ABSTRACT
Introduction:
Pegagan is a traditional medicinal plant with three major bioactive properties, triterpenoid, steroids, and saponin. It has the properties of antioxidant, antistress, and wound healing. Pegagan extract is prepared in self-nanoemulsifying drug delivery systems (SNEDDS) to overcome the problem of low water-solubility level.
Objectives:
This study aimed to observe the effect of pegagan ethanolic extract SNEDDS on the development of zebrafish embryos.
Materials and Methods:
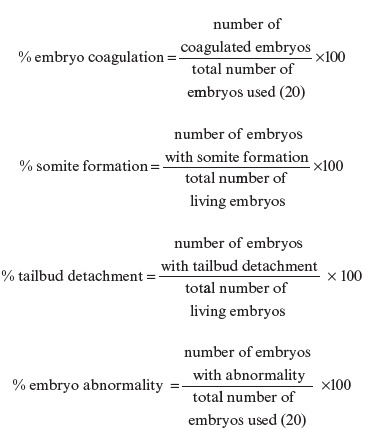
This study used 12 sets of zebrafish embryos presented in five sets of extract SNEDDS with different concentrations, that is, 20, 10, 5, 2.5, and 1.25 μg, five sets of SNEDDS without extract with different concentrations, that is, 20, 10, 5, 2.5, and 1.25 μg, a set of positive control (3.4-DCA 4 mg/L) with one control set (diluted with water), and a negative control (SNEDDS without extract). The procedure was conducted for 96 h with observations every 24 h. The parameters observed were embryonic coagulation, formation of somites, detachment of tail bud from the yolk, and abnormality of embryo
Results:
The results showed that in 96 h the 20ppm concentration caused 100% mortality. Embryo abnormality appeared as coagulation of embryo, somite malformation, and abnormal tail.
Discussion:
There is a correlation between the concentration of SNEDDS and the incidence of embryo coagulation. The malformation in the group of pegagan extract SNEDDS is characterized by cardiac edema, somite malformation, and abnormal tail.
Conclusion:
Pegagan ethanolic extract SNEDDS of 20ppm can inhibit the development of zebrafish embryos.
KEYWORDS: Centella asiatica L., coagulation, SNEDDS, zebrafish
INTRODUCTION
Pegagan is a herb widely studied and reported to have antihyperglycemic effects,[1,2] anti-inflammatory,[3] as well as neuroprotective, hepatoprotective, and cardioprotective effects.[3,4,5] Pegagan herb is also reported to contain bioactive compounds of triterpenoids, such as asiatic acid, madecassic acid, madecassoside, and asiaticoside on which a lot of research have been conducted to assess their anti-inflammatory activity and protective effects.[6,7] One of the common problems with herbal medicine is the low bioavailability, which can be resolved, among others, by formulating it into a nanopreparation.
The method to prepare nanoemulsion rapidly and easily by formulating an isotropic mixture of oil, surfactants, and cosurfactants mixed with water is known as self-nanoemulsifying drug delivery systems (SNEDDS).[8] The size of nanoemulsions formed ranges between 20 and 200nm.[9] Pegagan extract is prepared in SNEDDS to resolve the problem of low solubility in water. Among the benefits of SNEDDS are accelerating the dissolution time of lipophilic compounds, reducing the first-pass metabolism in the liver, and improving absorption.[10] To improve the SNEDDS of pegagan extract, a toxicity study, including screening of toxicity in zebrafish embryonic development, is required. Zebrafish have transparent embryos with rapid embryogenesis processes, which enable them to be used as a research model of chemical toxicity tests. Therefore, this study aimed to determine the effect of pegagan extract SNEDDS (PES) on the development of zebrafish embryos.
MATERIALS AND METHODS
Ethical clearance
The ethical clearance of this study has been obtained from the Medical and Health Research Ethics Committee of the Faculty of Medicine, Universitas Islam Indonesia (UII) No. 38/Ka.Kom.Et/70/KE/V/2019.
