Abstract
Background:
The objective of this study is to systematically evaluate the efficacy and safety of the calcitonin gene-related peptide (CGRP) receptor antagonist ubrogepant for the treatment of acute migraine.
Methods:
Randomized controlled trials (RCTs) of ubrogepant for treatment of acute migraine were identified in PubMed, MEDLINE, EMBASE, and the Cochrane Library from database establishment to June 2020; we also searched ClinicalTrials.gov manually during the same period. Then, RevMan 5.3 software was used to perform a meta-analysis on each outcome measure.
Results:
A total of 5 RCTs involving 4903 patients were included; there were 3358 cases in the ubrogepant group and 1545 cases in the placebo group. The meta-analysis showed the following results: at 2 hours postdose, the percentages of participants reporting pain relief and the absence of photophobia, nausea, and phonophobia were significantly higher in the ubrogepant group than in the placebo group (odds ratio [OR] = 1.71, 95%CI: 1.48–1.97, P < .00001; OR = 1.33, 95%CI: 1.22–1.45, P < .00001; OR = 1.07, 95%CI: 1.03–1.11, P = .0006; OR = 1.21, 95%CI: 1.14–1.28, P < .00001). The incidence of common adverse events was similar between the 2 groups (P > .05).
Conclusion:
Ubrogepant is effective and safe for the treatment of acute migraine.
Registration number:
PROSPERO CRD42019145286.
Keywords: acute migraine, calcitonin gene-related peptide receptor antagonist, meta-analysis, psychotherapy, ubrogepant
1. Introduction
Migraine is one of the most common nervous system diseases. It is characterized by recurrent unilateral pulsatile headache, with sensitivity to movement, visual stimulation, sound, and other sensory stimuli.[1] Most migraines cause discomfort for hours or days after the attack and are often accompanied by fatigue and other sequelae.[2] Migraines can occur at any time and commonly occur during sleep, upon awakening, or shortly after rising in the morning,[3,4] which is very inconvenient for patients.
About 1 billion people worldwide are affected by migraine, the ratio of female to male is 3:1 and the high incidence of migraine is between 35 and 39 years old.[5,6] In addition to adults, recurrent headache occurs in one-third to half of children and adolescents.[7]
The 5-hydroxytryptamine receptor agonist class triptans, discovered in the early 1990s, constitutes the only class of specific drugs developed and approved for the treatment of acute migraine in the past 20 years, and treatment with triptans is the standard protocol recommended by various guidelines.[8] However, triptans have adverse effects, and the use of triptans significantly increases the risk of cardiovascular and cerebrovascular events.[9,10] Calcitonin gene-related peptide (CGRP) can dilate the cerebral arteries and mediate neurogenic inflammation of the dura, which plays a key role in the pathophysiological mechanism underlying migraine.[11] As an oral CGRP receptor antagonist, ubrogepant mainly acts on the smooth muscle cells of the microvascular wall to control peripheral vascular resistance.[12,13] Ubrogepant may be able to meet the acute treatment needs of patients with migraine who are intolerant or unresponsive to triptans.[14] This study systematically evaluated the efficacy and safety of ubrogepant for the treatment of acute migraine to provide evidence that can serve as a reference in the subsequent clinical application of the drug.
2. Materials and methods
2.1. Inclusion and exclusion criteria
2.1.1. Type of study
Randomized controlled trial (RCT).
2.1.2. Type of subjects
-
(1)
Patients had at least a 1-year history of migraine with or without aura as defined by the International Headache Society (IHS) criteria 1.1 and/or 1.2.
-
(2)
Patients were 18 years old or older (sex and region were not considered).
-
(3)
Patients had moderate or severe migraine attack 2 to 8 times per month.
2.1.3. Intervention measures
-
(1)
Experimental group: single drug treatment with ubrogepant, divided into subgroups according to dose.
-
(2)
Placebo group: placebo single-drug control.
2.1.4. Outcome measures
The primary outcome measure was the percentage of subjects experiencing pain relief at 2 hours postdose. The secondary outcome measures were as follows: the percentage of subjects without photophobia at 2 hours postdose; the percentage of subjects without nausea at 2 hours postdose; and the percentage of subjects without phonophobia at 2 hours postdose. The safety outcome measure was the incidence of common adverse effects.
2.1.5. Study exclusion criteria
-
(1)
Multiple published studies with the same data;
-
(2)
reviews, retrospective studies, pharmacokinetics studies, etc;
-
(3)
cohort studies; and
-
(4)
open clinical trials without placebo control.
2.2. Search strategy
PubMed, MEDLINE, EMBASE, the Cochrane Library, and other databases were searched for clinical RCTs on acute migraine treated with ubrogepant from the establishment of the database to June 2020. The search terms used were “ubrogepant,” “MK-1602,” “migraine,” “Calcitonin gene-related peptide,” “CGRP,” “Calcitonin gene-related peptide receptor antagonist,” “CGRP receptor antagonist,” “randomized controlled trial,” “RCT,” and “controlled clinical trial.”
2.3. Literature screening and data extraction
Endnote X7 software was used to remove duplicates in the included literature. Two researchers read the titles, abstracts, and full texts independently, screened the RCTs and determined whether they met the standards. If there was any disagreement, a third researcher was consulted. The extracted data included the basic information of the included study, the data pertaining to the outcome measures and the quality indicators of the included studies. Then, the 2 researchers cross-checked the above information.
