Abstract
Vaccination-associated adenopathy is a frequent imaging finding after administration of COVID-19 vaccines that may lead to a diagnostic conundrum in patients with manifest or suspected cancer, in whom it may be indistinguishable from malignant nodal involvement. To help the medical community address this concern in the absence of studies and evidence-based guidelines, this paper offers recommendations developed by a multidisciplinary panel of experts from three of the leading tertiary care cancer centers in the United States. According to these recommendations, some routine imaging examinations, such as those for screening, should be scheduled before or at least 6 weeks after the final vaccination dose to allow for any reactive adenopathy to resolve. However, there should be no delay of other clinically indicated imaging (e.g., for acute symptoms, short-interval treatment monitoring, urgent treatment planning or complications) due to prior vaccination. The vaccine should be administered on the side contralateral to the primary or suspected cancer, and both doses should be administered in the same arm. Vaccination information (date(s) administered, injection site(s), laterality, and type of vaccine) should be included in every pre-imaging patient questionnaire, and this information should be made readily available to interpreting radiologists. Clear and effective communication between patients, radiologists, referring physician teams and the general public should be considered of the highest priority when managing adenopathy in the setting of COVID-19 vaccination.
Summary
COVID-19-vaccination–related adenopathy is a frequent imaging finding that may lead to a diagnostic conundrum in patients with manifest or suspected cancer, in whom it may be indistinguishable from malignant nodal involvement. This special report offers recommendations developed by a multidisciplinary panel of experts from three of the leading tertiary care cancer centers in the United States.
Introduction
The race to develop and execute effective COVID-19 vaccination programs is in full force, as the world grapples with staggering numbers of COVID-19 infections (1). In the U.S., two COVID-19 vaccines (Moderna and Pfizer/BioNTech), both belonging to a novel class of mRNA vaccines which stimulate immune cells to produce antigens, have received emergency use authorization (EUA) from the FDA. Several conventional vaccines using attenuated viral vectors to elicit an immune response are either in development or already approved and used in other countries. These include vaccines developed by Oxford-AstraZeneca, Johnson & Johnson, Sputnik V, and the CanSinoBIO-Beijing Institute of Biotechnology.
The overwhelmingly positive protective effect of vaccines is sometimes accompanied by unintended side effects, most of which are transient and minor. These include regional adenopathy, encountered in up to a third of cases with some conventional vaccines (2). Lymph node enlargement following vaccination is related to locally activated antigens that accumulate at the injection site and later migrate to the draining nodes (3). As COVID-19 vaccines are administered intramuscularly into the deltoid muscle, vaccination-associated adenopathy typically occurs in the axilla and supraclavicular region (4,5). Recognition of this association is crucial in patients with cancer, where it can lead to under- or overdiagnosis and under- or overtreatment as well as heightened anxiety. This is especially relevant for patients with certain cancers such as breast cancer, head and neck cancers, lymphoma, and melanoma of the back and upper extremities, which have a predilection for metastasizing to these lymph node stations. Multiple reports have also found increased nodal and splenic metabolic activity following influenza vaccine on 18FDG-PET, suggesting imaging findings related to regional and systemic immune response (6,7).
What is known about adenopathy after COVID-19 vaccination?
In the Phase 3 trial leading to Emergency Use Authorization for the Moderna vaccine, adenopathy was reported as an unsolicited event in 1.1% of patients (8). “Axillary swelling or tenderness” was listed separately and occurred in up to 16.0% in patients aged 18-64 years and up to 8.4% of patients over 65 years of age (vs. 4.3% and 2.5% in the corresponding placebo groups, respectively). Notably, in 0.3-0.6% of patients, symptoms were severe enough to require prescription medicine for relief. In the clinical trial leading to Emergency Use Authorization for the Pfizer-BioNTech vaccine, the self-reported rate of adenopathy in patients was 0.3% (9). Notwithstanding that the reported rate of adenopathy likely underestimates the true number (10), even at a presumed low relative rate, regional adenopathy will become a frequent incidental imaging finding as large numbers of the population undergo vaccinations against COVID-19 in the coming months. Clinically, axillary swelling or adenopathy manifested within 2-4 days after either dose and lasted on average 1-2 days (Moderna) and 10 days (Pfizer-BioNTech).