Materials
The material used was pegagan leaves including the blade (lamina) and stalk (petiole) obtained from Kalibawang, Kulonprogo. Zebrafish and their embryos were selected as the experimental animal. They were identified at LIPI Bogor (B-3853/IPH.1./KS.02.03 /XI/2017) with the inclusion criteria of 4–6 months of age and physically healthy zebrafish. Meanwhile, the zebrafish embryos should be fertile and aged younger than 6 h after fertilization. Other materials consisted of 96% ethanol, capryol 90, tween 20, and PEG 400 obtained from the Pharmaceutical Technology Laboratory of UII, as well as 3,4-DCA (dichloroaniline) from the Preclinical Test Laboratory of UII.
Extraction process
The collected pegagan leaves were dried in an oven at 40oC to reduce the water content and pulverized to obtain a fine powder. Centella asiatica ethanol extract was obtained by maceration using 96% ethanol solvent for 3 days with two times re-maceration. The maceration process is based on previous research with a slight modification in the maceration period.[11] This modification is based on research that pharmacological activity is influenced by a time-dependent maceration period.[12]
The macerated powder was filtered using a Buchner funnel to obtain filtrate that was then concentrated using a rotary evaporator at 40oC to produce the viscous extract.
SNEDDS PREPARATION: PROCESS AND EVALUATION
Formulation of pegagan extract SNEDDS
The selection of oils, surfactants, and cosurfactants has been conducted based on the solubility test on the previous study. The solubility test was performed on pegagan ethanolic extract in various types of oils (castor oil, sunflower oil, capryol, isopropyl myristate, oleic acid) surfactants (chremophor RH 40, labrafil M1944, labrasol, and tween 20), and cosurfactants (tween 80, PEG 400, and propylene glycol). The pseudoternary phase diagram of the oil, surfactant, and cosurfactant mixture without active ingredients were plotted, and each of them represents a self-nanoemulsion region. The oil, surfactant, and cosurfactant mixture were prepared with various concentrations of each component 10%–50% of oil, 20%–80% of surfactant, and 10%–30% of cosurfactant. All of the formulations were investigated by nanoemulsion formation after 100 dilution times; the visual observation was conducted to accessed nanoemulsion separation. Transmittant of each formula was measured at 630nm using a spectrophotometer (Shimadzu UV 1800, Japan). The formula in which transmittance more than 90% was accepted and carried out in this study.[13]
Based on the previous study, oil, surfactant, and cosurfactant with ratio 1:6:3 optimal formula was chosen in the study. As much as 1.6 mg of pegagan ethanolic extract was added to 1-mL capryol 90, 6-mL tween 20, and 3-mL PEG 400 followed by homogenization in an ultrasonicator. The SNEDDS was evaluated for the particle size using particle size analyzer (PSA), and polydispersity index (PDI).
Zebrafish embryos: preparation and selection
The breeding process was conducted using male zebrafish and female zebrafish at a ratio of 1:2 (male:female). The fish were placed in an aquarium with buffered water (pH 7.3) at 28oC in a cycle of 14 h:10 h (daylight:dark) and fed twice daily. The first stage began with the daylight cycle, in which male and female zebrafish were separated in different aquariums, and preceding the dark cycle, the male and female zebrafish were united in one larger aquarium and then left for 10 h.[14]
The medium for rearing zebrafish was RO (reverse osmosis) water added with ocean salt (0.1%) to fulfill the requirement of mineral contents, hardness, and acidity (pH). The fish were kept in a glass aquarium completed with an aerator, water filter, and digital thermometer to monitor the water temperature of 26ºC ± 1. The parameters to assess the water quality in zebrafish rearing included conductivity, acidity (pH), temperature, hardness, nitrate, nitrite, and oxygen.[15]
The lighting for zebrafish rearing was regulated using a photoperiod system with 14-h daylight and 10-h dark period for fish activities and egg laying in the daylight time and mating in the dark time. Separation was done near the dark time, and the eggs were observed in the next daylight. Feeding was given twice a day during the daylight and dark hours, and the feed for adult zebrafish was Tetramin flakes and hatched Artemia eggs. The embryo medium was E3 solution[16] dissolved in RO water and added with methylene blue.
Observation of zebrafish embryos
A total of 12 microwell plates each having 24 wells were prepared for 12 treatment groups (positive control 4 mg/L of 3,4-DCA, negative control dilution water, SNEDDS of pegagan extract in five concentrations, and SNEDDS without extract in five concentrations). During the period of embryo exposure, the temperature of the test solution was maintained at 26 ± 1ºC and the pH ranged from 6.8 to 8.5.
