Abstract
Pressurized liquid extraction (PLE) is a clean and environmentally friendly alternative for the recovery of bioactive compounds from fruit by-products. Herein we focused on PLE for the extraction of bioactive compounds from pomegranate peel using a combination of pressurized water and ethanol. The main aim was to determine the optimal PLE conditions, i.e., ethanol percentage and process temperature, to obtain a pomegranate peel extract (PPE) with maximum total phenolic content (TPC), punicalagin content, and antimicrobial activity (AMA). The experimental design was conducted using a central composite design with axial points. Response surface methodology was applied to optimize the response variables using the desirability function. Multiple response optimization indicated a process temperature of 200 °C and ethanol of 77% as optimal conditions. The TPC and the punicalagin content of PPE-PLE obtained under optimal conditions were 164.3 ± 10.7 mg GAE/g DW and 17 ± 3.6 mg/g DW, respectively. Our findings support the efficacy of PLE on TPC recovery but not in punicalagin recovery. The AMA against S. aureus was 14 mm. The efficacy of PPE-PLE in food applications must continue to be studied in order to achieve adequate information on its potential for developing new food additives.
Keywords: pomegranate by-products, pomegranate peel extract, polyphenols, punicalagin, antioxidant, RSM
1. Introduction
The pomegranate (Punica granatum L., Lythraceae) tree is native to northern India and other areas bordering the Himalayas, but over the centuries its cultivation has spread throughout the Mediterranean basin and the Americas [1]. Pomegranate is a balausta fruit consisting of a hard pericarp and a spongy mesocarp containing juicy arils. Pomegranate is consumed as fresh arils or as processed products, e.g., fresh or concentrated juice, infusions, or jam [2]. The juice yield is less than half of the total weight of the fruit [3]. Like other fresh fruits, pomegranate has an inedible fraction that is discarded although which exact part is disposed of depends on the fruit cultivar and cultural preferences [4,5]. The discarded fraction is used to calculate the unavoidable fruit waste intensity [5]. Thus, both the low yield in juice extraction and the inedible fraction (i.e., pomegranate mesocarp and peel) may explain why pomegranate fruit produces high levels of loss and waste along the food supply chain worldwide. In the first stages, fruit processing creates large amounts of pomegranate by-products as waste, while in the last stage, consumption of fresh pomegranate arils in households also results in high levels of unavoidable waste.
Food loss and waste results in a misuse of resources, e.g., water, land, energy, fertilizer, while also giving rise to methane and CO2 emissions from the natural decomposition of food [6]. Moreover, food loss and waste generate economic and social impacts [7,8]. The 2030 agenda for the United Nations sustainable development goals (SDGs) set food waste reduction targets (SDG 12) [9]. Due to this, several countries have adopted strategies to move toward a circular economy, where the food supply chain must take care of its by-products and food waste [10]. Because fruit by-products are considered food waste, their economic value is low [2]. However, pomegranate by-products have added value as a source of bioactive compounds, demonstrating the potential for developing new food additives and reducing waste in the agri-food industry.
Previous studies have shown that pomegranate peel is an important source of phenolic bioactive compounds such as phenolic acids, flavonoids, and hydrolysable tannins (ellagitannins) [11,12,13,14,15]. Among ellagitannins, punicalagin is the major compound found in pomegranate peel [16]. Phenolic compounds found in pomegranate peel have been associated with a wide range of biological activities in in vitro and animal models [17,18,19]. Antimicrobial, antioxidant, antidiabetic, anti-inflammatory, anticarcinogenic, and cardiovascular protective activities have been associated with ellagitannins such as punicalagin, punicalin, ellagic acid, and gallagic acid [20,21]. The antimicrobial activity of different types of pomegranate peel extract (PPE) has been studied mainly in vitro on pathogenic bacteria including L. monocytogenes, S. aureus, E. coli, Yersinia enterocolitica, B. cereus, B. subtilis, S. enteritidis, S. typhy and Pseudomonas fluorescens [22].
The profile and content of the bioactive compounds in PPE depend on the fruit cultivar, the pretreatment, and the extraction (e.g., solvent and method) procedure [18]. As a consequence, different types of PPE can show different degrees of antimicrobial activity.
Solid−liquid extraction is the most common procedure used to obtain bioactive compounds from pomegranate peel [16,23,24,25,26]. Specifically, methanol has been shown to be the most effective at extracting bioactive compounds from pomegranate peel in comparison to other organic solvents [16]. However, methanol extraction cannot be used in food applications [23].
Pressurized liquid extraction (PLE) has emerged as a novel technique to obtain bioactive compounds using both water and/or organic solvents in combination with elevated temperature and pressure [27]. PLE achieves fast, efficient, and selective extraction with a wide range of compound polarities, offering less extraction time and solvent consumption than conventional solid−liquid extraction [28]. Scarce information on the extraction of bioactive compounds from pomegranate peel using PLE has been reported [16,27]. In a first study, Cam and Hisil [16] showed that total phenolic content (TPC) using water in PLE was three-fold higher than using water in solid−liquid extraction (i.e., at atmospheric pressure conditions). A following study demonstrated that a combination of ultrasound and PLE was a clean and environmentally friendly alternative for extracting phenolic compounds from pomegranate peel [27].
The efficacy of PLE on the extraction of bioactive compounds from pomegranate peel using a combination of pressurized water and ethanol has not been evaluated. This study was therefore designed to gain new insight into this topic. The aim was to determine the optimal conditions, i.e., ethanol percentage and process temperature, to obtain a PPE-PLE with a maximum TPC, punicalagin content, and antimicrobial activity (AMA) using a multiple response optimization. The profile of phenolic compounds by high performance liquid chromatography–diode array detector–electrospray ionization–time of flight–mass spectroscopy (HPLC-DAD-ESI-TOF/MS), the antioxidant capacity (AC) and the cytotoxicity of the PPE-PLE obtained under optimal conditions were also characterized. This research contributes to the development of novel uses of pomegranate peel using a more environmentally friendly technique in order to reduce food waste in the pomegranate industry.
