Abstract
We present five cases of axillary lymphadenopathy which occurred after COVID-19 vaccination and that mimicked metastasis in oncologic patients. Initial radiologic diagnosis raised concerns for metastasis. However, further investigation revealed that patients received COVID-19 vaccinations in the ipsilateral arm prior to imaging. In two cases, lymph node biopsy confirmed vaccination related reactive lymphadenopathy. Ipsilateral axillary swelling / lymphadenopathy was reported based on symptoms and physical examination in COVID-19 vaccine trials. Knowledge of the potential for COVID-19 vaccine-related ipsilateral adenopathy is necessary to avoid unnecessary biopsy and change in therapy.
Summary
Reactive axillary lymphadenopathy following COVID-19 vaccination may mimic metastasis. Therefore, radiologist/clinicians should question recent history of COVID-19 vaccination in cases of unilateral axillary lymphadenopathy.
Introduction
The United States Food and Drug Administration issued emergency use authorization for two vaccines, Pfizer-BioNTech and Moderna COVID-19 vaccines, to help decrease COVID-19 infection and death in December 2020. As of February 6, 2021, about 28.9 million people in the United States had received one or more vaccine doses. (1-3). In conjunction with the rapid administration of these vaccines, we have started to observe secondary effects on diagnostic imaging.
In clinical studies, axillary lymphadenopathy was reported on the ipsilateral injection side (4, 5). Ipsilateral axillary swelling/tenderness was the second most frequently reported local reaction to the Moderna COVID-19 vaccine, occurring in 11.6% and 16.0% of recipients following first and second dose respectively. In the Moderna cohort, clinically detected axillary and supraclavicular lymphadenopathy was reported in 1.1% of study participants within 2-4 days after vaccination, as an unsolicited adverse event (4). In the Pfizer-BioNTech COVID-19 vaccine trial, the rate of ipsilateral axillary and supraclavicular lymphadenopathy was reported to be 0.3% among vaccine recipients versus <0.1% among placebo group (5).
In the above vaccine trials, abnormal lymphadenopathy was reported based on physical examination rather than using imaging. In our center, we have observed unilateral axillary lymphadenopathy in five patients using different imaging modalities including PET/CT, MRI and ultrasonography. Initial diagnosis was concerning for metastasis; however, further investigation revealed that these patients had received COVID-19 vaccinations prior to imaging. A description of these patients is below.
Materials and Methods: An institutional review board waiver was obtained for this HIPAA compliant retrospective case series. Cases were identified from our institution from 12.21.2020 to 1.27.2021. In two cases diagnosis was confirmed by histopathology. In three, diagnosis was made based on combination of clinical and radiologic information. For Cases 1-4, the lymphadenopathy was discovered incidentally on radiologic imaging. Case 5 presented for evaluation of left breast and left axillary pain.
