Abstract
We investigated the predictive value of the soluble fms-like tyrosine kinase-1 (sFlt-1)-to-placental growth factor (PlGF) ratio for poor neonatal outcomes of SGA neonates in the absence of preeclampsia.
This prospective cohort study included 530 singleton pregnant women who attended a prenatal screening program at a single institution. The sFlt-1/PlGF values at 24 to 28+6 weeks and 29 to 36+6 weeks of gestation were analyzed and compared between control and SGA group (subdivided as with normal neonatal outcomes and with poor neonatal outcomes).
After 22 preeclampsia cases were excluded, 47 SGA neonates and 461 control neonates were included. In the SGA group, 17 neonates had adverse neonatal outcomes (36.1%, 17/47). The mean (±D) sFlt-1/PlGF ratio of early third trimester was significantly higher in SGA with averse neonatal outcome group than in the control group (14.42 ± 23.8 vs 109.12 3.96, P = .041) and the ratio retained an independent and significant association with SGA with adverse neonatal outcomes (odds ratio = 1.017, P = .01). A sFlt-1/PlGF ratio cut-off of 28.15 at 29 to 36+6 weeks significantly predicted adverse outcomes among SGA neonates (sensitivity = 76.9%, specificity = 88%).
In this study, sFlt-1/PlGF ratio at 29 to 36 + 6wks of SGA with adverse neonatal outcome group was significantly higher than control group. This study suggests the feasibility of the sFlt-1/PlGF ratio as helpful objective measurement for predicting the adverse SGA neonatal outcome by providing sFlt-1/PlGF cut-off value.
Keywords: biomarker, fetal growth restriction, normotensive pregnancy, placental growth factor, small for gestational age, soluble fms-like tyrosine kinase-1
1. Introduction
Small-for-gestational-age (SGA) fetuses are defined as those having gestational weights below the tenth percentile. During the prenatal period, SGA fetuses are detected at a rate of approximately 5% to 8% of all late-phase pregnancies and usually combined clinically suspected preeclampsia (PE).[1] In cases of SGA fetuses combined with PE, the fetuses are carefully evaluated for intrauterine growth restriction (IUGR) status, which can lead to fetal death due to pathologic intrauterine fetal asphyxia and should do the immediate delivery. SGA fetuses in absence of IUGR generally receive routine prenatal care with full-term delivery and have long been considered to be constitutionally small babies with a good perinatal outcome.[2–4] However, as clinical study results have accumulated over recent decades, it has become clear that SGA fetuses may have poorer perinatal outcomes, from suboptimal neurodevelopment and higher postnatal cardiovascular risk to perinatal morbidity and mortality, than appropriate-for-gestational-age (AGA) newborns.[2–9] Although SGA can be detected through prenatal sonogram, it is difficult to predict the poor neonatal outcome and to decide appropriate delivery time.
A well-known diagnostic tool for pathologic fetal growth restriction is the uterine artery (UtA) Doppler index.[10–12] For earlier detection of fetal growth restriction, other Doppler US indices, such as increased umbilical artery pulsatility index (PI) and reduced values of middle cerebral artery (MCA) Doppler or cerebroplacental ratio (CPR), have been clinically applied.[13] There is growing interest in studying combined methods for the prediction and prevention of abnormal outcomes based upon multivariable models, including US and angiogenic biomarkers, to increase sensitivity and specificity.[14–16] The most widely studied angiogenic markers are placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1).[17] The sFlt-1/PlGF ratio is significantly higher in cases of uteroplacental insufficiency, such as PE originating from placental insufficiency.
However, clinicians must be concerned not only about fetal growth restriction combined with PE but also isolated SGA with no other abnormalities and the optimal management of such cases. There has been little research on biomarkers for poor perinatal outcomes in cases of isolated SGA in the absence of PE. A new approach and a different perspective are needed for the evaluation of isolated SGA because early recognition of SGA fetuses at increased risk of neonatal complications might enable more appropriate surveillance and, therefore, optimized management, which would subsequently reduce the risk of adverse fetal outcomes.
In the present study, we investigated the potential value of maternal serum levels of the sFlt-1/PlGF ratio for the prediction of adverse neonatal outcomes among isolated SGA fetuses in the absence of PE and investigated whether other factors can predict poor perinatal outcomes. Additionally, we reported the final analysis of clinically significant cut-off value of sFlt-1/PlGF for prediction of poor neonatal outcome in cases of isolated ultrasonic SGA fetuses.
2. Materials and methods
This was a prospective cohort study that included 530 singleton pregnant women who had attended a prenatal screening program at CHA Gangnam Medical Center in Seoul, Korea, between January 2011 and March 2012. Written consent was obtained from all of the participants, and the study was approved by the Institutional Review Board of CHA Gangnam Medical Center, CHA University. In our study, the control group included AGA neonates with no concerns regarding PE before or after delivery. Preeclampsia was defined as repeated systolic blood pressure measurements of ≥140 mm Hg (Korotkoff phase 1) or diastolic blood pressure measurements of ≥90 mm Hg (Korotkoff phase 5) in women who were normotensive before 20 weeks accompanied by proteinuria diagnosed as repeated ≥1 + proteinuria on dipstick urinalysis or ≥300 mg of protein in a 24-hour urine collection sample.[18] Our SGA group included infants with birth weights lower than the tenth percentile of the corresponding curves for Koreans after adjustment for gestational age.[19] Additionally, the SGA group was subdivided according to the neonatal outcome: SGA group with normal outcomes and SGA group with poor neonatal outcomes. The women were interviewed to obtain their obstetric data and medical history. The gestational age was assessed by embryo fetal crown-rump length in the first trimester. At a gestational age from 10 to 13 + 6 weeks, maternal serum levels of pregnancy-associated plasma protein-A (PAPP-A) were checked, and fetal nuchal translucency was measured between the gestational age of 11 and 13 + 6 weeks; subsequently, at the gestational ages of 15 to 20 + 6 weeks, 4 markers (alpha-fetoprotein (AFP), unconjugated estriol (uE3), inhibin-A, and human chorionic gonadotropin (hCG)) were measured. All of the markers were measured using a UniCel DxI 800 analyzer (Beckman Coulter Inc., Fullerton, CA, USA), and the values were transformed to multiples of the median (MoM) after adjusting for gestational age and maternal body mass index (BMI).