What is not known about adenopathy after COVID-19 vaccination?
First, clear information is not yet available regarding what proportion of patients will experience some form of adenopathy on imaging, whether the rate of adenopathy will vary between different doses and between different vaccines, the size, number, laterality and morphology of affected lymph nodes, or how long nodes will remain detectable as asymmetrically enlarged or otherwise abnormal in appearance on the various imaging modalities. Extrapolating from preliminary cases (such as the one presented in Figure 1) and other conditions, the findings may persist for a longer period on imaging, given the higher) sensitivity compared to physical exam (10), particularly on 18FDG-PET, in which inflammatory activity may be detected even in non-enlarged nodes (Figure 2) (11). The weight to be placed on the management and follow-up implications of specific imaging features (e.g. long-/short-axis dimensions, morphology of cortex, preservation of fatty hilum, doppler signal, SUV) remains unknown.
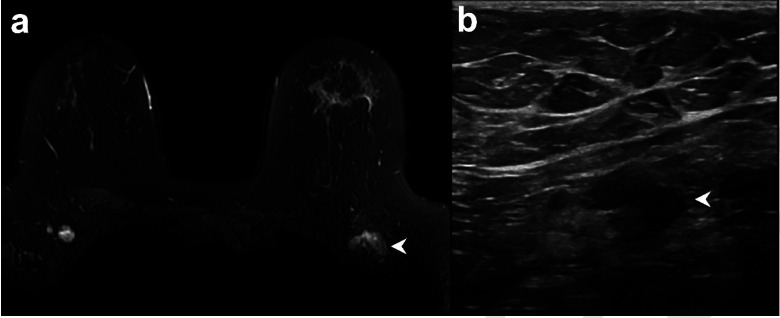
Figure 1:
Example of vaccine-associated adenopathy in a 41-year-old female patient undergoing breast MRI for follow up of focal lesion. (a) T2-weighted fat-saturated axial slice through the breasts and anterior chest, within 5 days of receiving COVID-19 vaccination in the left shoulder showing asymmetric left axillary adenopathy (3.0 x 1.7 cm, arrowhead) with preserved fatty hilum but irregular cortex. (b) 6-week follow-up axillary sonography of the same patient demonstrates decreased size (2.2 x 1.1 cm) and some residual cortical thickening.
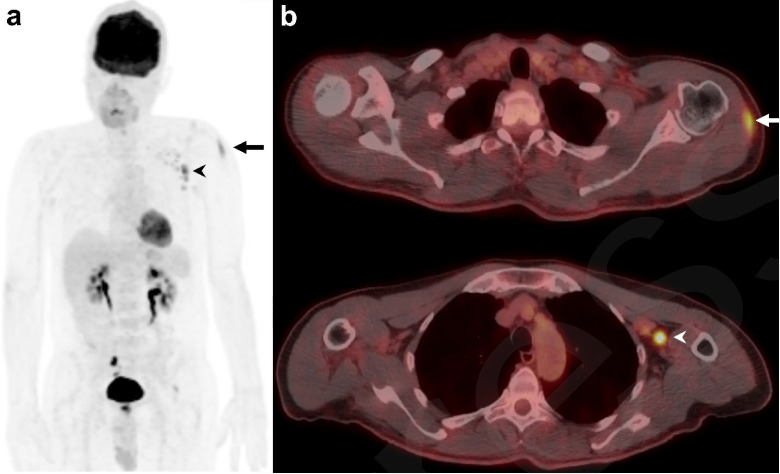
Figure 2:
Example of a 60-year-old male patient on surveillance for smoldering myeloma with typical vaccination-associated findings. (a) Coronal maximum intensity projection (MIP) of 18F-FDG-PET and (b) fused axial 18F-FDG-PET/CT showing FDG avidity in the left deltoid muscle (SUVmax 5.9 g/ml) from the injection site (arrowhead) and ipsilateral reactive FDG-avid draining axillary lymph nodes (SUVmax 9.6 g/ml) (arrow), which are normal-sized on CT.