2. Materials and Methods
2.1. Pomegranate Material Recovery
Pomegranate fruits (cv. Wonderful) were collected at the ripening stage (April 2017) from a commercial farm located in Vallenar (28°34′ South Latitude; 70°45′ West Longitude) in the Atacama Region of Chile. Fruit samples were taken randomly from the upper, middle and lower canopy, stored at 4 °C and processed within 24 h of collection. Pomegranate peel from fresh fruits was manually separated and dried by convection in an air-drying tunnel (no brand, built with a Tetlak motor) with a horizontal air flow rate of 2 m/s and 50% recirculation at 60 °C for 16 h. The dried product was ground in a knife mill (Wiley Mill, Model−2, A.H. Thomas Co., Swedesboro, NJ, USA) and passed through a 20 mesh (840 microns) sieve. The resulting pomegranate peel powder was stored in darkness and kept at room temperature until extraction. The proximate composition of pomegranate peel powder was determined, including moisture, proteins, ash and fat according to the AOAC official procedures [29]. The content of total dietary fiber was determined by enzymatic gravimetric method [30]. TPC was spectrophotometrically quantified using a Folin−Ciocalteu phenol reagent assay [31], and punicalagin content was determined by HPLC [32]. In TPC and punicalagin analysis, a pomegranate peel powder sample was treated with ethanol:water (40:60 v/v) for 3 h using a solid−liquid extraction.
2.2. Chemical Reagents
For the extraction procedure, double-deionized water with conductivity lower than 18.2 MV was obtained with a Milli-Q system (Millipore, Bedford, MA, USA) and ethanol (purity ≥ 99.9%) was obtained from Panreac (Barcelona, Spain).
In order to characterize phenolic compounds and antioxidant capacity, all chemicals used were of analytical reagent grade. HCl (purity ≥ 37%), NaOH, NaCl, KCl, NaH2PO4, KH2PO4, sodium carbonate anhydrous, acetic acid glacial, sodium acetate trihydrate, iron(III) chloride hexahydrate, Folin−Ciocalteu’s phenol reagent, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), were purchased from Merck (KGaA, Darmstadt, Germany). Gallic acid (purity ≥ 98%), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), 2,2′-Azobis(2-methylpropionamidine) dihydrochloride (AAPH), and Fluorescein sodium salt were purchased from Sigma-Aldrich (St. Louis, MO, USA).
For HPLC characterization, water (LC-MS grade LiChrosolv®), methanol, and acetonitrile (Liquid chromatography LiChrosolv®) were purchased from Merck S.A (Santiago, Chile). Standard of punicalagin (purity ≥ 98%) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Finally, for cytotoxicity analysis a Vybrant MTT Cell Proliferation Assay Kit (Thermo Fisher Scientific, Leicestershire, UK) was utilized.
2.3. Extraction of Phenolic Compounds from PPE by PLE
Extraction of the phenolic compounds by PLE was carried out in 34-mL extraction cells, containing a mixture of pomegranate peel powder (3.75 g) and sand (11.25 g) using a pressurized liquid extractor (Dionex ASE 350, Accelerated Solvent Extractor (Thermo Fisher Scientific, Leicestershire, UK)) at a pressure of 1500 psi for 20 min. The resulting extract (PPE-PLE) was filtered (0.22 μm PTFE membrane filters, VWR International, Atlanta, GA, USA) and stored absent of light at −80 °C.
2.4. Experimental Design for PPE-PLE
The extractions from pomegranate peel powder using PLE were performed using a central composite design (CCD) with axial points, following general Equation (1)
| (1) |
where Y was the response; subscripts i and j ranged from 1 to the number of variables (n = 2); b0 was the intercept term; bi values were the linear coefficients; bii values were the quadratic coefficients; bij values were the interaction of the cross-product coefficient, and Xi and Xj were the levels of independent variables.
Twelve experiments were performed using the independent variables of ethanol (10–90%) in the water:ethanol mixture and process temperature (55–185 °C). The dependent variables were TPC, punicalagin content, and AMA. Response surface methodology (RSM) was applied to optimize the response variables using the desirability function (DF), where 1 represented the maximization of each variable [33]. All experiments were conducted randomly to avoid systematic bias. The linear, quadratic, and interaction effects of the independent variables on the response variables were considered at a confidence level of 95% (Statgraphics Centurion XV, Version 15.1.02, StatPoint, Inc., Warrenton, VA, USA).
2.5. Characterization of PPE-PLE Obtained under Optimal Conditions
2.5.1. Determination of TPC
The TPC was spectrophotometrically quantified using a Folin−Ciocalteu phenol reagent assay [31]. The absorbance of samples was measured at 765 nm, and the results were expressed as milligrams of gallic acid equivalents per gram of pomegranate peel in dry weight (mg GAE/g DW), according to a calibration curve (100–800 mg GAE/L, R2: 0.9967). All analyses were performed in triplicate.
2.5.2. Determination of Punicalagin Content
Punicalagin was detected and quantified via high performance liquid chromatography (HPLC) using a Merck Hitachi L-6200 pump, a Waters 996 photodiode-array detector (DAD), and a C18 column (5 μm, 4.6 i.d. × 250 mm, Symmetry, Waters, Ireland) employing the method described by Zhang et al. [32] with some modifications. Briefly, to prepare the mobile phase, Solvent A (0.4% aqueous phosphoric acid) and Solvent B (acetonitrile) eluted according to the following multistep gradient: 0 min (5% B); 10 min (15% B); 30 min (25% B); 35 min (5% B). A measure of 20 µL of the sample was injected and the flow rate was 1.0 mL/min at room temperature.