We sequentially analyzed 2 periods of gestational age: 24 + 0 to 28 + 6 weeks of gestation and 29+0 to 36+6 weeks of gestation.
We also measured the maternal plasma levels of the sFlt-1 and PlGF values at both early-phase gestation and late-phase gestation as additional angiogenic biomarkers. After clotting, the samples were centrifuged, and plasma was stored at − 80°C. The sFlt-1 and PlGF levels of each of the samples were measured simultaneously using the fully automated Roche Diagnostics Elecsys assay (Roche Diagnostics, Penzberg, Germany), and the sFlt-1/PlGF ratio was calculated.
In each trimester, an US scan was also performed. Fetal biparietal diameter, femur length, and abdominal and head circumferences were measured using the ATL-5000 US system (Philips Medical Systems, Andover, MA, USA). UtA Doppler ultrasonography with color-flow mapping was performed at the gestational ages of 20 weeks and 24+6 weeks. Both left and right UtA blood flows were examined using color Doppler imaging. The Doppler gate was placed at the proximal UtA, according to the Fetal Medicine Foundation guidelines. The PI value was measured, and the average of the measurements from the left and right UtA was used for the analysis. The Doppler measurements were performed by 3 well-trained examiners.
Adverse neonatal outcomes regarded as requiring neonatal intensive care unit (NICU) admission were attributed to subcauses including jaundice, meconium aspiration syndrome, transient tachypnea of newborn, respiratory distress syndrome, necrotizing enterocolitis, sepsis, and the requirement of ventilation. Diagnoses up to 2 months of age were included in the study. The diagnosis of jaundice was made by measuring the serum bilirubin level in the blood; total bilirubin more than 19.5 mg/dl and increases in the level of total bilirubin by more than 0.5 mg/dl per hour or 5 mg/dl per 24 hours indicated jaundice. Meconium aspiration syndrome was diagnosed when darkly colored amniotic fluid was observed along with tachypnea and hypercapnia. Transient tachypnea of newborns was diagnosed with the exclusion of respiratory distress syndrome. The diagnosis of respiratory distress syndrome was made based on the clinical findings and the chest X-ray, which showed decreased lung volume, absence of the thymus and a ground-glass-appearance pattern. Necrotizing enterocolitis was diagnosed by both radiographic findings and clinical findings, such as poor feeding, bloating, decreased activity, blood in stool and vomiting of bile. The criteria of neonatal sepsis included a total leucocyte count <5000/mm3, bandemia 20%, CRP >10 ng per ml, and a decrease in erythrocyte sedimentation rate (ESR) > 10 mm in 1 hour. Hypoxia was defined according to the results of the vein blood analysis, pH<7.1 at birth.
Information on pregnancy outcomes, obstetric complications and fetal outcomes, including the sex of the infant, gestational age at delivery and birth weight, were obtained after delivery.
All statistical analyses were performed using SPSS for Windows version 21.0 (SPSS Inc., Chicago, IL, USA) software. Student t test or the Mann–Whitney U test and Pearson Chi-Squared test were used to conduct univariate comparisons between groups of quantitative and qualitative data, respectively. Categorical variables are given as total numbers (n) and percentages (%) and continuous variables in means ± SDs. The comparison of maternal characteristics, angiogenic factors and UtA PI values was performed between the following groups:
-
1.
control group vs total SGA,
-
2.
SGA with normal outcome vs SGA with adverse neonatal outcome, and
-
3.
control vs SGA with adverse neonatal outcome.
Values of P < .05 were considered statistically significant. To determine the significant factors for the prediction of SGA and SGA with adverse neonatal outcomes, logistic regression analysis with backward stepwise elimination by sequentially removing nonsignificant variables was used. Receiver operating characteristic (ROC) curves were constructed using logistic regression analysis to determine the clinically applicable cut-off value of sFlt-1/PlGF for the prediction of SGA neonates, especially those who showed adverse neonatal outcomes. The resulting areas under the ROC curves (AUCs) were compared by pairwise analysis.
3. Results
Among the 530 study participants, 22 (4.2%) pregnant women were diagnosed with PE. After excluding these 22 PE singletons, 508 sets of pregnancy data were analyzed and grouped as 47 SGA singletons and 461 control group subjects. The neonatal outcome data are presented in Figure 1.
Figure 1.
