Abstract
The subtypes of serous ovarian tumors (SOTs), including benign serous cystadenoma, serous borderline tumor (SBT), low-grade serous ovarian carcinoma (LGSC), and high-grade serous ovarian carcinoma (HGSC), remain poorly understood. Herein, we aimed to characterize the cell adhesion molecule 1 (CADM1)/signal transducer and activator of transcription 3 (STAT3)/human epidermal growth factor receptor 2 (HER2) axis and identify its clinical significance in patients with serous cystadenoma, SBT, LGSC, and HGSC.
The immunohistochemical expression of CADM1, HER2, and STAT3 was assessed in 180 SOT specimens, and its association with clinical data was determined.
High levels of CADM1 expression were detected in 100% of serous cystadenomas and 83.33% of SBTs, while a loss of CADM1 expression was observed in 44% of LGSCs and 72.5% of HGSCs. Relative to the levels in benign cystadenomas and SBTs, higher levels of HER2 and STAT3 expression were observed in LGSCs and aggressive HGSCs. Furthermore, the expression profile of the CADM1/HER2/STAT3 axis was significantly associated with histologic type, International Federation of Gynecology and Obstetrics stage, and lymph node metastasis in patients with SOT.
Our study identified the changes in the CADM1/HER2/STAT3 axis that were closely associated with the clinical behavior of SOTs. These molecular data may provide new insights into SOT carcinogenesis and aid in the diagnosis and treatment of patients with SOT.
Keywords: cell adhesion molecule 1, human epidermal growth factor receptor 2, serous ovarian tumor, signal transducer and activator of transcription 3
1. Introduction
Serous ovarian tumors (SOTs) are the most common histological tumor type of the extrauterine female genital tract. SOTs are subdivided into benign serous cystadenomas, serous borderline tumors (SBTs), low-grade serous ovarian carcinomas (LGSCs), and high-grade serous ovarian carcinomas (HGSCs) according to their malignant potential and clinical behavior.[1,2] Although the steady pace of scientific discovery has fueled recent improvements in the outcomes for patients with many other cancers, the overall survival of patients with malignant ovarian tumors has not fundamentally changed over the last 50 years.[3,4] Several emerging lines of evidence indicate that some traditional tenets of ovarian carcinogenesis and its cellular origin are fundamentally flawed.[5] Therefore, a better understanding of the molecular events associated with SOTs and their biological influence on clinical behavior is urgently needed to provide new insight into SOT carcinogenesis and clinical developments to improve the diagnosis and treatment of patients with SOTs.
Several critical molecular abnormalities, including mutations in the tumor suppressor genes tumor protein p53, breast cancer susceptibility gene 1/2, and phosphatase and tensin homolog, have been reported in HGSCs, leading to a paradigm shift in our conceptualization of the carcinogenesis and histogenesis of serous carcinomas.[6–8] However, the molecular events related to benign, borderline, and malignant SOTs remain ill-defined. Emerging evidence indicates that the cell adhesion molecule 1 (CADM1)/human epidermal growth factor receptor 2 (HER2)/signal transducer and activator of transcription 3 (STAT3) axis plays key roles in the tumor progression and metastasis of various epithelial cancers.[9,10] CADM1, also known as Necl2, TSLC1, IGSF4, RA175, and SynCam, is an immunoglobulin superfamily adhesion molecule that has been identified as a tumor suppressor. In squamous cell carcinomas (SqCC) of the lungs, head and neck, esophagus, and cervix, the extracellular domain of CADM1 forms a complex with HER2 at the cell surface to regulate downstream STAT3 activity, reducing tumor growth and metastases.[9] In breast and lung adenocarcinoma, studies have reported that abnormalities in the CADM1/HER2/STAT3 axis manifest as more aggressive biological behavior.[11] Despite these findings, information about the molecular events involving the CADM1/STAT3/HER2 axis among SOT subgroups is still lacking.