General Considerations and Preliminary Recommendations
As noted in the summary of recommendations provided in the Table, clear and effective communication between patients, radiologists, referring physician teams and the community in general should be considered of the highest priority when dealing with adenopathy in the setting of COVID-19 vaccination. Recommendations should be tailored to individual patients, based on risk-stratification and clinical context (including consideration of typical cancer spread patterns and other factors such as cancer stage).
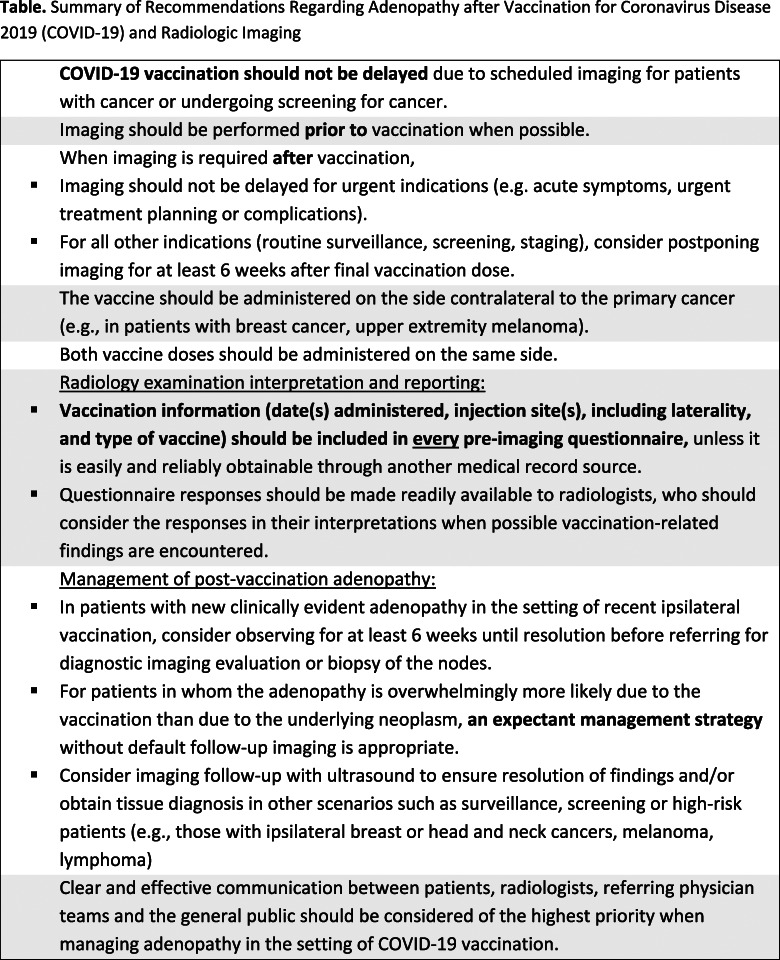
Table.
Summary of Recommendations Regarding Adenopathy after Vaccination for Coronavirus Disease 2019 (COVID-19) and Radiologic Imaging
As a general principle, the panel recommends against delaying COVID-19 vaccination due to imaging needs for patients with a history of cancer or for those undergoing screening for cancer. The estimated infection fatality risk of COVID-19 is orders of magnitude higher than the estimated mortality reduction achieved through effective cancer screening programs in the general population (12). Moreover, mortality from COVID-19 is likely worse in patients with cancer (13).