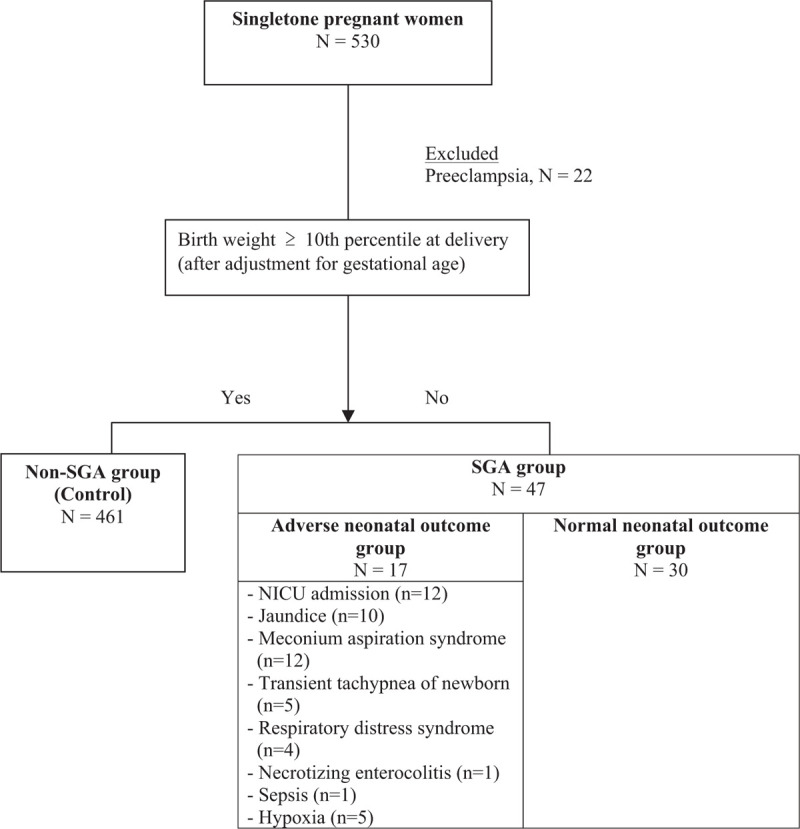
Flow diagram of participant through study.
The comparisons of basic characteristics among the 4 groups are summarized in Table 1. Prepregnancy maternal BMI was significantly lower in the SGA and SGA with adverse neonatal outcome groups, and the percentages of primigravidarum women was high in the SGA and SGA with adverse neonatal groups, compared to the control group.
Table 1.
Comparison of basic charateristics among the control, total SGA, SGA with normal neonatal outcome and SGA with adverse neonatal outcome groups.
| Control (N = 461) | Total SGA (N = 47) | P1 | SGA with normal neonatal outcome (N = 30) | SGA with adverse neonatal outcome (N = 17) | P2 | P3 | |
| Age, years (mean ± SD) | 32.26 ± 4.07 | 31.4 ± 3.63 | .169 | 31.23 ± 3.67 | 32.71 ± 3.65 | .673 | .583 |
| Prepregnancy BMI, kg/m2 (mean ± SD) | 20.21 ± 2.69 | 18.79 ± 1.94 | <.001∗ | 18.77 ± 1.99 | 18.82 ± 1.91 | .924 | .036∗ |
| Primigravida (N, %) | 64.6 | 80.9 | <.001∗ | 75.8 | 82.1 | .312 | <.001∗ |
| Gestational weeks at delivery (mean ± SD) | 38.43 ± 1.49 | 38.36 ± 1.58 | .783 | 38.33 ± 1.64 | 38.41 ± 1.50 | .872 | .971 |
| Gender of baby (N, %) | |||||||
| Male | 48.7 | 42.6 | .421 | 40 | 47.1 | .558 | .65 |
| Female | 51.3 | 57.4 | 60 | 52.9 | |||
| Hemoglobin in early pregnancy, g/dl (mean ± SD) | 12 ± 1.15 | 12.21 ± 0.90 | .161 | 12.16 ± 0.81 | 12.28 ± 1.05 | .667 | .326 |
| Gestational weeks at 1st serum test | 11.45 ± 0.62 | 11.57 ± 0.61 | .265 | 11.61 ± 0.58 | 11.5 ± 0.67 | .623 | .781 |
| Gestational weeks at 2nd serum test | 15.8 ± 1.9 | 15.79 ± 2.0 | .885 | 15.72 ± 0.75 | 15.92 ± 0.49 | .316 | .498 |
| Gestational weeks at sFlt-1/PlGF I measurement | 26.1 ± 0.97 | 26.02 ± 0.95 | .591 | 25.9 ± 1.01 | 26.2 ± 0.83 | .25 | .578 |
| Gestational weeks at sFlt-1/PlGF II mesurement | 36.27 ± 1.18 | 36.22 ± 1.28 | .817 | 36.14 ± 1.356 | 36.38 ± 1.14 | .557 | .715 |
| BP in early pregnancy, mm Hg (mean ± SD) | |||||||
| Systolic | 112.39 ± 15.3 | 110.22 ± 17.2 | .202 | 110.97 ± 10.93 | 108.94 ± 9.23 | .525 | .206 |
| Diastolic | 66.15 ± 10.2 | 64.76 ± 10.3 | .253 | 65.52 ± 7.67 | 63.47 ± 3.95 | .314 | .016∗ |
Table 2 compares the angiogenic factors and UtA Doppler results between the 4 groups. The MoM values of the PAPP-A from the 1st trimester screening test and the hCG and uE3 levels from the 2nd trimester screening test were significantly lower in the SGA groups. The sFlt-1/PlGF ratio at 29–36+6 weeks (late-phase gestation; ratio II), however, was significantly higher in the SGA groups. The UtA Doppler PI value of ultrasonography was significantly higher in the SGA groups.