Results:
Case Series
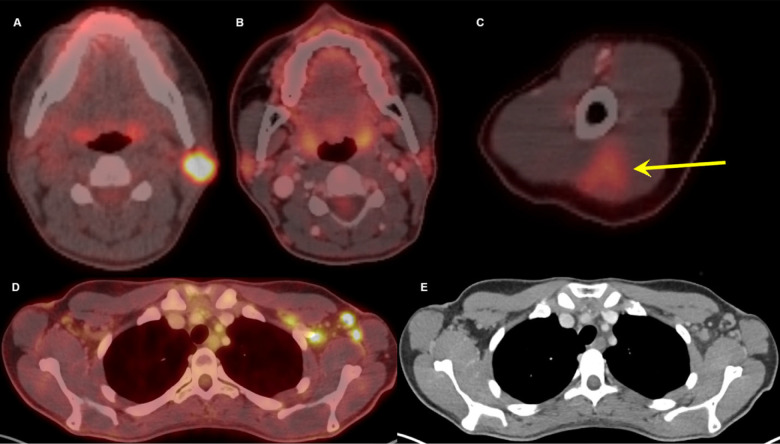
Case 1: A 32-year-old female presented with a left sided neck mass. Sonography revealed an indeterminant left intraparotid mass. Further evaluation with biopsy revealed metastatic lymph node with BRAF-V600E mutant malignant melanoma with unknown primary. Staging PET/CT demonstrated hypermetabolic left intraparotid lymphadenopathy with maxSUV of 16.6 and no other suspicious finding. As directed by an ongoing clinical trial, the patient started treatment with vemurafenib, cobimetinib and atezolizumab. Three-month follow-up 18-FDG PET/CT (per the clinical trial protocol) showed complete resolution of the known hypermetabolic metastatic intraparotid lymphadenopathy. However, PET/CT revealed multiple new, mildly enlarged, and hypermetabolic left axillary lymph nodes with fat stranding. The largest lymph node was measured 1.4 x 1.0 cm with maxSUV of 7.7. Initially, this was reported as progression vs. pseudoprogression secondary to immunotherapy. Given that the biopsy proven index tumor showed complete response to treatment and true progression could not be excluded, a biopsy was recommended. However, further investigation revealed that the patient received the second dose of Pfizer-BioNTech COVID-19 vaccine six days prior to PET/CT and there was triangular intramuscular FDG uptake in the left arm at the injection site (Figure 1). Based on this information, left axillary lymphadenopathy was attributed to recent vaccination. Findings were discussed with the patient, and the patient opted for observation instead of biopsy. Follow up of the lymphadenopathy will be obtained as part of the patient’s three-month follow-up PET/CT for melanoma surveillance.
Figure 1:
Thirty-two-year-old female. A, Axial fused 18-FDG PET/CT showed hypermetabolic biopsy proven intraparotid lymph node with metastatic malignant melanoma. B, Three-month follow-up axial fused 18-FDG PET/CT shows complete resolution of the neck mass following chemotherapy, C, while left arm shows hypermetabolic triangular shaped inflammation (arrow) at the COVID vaccine injection site. D, Axial fused images at the axilla level shows multiple new hypermetabolic lymph nodes. E, Axial contrast enhanced CT demonstrates mild fat stranding surrounding the ovoid lymph nodes with preserved fatty hilum.
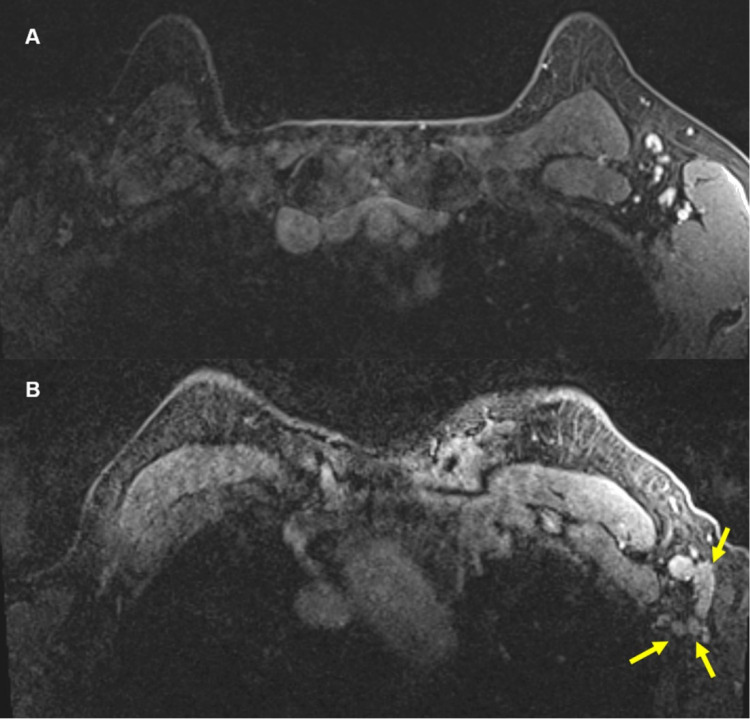
Case 2: A 57-year-old female with strong family history of breast cancer was undergoing annual MRI for high-risk breast cancer screening. The most recent contrast enhanced breast MRI showed increasing size and number of unilateral left axillary lymph nodes, concerning for malignancy. MRI from the previous year showed the longest lymph node diameter as 18 mm with cortical thickness of 2 mm (Figure 2A); while this year, the longest node measured 21 mm with abnormal cortical thickness of 5 mm (Figure 2B). However, further investigation revealed that the patient had received her second dose of Pfizer-BioNTech COVID-19 vaccine five days prior to the breast MRI. Thus, the findings were attributed to recent vaccination and biopsy was deferred with recommendation of axillary ultrasound follow-up in 4 to 6 weeks.
