Abstract
To compare visual function of 2-wall (medial and lateral) versus 3-wall (medial, lateral, and inferior) orbital decompression in patients with dysthyroid optic neuropathy (DON).
A total of 52 eyes of 37 patients underwent orbital decompression for DON between 2013 and 2019 were retrospectively reviewed. Two- or 3-wall decompression was performed in 31 eyes of 23 patients and 21 eyes of 14 patients, respectively. We examined best-corrected visual acuity (BCVA), visual field mean deviation (MD) and pattern standard deviation (PSD), pattern-reversed visual evoked potential (PVEP) for P100 latency and amplitude at 60 and 15 arcmin stimulation checkerboard size, as well as proptosis using Hertel exophthalmometry.
Whether 2-wall or 3-wall decompression, all parameters of visual function were improved after surgery (all P < .05). The improvement in BCVA, MD, and PSD was not statistically significant between groups (all P > .05). Proptosis reduction was higher after 3-wall decompression (P = .011). Mean increase in P100 amplitude after 3-wall decompression was statistically higher than that of after 2-wall decompression at 60 and 15 arcmin (P = .045 and .020, respectively), while the mean decrease in P100 latency was similar between the groups (P = .821 and .655, respectively). Six patients (66.67%) had persistent postoperative diplopia and 1 patient (20%) had new-onset diplopia in 3-wall decompression group, which were higher than in 2-wall decompression group (46.15% persistent postoperative diplopia and no new-onset diplopia).
Both 2-wall and 3-wall decompression can effectively improve visual function of patients with DON. Three-wall decompression provides better improvement in P100 amplitude and proptosis, however new-onset diplopia is more common with this surgical technique.
Keywords: 2-wall orbital decompression, 3-wall orbital decompression, dysthyroid optic neuropathy, pattern-reversed visual evoked potential
1. Introduction
Graves orbitopathy (GO) is an autoimmune disease involving intraorbital fat, extraocular muscles, and lacrimal glands. Its ocular signs and symptoms are complex, including proptosis, eyelid retraction, ocular dyskinesia, lagophthalmos, conjunctival congestion, diplopia, and exposure keratopathy. Dysthyroid optic neuropathy (DON) is a relatively uncommon but a serious complication of (GO), which can result in permanent vision loss if not treated appropriately.[1] It has an estimated incidence of 5% to 8.6% of patients with GO.[2–4] While the exact mechanism of DON remains elusive, direct compression of the optic nerve by enlarged extraocular muscles,[4,5] stretching of the optic nerve by proptosis,[6–8] orbital pressure,[9,10] and inflammation[11,12] have been proposed.
There is controversial evidence regarding the optimum management strategy. Based on the current literatures, widely accepted treatments include systemic corticosteroids, orbital radiotherapy, and orbital decompression, in which intravenous methylprednisolone being the first line of treatment, and orbital decompression becoming indispensable if steroid therapy fails.[13,14] Decompression surgery for DON is best performed after the disease has become inactive, but decompression may also be required in the active phase of GO for cases of DON that are refractory to medical treatment.[13] DON has been managed with orbital decompression by various techniques.[15–18] Orbital medial and inferior wall decompression for DON treatment is the earliest approved surgical method, because the ethmoid sinus and maxillary sinus have huge space, which can alleviate the compression effect of medial and inferior rectus muscle on optic nerve.[19] As the indications for surgical decompression for DON have expanded, new surgical approaches being developed for better postoperative cosmesis and fewer diplopia. Surgical techniques such as the deep lateral wall decompression,[20,21] balanced decompression,[22–25] and endoscopic medial decompression,[26,27] have all been widely used in the treatment of DON.
With the diversification of orbital decompression surgical methods, single surgical methods can no longer meet the requirements of surgery, and combined decompression of multiple surgical methods has gradually become a new development direction. However, there is no consensus among orbital surgeons about how much decompression should be performed to treat DON. Although orbital decompression is usually restorative in most cases, some patients do not respond well even with orbital decompression, and may still require either systemic immunosuppression or further decompression following initial surgery to improve optic nerve functions.[28,29]
Many studies compared different surgical techniques, such as lateral versus medial decompression, with or without inferior decompression or fat removal, and balanced decompression (medial and lateral) alone. But there are scanty evidences to elucidate the differences of visual function from a perspective of monitoring pattern-reversed visual evoked potential between 2-wall and 3-wall decompression. This retrospective study aimed to compare the therapeutic effects of 2-wall versus 3-wall orbital decompression on visual function in patients with DON.