Table 2.
Angiogenic factors and uterine artery Doppler results of the control, total SGA, SGA with normal neonatal outcome and SGA with adverse neonatal outcome groups.
| Control (N = 461) | Total SGA (N = 47) | P1 | SGA with normal neonatal outcome (N = 30) | SGA with adverse neonatal outcome (N = 17) | P2 | P3 | |
| 1st trimester screening testa | |||||||
| PAPP-A, MoM (mean ± SD) | 1.25 ± 0.7 | 1.00 ± 0.7 | .036∗ | 1.10 ± 0.66 | 0.80 ± 0.47 | .171 | .024∗ |
| Nuchal Translucency, MoM (mean ± SD) | 0.99 ± 0.35 | 1.02 ± 0.33 | .528 | 1.01 ± 0.32 | 1.04 ± 0.39 | .762 | .499 |
| 2nd trimester screening testb | |||||||
| AFP, MoM (mean ± SD) | 1.056 ± 0.49 | 1.085 ± 0.48 | .618 | 1.08 ± 0.38 | 1.08 ± 0.38 | .999 | .774 |
| hCG, MoM (mean ± SD) | 1.169 ± 0.38 | 0.93 ± 0.4 | .001∗ | 0.90 ± 0.35 | 1.00 ± 0.46 | .457 | .342 |
| Unconjugated estriol, MoM (mean ± SD) | 1.094 ± 0.29 | 0.99 ± 0.3 | .041∗ | 0.98 ± 0.29 | 1.01 ± 0.35 | .782 | .348 |
| Inhibin-A, MoM (mean ± SD) | 1.29 ± 0.82 | 1.34 ± 0.9 | .614 | 1.19 ± 0.55 | 1.68 ± 1.02 | .054 | .043∗ |
| Maternal angiogenic factors at late second trimesterc | |||||||
| sFlt-1, pg/ml (mean ± SD) | 1573.2 ± 958.1 | 1671.5 ± 1359.6 | .543 | 1777.84 ± 1619.7 | 1498.72 ± 792.21 | .525 | .759 |
| PlGF, pg/ml (mean ± SD) | 617.1 ± 333.52 | 563.0 ± 365.2 | .321 | 543.36 ± 317.24 | 594.99 ± 431.56 | .662 | .798 |
| sFlt-1/PlGF ratio (mean ± SD) | 3.74 ± 11.05 | 7.66 ± 27.2 | .36 | 10.24 ± 34.47 | 3.45 ± 2.42 | .438 | .917 |
| Maternal angiogenic factors at early third trimesterd | |||||||
| sFlt-1, pg/ml (mean ± SD) | 2831.9 ± 1599.92 | 3237.7 ± 2025.2 | .171 | 2820.70 ± 1266.52 | 4071.66 ± 2925.34 | .18 | .172 |
| PlGF, pg/ml (mean ± SD) | 399.8 ± 317.6 | 320.5 ± 321.7 | .164 | 372.74 ± 357.56 | 216.78 ± 210.34 | .174 | .052 |
| sFlt-1/PlGF ratio (mean ± SD) | 14.42 ± 23.8 | 28.62 ± 38.4 | .037∗ | 35.7 ± 56.4 | 109.12 ± 83.96 | .147 | .041∗ |
| Doppler ultrasonography pulsatility of uterine arterye | 0.93 ± 0.23 | 1.13 ± 0.5 | .025∗ | 1.13 ± 0.42 | 1.25 ± 0.46 | .397 | .027∗ |
Table 3 displays the results of the Logistic regression analysis. UtA Doppler PI and the sFlt-1/PlGF ratio II had an independent and significant association with SGA and SGA with complications.
Table 3.
Prediction of SGA and SGA with adverse neonatal outcome. Logistic regression analyses were used to estimate adjusted odds ratios (aOR) with 95% confidence intervals (CI).
| Model | |||||
| Variables | Control | SGA | SGA with adverse neonatal outcome | ||
| aOR (95% CI)a | P | aOR (95% CI)b | P | ||
| Doppler ultrasonography pulsatility of uterine artery | ref. | 2.455 (2.345–2.570) | .027 | 3.934 (1.193–12.977) | .024 |
| sFlt-1/PlGF ratio at 29 to 37+6 weeks | ref. | 1.012 (1.011–1.013) | .025 | 1.017 (1.004–1.030) | .01 |
Figure 2 (A) plots the ROC curves of the sFlt-1/PlGF ratio II for the prediction of SGA at birth. The best cut-off value was 11.25 with 60.0% sensitivity and 61.9% specificity (AUC area: 0.663 (95% CI, 0.564–0.762)). In Figure 2 (B), the ROC curve shows a much higher correlation between the sFlt-1/PlGF ratio II and those with SGA at birth with adverse neonatal outcomes among those subsequently admitted for NICU care. The cut-off value of the sFlt-1/PlGF ratio II was 28.15 with 76.9% sensitivity and 88% specificity (AUC area: 0.907 (95% CI, 0.829–0.985)).
Figure 2.