Figure 2:
57-year-old female. A, Axial contrast enhanced fat saturated T1 sequence of MRI from a year ago showed sub-centimeter, benign lymph nodes in the left axilla. B, Most recent contrast enhanced axial fat saturated T1 sequence at approximately same level showed multiple new, enlarged lymph nodes (yellow arrows) in the left axilla. There were no other suspicious findings to suggest a breast cancer primary.
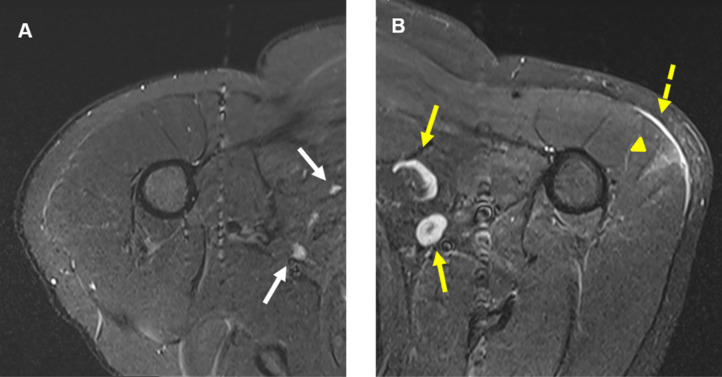
Case 3: A 41-year-old male with history of oligometastatic myxoid liposarcoma of the left thigh was evaluated. The patient was first diagnosed with his primary soft tissue tumor localized in the left hip three years ago. Patient underwent pre-operative radiotherapy, followed by surgical resection. After two-year of disease-free follow-up, patient was found to have an isolated 12 mm bone lesion within left iliac bone. Excisional biopsy confirmed metastasis. The patient started additional metastasis-directed stereotactic radiotherapy and subsequent follow-up imaging surveillance with whole body MRI. The most recent MRI showed no evidence of recurrence throughout the body, but there were new clusters of left axillary lymph nodes, largest reaching up to 20 mm with cortical thickness of 7 mm. Notably, there was new wedge-shaped intramuscular edema in the left deltoid muscle with subcutaneous edema overlying the muscle (Figure 3). This appearance was similar to muscle abnormalities described above in Case 1. Upon discussion with the patient, it was confirmed that patient received the second dose of Pfizer-BioNTech COVID-19 vaccine four days prior to the exam. The patient opted for clinical surveillance.
Figure 3:
41-year-old male. A, Axial T2-STIR MRI of the right shoulder shows normal looking muscular and osseous structures, and normal lymph nodes (white arrows). B, Axial T2-STIR MRI of the left shoulder correspondent to the level of A, shows new subcutaneous edema (dashed yellow arrow) overlying a wedge-shaped intramuscular edema (yellow arrowhead) in the left deltoid muscle. In addition, there are multiple prominent ovoid shaped lymph nodes (yellow arrows) in the ipsilateral axilla, asymmetric to the right and new compared with prior MRIs (not shown).