The goal of this study was to identify the spectrum of CADM1/STAT3/HER2 axis characteristics in benign, borderline, and malignant SOTs and analyze their relationship with patient clinicopathological characteristics. The expression profiles of CADM1, HER2, and STAT3 were established by immunohistochemical analysis of specimens from 180 patients, including those with benign serous cystadenomas (n = 20), borderline SBTs (n = 30), malignant LGSCs (n = 50), and HGSCs (n = 80).
2. Materials and methods
2.1. Patient samples
A total of 180 SOT specimens were collected from the Department of Surgery of Hunan Provincial Cancer Hospital from 2014 to 2017. The tumor samples used for this study were randomly obtained from primary tumors during primary surgery before patients had received any chemotherapy or radiotherapy. The clinical information of the patients was obtained from patient records. The histopathological diagnosis, including tumor grade and stage, was determined by independent staff pathologists as part of the clinical diagnosis. This study was approved by the ethics review committee of Hunan Provincial Cancer Hospital and the Second Affiliated Hospital of University of South China and was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. Written informed consent was collected from all participants before the study.
2.2. Immunohistochemistry
All SOT surgical specimens were fixed in 10% neutral buffered formalin and embedded in paraffin wax. Specimens were sliced into 4-μm-thick sections, stained with hematoxylin and eosin, and then reviewed by a senior pathologist. For immunohistochemical staining, the tissue sections were deparaffinized in xylol and rehydrated in graded alcohol. Antigen retrieval was performed at 95°C for 10 minutes in 10 mM citrate buffer (pH 6). Rabbit anti-human CADM1 antibody (clone ab3910; Abcam, Cambridge, MA), mouse anti-human HER2 antibody (clone 3B5; Abcam), and mouse anti-human STAT3 antibody (clone 3F4; Abcam) were diluted 1:1500, 1:1000, and 1:2000, respectively, and incubated with the tissue sections mounted on slides for 60 minutes at room temperature. Sections were subsequently washed, developed using the UltraSensitiveTM S-P Kit (KIT-9710, Fuzhou Maixin, Fujian, China), stained with DAB (Fuzhou Maixin), and counterstained with hematoxylin to highlight cell nuclei. Negative control slides were processed in the absence of primary antibody, secondary antibody, and chromogenic substrate, or with isotype control IgG to ensure specificity.
2.3. Immunohistochemical analysis
Immunohistochemical staining results were examined, and stained sections were photographed using an integrated microscope (Olympus BX43; Olympus, Tokyo, Japan). Proportional expression (0%–100%) and staining intensity (0–3+) in 5 random 40 × fields were examined by 3 observers in a blind fashion. The results were dichotomized into expression versus non-expression; a staining intensity of >1+ was considered indicative of protein expression. CADM1 positive expression was defined as the presence of cytoplasm or cell membrane staining in ≥30% of tumor cells. HER2 positive expression was defined as the presence of uniform intense cell membrane staining in ≥10% of tumor cells, and STAT3 positive expression was defined as the presence of cytoplasmic or nuclear staining in ≥25% of tumor cells.
2.4. Statistical analysis
All statistical analyses were performed using Prism Version 8.0 software (GraphPad Software, La Jolla, CA). Data are presented as numbers and/or percentages of each variable. Comparisons of categorical variables between groups were performed using the Chi-squared test. Pearson correlation analysis was used to estimate the relationships among immunohistochemical protein expression results. The threshold for statistical significance was set at P < .05.
3. Results
3.1. Baseline characteristics of the patients
In the current study, 180 SOT cases were analyzed, including 20 (11.11%) serous cystadenoma, 30 (16.67%) SBT, 50 (27.78%) LGSC, and 80 (44.44%) HGSC ovarian neoplasm cases. The patient ages ranged from 23 to 67 years, with a median age of 53 years. Patients’ characteristics are listed in Table 1.
Table 1.
