drug product not only meets the requirements but also satisfies requirements toward long-term stability and compatibility between drug and excipients. This comparison study was intended to investigate any possible changes in terms of drug release, physicochemical properties, and thermal parameters in generic drug that was claimed to be bioequivalent to branded drug.
MATERIALS AND METHODS
Materials
Generic drug, furosemide 40 mg tablets, and its reference product, Lasix 40 mg tablets (Hoechst AG, Frankfurt, Germany), were purchased from Al Ain Pharmacy, UAE.
Weight variation
As stated by the United States Pharmacopeia (USP)[38] and European Pharmacopeia,[39] instead of content uniformity, weight variation test applies if the tablet has 25 mg or more active pharmaceutical ingredient (API) or comprises 25% wt/wt. The test was implemented for illustrating the consistency of both dosage units (the declared content of FSD is 40 mg per tablet). Twenty tablets were weighed from each product individually using sensitive digital balance (Shimadzu, Kyoto, Japan), and the average was calculated; deviation of average weight of tablets was confirmed by calculating percentage weight.
Tablet friability
Tablet friability test for each product was performed following general USP method,[40] in which 10 tablets from each product were randomly picked, de-dusted, accurately weighed, loaded to the drum of friabilator TA 220 (Erweka, Heusenstamm, Germany), and tripped off in rotating drum (25rpm; 4 min). Tablets were then removed from friabilator and noticed for cracks or broken edges, de-dusted to remove any free particles on tablets surface, and reweighed for calculating the weight loss percentage.
Resistance to crushing of tablets
Ten tablets were tested from each FSD product to investigate the resistance to crushing using hardness tester TBH-225 TD (Erweka), which simultaneously records the tablet length. Tablets were placed individually on a testing chamber with a clean horizontal surface of apparatus and aligned in such a way that the platens compress parallel to the longest axis of tablets. The mean crushing force is usually measured in Newton (N), and standard deviation was calculated for both products. Moreover, the average of tablet length and standard deviation was calculated.
Chemical content of drug
For chemical content determination, 20 tablets from each product were weighed and triturated to fine powder. An approximate quantity of 0.04g of the powdered material was added into 50 mL distilled water. Sufficient volume of water was then added to obtain 100 mL solution of drug–water mixture. This drug–water mixture solution was filtered, and the final FSD concentration of 100 µg/mL was obtained. The procedure was executed in triplicate for both products.
Dissolution test
In vitro drug release of FSD from each product was evaluated using dissolution apparatus 2-DT-820 (Erweka). Pedal method with 100rpm rotational speed was used, and 900 mL of different pH media, namely pH 1.2, pH 3.0, and pH 6.5, were filled with media temperature set at 37°C ± 0.5°C. Samples (10 mL) were withdrawn from the dissolution vessels and replaced at the following times: 5, 10, 15, 20, 30, 45, 60, 120, and 180 min. Withdrawn samples were then filtered and prepared to analyze for drug content using ultraviolet (UV) spectrophotometer (measuring at 243nm). The dissolution testing was run for six tablets. Besides, various kinetic models were used, namely first-order, zero-order, Higuchi, and Korsmeyer–Peppas model, to find the release kinetics and mechanism of drug release.[41]
Tablet disintegration
Disintegration time for both FSD tablets (brand vs. generic) was determined using pharma test disintegration apparatus PTZ-Auto (Pharma Test Apparatebau, Hainburg, Germany). Distilled water was used as the disintegration media with temperature maintained at 37°C ± 0.5°C, and time duration set for 30 min. Six tablets of each product were examined, and the exact time of each tablet disintegration was noted.
Standard curve and drug content analysis
FSD stock solutions of 0.04% wt/vol were prepared using various pH media, namely pH 1.2, pH 3, and pH 6.5. Various FSD concentrations ranging from 0.4 to 14.6 µg/mL for the calibration curves were prepared to obtain the best fit line and regression equation for further drug analysis.
Sample preparation for TGA
Samples for TGA scans were obtained by scraping the surface of the content in the liquid filled capsules. The samples were placed in a non-hermetic aluminum pans (Perkin Elmer, Beaconsfield, UK), and the lids were crimped into their place to secure the samples within. This pan was selected as it provides satisfactory contact with the samples. Thermal evaluation was conducted on both products. As a reference, an empty covered pan was used during analysis of all samples.
Thermal analysis
Thermal transition behavior of both products was investigated to measure weight difference as function of time and temperature using TGA, TGA-Q500 (Thermal Analysis Instruments, Woodland, CA, USA). In platinum pans, 15 mg of prepared samples were placed and heated up to 600°C at a rate of 5°C/min. At 5 mL/min of flow rate, nitrogen gas was purged, and the scans for each prepared sample were performed in triplicates for authentication of results.