The risk of bias was evaluated by the Cochrane system evaluator in Handbook 5.1.0,[15] which evaluates
-
(1)
random sequence generation;
-
(2)
the hidden allocation scheme;
-
(3)
the blinding method;
-
(4)
the handling of incomplete data;
-
(5)
the selective reporting of results;
-
(6)
other biases.
RevMan 5.3 software provided by the Cochrane Collaboration Network was used for the statistical analysis. First, χ2 tests were used to assess heterogeneity, and the test level was α = 0.1. When there was no statistical heterogeneity among the studies (P > .1, I2 ≤ 50%), the fixed effect model was used for the meta-analysis. If statistical heterogeneity was found among the studies (P < .1, I2 > 50%), a random effect model was used, and subgroup and sensitivity analyses were carried out if necessary. For continuous data, the effect index was mean difference (MD) and its confidence interval (95%CI); for binary data, the effect index was relative risk (RR) or odds ratio (OR) and its 95%CI.
3. Results
3.1. Literature retrieval and basic information
A total of 189 articles were obtained. After screening, 5 studies were included in the quantitative analysis, with a total of 4903 patients. Ethical approval was not necessary, as all the included papers have passed the ethical review. There were 3358 cases in the ubrogepant group and 1545 cases in the placebo group. The flow chart of literature retrieval is shown in Figure 1, and the basic information of the literature is shown in Table 1.
Figure 1.
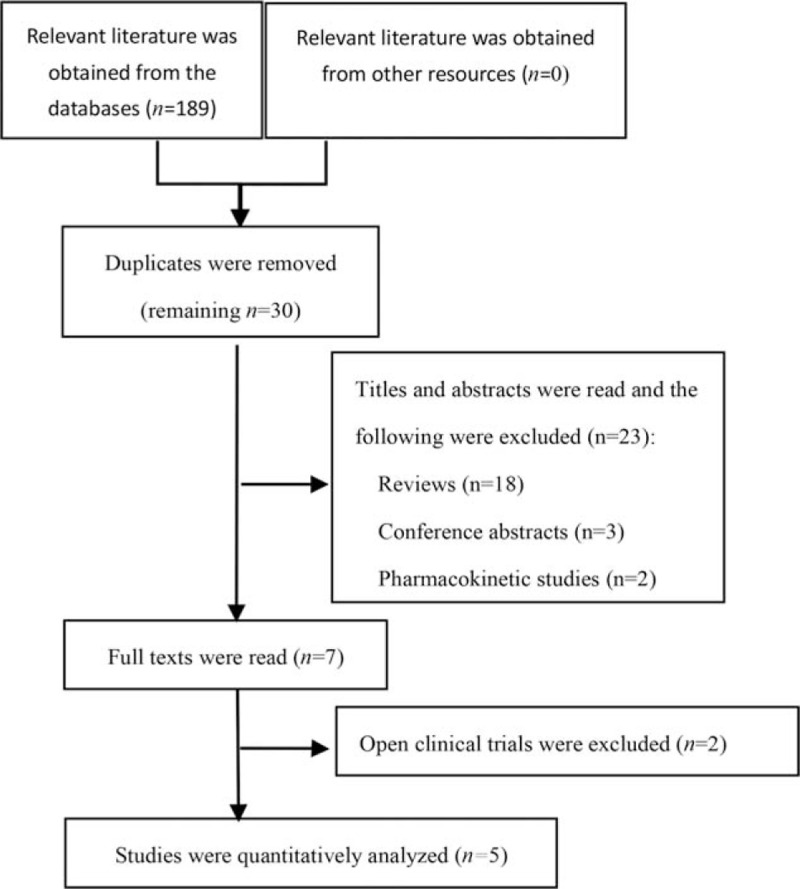
Study retrieval flow chart.
Table 1.
Basic information of the included literature.
| Study | Year | Group (dose, mg) | No. | Female/Male | Age, yr | Outcome measures |
| 01657370[13] | 2012 | Ub = 1 | 28 | 26/2 | NA | ①②③④⑤ |
| Ub = 10 | 26 | 22/4 | ||||
| Ub = 25 | 28 | 23/5 | ||||
| Ub = 50 | 28 | 26/2 | ||||
| Ub = 100 | 27 | 18/9 | ||||
| Placebo | 28 | 25/3 | ||||
| voss2016[14] | 2012 | Ub = 1 | 138 | 95/12 | 39.6 ± 10.7 | ①②③④⑤ |
| Ub = 10 | 139 | 92/16 | 41.1 ± 10.9 | |||
| Ub = 25 | 139 | 91/13 | 41.4 ± 11.5 | |||
| Ub = 50 | 139 | 92/14 | 40.7 ± 12.3 | |||
| Ub = 100 | 139 | 90/12 | 41.9 ± 11.0 | |||
| Placebo | 139 | 99/14 | 40.8 ± 11.4 | |||
| Lipton2019[15] | 2018 | Ub = 25 | 561 | 501/60 | 41.6 ± 12.3 | ①②③④⑤ |
| Ub = 50 | 562 | 497/65 | 41.0 ± 12.4 | |||
| Placebo | 563 | 494/69 | 41.5 ± 12.2 | |||
| Dodick2019[16] | 2017 | Ub = 50 | 556 | 493/63 | 40.2 ± 12.0 | ①②③④⑤ |
| Ub = 100 | 557 | 479/78 | 40.7 ± 12.4 | |||
| Placebo | 559 | 491/68 | 40.5 ± 12.2 | |||
| Goadsby2019[17] | 2018 | Ub = 100 | 260 | 140/116 | NA | ①②③④⑤ |
| Placebo | 256 | 141/119 |
NA = not available, Ub = ubrogepant; ① percentage of participants reporting pain relief at 2 h postdose; ② percentage of participant reporting absence of photophobia at 2 h postdose; ③ percentage of participants reporting absence of nausea at 2 h postdose; ④ percentage of participants reporting absence of phonophobia at 2 h postdose; ⑤ common adverse effects.