Baseline characteristics.
| Patients | n = 180 |
| Age at biopsy - yr (range) | 53 (23–67) |
| Serous cystadenoma | 20 (11.11) |
| SBT | 30 (16.67) |
| LGSC | 50 (27.78) |
| HGSC | 80 (44.44) |
| Histological grade | |
| Benign | 20 (11.11) |
| Borderline | 30 (16.67) |
| Low | 50 (27.78) |
| High | 80 (44.44) |
| FIGO Stage∗ | |
| I/II | 47 (26.11) |
| III/IV | 83 (46.11) |
| Lymph node matastasis∗ | |
| No | 52 (28.89) |
| Yes | 78 (43.33) |
Data are presented as n (%) or median (range).
Values include those from the low-grade serous ovarian carcinoma, and high-grade serous ovarian carcinoma patients (N = 130).
FIGO, International Federation of Gynecology and Obstetrics, HGSC = high-grade serous ovarian carcinoma, LGSC = low-grade serous ovarian carcinoma, SBT = serous borderline tumor.
3.2. CADM1 expression
We evaluated the expression patterns of CADM1 by analyzing its level in benign serous cystadenomas, borderline SBTs, malignant LGSCs, and HGSCs. Representative images of CAMD1 expression in SOTs are shown in Figure 1A. Abundant CADM1 expression was detected in 100% (20/20) of serous cystadenomas and 83.33% (25/30) of SBTs. In contrast, a loss of CADM1 expression was observed in 44% (22/50) of LGSCs and 72.5% (58/80) of HGSCs (Fig. 1B). A positive CADM1 expression ratio was twice as prevalent in benign and borderline cases as in malignant primary cases (P < .0001, Table 2), indicating that the loss of CADM1 expression was associated with pathological SOT stage.
Figure 1.
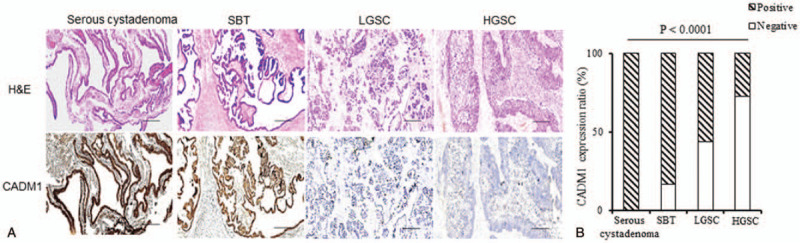
Characterization of CMDA1 expression in serous cystadenoma, serous borderline tumor, low-grade serous ovarian carcinoma, and high-grade serous ovarian carcinoma patients. (A) Representative histological images of Hematoxylin-Eosin staining and CMDA1 staining. Scale bars: 50 μM. (B) cell adhesion molecule 1 expression ratio in the serous ovarian tumor patients. Comparisons were performed using the Chi-squared test. P < .05 was considered statistically significant. HGSC = high-grade serous ovarian carcinoma, LGSC = low-grade serous ovarian carcinoma, SBT = serous borderline tumor.
Table 2.
Expression profiles of cell adhesion molecule 1, signal transducer and activator of transcription 3, and human epidermal growth factor receptor in serous cystadenoma, serous borderline tumor, low-grade serous ovarian carcinoma, and high-grade serous ovarian carcinoma patients.
| CADM1 n (%) | HER2 n (%) | STAT3 n (%) | ||||||||
| Clinical classification | n (total) | Negative | Positive | P-value | Negative | Positive | P value | Negative | Positive | P value |
| Serous cystadenoma | 20 | 0 (0) | 20 (100) | < .0001 | 20 (100) | 0 (0) | .002 | 18 (90) | 2 (10) | < .0001 |
| SBT | 30 | 5 (16.67) | 25 (83.33) | 28 (93.33) | 2 (6.67) | 22 (73.33) | 8 (26.67) | |||
| LGSC | 50 | 22 (44) | 28 (56) | 44 (88) | 6 (12) | 24 (48) | 26 (52) | |||
| HGSC | 80 | 58 (72.5) | 22 (27.5) | 57 (71.25) | 23 (28.75) | 16 (20) | 64 (80) | |||
Data are presented as n (%). Comparisons were performed using the Chi-squared test. P < .05 was considered statistically significant.