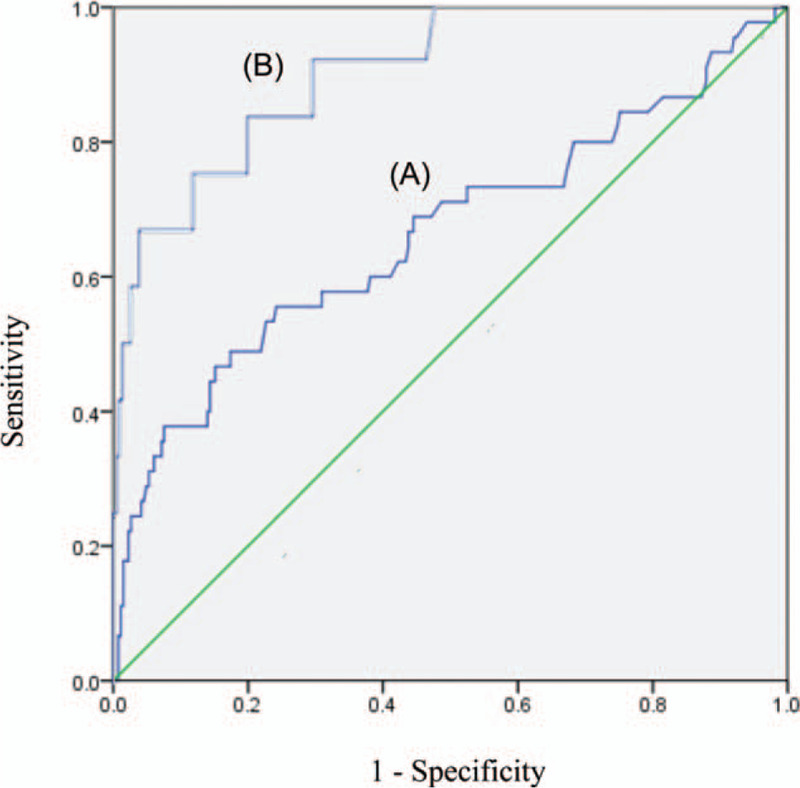
Receiver operating characteristics curves (A) for the prediction of small-for-gestational-age neonates among 508 pregnant women (461 in the control group and 47 in the small-for-gestational-age group) by the sFlt-1/PlGF ratio at 29 to 36+6 weeks of gestation, with a the cut-off value of 11.25. (B) for the prediction of small-for-gestational-age neonates with adverse neonatal outcomes among 478 pregnancy women (461 in the control group and 17 in the adverse outcomes neonatal group) by the sFlt-1/PlGF ratio at 29 to 36+6 weeks of gestation, with a cut-off value of 28.15.
4. Discussion
This study provides evidence that the sFlt-1/PlGF ratio measured at 29 to 36+6 weeks may predict SGA and SGA with adverse neonatal outcomes in addition to UtA Doppler. The sFlt-1/PlGF at 29 to 36+6 weeks of the SGA and SGA with adverse neonatal outcome groups was significantly higher than that of the AGA group.
Differential diagnoses between fetuses that were small due to “placental intrauterine growth restriction” and those that were “constitutionally small fetuses" have been considered a major area of interest in clinical obstetrics.[20–24] Typically, ultrasonic Doppler flow indices have been used to determine the risk of adverse neonatal outcomes. UtA plays a significant role in detecting poor neonatal outcomes based on the pathophysiology of poor maternal cardiovascular function that finally causes the UtA PI value to be elevated.[25,26] Our findings are in line with those of previous studies, as UtA PI was increased both in the SGA and SGA with adverse neonatal outcome groups. As another Doppler marker to predict adverse neonatal outcomes, some studies have reported that abnormal cerebral Doppler impedance is associated with poorer perinatal outcomes and neurobehavior in late-onset SGA fetuses.[10,13,27] In particular, the CPR, which combines MCA-PI and UA-PI, has been reported to correlate well with adverse outcomes.[28]
Impaired placentation and/or placental dysfunction reflected by reduced PlGF and increased sFlt-1, in addition to such Doppler indices, is associated with the subsequent development of PE and the birth of SGA neonates. Many results have shown that imbalances in angiogenic and antiangiogenic factors, as measured in maternal blood, are detectable prior to clinical diagnosis and that such measurements have high prognostic value.[29–35] The value of angiogenic biomarkers in the prediction and characterization of early-onset PE and FGR has been demonstrated in a large number of studies; already, in fact, a diagnostic serum kit based on the sFlt/PlGF ratio is being used in outpatient clinics. Gestation-age-specific sFlt/PlGF ratio cut-offs of >85 (20 + 0 to 33 + 6 weeks) and >110 (34 + 0 weeks to delivery) have been shown to be highly suggestive of PE. In the PROGNOSIS study, a single sFlt-1/PlGF ratio cut-off (<38) was validated as accurately and reliably ruling out PE within 1 week (negative predictive value >96%) and confirming PE (> = 38) within 4 weeks (positive predictive value >25%). As shown in the ASIA PROGNOSIS data, the sFlt-1/PlGF ratio is clearly meaningful for short-term prediction of PE in the suspected PE group. And even in the absence of PE, sFlt-1/PlGF ratio was associated with fetal adverse outcome. One study reported that among patients suspected of having SGA fetuses, abnormal maternal plasma concentrations of angiogenic/antiangiogenic factors were 5 to 9 and 8 to 9 times more likely to lead to PE or preterm delivery.[36] Ignacio et al reported the following median values of sFlt-1/PlGF: control group 11.0, fetal growth restriction group 116.8, PE 66.5, and PE combined with fetal growth restriction group 165.4.[37] In our study, we analyzed general population, not in the suspected PE group. In predicting SGA with poor neonatal outcome, the sFlt-/1PlGF ratio value showed meaningful results, lower than PE. This result shows that small increases in sFlt-1/PlGF associated with angiogenic inbalance have significance in predicting fetal weight gain and neonatal outcome.