Fourier transform infrared spectroscopy
Study was conducted using FTIR Spectrometer (Nicolet-380, Thermo Scientific, Madison, WA, USA) provisioned with attenuated total reflectance (ATR) attachment. The Thermo Smart Orbit ATR attachment was provided with a diamond crystal having single reflection in a horizontal configuration. Scanning of samples was performed with a 4cm−1 resolution from 500 to 4000cm−1, 64 times. On ATR crystal surface, 0.5g of the sample was placed and at 20°C–22°C, spectra were collected. Background intrusion was terminated, and OMNIC Professional software, version 8.3, was used for analyzing the recorded spectra. An average of spectral data was obtained by collecting four measurements from duplicate samples.[42]
Statistical analysis
One-way analysis of variance (ANOVA) was used for comparing the mean values of determined variables for all obtained data. Differences were assessed and considered statistically significant when P < 0.05. The Minitab program (version 15.0; Minitab Inc., State College, PA, USA) was used for analysis.
RESULTS
Tablets physical quality
The results from tablet friability, variation in weight, and hardness measurement are provided in Table 1. Only the branded product met the pharmacopeial standards for weight variation and friability tests of tablets. Weight variation results showed less than 3.5% for branded tablet with a total weight of 162.7 ± 0.5 mg (1.1% ± 0.4%), but it was above 3.5% for generic tablet with a total weight of 224.1 ± 5.2 mg (5.3 ± 3.8%). This was reflected by the data obtained for percent weight loss as less lost weight for branded tablet is measured (−0.42% ± 0.07%) in comparison to generic tablet (−3.29% ± 0.5%). The mechanical strengths measured for different products were lower for generic tablet (45.6 ± 5.3N) as compared to branded tablet (77.9 ± 8.5N) as specified by friability and hardness test. The difference between forces required for crushing the tablets between highest mean values and lowest mean values for branded and generic tablets was approximately 78 and 46N, respectively. ANOVA revealed significant difference among products with regard to hardness testing, weight variation, and friability (P < 0.05). Tablets from both products had different tablet lengths, as generic tablet (2.89 mm) was 31.8% longer than branded tablets (1.97 mm). Regarding chemical content determination results for tested tablets of both products, content deviation was <5% from the stated amount [Table 1]. Hence, the products pass USP chemical content test, allowing a range from 90% to 110% (USP, 2016).[2] The disintegration timing for both products in 200 mL of water was less than 15 min [Table 1].