3.2. Basic characteristics of the included studies and bias risk assessment results
The 5 included studies[16–20] were all in English and were randomized, double-blind RCTs. The follow-up time and outcome measures were generally comparable, and the samples were representative. Two papers[17,20] reported specific random sequence generation methods, while others only mentioned random grouping but did not report the specific methods. All studies[16–20] reported the specific numbers of missed visits and dropouts. The quality of the included studies is shown in Table 2, and the assessment of the risk of bias is shown in Figures 2 and 3.
Table 2.
Quality characteristics of the included literature.
| Study | Random sequence generation | Hidden allocation scheme | Blinding method | Incomplete results | Selective reporting of results | Other biases |
| 01657370[13] | Unclear | Unclear | Double blind | Low risk | Low risk | Low risk |
| Voss2016[14] | Cross voice response system | Low risk | Double blind | Low risk | Low risk | Low risk |
| Lipton2019[15] | Unclear | Low risk | Double blind | Low risk | Low risk | Low risk |
| Dodick2019[16] | Unclear | Low risk | Double blind | Low risk | Low risk | Low risk |
| Goadsby2019[17] | Computer generated randomization scheme | Low risk | Double blind | Low risk | Low risk | Low risk |
Figure 2.
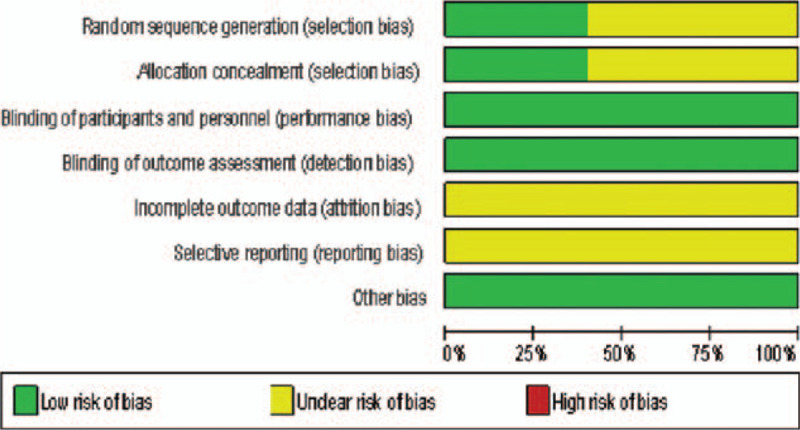
Risk of bias assessment of included studies.
Figure 3.
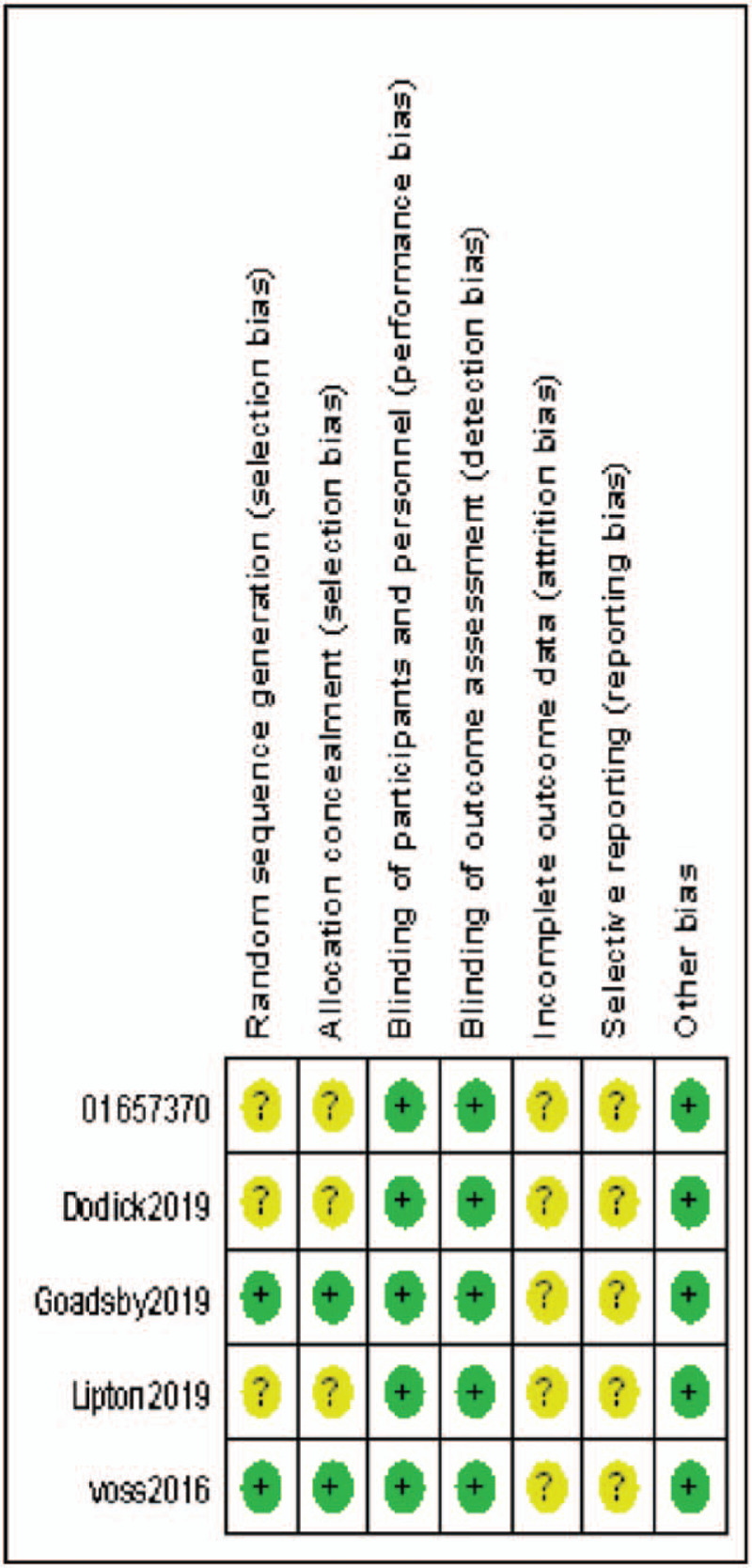
Risk of bias assessment of included studies.
3.3. Meta-analysis results
Primary outcome measures: The primary outcome measure was the percentage of subjects with pain relief at 2 hours postdose. Four RCTs[16–19] involving 4406 patients were included. Meta-analysis with a random effect model showed a significant difference between the experimental group and the placebo group (OR = 1.71, 95%CI: 1.48–1.97, P < .00001), subgroup analysis showed that there was no significant difference between the dose of 10 mg and 25 mg (P = .14, P = .03) compared with placebo group. When the dose was increased to 50 mg and 100 mg, the efficacy of the experimental group was significantly better than that of the placebo group (P = .0001, P < .0001) as shown in Figure 4.
Figure 4.
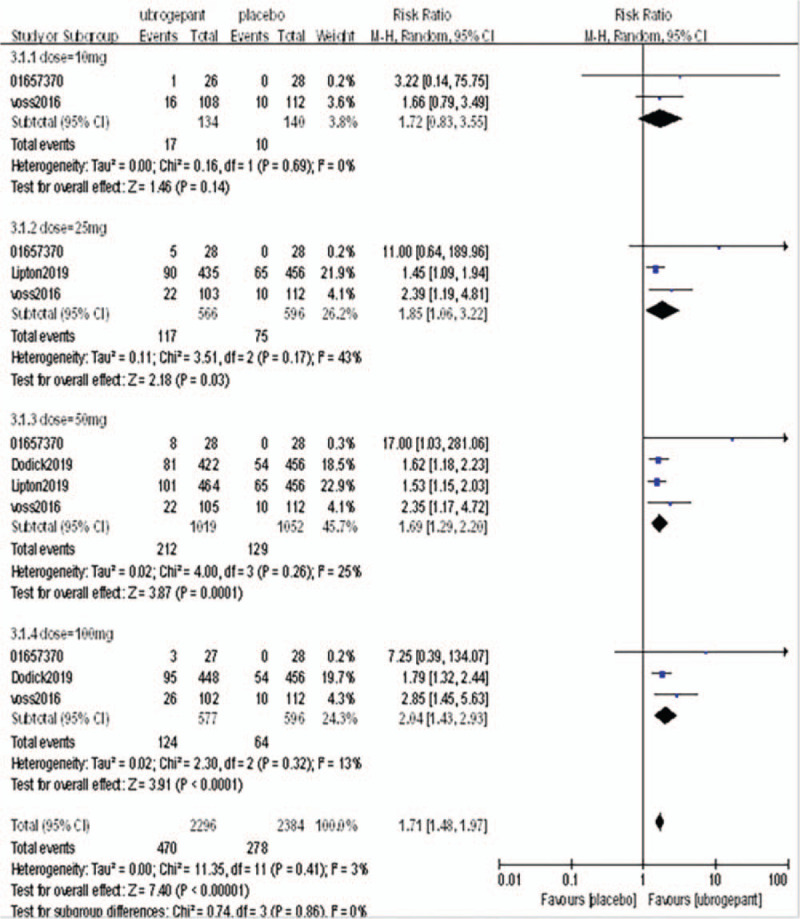
Comparison of the percentage of subjects with pain relief within 2 h after the first administration between the experimental group and the placebo group.