When comparing the results with respect to gestational weeks, the sFlt-1/PlGF ratio at 24 to 28 + 6 weeks showed no significant difference in the SGA group, but there was a tendency toward increased values in the SGA group. In a recent study of the population excluding the PE group, the PlGF value investigated at a gestational age of 19 to 24 weeks (adjusted as multiples of median) was significantly lower in women with SGA infants than in those without SGA infants.[38] In another paper on a population excluding those with PE, the sFlt-1/PlGR ratio was checked at a gestational age of 24 to 27 weeks, and the risk of SGA was 7.9-fold higher in women with sFlt-1/PlGF ≥90th percentile than in those with sFlt-1/PlGF <90th percentile.[39] The difference from our paper is that these papers measured sFlt-1 and PlGF value at 1 time, and we measured the value serially in the late-second phase and early-third phase in the same patient. Although there is a difference in gestational weeks, these studies suggest that PlGF and sFlt-1/PlGF might be candidate biomarkers for the prediction of SGA.
There is also growing interest in the topic of sFlt-1 and PlGF in the case of isolated SGA without PE. Previous studies revealed interesting results.[14–16] One case-control study reported that the combination of fetal weight, UA-PI, CPR, estriol and PlGF predicted 62% of adverse neonatal outcomes among SGA cases with a false positive rate (FPR) of 10%.[16] In our study, the sFlt-1/PlGF ratio at 29 to 26 + 6 weeks was strongly predictive of adverse neonatal outcomes, with a cut-off value of 28.15 (76.9% sensitivity and 88% specificity, AUC area: 0.907). Moreover, an increased sFlt-1/PlGF ratio, with a cut-off value of 11.25, checked at the same time was shown to be a clinically predictive marker of real SGA neonates in cases of ultrasonic SGA (60.0% sensitivity and 61.9% specificity; AUC area: 0.663; 95% CI, 0.564–0.762), providing predictive value beyond the clinical and ultrasonic values. These results suggest an optimal and novel approach that utilizes the sFlt-1/PlGF ratio as a highly effective prognostic indicator of adverse outcomes in cases of isolated SGA in the absence of PE.
It must be noted that in the third trimester, unlike the first and second trimesters, SGA and placental insufficiency might not be noticeable because SGA determination is routinely made only by US biometry. Thus, in addition to US biometry measurements for the prediction of SGA, objective measurements are needed.
A limitation of our study is the small number of patients, especially regarding the subgroup analysis. Therefore, the possibility that selection bias distorted the results to some extent could not be excluded. Although our results show significant P values based on the logistic regression analysis, further studies with larger patient groups and more follow up measures per patient are needed to confirm these findings. There is another limitation. When making multiple comparisons using individual Student t test applied to each comparison, there is a chance of a type I error increases with the number of comparisons. In our study, we compared the 3 groups. In the Bonferroni correction, Student t test method would have a significance level (alpha set) of P < .017 (0.05 divided by the number of comparisons). Thus, there is a loss of significance in several comparisons, including our primary outcomes (e.g., the sFlt-1/PlGF ratio and Doppler ultrasonography of the uterine artery). The regression model strengthens our analysis, but the results need to be interpreted carefully. Another limitation is that the difference in the multivariate model shows an approximately 1 to 3% deviation in the biochemical results. It is possible that increasing the sample size would result in a loss of this significance.
This study has important clinical implications. Effective screening for the detection of adverse perinatal outcomes in cases of isolated SGA is an area of unmet clinical need. In this study, we serially evaluated not only UtA Doppler but also sFlt-1/PlGF ratio data for cases of suspected SGA, determining that the sFlt-/PlGF ratio can be an independent marker of poor neonatal outcome. Such biomarkers can be identified from a simple blood test; thus, this approach might be helpful in areas where access to Doppler examination expertise is limited. If these results are corroborated by others, the strong potential of angiogenic biomarkers for risk stratification in cases of US-detected isolated SGA and the subsequent reduction in both morbidity and healthcare costs for the management of SGA fetuses will be confirmed.
In conclusion, the sFlt-1/PlGF ratio at 29 to 36+6 weeks in the SGA with adverse neonatal outcomes group was significantly higher than that in the control group. These results suggest the feasibility of sFlt/PlGF ratio, based on the cut-off value of 28.15, as an objective measurement and a possible useful predictor of SGA and adverse neonatal outcomes in addition to maternal Doppler indices.
Author contributions
Conceptualization: Sohyun Shim.
Data curation: Sohyun Shim, Haengjun Jeon, Hye Jin Ryu, So Hyun Kim, Seung Gi Min, Min Kyu Kang.
Formal analysis: Sohyun Shim, Seung Gi Min.
Methodology: Hee Jin Park, Donghyun Cha.
Project administration: Donghyun Cha.
Software: Hee Jin Park.
Supervision: Hee Jin Park, Donghyun Cha.
Validation: Donghyun Cha.
Visualization: Donghyun Cha.
Writing – original draft: Sohyun Shim.
Writing – review & editing: Hee Jin Park.