2. Methods
2.1. Patients
Our study is a retrospective case series. The medical records of all the patients affected by DON and treated with 2-wall or 3-wall orbital bony decompression combined with orbital fat removal performed at Department of Ophthalmology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology between 2013 and 2019 were evaluated. This study was approved by the Ethics Committee of Union Hospital affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, and was conducted in strict accordance with the Helsinki Declaration. All participants provided written informed consent.
Those patients that followed up for at least 3 months after the surgery were included in the study. One patient died and 5 were lost to follow-up. The last follow-up was either the last consultation in our clinic in those patients without further surgical interventions or the last consultation before the next surgical step in rehabilitative surgery (e.g., extraocular muscle surgery or lid surgery). The patients were excluded from study if they had the presence of any ocular disease affecting vision such as diabetic retinopathy, corneal ulcer, glaucoma, macular disease, and so on. Finally, the data from 37 cases (52 eyes) were recorded, including the age, sex, smoking status, thyroid function, previous therapy for GO, time elapsed from the first ocular symptoms to orbital surgery, presence of DON, orbital decompression technique, follow-up duration after orbital surgery, related surgical complications, and additional medical and/or surgical interventions after surgery.
All of the patients underwent full ophthalmic examinations both preoperatively and postoperatively, including clinical activity score (CAS), best-corrected visual acuity (BCVA), color vision, relative afferent pupillary defect (RAPD), fundoscopy, visual field by Humphrey 30-2 SITA-standard threshold strategy for mean deviation (MD) and pattern standard deviation (PSD), axial proptosis by Hertel exophthalmometry, and pattern-reversed visual evoked potential (PVEP) for P100 latency and amplitude at 60 and 15 arcmin stimulation checkerboard size. The diagnosis of DON was depended on the presence of any combination of visual deficits including visual acuity, visual field, and color vision. It was also supported by at least one of the apical crowding, optic disc edema, and afferent pupillary defect. Optic nerve dysfunction alone was responsible for all deficits.[4,30]
2.2. Orbital decompression surgery technique
The surgery was performed under general anesthesia by the same ophthalmologist. The deep lateral wall decompression was performed via lateral canthal incision or extended upper eyelid crease incision. The subcutaneous tissue was separated to expose the outer rim of the orbit, and separation upwards to the lateral margin of the superior orbital margin and downwards to the superior margin of the zygomatic arch. The periosteum of the orbital margin was cut open by unipolar electrotome, and the anterior margin of the temporalis muscle attached to the orbital margin was cut back. Then, the lateral orbital bone flap was cut off with a chainsaw, with the anterior orbital bone cut off. After that, the anterior temporal muscle was dissected posteriorly to fully expose the temporal fossa. Combined with bone chisel, bone biting forceps and high-speed grinding drill, all the posterior bone of lateral wall of orbit was removed, deep to the transition of the dura and periosteum of the outer edge of the superior orbital fissure, up to the sphenoid crest and lacrimal fossa, and down to the upper edge of the inferior orbital fissure. Exposure of most of the anterior temporal lobe meninges was a sign of termination of lateral wall decompression. An average of 3 cm3 of fat was removed from this region.
Medial orbital decompression was performed through a transcaruncular incision. Blunt dissection was carried posteriorly and medially with scissors to expose the medial orbital wall just behind the posterior lacrimal crest. The orbital periosteum was resected at 1.5 cm behind the crista lacrimalis posterior and the ethmoid paper template was bitten off by sinus forceps. It gone up to the level plate of the ethmoid bone, deep to the front of the inner edge of the optic canal, and down to the bone where the ethmoid bone meet the maxilla, namely orbital strut.[31] The bone here was hard and about 3 mm thick, having a supporting effect on the orbital tissue, which can reduce the probability of diplopia caused by the muscle cone shifting inward and downward. It should be reserved. In severe cases, it could be removed or compressed to shift inward and downward. Separate the muscle spacers and shift the medial rectus and fat toward the ethmoid sinus.
If floor decompression was planned, a transconjunctival incision was performed which was extended by a conjunctival incision in the medial wall. The posterior 1/2 structure at the strut was removed, extending downwards to the posterior 1/2 of the inferior orbital wall, deep to the apex of the orbit, and beyond to the inferior orbital fissure, retaining the inferior orbital nerve groove.
After satisfactory eyeball retraction without obvious displacement was observed, the orbital outer margin bone flap was reset and fixed with titanium nail and titanium plate. Then, the transcaruncular incision, periosteum, subcutaneous tissue, and skin incision were sutured successively. Postoperative compression bandaging was performed for 1 week, and intravenous broad-spectrum antibiotics and hemostatic drugs were routinely given at 3 days.