The monitored wavelength was 360 nm for the detection and quantification of total punicalagin (calculated by the sum of the peak areas of punicalagin A and B), according to a calibration curve (12–200 mg punicalagin/L extract, R2: 0.9942). The results were expressed as milligrams of punicalagin per gram of pomegranate peel in DW (mg/g DW). All analyses were performed in triplicate.
2.5.3. Determination of the Antioxidant Capacity (AC)
The Ferric Reducing Antioxidant Power (FRAP) assay was determined according to Benzie and Strain [34] with modifications. Briefly, a portion of an aqueous 10 mM solution TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) reagent in 40 mmol/L HCl was mixed with the same volume of 20 mmol L-1 FeCl3 · 6H2O and a 10-fold higher volume of acetate buffer, pH 3.6 (3.1 g sodium acetate and 16 mL acetic acid/L). The mixture was then incubated at 37 °C for 10 min. A portion (2700 μL) of the Fe3+-TPTZ mixture and 30 μL of each sample (or standard or water for blank) were combined and diluted to 270 μL with deionized water and then incubated at 37 °C for 30 min. Next, an aliquot of 225 µL of solution was transferred to a microplate (96 well, elisa plate) and the measured absorbance was 593 nm. Results were expressed as µmol of Trolox equivalents per g of pomegranate peel in DW (µmol TE/g DW), according to a calibration curve (200–1600 µM Trolox, R2: 0.9958). Each analysis was carried out in triplicate.
The Oxygen Radical Absorbance Capacity (ORAC) assay was determined according to Dávalos et al. [35]. Results were expressed as µmol of Trolox equivalents per g of pomegranate peel in DW (µmol TE/g DW), according to a calibration curve (6.25–100 µM Trolox, R2: 0.9958). Each analysis was carried out in triplicate.
2.5.4. Identification of Phenolic and Other Polar Compounds in PPE-PLE by HPLC-DAD-ESI-TOF/MS
Samples of PPE-PLE were analyzed in a high-performance resolution liquid chromatography (HPLC) system (Agilent Technologies, Waldbronn, Germany) equipped with a vacuum degasser, autosampler, binary pump, and diode-array-detector (DAD). This equipment was coupled to a time of flight mass spectrometer TOF (Bruker Daltonik, Bremen, Germany) equipped with an orthogonal electrospray (ESI) interface (model G1607 from Agilent Technologies, Palo Alto, CA, USA) operating in negative ion mode. The analytical column used was a C18 Zorbax Eclipse Plus (150 mm × 4.6 mm id, 1.8 µm, Agilent Technologies, Palo Alto, CA, USA).
The mobile phase was water acidified with 0.1% of formic acid (Solvent A) and methanol (Solvent B) eluted according to the following multistep gradient: 0 min (5% B); 42 min (95% B), 45 min (5% B) and 50 min (5% B). Then, 10 µL of the sample was injected and the flow rate was 0.4 mL/min at room temperature.
Polyphenols were detected by applying a mass range of 50–1500 m/z. The ESI source parameters were optimized and implemented as follows: capillary voltage of +4 kV; drying gas temperature, 210 °C; drying gas flow, 9 L min−1; and nebulizing gas pressure, 2.3 bar. The values of transfer parameters were: capillary exit, −120 V; skimmer 1, −40 V; hexapole 1, −23 V; RF hexapole, 80 V; and skimmer 2, −22.5 V. Moreover, to ensure proper calibration, the TOF mass spectrometer was externally calibrated using a 74900-00-05 Cole Palmer syringe pump (Vernon Hills, IL, USA) which was directly connected to the interface. The calibrant solution contained 10mM of sodium formate cluster. The mass data of the molecular ions acquired were managed using DataAnalysis 4.0 (Bruker Daltonics, Billerica, MA, USA) software, which uses a CHNO algorithm that increases the confidence in the suggested molecular formulas.
2.5.5. Antimicrobial Activity (AMA)
The agar-well diffusion method was employed using Mueller-Hinton agar. The agar plate surfaces were inoculated by spreading 1 mL of the microbial inoculum over the entire agar surface. The inoculums were prepared using Staphylococcus aureus subsp. aureus (ATCC 25923) or Escherichia coli (ATCC 25922) and were suspended in sterile water and diluted to 106 CFU/mL. Once the medium had solidified, wells with a diameter of 5 mm were punched aseptically with a sterile borer, and 70 µL of the PPE-PLE were placed into each well. Then, agar plates were incubated at 37 ± 1 °C for 24 h and AMA was evaluated by measuring the diameter of the inhibition zone of the tested bacteria. The results were expressed in mm.
2.5.6. Cytotoxicity Assay
To determine the cytotoxic activity of PPE-PLE, an in vitro cell proliferation assay with an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) kit was used on the human colorectal cancer cell line Caco-2 following the manufacturer’s instructions (Vybrant MTT). Cells were seeded onto a 96-well plate and incubated at 37 °C overnight with approximately 80% confluence, taking the cytotoxic compound SDS 0.2% as a positive control. Then, cells were incubated with 100 μL of PPE-PLE (in DW) at 3 different concentrations: 10, 50, 100 μg/mL, for 24 h. A portion of 10 µL MTT per well was added into the plate and incubated for an additional 3 h at 37 °C in the 5% CO2 incubator. The absorbance was measured in a microplate reader (Tecan Infinite® 200PRO, Männedorf, Switzerland), at a wavelength of 570 nm. Cell viability was expressed as the percentage of viable cells compared to the control group without treatment. Experiments were performed in triplicate.