Correction
The authors would like noted that Drs. Dong Hyun Cha and Hee Jin Park contributed equally. This has been updated in the footnote.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, AGA = appropriate for gestational age, hCG = human chorionic gonadotropin, IUGR = intrauterine growth restriction, MCA = middle cerebral artery, PAPP-A = pregnancy-associated plasma protein-A, PE = preeclampsia, PlGF = placental growth factor, sFlt-1 = soluble fms-like tyrosine kinase-1, SGA = small for gestational age, UA = umbilical artery, uE3 = unconjugated estriol, UtA = uterine artery.
How to cite this article: Shim SH, Jeon HJ, Ryu HJ, Kim SH, Min SG, Kang MK, Park HJ, Cha DH. Prenatal serum sFlt-1/PlGF ratio predicts the adverse neonatal outcomes among small-for-gestational-age fetuses in normotensive pregnant women: a prospective cohort study. Medicine. 2021;100:8(e24681).
DHC and HJP contributed equally.
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C-1839-010019). The funder had no role in the study design, data collection, interpretation of data or writing of the manuscript.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
1: comparison between control group and total SGA group. 2: comparison between SGA with normal neonatal outcome group and SGA with adverse neonatal outcome group. 3: comparison between control group and SGA with adverse neonatal outcome group.
P < .05.
BP = blood pressure, BMI = body max index, PlGF = placental growth factor, sFlt-1 = soluble fms-like tyrosine kinase-1, SGA = small for gestational age.
1: comparison between control group and total SGA group. 2: comparison between SGA with normal neonatal outcome group and SGA with adverse neonatal outcome group. 3: comparison between control group and SGA with adverse neonatal outcome group.
P < .05.
Evaluated between gestational ages 10+0 and 13+6 weeks.
Evaluated between gestational ages 15+0 and 20+6 weeks.
Evaluated between gestational ages 20+0 and 28+6 weeks.
Evaluated between gestational ages 29+0 and 36+6 weeks, e: evaluated between gestational ages 20+0 and 24+6 weeks, average value of the right and left value, data presented as the median.
AFP = alpha-fetoprotein, hCG = human chorionic gonadotropin, MoM = multiples of the median, PAPP-A = pregnancy-associated plasma protein-A, PlGF = placental growth factor, SGA = small for gestational age, sFlt-1 = soluble fms-like tyrosine kinase-1.
Adjusted for maternal prepregnancy BMI and parity.
Adjusted for maternal prepregnancy BMI, parity and diastolic BP.
aOR = adjusted odds ratio, CI = confidence interval, PlGF = placental growth factor, SGA = small for gestational age, sFlt-1 = soluble fms-like tyrosine kinase-1.
References
- [1].Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol 2011;204:288–300. [DOI] [PubMed] [Google Scholar]
- [2].Figueras F, Oros D, Cruz-Martinez R, et al. Neurobehavior in term, small-for-gestational age infants with normal placental function. Paper presented at: peds2009. [DOI] [PubMed] [Google Scholar]
- [3].Illa M, Coloma JL, Eixarch E, et al. Growth deficit in term small-for-gestational fetuses with normal umbilical artery. Doppler is associated with adverse outcome 2009;37:48–52. [DOI] [PubMed] [Google Scholar]
- [4].Savchev S, Figueras F, Cruz-Martinez R, et al. Estimated weight centile as a predictor of perinatal outcome in small-for-gestational-age pregnancies with normal fetal and maternal. Doppler Indices 2012;39:299–303. [DOI] [PubMed] [Google Scholar]
- [5].Comas M, Crispi F, Cruz-Martinez R, et al. Ajoo, gynecology, tissue doppler echocardiographic markers of cardiac dysfunction in small-for-gestational age fetuses. Am J Obstet Gynecol 2011;205:57.e1–6. [DOI] [PubMed] [Google Scholar]
- [6].Eixarch E, Meler E, Iraola A, et al. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. xxx 2008;32:894–9. [DOI] [PubMed] [Google Scholar]
- [7].Crispi F, Bijnens B, Figueras F, et al. Fetal growth restriction results in remodeled and less efficient hearts in children. xxx 2010. [DOI] [PubMed] [Google Scholar]
- [8].Crispi F, Figueras F, Cruz-Lemini M, et al. Cardiovascular programming in children born small for gestational age and relationship with prenatal signs of severity. xxx 2012;207:121.e121-121. e129. [DOI] [PubMed] [Google Scholar]
- [9].Barker DJ, Osmond C, Winter P, et al. Weight in infancy and death from ischaemic heart disease. xxx 1989;334:577–80. [DOI] [PubMed] [Google Scholar]
- [10].Ventura W, De Paco Matallana C, Prieto-Sanchez MT, et al. Uterine and umbilical artery Doppler at 28 weeks for predicting adverse pregnancy outcomes in women with abnormal uterine artery Doppler findings in the early second trimester. Prenat Diagn 2015;35:294–8. [DOI] [PubMed] [Google Scholar]
- [11].Gomez O, Figueras F, Martinez JM, et al. Sequential changes in uterine artery blood flow pattern between the first and second trimesters of gestation in relation to pregnancy outcome. Ultrasound Obstet Gynecol 2006;28:802–8. [DOI] [PubMed] [Google Scholar]
- [12].Stubert J, Ullmann S, Bolz M, et al. Prediction of preeclampsia and induced delivery at <34 weeks gestation by sFLT-1 and PlGF in patients with abnormal midtrimester uterine Doppler velocimetry: a prospective cohort analysis. BMC Pregnancy Childbirth 2014;14:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cruz-Martinez R, Figueras F, Oros D, et al. Cerebral blood perfusion and neurobehavioral performance in full-term small-for-gestational-age fetuses. Am J Obstet Gynecol 2009;201:474.e471-477. [DOI] [PubMed] [Google Scholar]
