Images in Radiology
A 37-year-old female presented with new, palpable left supraclavicular lymphadenopathy. Chart review was unremarkable, except for obtaining her first dose of the Moderna COVID-19 vaccine 12 days ago, in the left (ipsilateral) arm. On the day of vaccination, the patient was asymptomatic. However, five days later, she developed a palpable lump in the left supraclavicular region. A diagnostic mammogram demonstrated prominent left axillary and intramammary lymphadenopathy (Fig 1). Sonography confirmed prominent, cortically thickened nodes in the left axilla and left supraclavicular region, (Figure 2). Sonography of the right axilla was unremarkable. A 2-week follow-up ultrasound (performed prior to the second vaccine dose) revealed no significant change in the lymphadenopathy. An additional diagnostic ultrasound 3 months after the second dose will be performed to assess for resolution. This case highlights that if there is a history of recent vaccination (1, 2), a more conservative approach of short-term follow-up imaging, rather than immediate biopsy is recommended. Institutions should update their patient questionnaire to include the date(s) and site(s) of the vaccinations.
Figure 1:
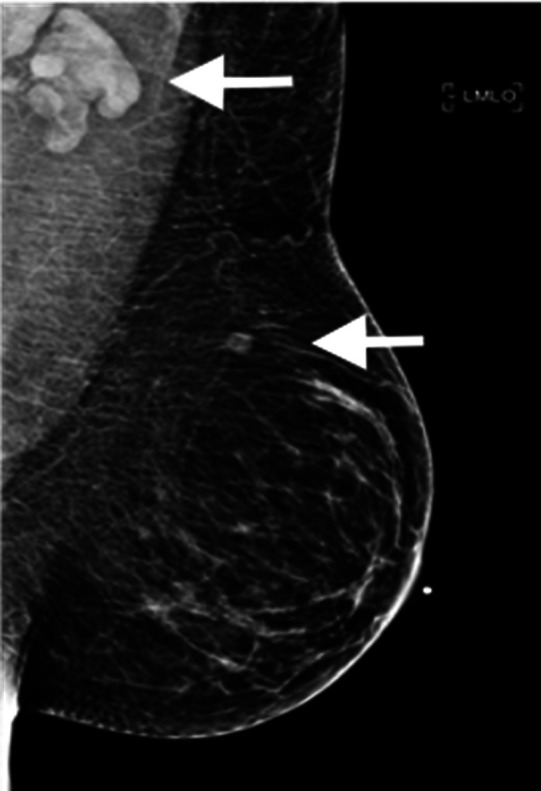
37-year-old female with palpable left supraclavicular adenopathy. Diagnostic mediolateral oblique mammogram of the left breast demonstrates prominent left axillary adenopathy and an intramammary lymph node (white arrows).
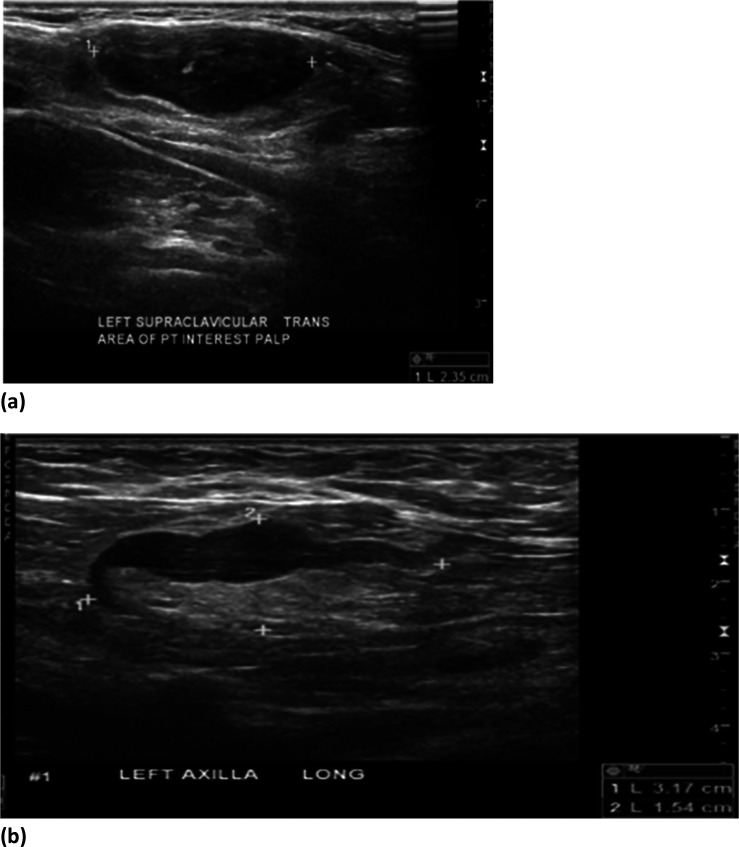
Figure 2:
(a) 37-year-old female with palpable left supraclavicular adenopathy. Grayscale ultrasound images of the left supraclavicular region demonstrates a single, cortically thickened lymph node correlating with the region of palpable interest. (b) 37-year-old female with palpable left axillary adenopathy. Grayscale ultrasound images of the left axilla demonstrate multiple, cortically thickened, level 1 axillary lymph nodes correlating with the region of palpable interest and mammographic abnormality.
References
- 1.Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events : Moderna COVID-19 Vaccine. Centers for Disease Control and Prevention; [January 16, 2021]; Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html. [Google Scholar]
- 2.Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events : Pfizer-BioNTech COVID-19 Vaccine. Centers for Disease Control and Prevention; [January 16, 2021]; Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html. [Google Scholar]