2.5.7. Statistical Analysis
The differences in cell viability using different PPE-PLE concentration was analyzed using a one-way ANOVA test for means comparison. When significant differences were found, the Tukey HSD (honest significant differences) multiple-comparison test (p ≤ 0.05) was applied. Analyses were performed with (Statgraphics Centurion XV, Version 15.1.02, StatPoint, Inc., Warrenton, VA, USA).
3. Results and Discussion
Pomegranate fruit processing is associated with unavoidable food waste, regardless of the manufacturers’ efficacy in managing and minimizing waste. Therefore, strategies to manage unavoidable food waste in the pomegranate industry are necessary to achieve SDGs and to move toward a circular economy [9,10]. Herein we focused on the treatment of a material (i.e., pomegranate peel) recycled from the pomegranate industry using PLE. Teigiserova et al. [36] proposed an updated hierarchy for food surplus and waste, where processing waste residues is equivalent to material recycling. Of note, material recycling actions appear in the center of the updated hierarchy [36]. Therefore, evaluating the efficacy of PLE on the recovery of bioactive compounds from pomegranate peel provides novel insights into alternative actions to mitigate food waste in the pomegranate industry. A previous study has reported that PLE is an environmentally friendly alternative for extracting phenolic compounds from pomegranate peel [27]. In this study, we showed new findings for the use of pomegranate peel powder as a recycled material, optimizing the PPE in order to obtain and characterize a PPE-PLE with maximum TPC, punicalagin content, and AMA.
3.1. Characterization of Pomegranate Peel Powder as Material Recycling
The unavoidable fruit waste intensity of pomegranate fruit was 57.4 ± 6.7%. The inedible fraction (i.e., pomegranate mesocarp plus pomegranate peel) can be used in material recycling. Specifically, pomegranate peel represented 10.5 ± 1.4% of the pomegranate-fruit fresh weight. After drying, the composition of the pomegranate peel powder was as follows: 3% moisture, 3.5% protein, 2.7% ash, 0.2% fat, and 60% available carbohydrates. Total dietary fiber was 30%, 14% soluble fiber and 16% insoluble fiber. Of note, the proximate composition of pomegranate peel was similar to that of pomegranate fruit, where the carbohydrate fraction predominated [37]. Nevertheless, pomegranate peel had a lower content of available carbohydrates than pomegranate fruit (i.e., 60% vs. 68%). In contrast, pomegranate peel contained higher dietary fiber content than pomegranate fruit (i.e., 30% vs. 18%). Indeed, following the daily recommended allowances (RDA) of dietary fiber in adults, pomegranate peel powder could therefore be considered a good source of dietary fiber [38]. TPC and punicalagin content were 125 mg GAE/g DW and 94 mg/g DW using solid−liquid extraction, respectively. These values were within the range described in the literature using solid−liquid extraction methods.
3.2. Optimization of Extraction of Phenolic Compounds from Pomegranate Peel by PLE Using RSM
PLE is a sample preparation technique that combines elevated temperature and pressure with liquid solvents to achieve fast and efficient analyte extraction from a solid matrix [27,28]. Thus, temperature [X1] and solvent extraction (i.e., ethanol percentage [X2] in water:ethanol mixture) were selected as independent variables because both variables have demonstrated a significant effect on the extraction of phenolic compounds from pomegranate peel [16,18,27,28]. Pressure was not considered as an independent variable, so a high pressure of 1500 psi was constant in all of the experiments based on previous research on PLE [16,27]. In order to find the optimal conditions for obtaining a PPE-PLE, an RSM analysis was applied. Of note, the desirability function was comprised of the maximization of each variable and a multiple response optimization. Results of dependent variables in PLE from pomegranate peel and ANOVA results are detailed in Table 1 and Table 2.
Table 1.
Central composite design and results of dependent variables in PLE from pomegranate peel.
| Run | X1 | X2 | Temperature [X1] | Ethanol [X2] | TPC | Punicalagin | AMA |
|---|---|---|---|---|---|---|---|
| (°C) | (%) | (mg GAE/g DW) | (mg/g DW) | S. aureus (mm) | |||
| 1 | −1 | −1 | 55 | 10 | 14.1 ± 0.7 | 7.7 ± 0.2 | 2.0 ± 0.4 |
| 2 | −1 | 1 | 55 | 90 | 75.9 ± 3.0 | 38.0 ± 1.9 | 7.5 ± 0.7 |
| 3 | 1 | −1 | 185 | 10 | 73.0 ± 1.0 | 5.0 ± 0.2 | 4.0 ± 0.7 |
| 4 | 1 | 1 | 185 | 90 | 129.6 ± 6.3 | 30.1 ± 0.9 | 7.0 ± 2.8 |
| 5 | −1.21 | 0 | 41.3 | 50 | 39.6 ± 1.6 | 36.1 ± 0.6 | 6.0 ± 0.0 |
| 6 | +1.21 | 0 | 198.6 | 50 | 149.0 ± 5.3 | 22.0 ± 0.3 | 8.3 ± 1.1 |
| 7 | 0 | −1.21 | 120 | 1.6 | 20.0 ± 3.5 | 4.0 ± 0.3 | 3.0 ± 0.7 |
| 8 | 0 | +1.21 | 120 | 98.4 | 40.9 ± 1.9 | 23.3 ± 1.3 | 5.5 ± 1.4 |
| 9 | 0 | 0 | 120 | 50 | 39.2 ± 0.2 | 38.6 ± 2.5 | 4.8 ± 1.1 |
| 10 | 0 | 0 | 120 | 50 | 27.7 ± 0.5 | 35.0 ± 1.5 | 3.0 ± 0.7 |
| 11 | 0 | 0 | 120 | 50 | 33.2 ± 1.0 | 31.4 ± 0.6 | 3.8 ± 0.4 |
| 12 | 0 | 0 | 120 | 50 | 39.0 ± 0.2 | 38.0 ± 1.1 | 4.8 ± 0.4 |
Data represent mean ± standard deviation. TPC, total phenolic content; AMA, antimicrobial activity.