Table 1.
Physical properties for branded drug as compared to generic drug
| Measurements | Generic tablet | Brand tablet |
|---|---|---|
| Average Weight (mg) | 224.1 ± 5.2* | 162.7 ± 0.5 |
| Weight Variation Range (%) | 5.3 ± 3.8* | 1.1 ± 0.4 |
| Tablet Friability (% Weight Loss) | -3.29 ± 0.5* | -0.42 ± 0.07 |
| Mean Resistance Force (N) | 45.6 ± 5.3* | 77.9 ± 8.5 |
| Mean Tablet’s Length (mm) | 2.89 ± 0.4* | 1.97 ± 0.9 |
| Chemical contents (%) | 99.5 ± 0.5 | 99.9. ± 0.1 |
| Disintegrating time (min) | 2.15 ± 0.6 | 1.30 ± 0.3 |
* p<0.05 vs Brand tablet
In vitro drug release evaluation
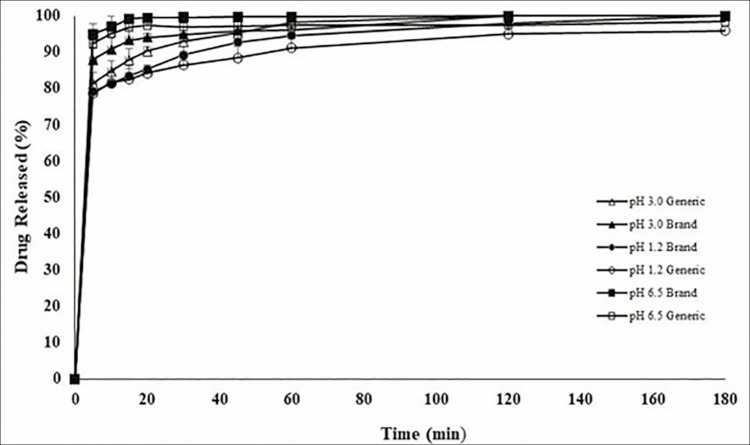
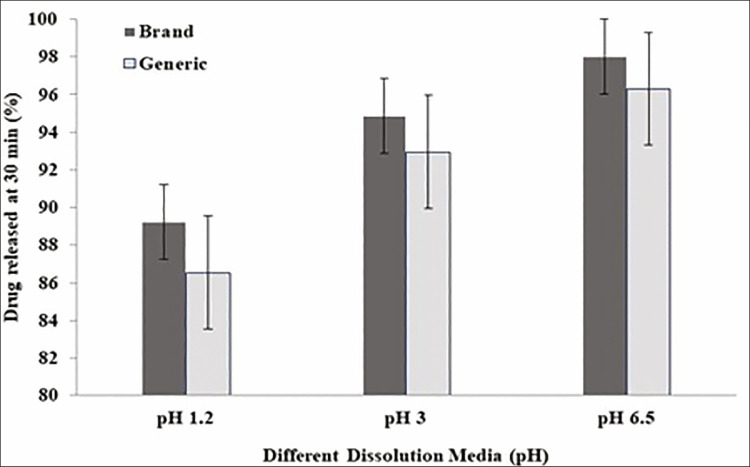
Figure 1 shows FSD release from branded and generic tablets in different pH media (pH 6.5, pH 3.0, and pH 1.2). More than 85% of FSD was released from both products in all examined pH media in less than 30 min [Figure 1]. Hence, the products passed the standard dissolution test as per USP requirements for FSD tablets (i.e., Q < 80% within 60 min).[43] However, drug release from both products was shown to be pH dependent. Drug release in pH 6.5 medium was considered faster than drug release in pH 3 and pH 1.2 media, respectively [Figure 2].
Figure 1.
Dissolution release profile of FSD for generic drug as compared to branded drug
Figure 2.
Dissolution release profile for branded drug as compared to generic drug at 30 min incubated in different pH media
Fourier transform infrared spectroscopy
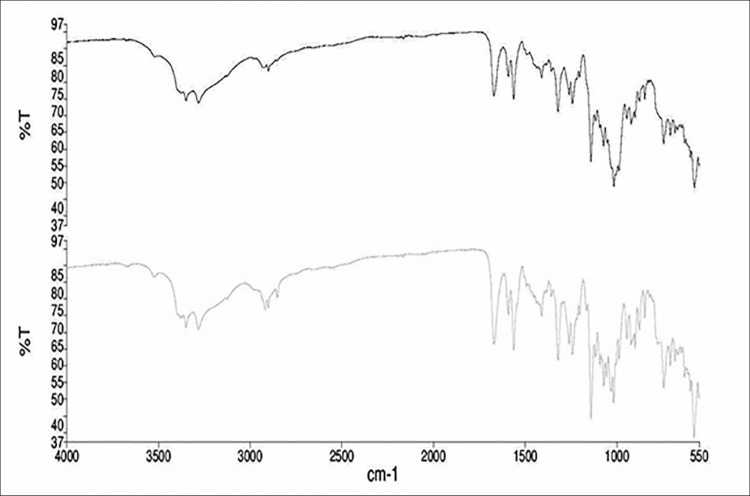
FTIR was performed on generic and branded FSD tablets products. Chemical structure of FSD is shown in Figure 3. The spectra were compared to check the presence of new bands in both samples. Any new band formation or difference can provide probability on the compatibility among the excipients in the formulations of both generic and branded FSD products. Figure 4 shows the comparison of FTIR spectra between generic and branded FSD. Both tablets showed the presence of main drug bands. The bands range of 1390–1290cm−1, 1190–1120cm−1, and 1060–1020cm-1, which fall at the fingerprint region, were originated by S=O asymmetrical stretching, symmetrical stretching, and stretching, respectively. The peak at 1320cm−1 indicated the presence of asymmetrical stretching of S=O group.[44] The presence of primary amine of sulfonamide group bending was also indicated at 1550cm−1. Two bands, namely 3400 and 3300cm−1, represented N–H stretching of primary amine, confirming the presence of primary amine in FSD.[45]
Figure 3.
Chemical structure of furosemide
Figure 4.