Secondary outcome measures: The secondary outcome measures were the percentages of subjects without photophobia, nausea, and phonophobia at 2 hours postdose.
A total of 4 RCTs[16–19] reported the percentages of subjects without photophobia, nausea, and phonophobia at 2 hours postdose. The above outcome measures were analyzed with a random effect model. After a single administration, the secondary outcome measures in the experimental group were better than those of the placebo group, and the differences were statistically significant (RR = 1.33, 95%CI = 1.22–1.45, P < .00001; RR = 1.07, 95%CI = 1.03–1.11, P = .0006; RR = 1.21, 95%CI = 1.14–1.28, P < .00001, respectively). The above 3 secondary outcome measures were divided into subgroups according to the dose. In the without photophobia outcome measure, there was no significant difference between the 2 groups (P = .06, P = .09) in the dose of 25 mg and 50 mg, but there was significant difference in the dose of 50 mg and 100 mg (P < .00001, P < .00001). In the without nausea outcome measure, the difference was not statistically significant when the dose was 10 mg, 25 mg, 50 mg (P = .45, P = .54, P = .02), and the difference was statistically significant when the dose was 100 mg (P = .07). In the phonophobia outcome measure, there was no significant difference when the dose was 25 mg (P = .06), but there was significant difference when the dose was 25 mg, 50 mg, 100 mg (P < .003, P < .00001, P < .00001). The results of the meta-analysis are shown in Figures 5–7.
Figure 5.
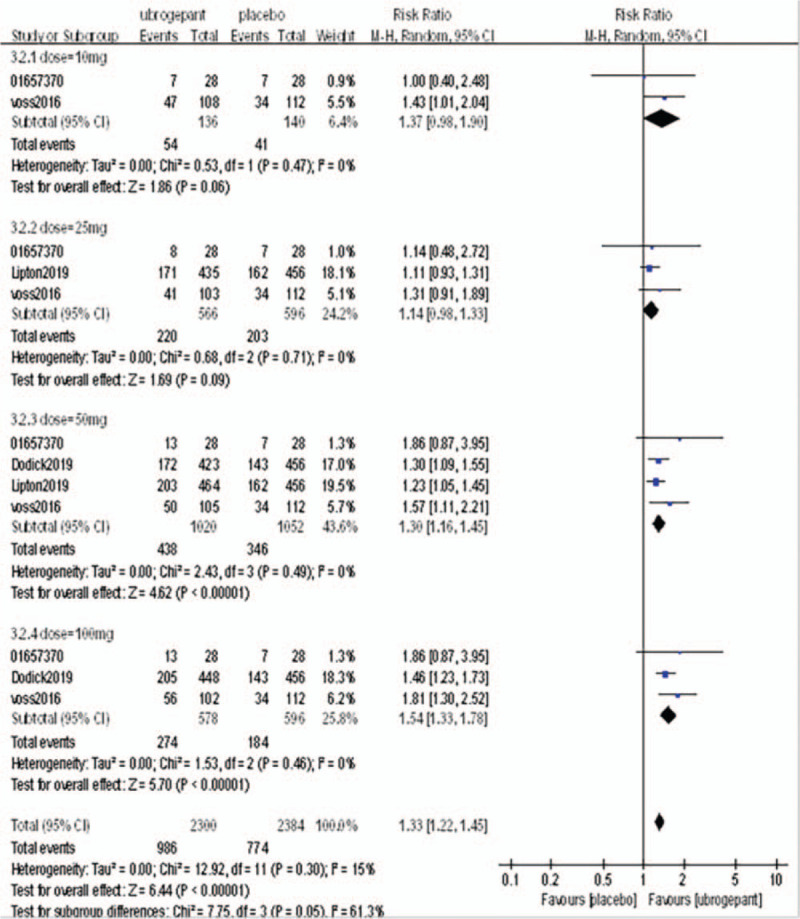
Comparison of the percentage of subjects with photophobia 2 h after initial administration between the experimental group and the placebo group.
Figure 7.
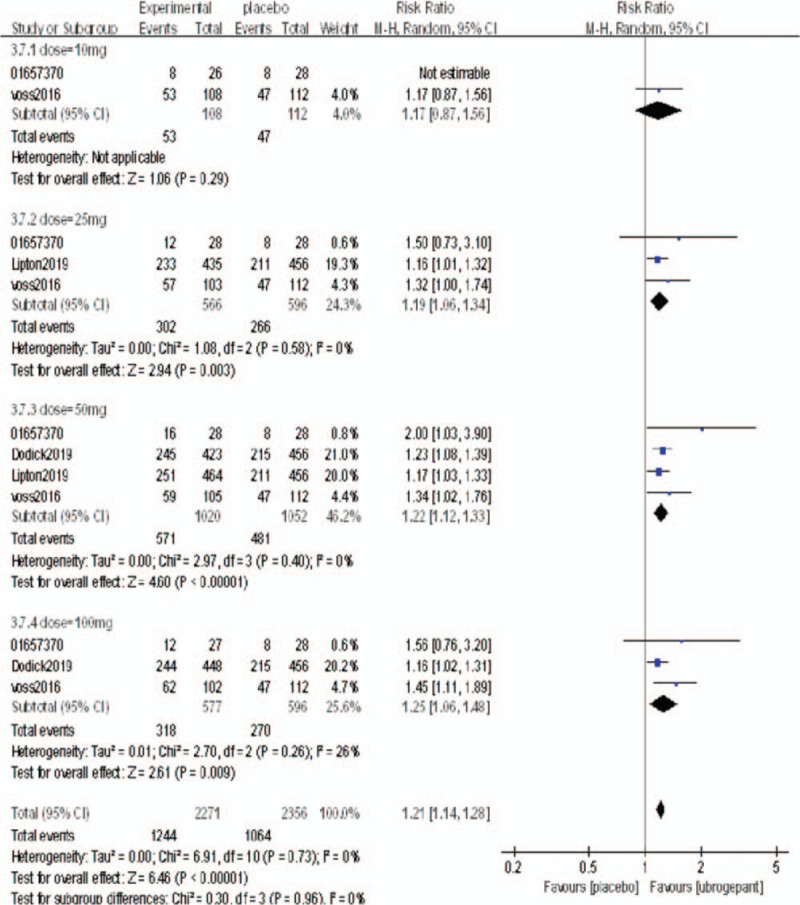
Comparison of the percentage of subjects without phonophobia 2 h after initial administration between the experimental group and the placebo group.