Table 2.
Analysis of variance (ANOVA) for the pomegranate peel extract obtained by PLE.
| Source | Sum of Squares | df | Mean Square | F-Ratio | p-Value | R 2 | R2 df.adj |
|---|---|---|---|---|---|---|---|
| TPC | |||||||
| X1: Temperature | 8662.03 | 1 | 8662.03 | 288.84 | 0.0004 * | 93.3 | 90.7 |
| X2: Ethanol | 2980.07 | 1 | 2980.07 | 99.37 | 0.0021 * | ||
| X1X1 | 7179.23 | 1 | 7179.23 | 239.39 | 0.0006 * | ||
| Lack of fit | 1273.17 | 5 | 254.635 | 8.49 | 0.0542 | ||
| Pure error | 899.675 | 3 | 299.892 | ||||
| Total (corr.) | 20184.5 | 11 | |||||
| Punicalagin | |||||||
| X1: Temperature | 110.437 | 1 | 110.437 | 10.14 | 0.0499 * | 91.1 | 87.7 |
| X2: Ethanol | 895.186 | 1 | 895.186 | 82.20 | 0.0028 * | ||
| X2X2 | 777.825 | 1 | 777.825 | 71.43 | 0.0035 * | ||
| Lack of fit | 142.147 | 5 | 284.294 | 2.61 | 0.2297 | ||
| Pure error | 32.67 | 3 | 10.89 | ||||
| Total (corr.) | 1958.27 | 11 | |||||
| AMA | |||||||
| X1: Temperature | 257.347 | 1 | 257.347 | 3.60 | 0.1539 | 79.6 | 80.1 |
| X2: Ethanol | 191.717 | 1 | 191.717 | 26.84 | 0.0140 * | ||
| X1X1 | 113.715 | 1 | 113.715 | 15.92 | 0.0282 * | ||
| Lack of fit | 635.145 | 5 | 127.029 | 1.78 | 0.3370 | ||
| Pure error | 21.425 | 3 | 0.714167 | ||||
| Total (corr.) | 416.106 | 11 | |||||
| Multiple Response Optimization | |||||||
| Desirability | 0.79896 | ||||||
| Factor | Low | High | Optimum | ||||
| X1: Temperature | 41.3 | 198.6 | 198.6 | ||||
| X2: Ethanol | 1.6 | 98.4 | 76.6 | ||||
TPC, total phenolic content; AMA, antimicrobial activity; df, degrees of freedom; df.adj, adjusted degrees of freedom. * p < 0.05.
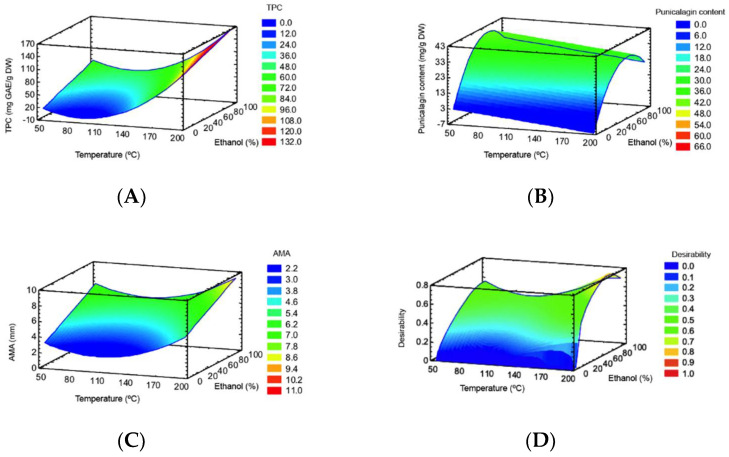
TPC ranged from 14.1 to 149.0 mg GAE/g DW (Table 1). The TPC was significantly affected by both lineal and quadratic forms (p < 0.05) of temperature, and the lineal form (p < 0.05) of ethanol (Table 2). The RSM plot (Figure 1A) showed that the highest TPC was obtained at the highest process temperature and intermediate ethanol percentage. This can be explained by the hydrolysis of polymeric compounds (e.g., ellagitannins) at high extraction temperatures resulting in monomeric phenolic compounds (e.g., ellagic acid) that were accounted in TPC measurement [39]. Moreover, ellagic acid glycosides that predominate in pomegranate peel are not affected much by temperature processing. For instance, thermal processing in red raspberry fruit did not greatly affect ellagic acid glycosides whereas free ellagic acid was more abundant after thermal processing [40]. The effect of ethanol percentage can be explained by the solvent polarity and the different polarity of bioactive compounds in the pomegranate peel matrix. This means that, as the ethanol percentage of the solvent increased, the extraction yield of polar compounds decreased [27]. Therefore, the recovery of compounds with a wide range of polarities present in pomegranate peels, ranging from polar (punicalagin and derivatives) to moderately polar (ellagic acid and derivatives) compounds may be accounted in TPC results.
Figure 1.
RSM plots for TPC (A), punicalagin (B), AMA (C) and multiple response optimization (D).