CADM1 = cell adhesion molecule 1, HER2 = human epidermal growth factor receptor 2, HGSC = high-grade serous ovarian carcinoma, LGSC = low-grade serous ovarian carcinoma, SBT = serous borderline tumor, STAT3 = signal transducer and activator of transcription 3.
3.3. HER2 expression
Next, we analyzed HER2 expression in the SOTs, and representative images are shown in Figure 2A. Immunohistochemically detectable HER2 protein expression (scored from >1+ to 3+) was noted in 12% of LGSCs (6/50) and in 28.75% of HGSCs (23/80), but only in 6.67% of SBTs (2/30) (Fig. 2B). A complete lack of HER2 staining was observed in benign serous cystadenoma biopsies (0/20) (Table 2), suggesting that an increased expression of HER2 might impart malignant characteristics to SOTs.
Figure 2.
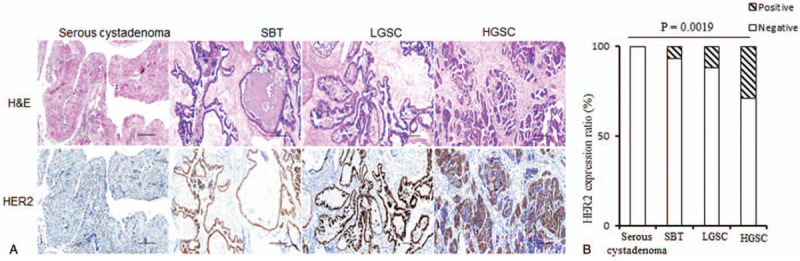
Characterization of human epidermal growth factor receptor 2 (HER2) expression in serous cystadenoma, serous borderline tumor , low-grade serous ovarian carcinoma, and high-grade serous ovarian carcinoma patients. (A) Representative histological images of Hematoxylin-Eosin staining and HER2 staining. Scale bars: 50 μM. (B) HER2 expression ratio in the serous ovarian tumor patients. Comparisons were performed using the Chi-squared test. P < .05 was considered statistically significant. HGSC = high-grade serous ovarian carcinoma, LGSC = low-grade serous ovarian carcinoma, SBT = serous borderline tumor.
3.4. STAT3 expression
Next, we examined whether STAT3 expression patterns were altered in patients with SOTs. We found that 90% of serous cystadenomas (18/20) and 73.33% of SBTs (22/30) lacked STAT3 expression (Figs. 3A and B). High levels of STAT3 staining were present in 52% of LGSCs (26/50) and 80% of progressive HGSCs (64/80) (Table 2). Chi-squared analysis confirmed the increasing trend of changes in STAT3 levels with tumor progression (P < .0001), indicating that STAT3 may play a role in the progression of SOTs.
Figure 3.
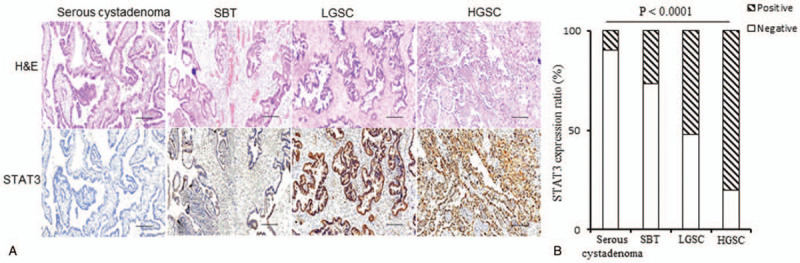
Characterization of signal transducer and activator of transcription 3 (STAT3) expression in serous cystadenoma, serous borderline tumor, low-grade serous ovarian carcinoma, and high-grade serous ovarian carcinoma patients. (A) Representative histological images of Hematoxylin-Eosin staining and STAT3 staining. Scale bars: 50 μM. (B) STAT3 expression ratio in the serous ovarian tumor patients. Comparisons were performed using the Chi-squared test. P < .05 was considered statistically significant. HGSC = high-grade serous ovarian carcinoma, LGSC = low-grade serous ovarian carcinoma, SBT = serous borderline tumor.