Data analysis
RESULTS
Formulation of pegagan extract SNEDDS
Characterization of nanoemulsion droplets was carried out using PSA to determine the particle size and PDI. The size distribution or PDI is defined as the standard deviation of the average particle size used as a parameter of uniformity and reliability of the nanoemulsion preparation method. The emulsified SNEDDS has a particle size of 101.27 ± 3.12nm, and PDI 0.257±0.07.
Effects of pegagan extract SNEDDS on zebrafish embryonic development
This study involved eight experimental groups, each with one positive control group, one negative control group using aquarium water, one solvent control using SNEDDS without extract, and five groups of test solution containing PES. The selected embryos were treated for 96 h and observation was conducted every 24 h.
DISCUSSION
The research began with the optimization of PES formulation followed by the evaluation of some supporting parameters, including the particle size and PDI. The physical stability studies of PES show that the SNEDDS preparation is stable as no separation, precipitation, creaming, or cracking are found. Our data show that the PDI value in PES is 0.257 ± 0.07. The PDI values of 0.3 and below are considered to be acceptable and indicated a homogenous of phospholipid vesicles. The results show that the PES nanoemulsion droplet size has been homogeneously distributed (monodisperse) and to be accepted as nanoemulsion criteria.[17]
This study examines the differences in the effects on embryos between SNEDDS of pegagan extract and SNEDDS without extract. Table 1 shows that the highest percentage of embryo coagulation is observed at the concentration of 20ppm, and this percentage continues to decrease at up to the lowest concentration of 1.25ppm. Therefore, higher concentration leads to more coagulation probably due to a compound in the ethanolic extract of pegagan leaves that can cause embryo coagulation. The positive control only has 15% coagulation until 96th h (day 4), whereas according to Organization for Economic Co-operation and Development (OECD), it should show a minimum of 30% mortality. Although the coagulation is only 15%, all the embryos in the positive control group experience abnormality [Table 2], and in fact, all the embryos die on day 5.
Table 1.
Percentage of coagulation embryo, somite formation, and detachment of tail bud
| Group | Percentage of coagulation |
Percentage of somite formation |
Percentage of detachment of tail bud |
||||
|---|---|---|---|---|---|---|---|
| 24 h | 96 h | 24 h | 96 h | 24 h | 96 h | ||
| Positive control | 5% | 15% | 100% | 100% | 80% | 100% | |
| Negative control | 5% | 5% | 100% | 100% | 100% | 100% | |
| SNEDDs of extract | 20 µg/mL | 100% | 100% | – | – | – | – |
| 10 µg/mL | 35% | 40% | 100% | 100% | 100% | 100% | |
| 5 µg/mL | 5% | 5% | 100% | 100% | 100% | 100% | |
| 2,5 µg/mL | 5% | 5% | 100% | 100% | 100% | 100% | |
| 1,25 µg/mL | 0% | 0% | 100% | 100% | 100% | 100% | |
| SNEDDs without extract | 20 µg/mL | 55% | 95% | 100% | 100% | 100% | 100% |
| 10 µg/mL | 45% | 75% | 100% | 100% | 100% | 100% | |
| 5 µg/mL | 15% | 15% | 100% | 100% | 100% | 100% | |
| 2,5 µg/mL | 5% | 5% | 100% | 100% | 100% | 100% | |
| 1,25 µg/mL | 10% | 10% | 100% | 100% | 100% | 100% | |
Table 2.