Punicalagin content ranged from 4.0 to 38.6 mg/g DW (Table 1). According ANOVA (Table 2), punicalagin content was significantly affected by both lineal and quadratic forms (p < 0.05) of ethanol. The lineal form (p < 0.05) of temperature also showed a significant effect on punicalagin content. The RSM plot (Figure 1B) shows that a high punicalagin content can be obtained at low process temperature at an intermediate ethanol percentage.
The results showed that TPC and punicalagin content were different among the experiments. This behavior is characteristic of the PLE method, which presents differences in the selectivity of extraction [28].The extraction factors such as temperature and water:ethanol ratio have been associated with the dielectric constant. Indeed, the lower the dielectric constant (i.e., higher ethanol in the water-ethanol extracting solvent), the higher the flavonoids content [41].
The AMA ranged from 2.0 to 8.3 mm (Table 1) and was significantly affected by the quadratic form (p < 0.05) of temperature, and the lineal form (p < 0.05) of ethanol (Table 2). The RSM plot (Figure 1C) showed that the highest AMA was obtained at the highest process temperature and an intermediate ethanol percentage.
The coefficients of determination (R2) and R2-adjusted, and a nonsignificant lack-of-fit indicated that the mathematical models fitted well with the experimental data. Therefore, the models are suitable as a predictor of the TPC, punicalagin, and AMA. The multiple response optimization (Figure 1D) indicated a process temperature of 198.6 °C (i.e., the major axial point) and an intermediate ethanol of 76.6% as optimal conditions to obtain a PPE-PLE. In this study, RSM did not involve the maximum punicalagin content value of the experimental design, because the optimal conditions considered the optimization of several dependent variables (i.e., TPC, punicalagin and AMA) simultaneously.
3.3. Characterization of PPE-PLE Obtained under Optimal Conditions
3.3.1. TPC and Punicalagin
The TPC and the punicalagin content of PPE-PLE obtained under optimal conditions was 164.3 ± 10.7 mg GAE/g DW and 17 ± 3.6 mg/g DW, respectively. Different values of TPC and punicalagin have been previously reported. Note, however, that the results among studies are not directly comparable, because of their different pomegranate genotypes, extraction and quantification methods. Based on solid−liquid extraction methods, Ambigaipalan et al. [42] reported a lower TPC (9.4 mg GAE/g of a pomegranate peel defatted sample) using acetone (70%). In addition, Fischer et al. [11] described a TPC of 101.9 mg GAE/g DW using aqueous methanol (80% v/v; 0.1% HCl) extraction. Contrarily, a higher TPC of 249.4 mg tannic acid equivalents/g DW using a mixture of methanol, ethanol, acetone and water, and a TPC of 432.7 mg GAE/g DW using ethanol:water (20:80 v/v) have been reported by Li et al. [43] and Rongai et al. [44], respectively. These studies showed the influence of extracting solvents on TPC, resulting in a wide range of TPC values. However, our results support the efficacy of PLE on the extraction of TPC from pomegranate peel using a combination of pressurized water and ethanol (76.6%).
Regarding punicalagin content, Rongai et al. [44] reported a much higher value (216.8 mg/g DW) using ethanol:water (20:80 v/v). Of note, another study has shown that a higher ethanol percentage in PLE extraction decreased solvent polarity, reducing the punicalagin extraction [27]. In our study, a combination of pressurized water and ethanol extended the use of PLE on the recovery of TPC from pomegranate peel under the experimental conditions, but did not enhance the recovery of punicalagin.
3.3.2. AC
The AC of PPE-PLE was 2265.6 ± 100.5 µmol TE/g DW measured by FRAP and 916.4 ± 102.0 µmol TE/g DW by ORAC. Polyphenol content in pomegranate peel extracts has been significantly correlated with AC, suggesting that the totality of polyphenols accounts for the AC observed [23,45]. Of note, the AC of PPE-PLE by FRAP was similar to the highest values obtained in pomegranate peel by Elfalleh et al. [24].
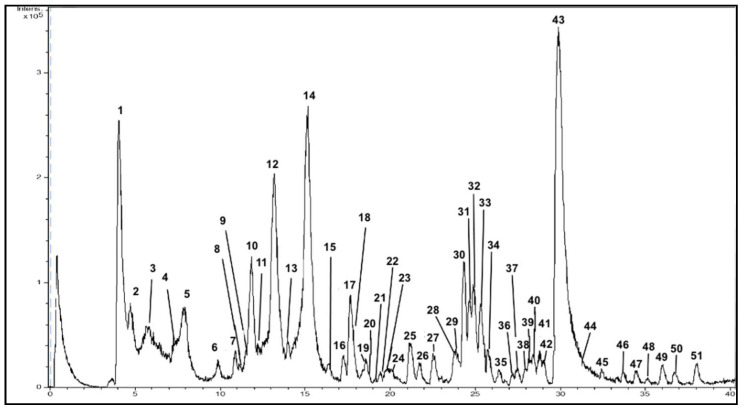
3.3.3. Qualitative Characterization of Phenolic and Other Polar Compounds in PPE-PLE by HPLC-ESI-TOF-MS
The chromatogram for PPE-PLE obtained by the HPLC-ESI-TOF-MS method is shown in Figure 2. The identification was based upon an interpretation of their MS spectra provided by TOF-MS and the information suitable in the literature. Table 3 shows the tentative identification of each peak with their retention times (RT), experimental and calculated m/z and molecular formula. These compounds have been numbered according their elution order. The HPLC-ESI-TOF-MS method allowed the detection of 51 compounds. Among these, a total of 42 compounds were identified, and then classified into five groups based on their chemical structure. Unfortunately, nine minor compounds could not be identified with the method used. Further analysis using improved identification methodologies should be performed in order to identify these unknown compounds.
Figure 2.