Fourier transform infrared analytical spectra of generic and branded furosemide tablets products
Thermal gravimetric analysis
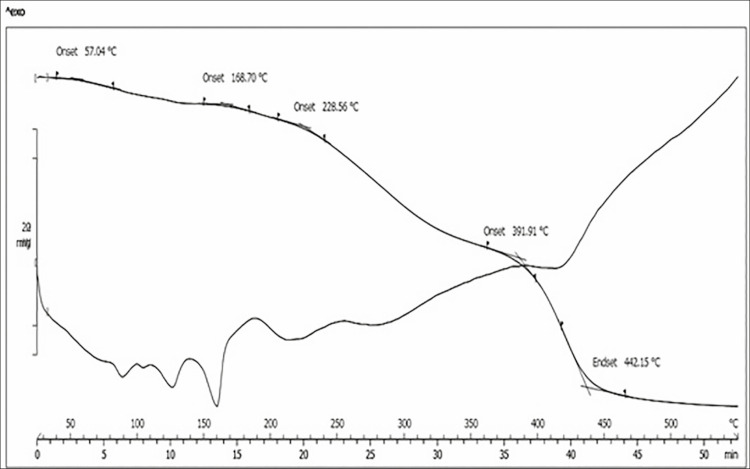
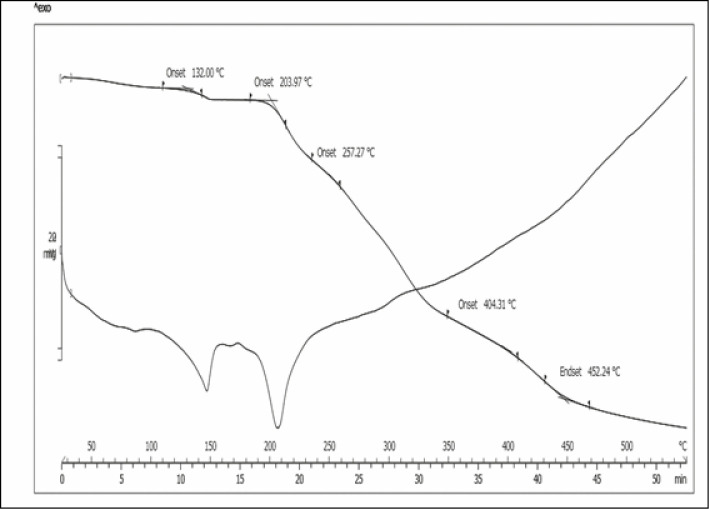
Figure 5 shows the results of combined TGA/Derivative Thermogravimetry (DTG) analysis of FSD from branded tablet product, whereas Figure 6 shows the results of combined TGA/DTG analysis of FSD from generic tablet product. TGA thermogram of FSD was in some way different, FSD from branded tablet showed its initial weight loss onset temperature (Tonset) at 57.04°C, whereas FSD from generic tablet showed its initial weight loss onset temperature (Tonset) at 132°C. Nevertheless, both FSD revealed approximately the same weight loss end set temperature (Tend set) [Figures 5 and 6]. Furthermore, DTG thermogram of FSD also differed in the number of endothermic decomposition peaks and temperature. As shown in Figure 5, FSD from branded tablet showed three endothermic decomposition peaks. The first two were partial decomposition peaks between 80°C–90°C and 120°C–130°C followed by third decomposition peak at 160°C. In contrast, FSD from generic tablet showed only two endothermic decomposition peaks [Figure 6], one partial decomposition peak at 150°C followed by a broad decomposition peak between 200°C and 210°C. Accordingly, these differences in either TGA or DTG analysis might correspond to different additives in the product.
Figure 5.
Thermal gravimetric analysis/DTG analytical spectra of furosemide from branded tablet product
Figure 6.
Thermal gravimetric analysis/DTG analytical spectra of furosemide from generic tablet product
DISCUSSION
Physical qualities, inclusive of tablets uniformity of weight and mechanical strengths, showed sufficiently good results by different FSD products [Table 1]; the latter was revealed by almost no loss from products resulting from friability testing. With advanced machinery and better quality formulation, production of tablets with an intended weight range is achievable, which usually substitutes minimum requirements of the USP.
Both tablets were simultaneously subjected to hardness test. Higher resistance to crushing strength was obtained for the brand drug which had lower tablet weight (162.7 mg) and length (197 mm) in comparison to the generic tablet (224.1 mg and 2.89 mm). Better resistance to crushing can slow down drug release from a solid unit dosage form. On the other hand, tablets with higher stability are preferable to control vibrations and following handling, inclusive of packaging, storage, transportation, and mainly the push of the tablets from its primary packaging; attentiveness is the key to cause no harm to the drug’s dissolution.