2.3. Statistical analysis
Data analysis was performed by using SPSS for Windows, version 20 (SPSS Inc., Chicago, IL). Best corrected Snellen acuity was converted to logarithm of minimal angle of resolution (LogMAR) units for statistical analyses. Eyes without formed visual acuity were assigned decimal visual acuity according to previous study.[32] Wilcoxon singed rank test, independent t test, paired t test were used when necessary. If there were bilateral DON in 1 patient, data of left and right eyes were both used for analysis. P < .05 was considered statistically significant.
3. Results
3.1. Demographic characteristics
A total of 52 eyes of 37 patients who underwent orbital decompression for the treatment of DON were included in the study. All bilateral decompressions were of identical type on each side. All patients were previously treated either by radioiodine (n = 12) or thyroidectomy (n = 5) or antithyroid drug therapy (n = 36) and had normal serum fT3 and fT4 values at the time of decompression surgery as well as during follow-up. All patients previously received systemic steroids and 2 patients underwent additional orbital irradiation without sufficient or definite remission of GO prior to decompression. Because in most cases pretreatment was not coordinated by our hospital, both orbital irradiation and steroid therapy were administered at variable treatment and dosing regimes.[33] There were 9 men and 28 women. The mean age at decompression was 48 years (range, 28–71 years). Two-wall decompression was performed in 31 eyes (23 patients) and 3-wall in 21 eyes (14 patients). The mean follow-up time was 22 months (range, 3–71 months). There were no differences between the groups with respect to demographics, smoking habits, I131 treatment, duration of GD, GO and DON, and thyroid function at preoperative visit (all P > .05).
3.2. Clinical outcome measures
Preoperative and postoperative clinical outcome measures were summarized in Table 1. There was no differences between the groups about preoperative value of LogMAR BCVA, MD, PSD (all P > .05), except for proptosis, which was greater in 3-wall decompression group (P < .001). Whether 2-wall decompression or 3-wall decompression, BCVA, MD, PSD, and proptosis were significantly improved after decompression surgery (all P < .001). Moreover, there were no significant differences between the 2 groups in improvement of these 3 parameters (all P > .05), except in proptosis (P = .011).
Table 1.
Comparison of pre- and postoperative clinical outcome measures.
| Outcome measures | Preoperative (mean ± SD) | Postoperative (mean ± SD) | △ (mean ± SD) | P∗ value | △P∗ value |
| BCVA, LogMAR | |||||
| 2-wall | 0.94 ± 0.62 | 0.30 ± 0.52 | −0.64 ± 0.45 | <.001 | .504 |
| 3-wall | 0.70 ± 0.40 | 0.09 ± 0.15 | −0.61 ± 0.43 | <.001 | |
| Proptosis, mm | |||||
| 2-wall | 18.84 ± 2.72 | 13.68 ± 1.12 | −5.16 ± 2.34 | <.001 | .011‡ |
| 3-wall | 22.05 ± 3.24 | 14.52 ± 1.53 | −7.53 ± 3.40 | <.001∗∗ | |
| MD, dB | |||||
| 2-wall | −13.73 ± 9.15 | −6.79 ± 6.95 | 6.94 ± 6.82 | <.001 | .556‡ |
| 3-wall | −9.45 ± 6.85 | −3.68 ± 3.44 | 5.77 + 6.15 | <.001 | |
| PSD, dB | |||||
| 2-wall | 6.05 ± 2.64 | 3.30 ± 1.72 | −2.75 ± 2.44 | <.001 | .544 |
| 3-wall | 5.07 ± 2.49 | 2.58 ± 1.48 | −2.49 ± 2.16 | <.001 | |
△ = the difference between post- and post- and pre-surgery, BCVA = best-corrected visual acuity, LogMAR = logarithm of minimal angle of resolution, MD = mean deviation, PSD = pattern standard deviation.
Mann–Whitney U test or otherwise, as indicated.
Paired t test.
Independent t test.
3.3. PVEP
The changes of PVEP after decompression surgery were shown in Table 2. Mean P100 amplitude increased significantly in 2-wall decompression group by 2.30 and 2.43 μV at 60 and 15 arcmin stimulation checkerboard size, respectively, corresponding to increases significantly in 3-wall decompression group by 4.89 and 4.49 μV at 60 and 15 arcmin stimulation checkerboard size, respectively. Mean increase in P100 amplitude after 3-wall decompression was statistically higher than that of after 2-wall decompression at 60 and 15 arcmin (P = .046 and .020, respectively) (shown in Fig. 1).
Table 2.