Representative HPLC-DAD-ESI-TOF/MS chromatogram of polar compounds in PPE-PLE.
Table 3.
Tentative identification of polar compounds and their derivatives in PPE-PLE by HPLC-DAD-ESI-TOF/MS.
| Peak | RT (min) | m/z exp | m/z cal | Molecular Formula | Tentative Compound |
| 1 | 4.2 | 181.0724 | 181.0718 | C6H13O6 | Sugar |
| 2 | 4.8 | 353.0721 | 353.0725 | C12H17O12 | Sugar |
| 3 | 5.9 | 353.0724 | 353.0878 | C16H17O9 | Chlorogenic acid |
| 4 | 7.5 | 191.0195 | 191.0197 | C6H7O7 | Citric acid |
| 5 | 8.0 | 191.0204 | 191.0197 | C6H7O7 | Isocitric acid |
| 6 | 10.1 | 331.0657 | 331.0671 | C13H15O10 | Galloyl hexose isomer 1 |
| 7 | 10.9 | 125.0247 | 125.0244 | C6H5O3 | Phloroglucinol |
| 8 | 11.3 | 331.0655 | 331.0.671 | C13H15O10 | Galloyl hexose isomer 2 |
| 9 | 11.7 | 541.0282 | 541.0260 | C24H13O15 | Punicalagin α |
| 10 | 12.0 | 169.0143 | 169.0142 | C7H5O5 | Gallic acid |
| 11 | 12.8 | 289.0869 | 289.0929 | C12H17O8 | Sugar derivative |
| 12 | 13.3 | 541.0284 | 541.0260 | C24H13O15 | Punicalagin β |
| 13 | 14.2 | 289.0927 | 289.0929 | C12H17O8 | Sugar derivative |
| 14 | 15.2 | 541.0295 | 541.0260 | C24H13O15 | Punicalagin γ |
| 15 | 16.5 | 469.0029 | 469.0049 | C21H9O13 | Valonic acid bilactone |
| 16 | 17.3 | 461.0916 | 461.0937 | C18H21O14 | Unknown 1 |
| 17 | 17.7 | 219.0514 | 219.0510 | C8H11O7 | Citric acid, dimethyl ester |
| 18 | 17.7 | 633.0726 | 633.0733 | C27H21O18 | Galloyl-HHDP-hexose |
| 19 | 18.6 | 289.0718 | 289.0718 | C15H13O6 | Catechin |
| 20 | 18.7 | 483.0768 | 483.0780 | C20H19O14 | Digalloyl hexoside |
| 21 | 19.3 | 443.1938 | 443.1711 | C24H27O8 | Unknown 2 |
| 22 | 19.5 | 453.0963 | 453.0827 | C23H17O10 | Unknown 3 |
| 23 | 19.8 | 575.0125 | 575.0103 | C27H11O15 | Unknown 4 |
| 24 | 20.1 | 325.0915 | 325.0929 | C15H17O8 | p-Coumaric acid hexoside |
| 25 | 21.3 | 633.0740 | 633.0463 | C27H21O18 | Corilagin |
| 26 | 21.8 | 291.0168 | 291.0146 | C13H7O8 | Brevifolin carboxylic acid |
| 27 | 22.6 | 431.1941 | 431.1711 | C23H27O8 | Unknown 5 |
| 28 | 24.1 | 345.0826 | 345.0827 | C14H17O10 | Methyl gallate hexoside |
| 29 | 24.1 | 600.9896 | 600.9896 | C28H9O16 | Gallagyldilactone |
| 30 | 24.4 | 197.0462 | 197.0455 | C9H10O5 | Gallic acid dimethyl ether |
| 31 | 24.8 | 463.0526 | 463.0518 | C20H15O13 | Ellagic acid hexoside |
| 32 | 24.9 | 275.0208 | 275.0197 | C13H7O7 | Decarboxyellagic acid |
| 33 | 25.5 | 247.0258 | 247.0096 | C12H7O6 | Brevifolin |
| 34 | 25.7 | 517.1526 | 517.1563 | C22H29O14 | Feruloyl sucrose |
| 35 | 26.4 | 293.1034 | 293.1031 | C15H17O6 | Cinnamoyl rhamnoside |
| 36 | 27.2 | 319.0446 | 319.0459 | C15H11O8 | Brevifolincarboxylic acid ethyl ester |
| 37 | 27.6 | 491.0845 | 491.0620 | C25H15O11 | Unknown 6 |
| 38 | 27.9 | 425.0161 | 425.0150 | C20H9O11 | Monodecarboxyvaloneic acid dilactone |
| 39 | 28.1 | 463.0906 | 463.0882 | C21H19O12 | Hyperoside |
| Peak | RT (min) | m/z exp | m/z cal | Molecular Formula | Tentative Identification |
| 40 | 28.3 | 433.0418 | 433.0412 | C19H13O12 | Ellagic acid pentoside |
| 41 | 29.0 | 447.0594 | 447.0569 | C20H15O12 | Ellagic acid-deoxyhexoside |
| 42 | 29.0 | 181.0505 | 181.0506 | C9H9O4 | Homovanillic acid |
| 43 | 30.0 | 300.9998 | 300.9990 | C14H5O8 | Ellagic acid |
| 44 | 31.9 | 447.0908 | 447.0933 | C21H19O11 | Luteolin hexoside |
| 45 | 32.4 | 321.1344 | 321.1344 | C17H21O6 | Unknown 7 |
| 46 | 33.9 | 417.0822 | 417.0827 | C20H17O10 | Luteolin pentoside |
| 47 | 34.5 | 285.0431 | 285.0557 | C15H9O6 | Kaempferol |
| 48 | 35.1 | 329.0305 | 329.0303 | C16H9O8 | Ellagic acid dimethyl ether |
| 49 | 36.2 | 285.0419 | 285.0405 | C15H9O6 | Luteolin |
| 50 | 36.7 | 317.0406 | 371.0409 | C18H11O9 | Unknown 8 |
| 51 | 38.0 | 463.0654 | 463.0671 | C24H15O10 | Unknown 9 |
Sugars
The method applied allowed for the identification of four sugars in PPE-PLE (compounds 1, 2, 11 and 13). These compounds were eluted earlier in the chromatographic run due to their highly hydrophilic character. Some studies have attributed the sweetener property of fruits to glucose, fructose, and sucrose [46,47]. Furthermore, two sugar derivatives, compounds 11 and 13, were found at 12.8 and 14.2 min, respectively. These are related to hexose or pentose derivatives. Unfortunately, an accurate identification could not be achieved because while the MS equipment used was able to provide molecular formulas, it could not give functional group positions.