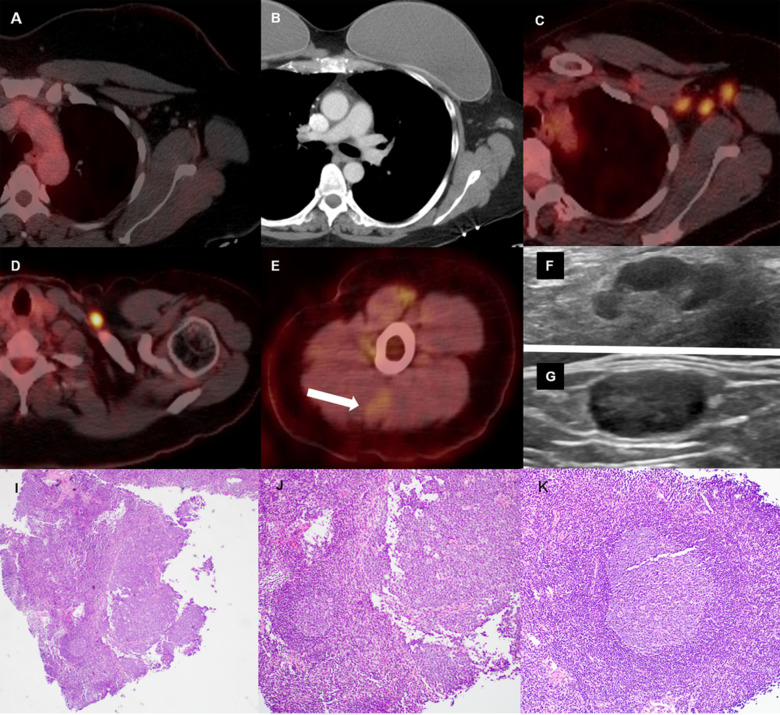
Case 4: A 46-year-old female with history of triple negative, poorly differentiated infiltrating ductal carcinoma of the left breast. She underwent bilateral mastectomies with complete pathological response after neoadjuvant chemotherapy. A routine follow-up chest CT showed new mild left axillary and left supraclavicular lymphadenopathy (Figure 4B). Therefore, 18-FDG PET/CT was performed for further evaluation. PET/CT demonstrated multiple enlarged hypermetabolic lymph nodes in the left axilla, largest is 20x12mm with maxSUV of 9, and left supraclavicular region measuring 13x8mm with maxSUV of 13.4 (Figure 4C, 4D). The radiologist initially reported the PET/CT as “concerning for metastasis.” Later, it was found that the patient received the first dose of Pfizer-BioNTech COVID-19 vaccine 15 days prior to the chest CT and the second dose, seven days prior to the PET/CT. Retrospective evaluation of the PET/CT showed a vague wedge-shaped metabolic activity in the left deltoid muscle with mild heterogeneity on CT, similar to the Case 1 and 3 (Figure 4E). Therefore, findings were more attributed to recent COVID-19 vaccination. However, patient opted for ultrasound guided core needle biopsy of axillary and supraclavicular lymph nodes, which occurred 35 days after the first and 16 days after the second dose of COVID-19 vaccine. On histopathologic exam, both nodes were consistent with benign reactive lymph nodes, without any evidence of breast cancer metastasis (Figure 4I, 4J, 4K).
Figure 4:
46-year-old female with triple negative left breast cancer, disease free for three years. A, Axial fused 18-FDG PET/CT three years earlier with no concerning lymph node in the left axilla. B, Surveillance contrast enhanced axial chest CT showed new left axillary lymphadenopathy with fat stranding 15 days after the first Covid-19 vaccine. Further evaluation with PET/CT six days after the 2nd dose of vaccine, demonstrated, C, multiple enlarged hypermetabolic left axillary lymph nodes and, D, a hypermetabolic round shaped left supraclavicular lymph node in axial fused 18-FDG PET/CT images. E, A subtle wedge-shaped intramuscular hypermetabolism (white arrow) was also noted in this case, similar to first and third cases. Ultrasonography guided core needle biopsy was performed. F, On ultrasonography, axillary lymph nodes had thickened cortex while the supraclavicular lymph node demonstrated, G, thickened cortex with loss of normal fatty hilum. I, Hematoxylin and eosin staining under 40x magnification shows enlarged germinal center with interfollicular expansion by small lymphocytes. 100x magnification images show, J, prominent germinal center with tingible body macrophage and, K, reactive germinal center with expansion of interfollicular regions by small lymphocytes and focally prominent endothelial cells.