Percentage of abnormality of zebrafish embryo (n = 20)
| Groups | Variation of treatment | Abnormal embryo |
Coagulation | Normal embryo | |
|---|---|---|---|---|---|
| Death | Alive | ||||
| Control groups | Positive control | 10% | 85% | 5% | 0% |
| Cardio edema | 10% | 85% | – | – | |
| Somite malformation | 0% | 55% | – | – | |
| Tail abnormal | 0% | 0% | – | – | |
| Others | 0% | 0% | – | – | |
| Negative control | 0% | 0% | 5% | 95% | |
| SNEDDs extract of Centella asiatica | 20 µg/mL | 0% | 0% | 100% | 0% |
| 10 µg/mL | 5% | 20% | 35% | 40% | |
| Cardio edema | 5% | 10% | – | – | |
| Somite malformation | 0% | 20% | – | – | |
| Tail abnormal | 0% | 10% | – | – | |
| Others | 0% | 0% | – | – | |
| 5 µg/mL | 0% | 0% | 5% | 95% | |
| 2,5 µg/mL | 0% | 0% | 5% | 95% | |
| 1,25 µg/mL | 0% | 0% | 0% | 100% | |
| SNEDDs without extract | 20 µg/mL | 30% | 0% | 65% | 5% |
| Cardio edema | 0% | 0% | – | – | |
| Somite malformation | 0% | 0% | – | – | |
| Tail abnormal | 0% | 0% | – | – | |
| Others | 30% | 0% | – | – | |
| 10 µg/mL | 30% | 15% | 45% | 10% | |
| Cardio edema | 10% | 5% | – | – | |
| Somite malformation | 0% | 15% | – | – | |
| Tail abnormal | 0% | 0% | – | – | |
| Others | 0% | 0% | – | – | |
| 5 µg/mL | 0% | 0% | 15% | 85% | |
| 2,5 µg/mL | 0% | 0% | 5% | 95% | |
| 1,25 µg/mL | 0% | 0% | 10% | 90% | |
Somite is a tissue that will differentiate into the spine and skeletal muscle; therefore, a problem in somite development can lead to abnormalities of the body axis and can inhibit the hatching process. Somites are formed after 24 h of fertilization, and Table 1 shows that nothing inhibits the growth of somites in the living embryos. All somites develop at 24th h, but some of them experience malformation [Table 2]. The most frequent malformation occurs in the positive control group (DCA) although the somite development remains unobstructed.
Tailbud detachment from the yolk indicates that an embryo can develop appropriately and affect the egg hatching. Table 1 shows that in the positive control group there are only 80% detachment of the tail bud after 24 h of exposure. Meanwhile, in the group of PES and group of SNEDDS without extract, all the living embryos experience tailbud detachment from the yolk. However, tail abnormalities occur in the group of 10 µg/mL dose of PES although in a relatively small number.
The malformation in the group of PES is characterized by cardiac edema, somite malformation, and abnormal tail. The results show that one of the components of SNEDDS can apparently cause toxicity to zebrafish embryos. SNEDDS is an alternative nanoparticle preparation that has been widely studied and proven to increase the effectiveness of research on natural ingredients.[18,19] One of the components suspected of having potential toxicity is capryol.[20] In fact, a nanocompound with a size <200 µg is able to penetrate into the chorion, thus affecting the growth of zebrafish embryos.
CONCLUSION
Pegagan ethanolic extract SNEDDS of 20ppm can inhibit the development of zebrafish embryos.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to acknowledge the support from the Department of Pharmacy UII and the Ministry of Research, Technology, and Higher Education of the Republic of Indonesia and for the research facilities provided through the Excellent Basic Research Grant for Higher Education 2020 No.1627.1/LL5/PG/2020.