- [14].Valino N, Giunta G, Gallo DM, et al. Biophysical and biochemical markers at 35-37 weeks’ gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet Gynecol 2016;47:203–9. [DOI] [PubMed] [Google Scholar]
- [15].Lobmaier SM, Figueras F, Mercade I, et al. Angiogenic factors vs Doppler surveillance in the prediction of adverse outcome among late-pregnancy small-for- gestational-age fetuses. Ultrasound Obstet Gynecol 2014;43:533–40. [DOI] [PubMed] [Google Scholar]
- [16].Miranda J, Triunfo S, Rodriguez-Lopez M, et al. Performance of third-trimester combined screening model for prediction of adverse perinatal outcome. Ultrasound Obstet Gynecol 2017;50:353–60. [DOI] [PubMed] [Google Scholar]
- [17].Agrawal S, Cerdeira AS, Redman C, et al. Meta-analysis and systematic review to assess the role of soluble FMS-like tyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: the SaPPPhirE study. Hypertension 2018;71:306–16. [DOI] [PubMed] [Google Scholar]
- [18].American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- [19].Lim JS, Lim SW, Ahn JH, et al. New Korean reference for birth weight by gestational age and sex: data from the Korean Statistical Information Service (2008-2012). Ann Pediatr Endocrinol Metab 2014;19:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang J, Merialdi M, Platt LD, et al. Defining normal and abnormal fetal growth: promises and challenges. Am J Obstet Gynecol 2010;202:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ananth CV, Vintzileos AM. Distinguishing pathological from constitutional small for gestational age births in population-based studies. Early Hum Dev 2009;85:653–8. [DOI] [PubMed] [Google Scholar]
- [22].Xu H, Simonet F, Luo ZC. Optimal birth weight percentile cut-offs in defining small- or large-for-gestational-age. Acta Paediatr 2010;99:550–5. [DOI] [PubMed] [Google Scholar]
- [23].Galan HL. Timing delivery of the growth-restricted fetus. Semin Perinatol 2011;35:262–9. [DOI] [PubMed] [Google Scholar]
- [24].von Beckerath AK, Kollmann M, Rotky-Fast C, et al. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol 2013;208:130.e131-136. [DOI] [PubMed] [Google Scholar]
- [25].Ridder A, Giorgione V, Khalil A, et al. Preeclampsia: the relationship between uterine artery blood flow and trophoblast function. Int J Mol Sci 2019;20:3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martinez-Portilla RJ, Caradeux J, Meler E, et al. Third-trimester uterine-artery Doppler for prediction of adverse outcome in late small-for-gestational-age fetuses: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2020;55:575–85. [DOI] [PubMed] [Google Scholar]
- [27].Severi FM, Bocchi C, Visentin A, et al. Uterine and fetal cerebral Doppler predict the outcome of third-trimester small-for-gestational age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 2002;19:225–8. [DOI] [PubMed] [Google Scholar]
- [28].Bahado-Singh RO, Kovanci E, Jeffres A, et al. The Doppler cerebroplacental ratio and perinatal outcome in intrauterine growth restriction. Am J Obstet Gynecol 1999;180(3 Pt 1):750–6. [DOI] [PubMed] [Google Scholar]
- [29].Molvarec A, Szarka A, Walentin S, et al. Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens Res 2010;33:892–8. [DOI] [PubMed] [Google Scholar]
- [30].Garovic VD. The role of angiogenic factors in the prediction and diagnosis of preeclampsia superimposed on chronic hypertension. Hypertension 2012;59:555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ghosh SK, Raheja S, Tuli A, et al. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch Gynecol Obstet 2012;285:417–22. [DOI] [PubMed] [Google Scholar]
- [32].Hagmann H, Thadhani R, Benzing T, et al. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem 2012;58:837–45. [DOI] [PubMed] [Google Scholar]
- [33].Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci (Lond) 2012;122:43–52. [DOI] [PubMed] [Google Scholar]
- [34].Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 2013;209:544.e541-544 e512. [DOI] [PubMed] [Google Scholar]
- [35].Bian X, Biswas A, Huang X, et al. Short-term prediction of adverse outcomes using the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) ratio in Asian women with suspected preeclampsia. Hypertension 2019;74:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chaiworapongsa T, Romero R, Whitten AE, et al. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. J Matern Fetal Neonatal Med 2016;29:1214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Herraiz I, Dröge LA, Gómez-Montes E, et al. Characterization of the soluble fms-like tyrosine kinase-1 to placental growth factor ratio in pregnancies complicated by fetal growth restriction. xxx 2014;124:265–73. [DOI] [PubMed] [Google Scholar]
- [38].Poon LC, Lesmes C, Gallo DM, et al. Prediction of small-for-gestational-age neonates: screening by biophysical and biochemical markers at 19-24 weeks. Ultrasound Obstet Gynecol 2015;46:437–45. [DOI] [PubMed] [Google Scholar]
- [39].Furuta I, Umazume T, Kojima T, et al. Serum placental growth factor and soluble fms-like tyrosine kinase 1 at mid-gestation in healthy women: association with small-for-gestational-age neonates. J Obstet Gynaecol Res 2017;43:1152–8. [DOI] [PubMed] [Google Scholar]