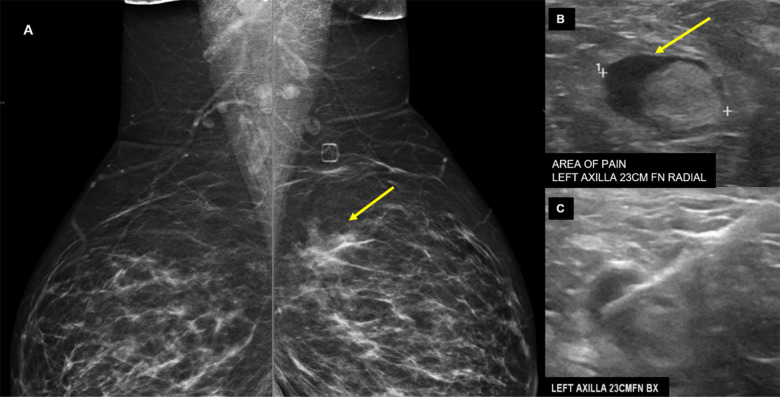
Case 5: A 38-year-old female, with family history for breast cancer, was evaluated for left breast and left axillary pain with baseline diagnostic mammogram and ultrasound. Mammographic views demonstrated asymmetry in left superior breast without ultrasound correlate (Figure 5A). Ultrasound for axillary pain demonstrated lymph node with abnormal cortical thickening of 6 mm (Figure 5B). Stereotactic biopsy of the left breast asymmetry revealed fibrocystic change with pseudoangiomatous stromal hyperplasia, concordant with radiology findings. Ultrasound guided core needle biopsy of the left axillary lymph node revealed reactive follicular hyperplasia without any evidence of malignancy. Retrospective chart review demonstrated that the patient had received the first dose of Pfizer-BioNTech COVID-19 vaccine in the left arm eight days prior to the initial mammogram, and the second dose at the day of the biopsy.
Figure 5:
38-year-old presented for evaluation of left breast and left axillary pain. A, Bilateral MLO views demonstrate an asymmetry (yellow arrow) in the left superior breast which was biopsied as pseudoangiomatous stromal hyperplasia. B, Ultrasound evaluation of the left axilla demonstrates a lymph node with abnormally thickened cortex (yellow arrow). C, Ultrasound guided core needle biopsy for lymph node revealed reactive follicular hyperplasia.
Discussion
We have highlighted five cases with ipsilateral axillary lymphadenopathy following administration of Pfizer-BioNTech vaccine. Ipsilateral axillary lymphadenopathy following vaccinations has been previously reported with vaccinations other than COVID-19. For example, ipsilateral axillary and supraclavicular lymphadenopathy was clinically reported in a patient following HPV vaccine administration (6). Radiologically, hypermetabolism in the ipsilateral lymph nodes was reported with 18-FDG PET/CT exams following seasonal H1N1 Influenza-A vaccines (7-9). Shirone et al reported hypermetabolic ipsilateral lymphadenopathy in 4 of 83 patients (4.8%) following seasonal influenza vaccination and all these reported cases were within seven days following vaccination (9). On January 22, 2021, the Society of Breast Imaging issued a statement for management recommendations of ipsilateral axillary lymphadenopathy following COVID-19 vaccinations. A recent ultrasonography case series of four patients, demonstrated ipsilateral asymmetrical lymphadenopathy following vaccination with both Pfizer-BioNTech and Moderna vaccines (10, 11). None of these were confirmed as reactive lymphadenopathy on histopathology; but based on clinical and radiological findings, follow-up was recommended. Although lymphadenopathy related to vaccination has been known in breast cancer imaging, the unprecedented administration of COVID-19 vaccines is affecting almost all modalities of imaging. To our knowledge, a single PET/CT case was published similar to our fourth patient, although this case was without pathologic confirmation (12).