Planning and Scheduling
When feasible, the panel recommends performing all cancer-related imaging prior to vaccination. After vaccination, imaging for urgent clinical indications (e.g., acute symptoms, short-interval treatment monitoring, urgent treatment planning or complications) should not be delayed. For all other indications (routine surveillance, screening, staging), postponement of imaging for at least 6 weeks after completion of recommended vaccinations should be considered. These recommendations are in line with those from the Society of Breast Imaging, which recently recommended that breast imaging may be postponed at least 4-6 weeks following the second dose of a COVID-19 vaccination “when it does not unduly delay care” (14). Due to our preliminary experience of some nodes remaining enlarged after 4 weeks (Figure 1), a longer interval is desirable, but this needs to be weighed against the patient’s risk factors and the overall context. Special consideration should be given to modelling studies suggesting a large proportion of missed cancer diagnoses due to the pandemic (15), with an expected rise in otherwise avoidable cancer deaths in the coming years (16).
In general, the vaccine should be administered on the side contralateral to the primary cancer site, if applicable, and both vaccine doses should be administered on the same side. CDC guidelines recommend intramuscular injection of Pfizer and Moderna vaccine into the deltoid muscle. Prior studies on other vaccines showed that injection in the thigh or gluteal muscles may adversely affect immunity response. Hence, the panel recommends following CDC guidance and not modifying the injection site based on cancer type or site.
Imaging Interpretation and Reporting
Vaccination information (date of each administered dose, injection site including laterality, and type of vaccine) should be included in all pre-imaging questionnaires, unless it is easily and reliably obtainable through another medical record source. This information should be made readily available to radiologists, who should consider it in their interpretation when possible vaccination-related findings are encountered. Depending on the practice setting, it may be desirable to include the full vaccination questionnaire information in all radiology reports containing possible vaccination-related findings. This may be particularly desireable when, for example, the providers involved in the patient’s care are not part of integrated medical systems or when referrers may otherwise not have access to vaccination details.
It is acknowledged that a single definition for “adenopathy,” whether related to cancer or other etiologies (such as vaccination), is not widely agreed upon. In the cancer setting, different criteria have been employed depending on several factors including the primary tumor site. It is beyond the scope of this manuscript to recommend a specific definition, and until more data become available, the committee recommends reporting morphologic (e.g. size, number, shape), functional (e.g. apparent diffusion coefficient value on MRI) and metabolic (e.g. standardized uptake values on PET) features of adenopathy encountered on imaging following vaccination. A standardized macro in the dictation software may be used, for example: “New [right/left] [axillary/supraclavicular/axillary and supraclavicular] adenopathy in the setting of recent ipsilateral COVID-19 vaccination, probably reactive. If clinically warranted, consider follow-up ultrasound for further evaluation.”
Management
Adenopathy after vaccination, identified clinically or radiologically, should be interpreted in the context of the time since vaccination and overall risk related to the cancer diagnosis (cancer type, location, stage, etc.). For patients at low risk of axillary or supraclavicular nodal metastases in whom the adenopathy is overwhelmingly more likely due to the vaccination than due to the underlying neoplasm (considering time frame, pain, type and location of cancer), an expectant management strategy without default follow-up imaging is appropriate. Short-interval follow-up imaging with ultrasound at least 6 weeks later may be obtained if there is a higher risk of metastatic adenopathy (e.g., breast, head and neck, upper extremity/trunk melanoma or lymphoma). Tissue biopsy should be considered in the setting of high nodal metastatic risk when immediate histopathologic confirmation is necessary for timely patient management. Multi-disciplinary discussion may be helpful in uncertain cases.