Figure 6.
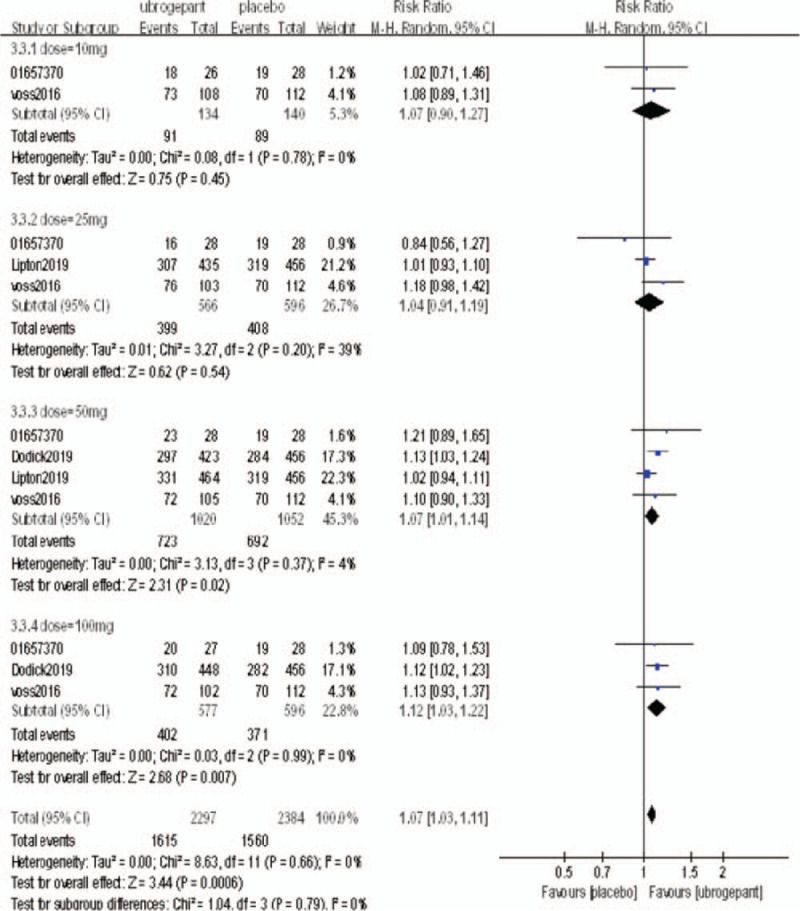
Comparison of the percentage of subjects with no nausea 2 h after the initial administration between the experimental group and the placebo group.
3.4. Meta-analysis of safety indicators
The incidence of common adverse reactions was reported in 3 studies,[16,17,20] including headache (31/393 vs 25/288), oropharyngia (36/392 vs 10/288), nasopharynx (18/393 vs 18/288), nausea (49/920 vs 16/401), dizziness (39/920 vs 9/401), diarrhea (11/393 vs 8/288), fatigue (8/393 vs 7/288) had no significant difference compared with placebo group (P > .05) (Table 3).
Table 3.
Meta-analysis results of comparison of common adverse effects between the 2 groups.
| Incidence, % | Effect estimates | ||||||
| Outcome measures | Model | Ubrogepant | Placebo | RR | 95%CI | P | I2 (%) |
| Headache | Random | 7.89% | 8.68% | 1.17 | 0.71–1.93 | .53 | 0 |
| Oropharyngeal pain | Random | 9.18% | 3.47% | 2.10 | 0.99–4.48 | .30 | 42 |
| Nasopharyngitis | Random | 4.58% | 6.25% | 0.83 | 0.43–1.60 | .59 | 0 |
| Nausea | Random | 5.33% | 3.99% | 1.24 | 0.67–2.29 | .55 | 0 |
| Dizziness | Random | 4.24% | 2.24% | 1.38 | 0.25–7.70 | .71 | 78 |
| Diarrhea | Random | 2.80% | 2.78% | 1.06 | 0.42–2.65 | .91 | 0 |
| Fatigue | Random | 2.04% | 2.43% | 0.88 | 0.32–2.40 | .77 | 0 |
95%CI = confidence interval, RR = relative risk.