Comparison of pre- and post-operative parameters of pattern-reversed visual evoked potential.
| 60 arcmin | 15 arcmin | ||||
| PVEP | P100 latency, ms | P100 amplitude, μV | P100 latency, ms | P100 amplitude, μV | |
| 2-wall | Preoperative (mean ± SD) | 118.33 ± 16.41 | 4.22 ± 3.01 | 122.29 ± 17.02 | 4.59 ± 3.08 |
| Postoperative (mean ± SD) | 112.17 ± 9.47 | 6.52 ± 2.17 | 116.53 ± 13.77 | 7.03 ± 2.36 | |
| △(mean ± SD) | −6.16 ± 13.04 | 2.30 ± 2.26 | −5.76 ± 17.05 | 2.43 ± 2.63 | |
| P (pre vs post) | .037∗∗ | <.001∗∗ | .021∗ | <.001∗∗ | |
| 3-wall | Preoperative (mean ± SD) | 115.89 ± 8.70 | 4.62 ± 2.26 | 125.38 ± 16.12 | 4.31 ± 2.02 |
| Postoperative (mean ± SD) | 108.87 ± 7.91 | 9.51 ± 3.79 | 116.26 ± 17.53 | 8.80 ± 2.25 | |
| △(mean ± SD) | −7.02 ± 10.06 | 4.89 ± 4.67 | −9.12 ± 12.46 | 4.49 ± 2.62 | |
| P (pre vs post) | .010∗∗ | <.001∗∗ | .004∗ | <.001∗∗ | |
| △P | .821‡ | .046‡ | .655∗ | .020‡ | |
△ = the difference between post- and pre-surgery, PVEP = pattern-reversed visual evoked potential.
Mann–Whitney U test or otherwise, as indicated.
Paired t test.
Independent t test.
Figure 1.
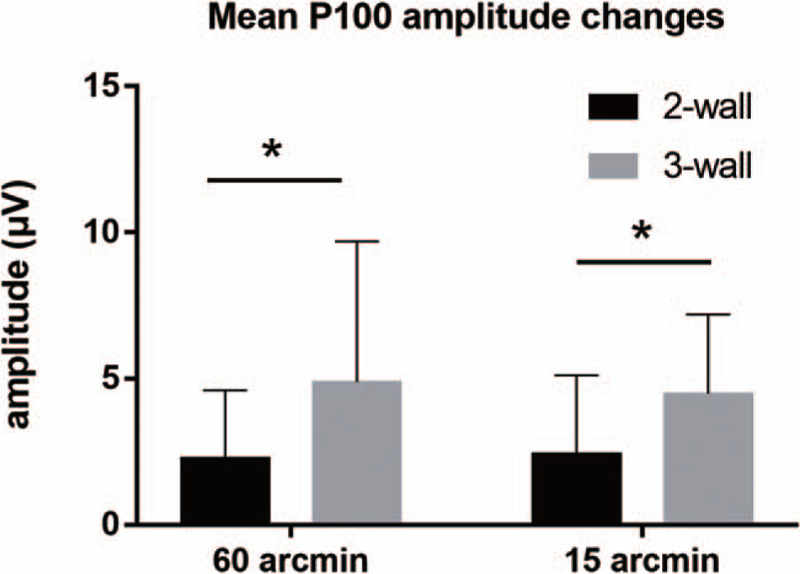
Postoperative improvement of P100 amplitude in 2-wall and 3-wall decompression at different stimulation pattern sizes (60 and 15 arcmin). ∗, P < .05.
Mean P100 latency shortened significantly in 2-wall decompression group by 6.16 and 5.76 ms at 60 and 15 arcmin stimulation checkerboard size, respectively, corresponding to decreases significantly in 3-wall decompression group by 7.02 and 9.12 ms at 60 and 15 arcmin stimulation checkerboard size, respectively. Mean decrease in P100 latency was not statistically significantly different between the 2 groups (P = .821 and .655, respectively) (shown in Fig. 2).
Figure 2.
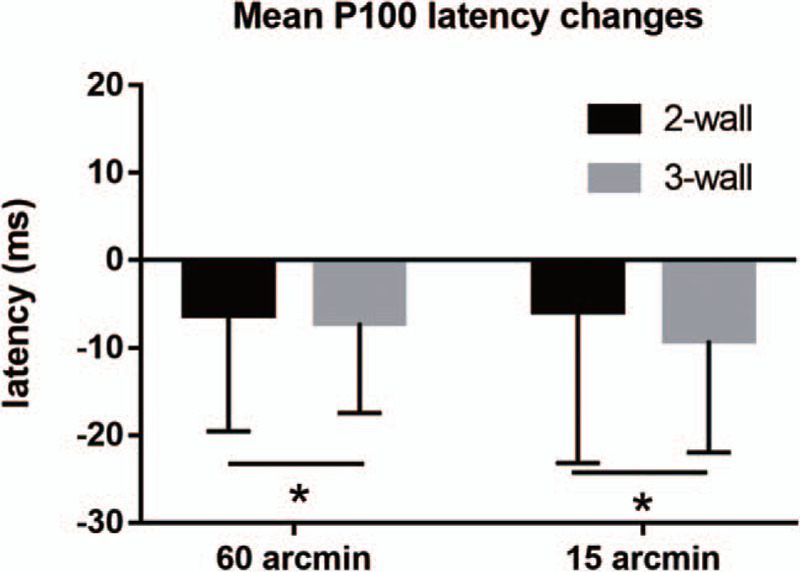
Postoperative improvement of P100 latency in 2-wall and 3-wall decompression at different stimulation pattern sizes (60 and 15 arcmin). ∗, P > .05.