3.5. Relationships among the expression profiles of CADM1, HER2, and STAT3
Previous studies have reported that the HER2-CADM1 complex at the cell surface mediates STAT3 activity in various cancers.[9] In our cohort of 180 patients with serous cystadenoma, SBT, LGSC, and HGSC, CADM1 expression was detected in 100%, 83.33%, 56%, and 27.5% of cases, respectively; HER2 was detected in 0%, 6.67%, 12%, and 28.75% of cases, respectively; and STAT3 was detected in 10%, 26.67%, 52%, and 80% of cases, respectively (Tables 2 and 3). We found that the CADM1 expression ratio had a significant negative relationship with that of HER2 (Pearson r = –0.9762; P = .02), and STAT3 (Pearson r = –0.999; P < .0001). In contrast, a significant positive relationship was observed between the expression ratios of HER2 and STAT3 (Pearson r = 0.9784; P = .02).
Table 3.
Association between the cell adhesion molecule 1/human epidermal growth factor receptor 2/signal transducer and activator of transcription 3 axis and clinico-pathological factors.
| CADM1 n (%) | HER2 n (%) | STAT3 n (%) | ||||||||
| Clinical features | n (total) | Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
| Age (yr) | .45 | .12 | .18 | |||||||
| ≤ 50 | 93 | 41 (44.09) | 52 (55.91) | 81 (87.1) | 12 (12.9) | 46 (49.46) | 47 (50.54) | |||
| > 50 | 87 | 44 (50.57) | 43 (49.43) | 68 (78.16) | 19 (21.84) | 34 (39.08) | 53 (60.92) | |||
| Histological grade | < .0001 | .002 | < .0001 | |||||||
| Benign | 20 | 0 (0) | 20 (100) | 20 (100) | 0 (0) | 18 (90) | 2 (10) | |||
| Borderline | 30 | 5 (16.67) | 25 (83.33) | 28 (93.33) | 2 (6.67) | 22 (73.33) | 8 (26.67) | |||
| Low | 50 | 22 (44) | 28 (56) | 44 (88) | 6 (12) | 34 (48) | 26 (52) | |||
| High | 80 | 58 (72.5) | 22 (27.5) | 57 (71.25) | 23 (28.75) | 16 (20) | 64 (80) | |||
| FIGO Stage | .005 | .02 | < .0001 | |||||||
| I/II | 47 | 21 (44.68) | 26 (55.32) | 42 (89.36) | 5 (10.64) | 26 (55.32) | 21 (44.68) | |||
| III/IV | 83 | 59 (71.08) | 24 (28.92) | 59 (71.08) | 24 (28.92) | 14 (16.87) | 69 (83.13) | |||
| Lymph node metastasis | .004 | .009 | < .0001 | |||||||
| No | 78 | 40 (51.28) | 38 (48.72) | 67 (85.90) | 11 (14.10) | 34 (43.59) | 44 (56.41) | |||
| Yes | 52 | 40 (76.92) | 12 (23.08) | 34 (65.38) | 18 (34.62) | 6 (11.54) | 46 (88.46) | |||
Data are presented as n (%). Comparisons were performed using the Chi-squared test. P < .05 was considered statistically significant.
CADM1 = cell adhesion molecule 1, FIGO, International Federation of Gynecology and Obstetrics, HER2 = human epidermal growth factor receptor 2, HGSC = high-grade serous ovarian carcinoma, LGSC = low-grade serous ovarian carcinoma, SBT = serous borderline tumor, STAT3 = signal transducer and activator of transcription.