The USP permits an extensive range of FSD (active principle) in the tablets with as high as 110% and lower range of 90% in contrast to British Pharmacopeia (BP) where the upper and lower limit are given as 105% and 95%, respectively. Regardless of the differences, in accordance with both pharmacopeial standards, all products passed the test [Table 1]. Considering the percentage of the chemical contents, that is, almost 100% for both generic and brand, comparative dissolution experiments calculating % dissolved drug are the justification for the use of labeled amount of drug instead of chemical content test.
Dissolution testing is considered as an aid to predict the in vivo bioavailability and release behavior of drugs and is being used to show bioequivalence, enabling interchangeability. Generally, FDA examines in vitro dissolution to be better perceptive than in vivo study.[46] FSD is considered under the group IV of biopharmaceutics classification system (BCS), indicating less solubility and permeability.[47,48] FSD is considered under the group IV of biopharmaceutics classification system (BCS), indicating poor solubility and permeability.[47,48] Hence, the drug is not eligible for FDA bio-waiver approval.[35] It is also concluded that current regulatory guidance does not allow bio-waivers for BCS Class IV APIs.[35] This study concealed the tablet dissolution, including only one strength (40 mg) oral formulation, in contrasting pH media. Dissolution of FSD of both products was instant with 85% release of labeled amount in 20–30 min [Figure 3]. Specifications in USP 32 for FSD drug dissolution are not 80% (Q) within 60 min in phosphate buffer in 900 mL.[49] It can be observed in Figure 2 that FSD release showed pH-dependent behavior, as at pH 6.5, the drug was released immediately within 20 min of starting dissolution experiment. However, at pH 3 and pH 1.2, drug fulfills the USP requirements of dissolution but still showed less release than at pH 6.5. So one can say that FSD tablets (40 mg), both branded and generic products passed the dissolution test at all pH used. Drug release followed Hixson–Crowell model, which describes that the drug releases from the tablets by surface degradation via erosion.
Figure 4 shows a typical representative for the FTIR spectral comparison between the generic and branded FSD available products. Spectra of both formulations signified the presence of major FSD bands. The sulfur group gives specificity to the structure of FSD. The symmetrical and asymmetrical stretches accountable for SO2, appeared at the fingerprint region within band horizon of 1190–1120 and 1390–1290cm−1. Another stretching observed at band range 1060–1020cm−1 indicated S=O stretch. Peak at 1320cm−1 specified the asymmetrical S=O group stretching.[44] A bend at 1550cm−1 indicated the primary amine presence in the sulfonamide group. Moreover, peaks at 3300 and 3400cm−1 further confirmed the presence of primary amine in FSD by N–H stretch.[45] Peak that appeared at 1700cm−1 in FSD indicated carbonyl existence in carboxylate group. New bands did not appear or disappear on FTIR spectra for both the sample products. However, visible difference in the intensity between both samples was observed owing to the presence of distinct excipients, signaling the drug–excipients interactions. Both products of FSD contains alike excipients such as talc, lactose, starch, aerosol (also known as colloidal silicon dioxide), and magnesium stearate, besides stearic acid that is present in generic product only. The presence of stearic acid belonged to –CH2 groups, introducing peaks at 2900cm−1, whereas peak at 1700cm−1 attributed to the carbonyl groups stretching.[50] Main peaks for talc were recorded as follows: at 3600–3400cm−1 to represent hydroxyl group vibrations linked to Si (Si–OH) and Mg (Mg–OH),[51] and at 1050 and 680cm−1 to indicate stretching vibrational bands for the siloxane group (Si–O–Si) and Si–O–Mg bond, respectively. Peaks at 1150 and 2900cm−1 presented the C–O and C–H stretch in starch, although a wide band at 3400cm−1 was due to the stretch of –OH group of starch. An evident predictive band observed at 1100cm−1 was an indication of aerosol because of Si–O linkage. Prominent CH2–CH3 trembling peaks were evident for magnesium stearate at 2900–2800cm−1, and in the band region of 1600–1500cm−1, COO– group was revealed by asymmetric stretching bands. Appearance of stretching vibrations of –OH (hydroxyl group) bands at 3600–3400cm−1 represented the presence of lactose, and vibrational peaks at 1200–1100cm−1 were due to C–O–C presence, indicating the glucose and galactose.[52]
On the basis of TGA thermogram of FSD from brand product [Figures 5 and 6], the initial weight loss onset that appeared at 57°C indicated the loss of volatile material. Hence, it was proved by the first decomposition peak that appeared between 80°C–90°C due to the evaporation of water present in the tablet. This is, however, not visible in FSD from generic product, which can be attributed to the presence of stearic acid, which is absent in the brand-name FSD. Containing additional hydrophobic excipient most likely hinders the absorption of water vapor into the tablet. FSD from generic tablets showed a strong endothermic peak that appeared around 210°C, which indicated the melting point of the drug. This peak, however, was not very strong in brand-name drug with a concomitant reduction of peak size suggesting interactions between the drug and the excipients, but not necessarily an incompatibility. Apart from that, different endothermic peaks observed on both products [Figures 5 and 6] are attributable to the excipients melting. However, the peak shape, intensity, and the onset temperature are not identical for both, perhaps due to overlapping of endothermic excipients melting peaks and the dissolution of the excipients.