REFERENCES
- 1.Maulidiani , Abas F, Khatib A, Perumal V, Suppaiah V, Ismail A, et al. Metabolic alteration in obese diabetes rats upon treatment with Centella asiatica extract. J Ethnopharmacol. 2016;180:60–9. doi: 10.1016/j.jep.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Palupi FD, Wasita B, Nuhriawangsa AMP. Pengaruh Dosis dan Lama Pemberian Ekstrak Etanol Pegagan (Centella Asiatica) Terhadap Kadar Gula Darah dan Derajat Insulitis Tikus Model Diabetes Melitus Tipe 2. MGMI. 2019;10:111–24. [Google Scholar]
- 3.Orhan IE. Centella asiatica (L.) urban: from traditional medicine to modern medicine with neuroprotective potential. Evidence-based Complement Altern Med. 2012;12:1–7. doi: 10.1155/2012/946259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdou HM, Mohamed NA. Centella asiatica ameliorates diabetic complications and oxidative stress in streptozotocin-induced diabetes mellitus in male rats. Int J Pharm Sci Rev Res. 2016;36:61–70. [Google Scholar]
- 5.Choi M, Zheng H, Kim JM, Lee KW, Park YH, Lee DH. Protective effects of Centella asiatica leaf extract on dimethylnitrosamine-induced liver injury in rats. Mol Med Rep. 2016;14:4521–8. doi: 10.3892/mmr.2016.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huo L, Shi W, Chong L, Wang J, Zhang K, Li Y. Asiatic acid inhibits left ventricular remodeling and improves cardiac function in a rat model of myocardial infarction. Exp Ther Med. 2016;11:57–64. doi: 10.3892/etm.2015.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Ma J, Feng R, Wang Z. Centella asiatica inhibits renal interstitial fibrosis by regulating Smad3 and Smad7 expression in the TGFβ signaling pathway. Int J Clin Exp Pathol. 2018;11:1009–17. [PMC free article] [PubMed] [Google Scholar]
- 8.Patel J, Kevin G, Patel A, Raval M, Sheth N. Design and development of a self-nanoemusifying drug delivery system for telmisartan for oral drug delivery. Int J Pharm Investig. 2011;1:112–8. doi: 10.4103/2230-973X.82431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutiérrez JM, González C, Maestro A, Solè I, Pey CM, Nolla J. Nano-emulsions: new applications and optimization of their preparation. Curr Opin Colloid Interface Sci. 2008;13:245–51. [Google Scholar]
- 10.Kyatanwar AU, Jadhav KR, Kadam VJ. Self micro-emulsifying drug delivery system (SMEDDS): review. J Pharm Res. 2010;3:75–83. [Google Scholar]
- 11.Azis HA, Taher M, Ahmed AS, Sulaiman WMAW, Susanti D, Chowdhury SR, et al. In vitro and in vivo wound healing studies of methanolic fraction of Centella asiatica extract. South Afr J Bot. 2017;108:163–74. [Google Scholar]
- 12.Yeo YL, Chia YY, Lee CH, Sow HS, Yap WS. Effectiveness of maceration periods with different extraction solvents on in-vitro antimicrobial activity from fruit of Momordica charantia L. J Appl Pharm Sci. 2014;4:016–23. [Google Scholar]
- 13.Syukri Y, Martien R, Lukitaningsih E, Nugroho AE. Novel self-nano emulsifying drug delivery system (SNEDDS) of andrographolide isolated from Andrographis paniculata nees: characterization, in-vitro and in-vivo assessment. J Drug Delivery Sci Technol. 2018;47:514–20. [Google Scholar]
- 14.Organization for Economic Co-operation and Development (OECD) Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals, Section 2. Paris: OECD Publishing; 2013. https://doi.org/10.1787/9789264203709-en. [Google Scholar]
- 15.Avdesh A, Chen M, Martin-Iverson MT, Mondal A, Ong D, Rainey-Smith S, et al. Regular care and maintenance of a Zebrafish (Danio rerio) laboratory: an introduction. J Vis Exp. 2012;69:1–8. doi: 10.3791/4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman CK, White RM, Zon L. Chemical genetic screening in the zebrafish embryo. Nat Protoc. 2009;4(10):32. doi: 10.1038/nprot.2009.144. 10.1038/nprot.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danaei M, Dehghankhold M, Ataei S, Davarani FH, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayati F, Lutfi C, Darma DD. Antihyperglycemia activity of self-nano emulsifying drug-delivery systems (SNEDDS) of Ipomoea reptans, Poir leaf ethanolic extract in Zebrafish (Danio rerio) AIP Conf Proc. 2018;2026:020026. https://doi.org/10.1063/1.5064986. [Google Scholar]
- 19.Jumaryatno P, Chabib L, Hayati F, Awaluddin R. Stability study of Ipomoea reptans extract self-nanoemulsifying drug delivery system (SNEDDS) as anti-diabetic therapy. J Appl Pharma Sci. 2018;8:011–4. [Google Scholar]
- 20.Ujhelyi Z, Fenyvesi F, Váradi J, Fehér P, Kiss T, Veszelka S, et al. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur J Pharm Sci. 2012;47:564–73. doi: 10.1016/j.ejps.2012.07.005. [DOI] [PubMed] [Google Scholar]