3.6. Association between the CADM1/HER2/STAT3 axis and clinicopathological factors
In SOTs, the expression level of the CADM1/HER2/STAT3 axis was associated with the histological type, International Federation of Gynecology and Obstetrics (FIGO) stage, and lymph node metastasis (Table 3). No significant association was identified between the expression profile of the axis and patient age at diagnosis. The positive expression ratios of CADM1 protein in patients with FIGO stage I/II tumors were significantly higher than those in patients with FIGO stage III/IV tumors, and low CADM1 expression levels were associated with lymph node metastases (Table 3). In contrast, the positive expression ratios of HER2 and STAT3 were significantly higher in patients with aggressive SOTs and lymph node metastases.
4. Discussion
In this study, we presented the spectrum of molecular changes in the CADM1/STAT3/HER2 axis in SOTs and their clinical significance by analyzing a large number of patients with various subtypes of SOTs. To summarize the main findings, the benign serous cystadenoma and borderline SBT cases exhibited high levels of CADM1 expression but absent or low levels of HER2 and STAT3 expression. Malignant LGSC or HGSC showed the reverse expression patterns, with low levels of CADM1 expression but high levels of HER2 and STAT3 expression. Moreover, the expression profiles of the CADM1/HER2/STAT3 axis were significantly associated with histologic type, FIGO stage, and lymph node metastasis in the SOT subgroups.
Recent morphologic and molecular event studies have led to a shift in our understanding of the carcinogenesis and histogenesis of SOTs.[12] Although histologic pathology is a powerful tool for unraveling the origins of SOTs, detailed molecular studies are needed to reduce ovarian cancer-related mortality. Progress is being made toward a better understanding of the fundamental biology of this disease. For example, studies have shown that SBTs and LGSCs have relatively frequent point mutations in kirsten rat sarcoma 2 viral oncogene homolog and v-raf murine sarcoma viral oncogene homolog B1 genes,[13] while tumor protein p53 and breast cancer susceptibility gene 1/2 mutations in HGSCs are associated with tumor-infiltrating lymphocytes and poor prognosis.[6,14] CADM1 is a neural tissue-specific cell-cell adhesion molecule that mediates hemophilic and heterophilic cell-cell adhesion in a Ca2+-independent manner. In glioblastoma and SqCC of the lungs, head and neck, esophagus, and cervix, CADM1 has been shown to be downregulated, and this reduction is associated with cancer progression and poor prognosis.[11,15] This finding was supported by our observation of high CADM1 expression in almost all serous cystadenomas and SBTs. In contrast, a decrease or loss of CADM1 expression was identified in malignant tumor samples from patients with LGSC and HGSC (Fig. 1), and a negative correlation between CADM1 expression and HER2 or STAT3 expression was detected in patients with SOTs. In addition, our study found that the loss of CADM1 expression was associated with aggressive tumor behavior and lymph node metastases (Tables 2 and 3). These findings indicated that a loss of CADM1 expression may play a role in SOT carcinogenesis and suggested that CADM1 may be a novel molecular biomarker for identifying and monitoring disease progression in patients with SOTs.
Clinical studies have shown high levels of HER2 and STAT3 expression in human HGSCs as well as in cancers of the breast, colon, lung, prostate, and cervix and that they are associated with tumor progression and decreased survival.[16–18] HER2 is a membrane tyrosine kinase and oncogene that is activated by forming complexes with other membrane receptors. Constitutively active STAT3 has been shown to cause tumor resistance to radiation, chemotherapy, and immunotherapy.[19] Various HER2-based radiotheranostic strategies (a combination of non-invasive diagnostic imaging and radionuclide therapy with the same, but differently radio-labeled, carrier agent) and STAT3 inhibitors have been developed and were shown to have excellent efficacy for inhibiting tumor growth and metastasis in vitro and in vivo.[20–23] Therefore, given the existing evidence indicating that HER2 and STAT3 are overexpressed in aggressive HGSCs, we considered it imperative to understand the changes in these 2 molecules in patients with premalignant and malignant SOTs. By analyzing tissue specimens from benign, borderline, and malignant SOTs, we found that the benign and borderline cases lacked or had low levels of HER2 and STAT3 expression. However, high HER2 and STAT3 expression levels were present in LGSCs and HGSCs, which aligned with the diminished CADM1 levels in these cases. This is partially consistent with the findings of a recent study indicating that CADM1 interactions with HER2 and ITGα6β4 regulate downstream STAT3 activity in SqCC.[11,15] Furthermore, we found a close, positive correlation between HER2 and STAT3 expression, and the expression level of the CADM1/HER2/STAT3 axis was found to be associated with histological type, FIGO stage, and lymph node metastasis in patients with SOTs. Based on these results, we suggest that the CADM1/HER2/STAT3 axis might offer promising diagnostic biomarkers and therapeutic targets that may contribute to the development of precision medicine for SOTs.