CONCLUSION
Thermal variations between FSD from branded and generic tablets were determined and supported by variations in quality control tests. This can be attributed either to the ingredient contents, ingredient purity, and quality, or the manufacturing process of the manufacturer. This study is a trendsetting example for the independent investigations using thermal analysis to confirm any hidden variations that could not be shown in the bioequivalence studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We acknowledge Al Ain University for providing support for conducting this study. And we would like to thank everyone who contributed for the completion of this work.
REFERENCES
- 1.Kesselheim AS, Stedman MR, Bubrick EJ, Gagne JJ, Misono AS, Lee JL, et al. Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis. Drugs. 2010;70:605–21. doi: 10.2165/10898530-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arafat M, Ahmed Z, Arafat O. Comparison between generic drugs and brand name drugs from bioequivalence and thermoequivalence prospective. Int J Pharm Pharm Sci. 2017;9:1–4. [Google Scholar]
- 3.El-Dahiyat F, Kayyali R. Evaluating patients’ perceptions regarding generic medicines in Jordan. J Pharm Policy Pract. 2013;6:3. doi: 10.1186/2052-3211-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Dahiyat F, Kayyali R. Community pharmacists’ perceptions towards generic medicines and their opinions on future generic substitution policy implementation: a descriptive study from Jordan. J Generic Med. 2013;10:4. [Google Scholar]
- 5.El-Dahiyat F, Kayyali R, Bidgood P. Physicians’ perception of generic and electronic prescribing: a descriptive study from Jordan. J Pharm Policy Pract. 2014;7:7. doi: 10.1186/2052-3211-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Dahiyat F, Kayyali R, Abbadi I. A comparison of generic and originator brand drug prices. Jor J Pharm Sci. 2001;4:1. [Google Scholar]
- 7.Zheng J, Chow SC, Yuan M. On assessing bioequivalence and interchangeability between generics based on indirect comparisons. Stat Med. 2017;36:2978–93. doi: 10.1002/sim.7326. [DOI] [PubMed] [Google Scholar]
- 8.Benet LZ. Pharmacokinetics/pharmacodynamics of furosemide in man: a review. J Pharmacokinet Biopharm. 1979;7:1–27. doi: 10.1007/BF01059438. [DOI] [PubMed] [Google Scholar]
- 9.Arafat M. Approaches to achieve an oral controlled release drug delivery system using polymers: a recent review. Int J Pharm Pharm Sci. 2015;7:16–21. [Google Scholar]
- 10.Johnston A. Challenges of therapeutic substitution of drugs for economic reasons: focus on CVD prevention. Curr Med Res Opin. 2010;26:871–8. doi: 10.1185/03007990903578462. [DOI] [PubMed] [Google Scholar]
- 11.Bollmann A, Husser D, Cannom DS. Antiarrhythmic drugs in patients with implantable cardioverter-defibrillators. Am J Cardiovasc Drugs. 2005;5:371–8. doi: 10.2165/00129784-200505060-00004. [DOI] [PubMed] [Google Scholar]
- 12.Ferner RE, Lenney W, Marriott JF. Controversy over generic substitution. BMJ. 2010;340:c2548. doi: 10.1136/bmj.c2548. [DOI] [PubMed] [Google Scholar]
- 13.Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514–26. doi: 10.1001/jama.2008.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talati R, Scholle JM, Phung OP, Baker EL, Baker WL, Ashaye A, et al. Efficacy and safety of innovator versus generic drugs in patients with epilepsy: a systematic review. Pharmacotherapy. 2012;32:314–22. doi: 10.1002/j.1875-9114.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 15.Crawford P, Feely M, Guberman A, Kramer G. Are there potential problems with generic substitution of antiepileptic drugs? A review of issues. Seizure. 2006;15:165–76. doi: 10.1016/j.seizure.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Dunne S, Shannon B, Dunne C, Cullen W. A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol Toxicol. 