3.5. Sensitivity analysis of each index
Sensitivity analyses were carried out on the indexes of effectiveness and safety. After changing the effect model (fixed or random) and removing the maximum or minimum weight proportion, the results of the meta-analysis were not significantly different from those of the original analysis, indicating low sensitivity and high stability of the research results (Table 4).
Table 4.
Sensitivity analysis of effectiveness indicators.
| Fixed effect model | Excluded the RCT with maximum weight | Excluded the RCT with minimum weight | |||||||
| Outcome measures | RR | 95%CI | P | RR | 95%CI | P | RR | 95%CI | P |
| Pain relief | 1.75 | 1.52–2.00 | <.00001 | 1.78 | 1.50–2.11 | <.00001 | 1.70 | 1.47–1.96 | <.00001 |
| Absence of photophobia | 1.32 | 1.22–1.42 | <.00001 | 1.36 | 1.23–1.51 | <.00001 | 1.34 | 1.22–1.47 | <.00001 |
| Absence of nausea | 1.07 | 1.03–1.12 | .0004 | 1.09 | 1.04–1.14 | .002 | 1.07 | 1.03–1.12 | .0004 |
| Absence of phonophobia | 1.21 | 1.14–1.28 | <.00001 | 1.20 | 1.13–1.29 | <.00001 | 1.21 | 1.14–1.28 | <.00001 |
95%CI = confidence interval, RCTs = randomized controlled trials, RR = relative risk.
4. Discussion
A total of 5 RCTs were included in this study. The baseline and outcome data were relatively complete and balanced, with high comparability and quality of the included literature. The aim of this study was to evaluate the efficacy and safety of ubrogepant for the treatment of acute migraine. The analysis results of 5 RCTs showed that the percentages of subjects with pain relief and the absence of photophobia, nausea, and phonophobia at 2 hours postdose were significantly higher in the experimental group than in the placebo group (P < .05). However, the effect of different doses of ubrogepant was not stable. Subgroup analysis by dose showed that when the dose was 10 mg and 25 mg, there were no significant differences in the 4 effective outcome measures between the experimental group and the placebo group. When the dose was increased to between 50 mg and 100 mg, the outcome measures in the experimental group were significantly better than those in the placebo group, which is consistent with the research results obtained by Do's group.[21] It is recommended that the starting dose of ubrogepant should be at least 25 mg, and the analgesic effect is enhanced as the dose increases.
We analyzed the safety of the included studies, that is, the incidence of common adverse reactions and serious adverse reactions. The common adverse effects, such as headache, oropharyngeal pain, nasopharyngitis, nausea, dizziness, diarrhea and fatigue, in the experimental group were similar to those in the placebo group, and the incidence rates were low, suggesting that patients tolerated ubrogepant treatment well. As severe adverse effects, Lipton[18] reported 1 patient in the experimental group had severe adverse effects on the nervous and urinary systems, while the placebo group had no severe adverse effects; Voss[17] reported that 1 patient in the experimental group had severe adverse effects (myoclonus), while the placebo group had no severe adverse effects; Goasby[20] reported that 2 patients in the experimental group had severe adverse effects (1 subject had a selective abortion, and the other subject had abdominal pain, arthralgia, back pain, musculoskeletal pain, and neck pain related to a motor vehicle accident that occurred on day 55), while one severe adverse effect (selective abortion) occurred in the placebo group, but the above severe adverse effects were not considered to be directly related to the interventions. The results of a long-term open clinical trial[22] showed that the incidence of severe adverse effects in the experimental group (2.58%) was lower than that in the conventional treatment group (4.08%). There was no significant difference between the incidence of common adverse effects in the experimental group (32.35%) and the conventional treatment group (31.65%). This conclusion is similar to the research results in this paper, indicating that the safety and tolerability of ubrogepant are good.
However, this study also has limitations:
-
(1)
Due to the limitation of the number and language of the included literature, the sample size is small, which may have led to publication bias, affecting the reliability of the results.
-
(2)
One of the RCTs in this study had no relevant published literature and lacked descriptions of the randomization scheme and allocation concealment method. Thus, there may have been implementation bias or other bias, which could reduce the reliability of the results.
-
(3)
Due to the low incidence of adverse effects, we did not conduct a subgroup analysis of adverse effects. It is unknown whether an increase in the dose of ubrogepant increases the incidence of common adverse effects.
-
(4)
None of the participants in the studies had cardiovascular or cerebrovascular diseases; therefore, the safety of ubrogepant in this group of people cannot be determined.
-
(5)
The intervention measures in 5 studies were the single administration of ubrogepant, and the follow-up time was short; therefore, it was difficult to evaluate the long-term efficacy and safety of ubrogepant objectively. The results of this study should serve as a reference only. To obtain more stable results, more high-quality studies are needed.
Author contributions
Conceptualization: Zhizhen Zhang.
Data curation: Yunfeng Shu.
Formal analysis: Yunfeng Shu.
Funding acquisition: Biao Du.
Investigation: Yun Diao.
Methodology: Yun Diao.
Project administration: Yang Du.
Resources: Yang Du.