The present study has several limitations that should be mentioned. First, the lack of clinical information after treatment intervention meant that we could not fully assess the possible roles of the CADM1/HER2/STAT3 axis; thus, we could not examine the potential clinical benefits of modifying the molecular events. Second, the exact mechanism underlying the changes in the CADM1/HER2/STAT3 axis remains elusive. Third, common to all observational studies, we could not infer a causal relationship between the alterations in the CADM1/HER2/STAT3 axis and the clinical behavior of the SOTs. Decisive illustration of the underlying mechanism and relationships might strengthen our understanding of SOT carcinogenesis and enable us to exploit the full potential of the molecular events. Nevertheless, our findings expand our knowledge and extend molecular networks in the gene regulation of SOTs. Further exploration into these basic aspects is ongoing and will be reported in the near future.
5. Conclusion
We showed that the molecular events involving the CADM1/HER2/ STAT3 axis in patients with progressing or metastasizing SOTs were HER2 and STAT3 activation and CADM1 suppression or loss. These findings represent an important step toward a better understanding of the fundamental biology of SOTs, and provide a basis for the development of novel diagnostic and therapeutic strategies. A greater understanding of the molecular events involved in the pathogenic transformation of benign to malignant SOTs will improve our ability to predict the behavior of SOTs and develop precision diagnostic and treatment systems to improve the survival rate of patients with SOTs.
Acknowledgments
The authors thank the staff at Hunan Provincial Cancer Hospital for collecting high-quality samples and excellent technical assistance. This study was partially supported by the Research Fund of Hunan Health Commission of China (No. C2019105).
Author contributions
Conceptualization: Dan Wu, Lin Xie, Jianbin Zhou.
Data acquisition and Formal analysis: Dan Wu, Yuzhou Lei, Qin Liu, Hua Hu, Huanhuan Li.
Data curation: Dan Wu, Yuzhou Lei, Qin Liu, Huanhuan Li, Lin Xie.
Formal analysis: Dan Wu, Yuzhou Lei, Lin Xie.
Funding acquisition: Jianbin Zhou.
Investigation: Yuzhou Lei, Qin Liu, Hua Hu, Huanhuan Li, Jianbin Zhou.
Methodology: Qin Liu.
Project administration: Dan Wu, Yuzhou Lei, Qin Liu, Hua Hu, Huanhuan Li, Jianbin Zhou.
Supervision: Lin Xie, Jianbin Zhou.
Validation: Qin Liu, Hua Hu, Huanhuan Li, Lin Xie, Jianbin Zhou.
Visualization: Yuzhou Lei, Qin Liu, Hua Hu, Huanhuan Li.
Writing – original draft: Dan Wu, Lin Xie.
Writing – review & editing: Dan Wu, Yuzhou Lei, Qin Liu, Hua Hu, Huanhuan Li, Lin Xie, Jianbin Zhou.
Footnotes
Abbreviations: CADM1 = cell adhesion molecule 1, FIGO = International Federation of Gynecology and Obstetrics, HER2 = human epidermal growth factor receptor 2, HGSC = high-grade serous ovarian carcinoma, LGSC = low-grade serous ovarian carcinoma, SBT = serous borderline tumor, SOT = serous ovarian tumor, SqCC = squamous cell carcinomas, STAT3 = signal transducer and activator of transcription 3.