2013;14:1. doi: 10.1186/2050-6511-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paveliu MS, Bengea S, Paveliu FS. Generic substitution issues: brand-generic substitution, generic-generic substitution, and generic substitution of narrow therapeutic index (NTI)/critical dose drugs. Maedica (Buchar) 2011;6:52–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston A, Asmar R, Dahlöf B, Hill K, Jones DA, Jordan J, et al. Generic and therapeutic substitution: a viewpoint on achieving best practice in Europe. Br J Clin Pharmacol. 2011;72:727–30. doi: 10.1111/j.1365-2125.2011.03987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mumoli N, Cei M, Luschi R, Carmignani G, Camaiti A. Allergic reaction to croscarmellose sodium used as excipient of a generic drug. QJM. 2011;104:709–10. doi: 10.1093/qjmed/hcq175. [DOI] [PubMed] [Google Scholar]
- 20.Zarbock SD, Magnuson B, Hoskins L, Record KE, Smith KM. Lactose: the hidden culprit in medication intolerance? Orthopedics. 2007;30:615–7. doi: 10.3928/01477447-20070801-10. [DOI] [PubMed] [Google Scholar]
- 21.Davit BM, Nwakama PE, Buehler GJ, Conner DP, Haidar SH, Patel DT, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583–97. doi: 10.1345/aph.1M141. [DOI] [PubMed] [Google Scholar]
- 22.Davit B, Braddy AC, Conner DP, Yu LX. International guidelines for bioequivalence of systemically available orally administered generic drug products: a survey of similarities and differences. AAPS J. 2013;15:974–90. doi: 10.1208/s12248-013-9499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallelli L, Palleria C, De Vuono A, Mumoli L, Vasapollo P, Piro B, et al. Safety and efficacy of generic drugs with respect to brand formulation. J Pharmacol Pharmacother. 2013;4:S110–4. doi: 10.4103/0976-500X.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avet Pharmaceuticals Inc. Avet Pharmaceuticals Inc. issues voluntary nationwide recall of tetracycline HCl capsules USP, 250 mg and 500 mg due to failed dissolution specifications. USA: FDA, U.S. Food & Drug Administration; 2020. [Google Scholar]
- 25.Storrs C. New York City, USA: MedicineNet, Inc., Health Day Reporter; 2012. FDA pulls one generic form of Wellbutrin off the market. [Google Scholar]
- 26.Singh S, Wu C, Williams PT. Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J Anal Appl Pyrolysis. 2012;94:99–107. [Google Scholar]
- 27.Wendlandt WW, George TD. The thermal decomposition of metal complexes-I: thermogravimetric and differential thermal analysis. J Inorg Nucl Chem. 1961;21:69–76. [Google Scholar]
- 28.Dordunoo SK, Ford JL, Rubinstein MH. Solidification studies of polyethylene glycols, gelucire 44/14 or their dispersions with triamterene or temazepam. J Pharm Pharmacol. 1996;48:782–9. doi: 10.1111/j.2042-7158.1996.tb03974.x. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira GGG, Ferraz HG, Matos JSR. Thermoanalytical study of glibenclamide and excipients. J Therm Anal Calorim. 2005;79:267–70. [Google Scholar]
- 30.Gutch PK, Jitendra S, Alankar S, Anurekha J, Ganesan K. Thermal analysis of the interaction between 2-PAM chloride and various excipients in some binary mixtures by TGA and DSC. J Therm Anal Calorim. 2013;111:1953–8. [Google Scholar]
- 31.Arafat M, Golocorbin KS, Mikov M. The measurement of cefotaxime sodium in rat plasma after oral administration: a sensitive HPLCUV method. Int J Pharm Pharm Sci. 2015;7:343–6. [Google Scholar]
- 32.Arafat M. Simple HPLC validated method for determination of diltiazem hydrochloride in human. Int J Pharm Pharm Sci. 2014;6:213–6. [Google Scholar]
- 33.Arafat M, Zahaa A, Mikov M. Determination of nifedipine in rat plasma using HPLC-UV detector: a simple method for pharmacokinetics and oral bioavailability studies. Int J Pharm Pharm Sci. 2016;8:98–102. [Google Scholar]