Patient and Physician Communication
New unexplained asymmetric “bumps and lumps” – incidental, self-detected or revealed during a physical exam – may generate anxiety and may prompt additional imaging and histopathologic assessment due to the possibility of cancerous involvement. It is of paramount importance to educate patients about the expected side-effects of COVID-19 vaccination and their significance. This can be achieved for example with a flyer in the waiting room or accompanying the appointment letter. The information should use lay terms and be understandable on an 8th grade reading level. In a breast imaging service or practice, an example would be the following text: “Lymph nodes in the underarms of people who were recently vaccinated for COVID-19 sometimes may become enlarged. This is an expected response as our bodies begin to develop antibodies to the vaccination. As a result, we recommend consulting with your health care provider and scheduling your annual mammogram either before or at least 6 weeks after completion of vaccination to reduce the need for additional testing. This recommendation is for people who do not have any breast symptoms. If you are having any problems with your breasts, you should not delay your visit with us.” Similar communications could be drafted in other clinical scenarios, as appropriate.
Final remarks
Weeks before mass vaccinations against COVID-19 commenced, new strains of the virus had been identified (17). It is only a question of time before new variants escape established vaccine coverage, requiring the development and administration of new vaccines. This cycle may well continue into the near future. Hence, COVID-19-vaccination-related phenomena such as onset, duration and size limits of associated adenopathy deserve further scientific investigation to inform future clinical guidelines.
Acknowledgments
Acknowledgment
The authors thank Ada Muellner, MS, for assistance in editing the manuscript.
Footnotes
Funding: ASB, RPJ, ME-H, KNF, KG, ATI, RY, MM, HH, HAV were in part funded through the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Worldometers.info COVID-19 . https://www.worldometers.info/coronavirus/worldwide-graphs/. Accessed February 15, 2021.
- 2.Frey SE, Couch RB, Tacket CO, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med 2002;346(17):1265–1274. doi:10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- 3.Youn H, Hong K-J. Non-invasive molecular imaging of immune cell dynamics for vaccine research. Clin Exp Vaccine Res 2019;8(2):89–93. doi:10.7774/cevr.2019.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta N, Sales RM, Babagbemi K, et al. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin Imaging 2021;75:12–15. doi:10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn RW, Mootz AR, Brewington CC, Abbara S. Axillary Lymphadenopathy After mRNA COVID-19 Vaccination. Radiology: Cardiothoracic Imaging 2021;3(1):e210008. doi:10.1148/ryct.2021210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panagiotidis E, Exarhos D, Housianakou I, Bournazos A, Datseris I. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1). Eur Radiol 2010;20(5):1251–1253. doi:10.1007/s00330-010-1719-5. [DOI] [PubMed] [Google Scholar]
- 7.Mingos M, Howard S, Giacalone N, Kozono D, Jacene H. Systemic Immune Response to Vaccination on FDG-PET/CT. Nucl Med Mol Imaging 2016;50(4):358–361. doi:10.1007/s13139-015-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events : Moderna COVID-19 Vaccine | CDC. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html. Accessed February 2, 2021.
- 9.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum A, Schlagenhauff B, Stroebel W, Breuninger H, Rassner G, Garbe C. Ultrasound examination of regional lymph nodes significantly improves early detection of locoregional metastases during the follow-up of patients with cutaneous melanoma. Cancer 2000;88(11):2534–2539. doi:10.1002/1097-0142(20000601)88:11<2534::AID-CNCR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Eifer M, Eshet Y. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology 2021;210030. doi:10.1148/radiol.2020210030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: A systematic review for the U.S. preventive services task force. Rockville (MD): Agency for Healthcare Research and Quality (US), 2016. [PubMed] [Google Scholar]
- 13.Lee LYW, Cazier J-B, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol 2020;21(10):1309–1316. doi:10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Society of Breast Imaging . SBI recommendations for managing axillary adenopathy post COVID vaccination. https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf. Accessed February 7, 2021.
- 15.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clin Cancer Inform 2020;4:657–665. doi:10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21(8):1023–1034. doi:10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise J. Covid-19: New coronavirus variant is identified in UK. BMJ 2020;371:m4857. doi:10.1136/bmj.m4857. [DOI] [PubMed] [Google Scholar]