Software: Lizhi Chen.
Supervision: Zhizhen Zhang.
Validation: Lizhi Chen.
Visualization: Ying Liu.
Writing – original draft: Zhizhen Zhang.
Writing – review & editing: Ying Liu.
Footnotes
Abbreviations: 95%CI = confidence interval, CGRP = Calcitonin gene-related peptide, His = International Headache Society, MD = mean difference, OR = odds ratio, RCTs = randomized controlled trials, RR = relative risk.
How to cite this article: Zhang Z, Shu Y, Diao Y, Du Y, Chen L, Liu Y, Du B. Calcitonin gene-related peptide receptor antagonist ubrogepant for the treatment of acute migraine: A meta-analysis. Medicine. 2021;100:8(e24741).
This article was supported by Chongqing Science and Technology Commission (Grant No. cstc2017shmsA130105) and Chongqing Wanzhou Science and Technology Commission/Chongqing Wanzhou Health and Family Planning Commission (Grant No wzstc-2017002).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: a disorder of sensory processing. J Physiol Rev 2017;97:553–622. DOI 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kelman L. The postdrome of the acute migraine attack. J Cephalalgia 2006;26:214–20. DOI 10.1111/j.1468-2982.2005.01026.x. [DOI] [PubMed] [Google Scholar]
- [3].Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. J Headache 2005;45:904–10. DOI 10.1111/j.1526-4610.2005.05159.x. [DOI] [PubMed] [Google Scholar]
- [4].Gori S, Lucchesi C, Balacci F, et al. Preferential occurrence of attacks during night sleep and/or upon awakening negatively affects migraine clinical presentation. J Funct Neurol 2015;30:119–23. DOI 10.11138/fneur/2015.30.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017;16:877–97. DOI 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ashina M. Migraine. N Engl J Med 2020;383:1866–76. DOI 10.1056/NEJMra1915327. [DOI] [PubMed] [Google Scholar]
- [7].Youssef PE, Mack KJ. Episodic and chronic migraine in children. Dev Med Child Neurol 2020;62:34–41. DOI 10.1111/dmcn.14338. [DOI] [PubMed] [Google Scholar]
- [8].Negro A, Martelletti P. Gepants for the treatment of migraine. J Expert Opin Investig Drugs 2019;28:555–67. DOI 10.1080/13543784.2019.1618830. [DOI] [PubMed] [Google Scholar]
- [9].Dodick D, Lipton RB, Martin V, et al. Consensus statement: cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. J Headache 2004;44:414–25. DOI 10.1111/j.1526-4610.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- [10].Negro A, Martelletti P. Chronic migraine plus medication overuse headache: two entities or not? J Headache Pain 2011;12:593–601. DOI 10.1007/s10194-011-0388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang CB, Zhong XY, Li MY, et al. Meta analysis of the efficacy and safety of galcanezumab in the prophylactic treatment of migraine. J Chin J New Drugs Clin Med 2019;38:367–72. DOI 10.14109/j.cnki.xyylc.2019.06.011. [Google Scholar]
- [12].Gupta S, Mehrotra S, Villalón CM, et al. Characterisation of CGRP receptors in human and porcine isolated coronary arteries: evidence for CGRP receptor heterogeneity. J Eur J Pharmacol 2006;530:107–16. DOI 10.1016/j.ejphar.2005.11.020. [DOI] [PubMed] [Google Scholar]
- [13].Smillie SJ, Brain SD. Calcitonin gene-related peptide (CGRP) and its role in hypertension. J Neuropeptides 2011;45:93–104. DOI 10.1016/j.npep.2010.12.002. [DOI] [PubMed] [Google Scholar]
- [14].Chan C, Goadsby PJ. Recent advances in pharmacotherapy for episodic migrain. J CNS Drugs 2019;33:1053–71. DOI 10.1007/s40263-019-00665-9. [DOI] [PubMed] [Google Scholar]
- [15].Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. Section 8:174–218. [Google Scholar]
- [16].Allergan. A pharmacokinetic Study of MK-1602 in the Treatment of Acute Migraine (MK-1602-007). 2012;https://clinicaltrials.gov/ct2/show/NCT01657370 [Google Scholar]
- [17].Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. J Cephalalgia 2016;36:887–98. DOI 10.1177/0333102416653233. [DOI] [PubMed] [Google Scholar]
- [18].Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA 2019;322:1887–98. DOI 10.1001/jama.2019.16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. J N Engl J Med 2019;381:2230–41. DOI 10.1056/NEJMoa1813049. [DOI] [PubMed] [Google Scholar]
- [20].Goadsby PJ, Tepper SJ, Watkins PB. Safety and tolerability of ubrogepant following intermittent, high-frequency dosing: randomized, placebo-controlled trial in healthy adults. J Cephalalgia 2019;39:1753–61. DOI 10.1177/0333102419869918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Do TP, Guo S, Ashina M. Therapeutic novelties in migraine: new drugs, new hope? J Headache Pain 2019;20:37.DOI 10.1186/s10194-019-0974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Allergan. An extension study to evaluate the long-term safety and tolerability of ubrogepant in the treatment of migraine. https://clinicaltrials.gov/ct2/show/NCT02873221, 2018. [Google Scholar]