3.4. Diplopia
Of the 23 patients with 2-wall decompression, 13 patients (56.52%) complained preoperative diplopia and of the 14 patients with 3-wall decompression, 9 patients (64.29%) complained preoperative diplopia, with no statistical difference between the 2 groups (P = .738). Six patients (66.67%) had persistent postoperative diplopia and 1 patient (20%) had new-onset diplopia in 3-wall decompression group, which were higher than in 3-wall decompression group (46.15% persistent postoperative diplopia and no new-onset diplopia). As to the resolution rate of diplopia, 2-wall decompression group (53.85%) was higher than 3-wall decompression group (33.33%).
4. Discussion
In this study, we demonstrated that both 2-wall and 3-wall decompression techniques could effectively improve proptosis, visual acuity impairment, visual field defect, and PVEP abnormality in patients with DON. However, 3-wall decompression provided better improvement of visual functions regarding the parameters of P100 amplitude. To the best of our knowledge, this is the first study that showed 3-wall decompression was superior to 2-wall in improving P100 amplitude. The results could provide theoretical guidance to the ophthalmologist for the choice of orbital decompression technique.
PVEP reflects the transmission function of visual signals from retinal ganglion cells to occipital visual cortex. Previous studies showed that PVEP was a valuable tool in the diagnosis and follow-up of DON, as DON was significantly associated with P100 amplitude loss and delayed deflection.[4,34,35] In addition, PVEP was able to identify even subclinical courses of DON with high sensitivity.[36] Several studies suggested that orbital decompression exert beneficial effects on improvement of P100 latency and amplitude in patients with DON.[35,37,38] Moreover, Tsaloumas et al[35] argued that P100 latency was shortened and P100 amplitude was increased after orbital decompression, but the improvement of P100 amplitude was more obvious than that of P100 latency, providing parallel results with our works, which indicated that P100 amplitude was more sensitive to the change of orbital pressure. Our study demonstrated that both surgical techniques significantly improved PVEP in patients with DON, in which the increase of P100 amplitude at 15 and 60 arcmin stimulation checkerboard size after 3-wall decompression was significantly higher than that after 2-wall decompression (P = .046 and .020, respectively).
As for the mechanism of DON under the crowded orbital condition, it has been demonstrated that the compression lesions of DON could have double effects of demyelination and axonal damage.[34,35] Because the loss of myelin sheath and the restored myelin sheath are extremely thin, uneven, and underdeveloped in insulation, the conduction of nerve impulse changes from jump conduction between Ranvier's nodes to crawling conduction along the repaired myelin sheath, thus slowing down the conduction and prolonging P100 latency. Another reason for P100 latency delay is the lack of nerve fibers with thicker diameters and faster conduction. In addition, the degeneration and necrosis of optic nerve axons result in the decrease of axonal number, consequently triggering the decrease of electrical activity and VEP amplitude. Both 2-wall and 3-wall orbital decompression effectively enlarges the orbital volume and decreases the intra-orbital pressure, which is more distinct in 3-wall decompression. Orbital decompression relieves the compression of the remaining axons and is beneficial to the regeneration of myelin sheath, and also the reconstruction of impulse conduction, thus could improve the optic nerve function and also relieve PVEP abnormalities. To our delight, the present study showed for the first time that 3-wall decompression was superior to 2-wall in improving P100 amplitude.
The main pathological changes of GO are inflammatory cell infiltration, edema, or extraocular muscle thickening of the extraocular muscles, which leads to the increased orbital contents not compatible with the volume of the orbital cavity, with the proptosis and the increase of the orbital pressure appearing.[39] Proptosis is an important index in the clinical diagnosis of GO, and also the evaluation of the therapeutic effect. The improvement of proptosis is dependent on the number of orbital bone walls decompressed and the amount of fat removed. It has been demonstrated that proptosis reduction was 7.2 ± 1.9 mm for 3-wall decompression, compared with 5.1 ± 1.3 mm for 2-wall decompression.[40] This difference was found to be even more marked in the present study, with an average 7.53 ± 3.40 mm of proptosis reduction following 3-wall decompression versus 5.16 ± 2.34 mm after 2-wall decompression (P = .011). Although the main goal of orbital decompression is not to relieve proptosis but to reserve the visual function, better cosmesis, and more eyeball retropulsion could be achieved by 3-wall decompression if the preoperative proptosis is severe.