How to cite this article: Wu D, Lei Y, Liu Q, Hu H, Li H, Xie L, Zhou J. Characterization and clinical significance of the CADM1/HER2/STAT3 axis in serous ovarian tumors. Medicine. 2021;100:8(e23777).
This study was partially supported by the Research Fund of Hunan Health Commission of China (No. C2019105) and the Initiative for Realizing Diversity in the Research Environment.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Matulonis UA, Sood AK, Fallowfield L, et al. Ovarian cancer. Nat Rev Dis Primers 2016;2:16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vaughan S, Coward JI, Bast RC, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 2011;11:719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dabi Y, Huchon C, Ouldamer L, et al. Patients with stage IV epithelial ovarian cancer: understanding the determinants of survival. J Transl Med 2020;18:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943–53. [DOI] [PubMed] [Google Scholar]
- [5].Ricci F, Affatato R, Carrassa L, et al. Recent insights into mucinous ovarian carcinoma. Int J Mol Sci 2018;19:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bougeard G, Renaux-Petel M, Flaman JM, et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol 2015;33:2345–52. [DOI] [PubMed] [Google Scholar]
- [7].Soslow RA, Han G, Park KJ, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol 2012;25:625–36. [DOI] [PubMed] [Google Scholar]
- [8].Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol 2011;42:918–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vallath S, Sage EK, Kolluri KK, et al. CADM1 inhibits squamous cell carcinoma progression by reducing STAT3 activity. Sci Rep 2016;6:24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Momose K, Minami A, Shimono Y, et al. miR-214 and hypoxia down-regulate Necl-2/CADM1 and enhance ErbB2/ErbB3 signaling. Genes Cells 2013;18:195–202. [DOI] [PubMed] [Google Scholar]
- [11].Mao X, Seidlitz E, Ghosh K, et al. The cytoplasmic domain is critical to the tumor suppressor activity of TSLC1 in non-small cell lung cancer. Cancer Res 2003;63:7979–85. [PubMed] [Google Scholar]
- [12].Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol 2004;28:496–504. [DOI] [PubMed] [Google Scholar]
- [13].Ho CL, Kurman RJ, Dehari R, et al. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res 2004;64:6915–8. [DOI] [PubMed] [Google Scholar]
- [14].Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol 2014;32:1547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Faraji F, Pang Y, Walker RC, et al. Cadm1 is a metastasis susceptibility gene that suppresses metastasis by modifying tumor interaction with the cell-mediated immunity. PLoS Genet 2012;8:e1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. [DOI] [PubMed] [Google Scholar]
- [17].Zhong Z, Wen Z, Darnell JE, Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994;264:95–8. [DOI] [PubMed] [Google Scholar]
- [18].Silver DL, Naora H, Liu J, et al. Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res 2004;64:3550–8. [DOI] [PubMed] [Google Scholar]
- [19].Lee HJ, Zhuang G, Cao Y, et al. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell 2014;26:207–21. [DOI] [PubMed] [Google Scholar]
- [20].Gebhart G, Flamen P, De Vries EG, et al. Imaging diagnostic and therapeutic targets: human epidermal growth factor receptor 2. J Nucl Med 2016;57: Suppl 1: 81S–8S. [DOI] [PubMed] [Google Scholar]
- [21].Rousseau C, Goldenberg DM, Colombié M, et al. Initial clinical results of a novel immuno-PET theranostic probe in human epidermal growth factor receptor 2-negative breast cancer. J Nucl Med 2020;61:1205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 2018;15:234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huynh J, Chand A, Gough D, et al. Therapeutically exploiting STAT3 activity in cancer - using tissue repair as a road map. Nat Rev Cancer 2019;19:82–96. [DOI] [PubMed] [Google Scholar]