In two cases, we had pathologic confirmation of benign reactive lymphadenopathy secondary to vaccination and to our knowledge, these are the first pathologically proven cases in the literature. Although, the remaining three cases are not confirmed as reactive benign lymph nodes with histopathological evaluation, as we described above, it is more plausible to attribute these findings to recent vaccine administration. At present, no data is available regarding the duration of radiologically evident lymphadenopathy or appropriate follow-up intervals. Until data becomes available, management will be guided by clinical context, particularly in patients with history of cancers with specific propensity towards axillary lymphadenopathy.
Due to widescale vaccination of the majority of the U.S. population, axillary lymphadenopathy due to COVID-19 vaccination is likely to be encountered in oncologic patients. In addition, in this limited series, a triangle of intramuscular inflammation was demonstrated at the injection site on MRI and PET/CT, as also described by Eifer and Eshet (12), suggesting vaccine-related inflammation.
Overall, our findings are important, particularly for cancer patients. Radiologists, oncologists, and internists should be aware of this secondary effect of vaccination to obviate unnecessary changes in management, unnecessary patient emotional stress or biopsy.
Acknowledgments
Acknowledgement
We would like to thank Dr Bruce C. Trautman for his help with the histopathologic evaluation, and Dr Katherine Chang for informing us about one of the cases.
References
- 1.Moderna COVID-19 Vaccine . US Food & Drug Administration Web site. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine. Accessed February 06, 2021.
- 2.Pfizer-BioNTech COVID-19 Vaccine . US Food & Drug Administration Web site. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine. Accessed February 06, 2021.
- 3.Covid data tracker . US Center for Disease Control and Prevention Web site. https://covid.cdc.gov/covid-data-tracker/#vaccinations. Accessed February 06, 2021.
- 4.Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine . US Center for Disease Control and Prevention Web site. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html. Accessed February 06, 2021.
- 5.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr., Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Studdiford J, Lamb K, Horvath K, Altshuler M, Stonehouse A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy 2008;28:1194-1197. doi: 10.1592/phco.28.9.1194 [DOI] [PubMed] [Google Scholar]
- 7.Panagiotidis E, Exarhos D, Housianakou I, Bournazos A, Datseris I. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1). Eur Radiol 2010;20:1251-1253. doi: 10.1007/s00330-010-1719-5 [DOI] [PubMed] [Google Scholar]
- 8.Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A, Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging 2011;38:894-898. doi: 10.1007/s00259-011-1729-9 [DOI] [PubMed] [Google Scholar]
- 9.Shirone N, Shinkai T, Yamane T, Uto F, Yoshimura H, Tamai H, Imai T, Inoue M, Kitano S, Kichikawa K, Hasegawa M. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med 2012;26:248-252. doi: 10.1007/s12149-011-0568-x [DOI] [PubMed] [Google Scholar]
- 10.Lars Grimm SD, Basak Dogan, Brandi Nicholson, Brian Dontchos, Emily, Sonnenblick HM, JoAnn Pushkin, John Benson, Katia Dodelzon, Neha Modi, Roger, Yang VD, Vidushani Perera. SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination. Society of Breast Imaging Web site. https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf. Published 2021. Accessed February 06, 2021.
- 11.Mehta N, Sales RM, Babagbemi K, Levy AD, McGrath AL, Drotman M, Dodelzon K. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clinical Imaging 2021;75:12-15. doi: 10.1016/j.clinimag.2021.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michal Eifer YE. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology 2021. 10.1148/radiol.2020210030 [DOI] [PMC free article] [PubMed] [Google Scholar]