According to the results of Korkmaz and Konuk,[40] 3-wall decompression was superior to 2-wall in improving both MD and PSD, while the vision acuity improvement is comparable. However, we demonstrated that 2-and 3-wall decompression effectively improved the MD, PSD, and vision acuity in a similar manner. Visual field is a subjective examination, and there are some patients whose accuracy is affected by poor cooperation. This is similar to the visual acuity test, so there is no doubt that the results are different from other researchers. Orbital decompression effectively decreases the intra-orbital pressure, showing beneficial effect on improvement of visual field in patients with DON. This is parallel with several studies.[37,40]
A large number of clinical evidences demonstrated that orbital inferior wall decompression, especially combined with medial wall decompression, was inclined to destroy the “orbital strut” of the supporting structure of the orbital inferomedial wall, resulting in the occurrence of diplopia and the displacement of the eyeball after surgery.[25,31] It was suggested that balanced decompression technique was associated with more favorable results regarding the postoperative new diplopia rates.[16,25] Our study also analyzed the occurrence of diplopia before and after surgery in both groups. We found that the resolved diplopia rate of 2-wall decompression (53.85%) was higher than that of 3-wall decompression (33.33%), and the new-onset diplopia rate of 3-wall decompression (20%) was significantly higher than that of 2-wall decompression (0%). In 3-wall decompression group, the development of postoperative significant new-onset diplopia could have been induced by inferomedial shifting of the orbital contents after simultaneous removal of the medial and inferior orbital walls. Preservation of the inferior orbital wall provides support for orbital soft tissues from below and prevents inferomedial shifting of the muscle cone and orbital fibro-connective septal system. This might be the reason for the absence of postoperative new-onset diplopia in 2-wall decompression group.
Interpretation of the results of this study must take into account its limitations. First, its retrospective nonrandomized study design results in incomplete and inaccurate data, such as the statistics of dyschromatopsia and subjective diplopia which is absent of objective data for color vision and diplopia assessment. Second, there were statistically significant differences in the amount of preoperative proptosis due to this design. While these preoperative differences make direct outcome comparisons difficult, we contend that the most critical determinant of operative success is the degree of postoperative change which we have showed in the results. Third, the sample size is relatively small. Because of the relative infrequency of DON, surgeon-dependent variations in surgical technique, and difficult follow up, the number of cases is relatively small. More cases can be accumulated unless the number of cases is selected for a longer period of time. To design a large multicenter prospective randomized comparative clinical trial is a pleasurable choice, which can also address the first problem above.
We conclude that 2-wall and 3-wall decompression effectively treated patients with DON. Both techniques significantly improved the visual acuity, visual field, and P100 latency in a similar manner. However, 3-wall decompression provides better improvement in P100 amplitude and proptosis, while the resolved diplopia rate is lower and new onset diplopia is more common with this surgical technique. Afterwards, preoperative ocular evaluation of patients with DON is essential and indispensable to the choice of surgical techniques and the optimal treatment.
Author contributions
Conceptualization: Sheng-Nan Cheng, Xing-Hua Wang, Fa-Gang Jiang.
Data curation: Sheng-Nan Cheng, Yue-Qi Yu, Ya-Yan You, Jin Chen, Xiao-Huan Pi, Xing-Hua Wang.
Formal analysis: Sheng-Nan Cheng.
Funding acquisition: Xing-Hua Wang.
Investigation: Ya-Yan You, Jin Chen.
Methodology: Sheng-Nan Cheng, Fa-Gang Jiang.
Software: Yue-Qi Yu, Xiao-Huan Pi.
Supervision: Fa-Gang Jiang.
Writing – original draft: Sheng-Nan Cheng.
Writing – review & editing: Sheng-Nan Cheng, Fa-Gang Jiang.
Footnotes
Abbreviations: BCVA = best-corrected visual acuity, DON = dysthyroid optic neuropathy, MD = mean deviation, PSD = pattern standard deviation, PVEP = pattern-reversed visual evoked potential.
How to cite this article: Cheng SN, Yu YQ, You YY, Chen J, Pi XH, Wang XH, Jiang FG. Comparison of 2-wall versus 3-wall orbital decompression against dysthyroid optic neuropathy in visual function: a retrospective study in a Chinese population. Medicine. 2021;100:8(e24513).