- 34.Bostanudin MF, Arafat M, Sarfraz M, Górecki DC, Barbu E. Butylglyceryl pectin nanoparticles: synthesis, formulation and characterization. Polymers. 2019;11:789. doi: 10.3390/polym11050789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turk MJ, Ansari AS, Alston WB, Gahn GS, Frimer AA. Evaluation of the thermal oxidative stability of polyimides via TGA techniques. Inc J Polym Sci A: Polym Chem. 1999;37:3943–56. [Google Scholar]
- 36.Al Hanbali OA, Khan HMS, Sarfraz M, Arafat M, Ijaz S, Hameed A. Transdermal patches: design and current approaches to painless drug delivery. Acta Pharm. 2019;69:197–215. doi: 10.2478/acph-2019-0016. [DOI] [PubMed] [Google Scholar]
- 37.Arafat M. The effect of intestinal bile on the stability of lipid-based vesicular system used as oral drug carriers. Glob Drugs Therap. 2016;2:1–2. [Google Scholar]
- 38.United States Pharmacopeial Convention. USP 39-NF 34. Vol. 1. Rockville, MD: United States Pharmacopeial Convention; 2016. Uniformity of dosage unit; p. 736. [Google Scholar]
- 39.European Directorate for the Quality of Medicines & Health Care. 2.9.40. European Pharmacopoeia. 9th ed. Strasbourg, France: Council of Europe; 2017. Uniformity of dosage units; p. 372. [Google Scholar]
- 40.United States Pharmacopeial Convention. USP 39-NF 34. Vol. 1. Rockville, MD: United States Pharmacopeial Convention; 2016. Tablet friability; p. 1609. [Google Scholar]
- 41.Al-Hanbali OA, Hamed R, Arafat M, Bakkour Y, Al-Matubsi H, Mansour R, et al. Formulation and evaluation of diclofenac controlled release matrix tablets made of HPMC and poloxamer 188 polymer: an assessment on mechanism of drug release. Pak J Pharm Sci. 2018;31:345–51. [PubMed] [Google Scholar]
- 42.Kamal T, Sarfraz M, Arafat M, Mikov M, Rahman N. Cross-linked guar gum and sodium borate based microspheres as colon-targeted anticancer drug delivery systems for 5-fluorouracil. Pak J Pharm Sci. 2017;30:2329–36. [PubMed] [Google Scholar]
- 43.United States Pharmacopeial Convention. USP39-NF34. Vol. 2. Rockville, MD: United States Pharmacopeial Convention; 2016. Monograph for amoxicillin and clavulanate potassium tablets; p. 2526. [Google Scholar]
- 44.Pereira RN, Valente BR, Cruz AP, Foppa T, Murakami FS, Silva MAS. Thermoanalytical study of atenolol and commercial tablets. Lat Am J Pharm. 2007;26:382–6. [Google Scholar]
- 45.The Merck index; an encyclopedia of chemicals, drugs, and biological. 13 ed. Readington, New Jersey: Whitehouse Station; 2001. p. 147. [Google Scholar]
- 46.Uppoor VR. Regulatory perspectives on in vitro (dissolution)/in vivo (bioavailability) correlations. J Control Release. 2001;72:127–32. doi: 10.1016/s0168-3659(01)00268-1. [DOI] [PubMed] [Google Scholar]
- 47.Granero GE, Longhi MR, Mora MJ, Junginger HE, Midha KK, Shah VP, et al. Biowaiver monographs for immediate release solid oral dosage forms: furosemide. J Pharm Sci. 2010;99:2544–56. doi: 10.1002/jps.22030. [DOI] [PubMed] [Google Scholar]
- 48.Khalid N, Sarfraz M, Arafat M, Akhtar M, Löbenberg R, Ur Rehman N. Nano-sized droplets of self-emulsifying system for enhancing oral bioavailability of chemotherapeutic agent VP-16 in rats: a nano lipid carrier for BCS class IV drugs. J Pharm Pharm Sci. 2018;21:398–408. doi: 10.18433/jpps30097. [DOI] [PubMed] [Google Scholar]
- 49.Becker C, Dressman JB, Junginger HE, Kopp S, Midha KK, Shah VP, et al. Biowaiver monographs for immediate release solid oral dosage forms: rifampicin. J Pharm Sci. 2009;98:2252–67. doi: 10.1002/jps.21624. [DOI] [PubMed] [Google Scholar]
- 50.Shin S, Oh I, Lee Y, Choi H, Choi J. Enhanced dissolution of furosemide by coprecipitating or cogrinding with crospovidone. Int J Pharm. 1998;175:17–24. [Google Scholar]
- 51.da Silva DC, Semaan FS, Novák C, Cavalheiro ETG. Thermal behavior of furosemide. J Thermal Analysis Calorimetry. 2011;111:1933–7. [Google Scholar]
- 52.Cides L, Araújo A, Santos-Filho M, Matos J. Thermal behaviour, compatibility study and decomposition kinetics of glimepiride under isothermal and non-isothermal conditions. J Thermal Analysis Calorimetry. 2006;84:441–5. [Google Scholar]