X-HW and F-GJ authors have contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (No.81900912). The funding organization had no role in the design or conduct of this study.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Trobe JD. Optic nerve involvement in dysthyroidism. Ophthalmology 1981;88:488–92. [DOI] [PubMed] [Google Scholar]
- [2].Bartalena L, Wiersinga WM, Pinchera A. Graves’ ophthalmopathy: state of the art and perspectives. J Endocrinol Invest 2004;27:295–301. [DOI] [PubMed] [Google Scholar]
- [3].Khong JJ, Finch S, De Silva C, et al. Risk factors for Graves’ orbitopathy; the australian thyroid-associated orbitopathy research (ATOR) study. J Clin Endocrinol Metab 2016;101:2711–20. [DOI] [PubMed] [Google Scholar]
- [4].Neigel JM, Rootman J, Belkin RI, et al. Dysthyroid optic neuropathy. Ophthalmology 1988;95:1515–21. [DOI] [PubMed] [Google Scholar]
- [5].Soni CR, Johnson LN. Visual neuropraxia and progressive vision loss from thyroid-associated stretch optic neuropathy. Eur J Ophthalmol 2010;20:429–36. [DOI] [PubMed] [Google Scholar]
- [6].Jou IM, Lai KA, Shen CL, et al. Changes in conduction, blood flow, histology, and neurological status following acute nerve-stretch injury induced by femoral lengthening. J Orthop Res 2000;18:149–55. [DOI] [PubMed] [Google Scholar]
- [7].Trobe JD, Glaser JS, Laflamme P. Dysthyroid optic neuropathy. Clinical profile and rationale for management. Arch Ophthalmol 1978;96:1199–209. [DOI] [PubMed] [Google Scholar]
- [8].Regensburg NI, Wiersinga WM, Berendschot TT, et al. Do subtypes of graves’ orbitopathy exist? Ophthalmology 2011;118:191–6. [DOI] [PubMed] [Google Scholar]
- [9].Riemann CD, Foster JA, Kosmorsky GS. Direct orbital manometry in patients with thyroid-associated orbitopathy. Ophthalmology 1999;106:1296–302. [DOI] [PubMed] [Google Scholar]
- [10].Otto AJ, Koornneef L, Mourits MP, et al. Retrobulbar pressures measured during surgical decompression of the orbit. Br J ophthalmol 1996;80:1042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yanik B, Conkbayir I, Acaroglu G, et al. Graves’ Ophthalmopathy: comparison of the Doppler sonography parameters with the clinical activity score. J Clin Ultrasound 2005;33:375–80. [DOI] [PubMed] [Google Scholar]
- [12].Perez-Lopez M, Sales-Sanz M, Rebolleda G, et al. Retrobulbar ocular blood flow changes after orbital decompression in Graves’ ophthalmopathy measured by color Doppler imaging. Invest Ophthalmol Vis Sci 2011;52:5612–7. [DOI] [PubMed] [Google Scholar]
- [13].Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the management of Graves’ orbitopathy. Eur Thyroid J 2016;5:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tagami M, Honda S, Azumi A. Preoperative clinical factors and visual outcomes following orbital decompression with dysthyroid optic neuropathy. BMC Ophthalmol 2020;20:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Blandford AD, Zhang D, Chundury RV, et al. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol 2017;12:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cubuk MO, Konuk O, Unal M. Orbital decompression surgery for the treatment of Graves’ ophthalmopathy: comparison of different techniques and long-term results. Int J Ophthalmol 2018;11:1363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jefferis JM, Jones RK, Currie ZI, et al. Orbital decompression for thyroid eye disease: methods, outcomes, and complications. Eye (Lond) 2018;32:626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liang QW, Yang H, Luo W, et al. Effect of orbital decompression on dysthyroid optic neuropathy: a retrospective case series. Medicine (Madr) 2019;98:e14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang EL, Bernardino CR, Rubin PA. Transcaruncular orbital decompression for management of compressive optic neuropathy in thyroid-related orbitopathy. Plast Reconstr Surg 2003;112:739–47. [DOI] [PubMed] [Google Scholar]
- [20].Ben Simon GJ, Wang L, McCann JD, et al. Primary-gaze diplopia in patients with thyroid-related orbitopathy undergoing deep lateral orbital decompression with intraconal fat debulking: a retrospective analysis of treatment outcome. Thyroid 2004;14:379–83. [DOI] [PubMed] [Google Scholar]
- [21].Ben Simon GJ, Syed HM, Lee S, et al. Strabismus after deep lateral wall orbital decompression in thyroid-related orbitopathy patients using automated hess screen. Ophthalmology 2006;113:1050–5. [DOI] [PubMed] [Google Scholar]
- [22].Goldberg RA, Perry JD, Hortaleza V, et al. Strabismus after balanced medial plus lateral wall versus lateral wall only orbital decompression for dysthyroid orbitopathy. Ophthalmic Plast Reconstr Surg 2000;16:271–7. [DOI] [PubMed] [Google Scholar]
- [23].Leone CR, Jr, Piest KL, Newman RJ. Medial and lateral wall decompression for thyroid ophthalmopathy. Am J Ophthalmol 1989;108:160–6. [DOI] [PubMed] [Google Scholar]
- [24].Graham SM, Brown CL, Carter KD, et al. Medial and lateral orbital wall surgery for balanced decompression in thyroid eye disease. Laryngoscope 2003;113:1206–9. [DOI] [PubMed] [Google Scholar]
- [25].Unal M, Leri F, Konuk O, et al. Balanced orbital decompression combined with fat removal in Graves ophthalmopathy: do we really need to remove the third wall? Ophthalmic Plast Reconstr Surg 2003;19:112–8. [DOI] [PubMed] [Google Scholar]
- [26].Schaefer SD, Soliemanzadeh P, Della Rocca DA. Endoscopic and transconjunctival orbital decompression for thyroid-related orbital apex compression. Laryngoscope 2003;113:508–13. [DOI] [PubMed] [Google Scholar]
- [27].Neugebauer A, Nishino K, Neugebauer P, et al. Effects of bilateral orbital decompression by an endoscopic endonasal approach in dysthyroid orbitopathy. Br J Ophthalmol 1996;80:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fatourechi V, Bartley GB, Garrity JA, et al. Transfrontal orbital decompression after failure of transantral decompression in optic neuropathy of Graves’ disease. Mayo Clin Proc 1993;68:552–5. [DOI] [PubMed] [Google Scholar]
- [29].Bartalena L, Baldeschi L, Dickinson AJ, et al. Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid 2008;18:333–46. [DOI] [PubMed] [Google Scholar]
- [30].McKeag D, Lane C, Lazarus JH, et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol 2007;91:455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goldberg RA, Shorr N, Cohen MS. The medical orbital strut in the prevention of postdecompression dystopia in dysthyroid ophthalmopathy. Ophthalmic Plast Reconstr Surg 1992;8:32–4. [DOI] [PubMed] [Google Scholar]
- [32].Ferris FL, Kassoff A, Bresnick GH, et al. New visual acuity charts for clinical research. Am J Ophthalmol 1982;94:91–6. [PubMed] [Google Scholar]
- [33].Johnson KTM, Wittig A, Loesch C, et al. A retrospective study on the efficacy of total absorbed orbital doses of 12, 16 and 20 Gy combined with systemic steroid treatment in patients with Graves’ orbitopathy. Graefes Arch Clin Exp Ophthalmol 2010;248:103–9. [DOI] [PubMed] [Google Scholar]
- [34].Setala K, Raitta C, Valimaki M, et al. The value of visual evoked potentials in optic neuropathy of Graves’ disease. J Endocrinol Invest 1992;15:821–6. [DOI] [PubMed] [Google Scholar]
- [35].Tsaloumas MD, Good PA, Burdon MA, et al. Flash and pattern visual evoked potentials in the diagnosis and monitoring of dysthyroid optic neuropathy. Eye (Lond) 1994;8:638–45. [DOI] [PubMed] [Google Scholar]
- [36].Salvi M, Spaggiari E, Neri F, et al. The study of visual evoked potentials in patients with thyroid-associated ophthalmopathy identifies asymptomatic optic nerve involvement. J Clin Endocrinol Metab 1997;82:1027–30. [DOI] [PubMed] [Google Scholar]
- [37].Liao SL, Chang TC, Lin LL. Transcaruncular orbital decompression: an alternate procedure for graves ophthalmopathy with compressive optic neuropathy. Am J Ophthalmol 2006;141:810–8. [DOI] [PubMed] [Google Scholar]
- [38].Lipski A, Eckstein A, Esser J, et al. Course of pattern-reversed visual evoked cortical potentials in 30 eyes after bony orbital decompression in dysthyroid optic neuropathy. Br J Ophthalmol 2011;95:222–6. [DOI] [PubMed] [Google Scholar]
- [39].Miskiewicz P, Rutkowska B, Jablonska A, et al. Complete recovery of visual acuity as the main goal of treatment in patients with dysthyroid optic neuropathy. Endokrynol Pol 2016;67:166–73. [DOI] [PubMed] [Google Scholar]
- [40].Korkmaz S, Konuk O. Surgical treatment of dysthyroid optic neuropathy: long-term visual outcomes with comparison of 2-wall versus 3-wall orbital decompression. Curr Eye Res 2015;41:1–6. [DOI] [PubMed] [Google Scholar]