Organic acids
According to the MS spectra provided by TOF-MS and the elution profile, three organic acids were identified: citric acid (compound 4) and two derivatives. Compound 5 showed an elution time of 8.0 min enabling it to be identified as isocitric acid (m/z 191.0197) and compound 17 eluted at 17.7 min which provided a m/z at 219.0510. MS data characterized it as dimethyl ester citric acid [48].
Phenolic acids
A total of eight phenolic acids were detected after examining the MS spectra, elution time, and available bibliography. Chlorogenic acid (compound 3) with the molecular formula (C16H18O9) and a retention time of 5.9 min was identified. Compound 7 showed a m/z of 125.0244 and a deprotonated molecular formula C6H5O3 which identified it as phloroglucinol [49]. Compound number 10 was related to gallic acid, as previously described in other studies [11,50]. Additionally, another gallic acid derivative was found and associated to compound 30. Specifically, this compound was related to gallic acid dimethyl ether since it gave the molecular formula C9H10O5. Compound 34 is a ferulic acid derivative (feruloyl sucrose), which was previously identified in other studies [11]. Furthermore, other phenolic acids corresponding to compounds 24, 35 and 42 were also detected. The first compound (24) was identified as p-coumaric acid hexoside, the first time this compound has been detected in pomegranate. Additionally, compound 35 presented a m/z of 293.1031 and a retention time of 26.4 min being identified as cinnamoyl rhamnoside. Compound 42 was also identified as homovanillic acid (C9H10O4). This compound was characterized on the basis that cinnamic acid and its derivatives were previously found in pomegranate peel [24,51,52].
Phenolic acids from pomegranate peel have shown the capacity to inhibit the activity of the angiotensin converting enzyme (ACE) that is implicated in blood pressure [21]. Gallic acid, has been associated with antidiabetic, anti-inflammatory, antioxidant and anticarcinogenic properties. Additionally, caffeic acid increases glucose uptake by rat adipocytes and mouse myoblasts [53].
Flavonoids
Overall, six flavonoids were detected in PPE-PLE. The first eluted flavonoid (18.6 min) was catechin which was related to compound 19. [54,55]. Compound 39 (28.1 min), which gave a deprotonated molecular formula of C21H19O12 enabled its identification as hyperoside. This flavonoid has previously been found in different pomegranate cultivars [50,56]. In addition, kaempferol (compound 47) and two luteolin derivatives were detected and attributed to compounds 44 and 46. They gave similar molecular formulas of C21H19O11 and C20H17O10, respectively. Therefore, it could be established that they were joined to a hexose and a pentose, characterizing compound 44 as luteolin hexoside and compound 46 as luteolin pentoside. Both luteolin hexoside and luteolin pentoside have previously been found in pomegranate [11]. Additionally, luteolin was tentatively identified as compound 49, since it displayed a deprotonated molecular formula of C15H9O6 and has previously been described in the literature [50].
Flavonoids of pomegranate peel are associated with antioxidant properties and improving oral health, particularly in relation to gingivitis development [22,54].
Hydrolysable tannins
The analytical data provided enabled 21 hydrolysable tannins to be identified in PPE-PLE, the most abundant chemical group found. Because of the broad variety of compounds belonging to this group, they were classified in two groups: gallotannins and ellagitannins. Regarding gallotannins, compounds 6 and 8 presented the same deprotonated molecular formula (C13H15O10) and a m/z ratio of 331.0671, characterizing them as galloyl-hexoside isomers. Isomer 1 and 2 were detected. In addition to these compounds, four additional tannins derived from gallic acid were also characterized. Thus, compound 18, which displayed a molecular formula C27H22O18, was related to galloyl-HHDP-hexose [11]. Compound 20, which presented a m/z of 483.0780, was tentatively identified as digalloyl hexoside. Additionally, another gallotannin was found with a retention time of 24.1 min. This compound was characterized as methyl gallate hexoside (compound 28). Finally, the last gallotannin was compound 29, related to gallagyldilactone [57].
Ellagitannins are predominant in pomegranate. Among them, some isomers of punicalagin were found. Indeed, compounds 9, 12 and 14, which showed the same molecular formula (C24H14O15), were related to punicalagin α, β and γ, respectively. Isomers α and β were identified earlier by following their elution order, as has been described in other studies [27,58]. However, isomer γ was identified for the first time in this study. In addition, compound 15 with a m/z of 469.0049 was assigned to valonic acid bilactone [11,51]. Compound 38 was associated with a valonic acid derivative, particularly, monodecarboxyvaloneic acid dilactone. Compound 25 gave a deprotonated molecule of m/z 633.0463 with the molecular formula C27H21O18 and eluted at 21.3 min. This peak was associated with corilagin, a compound previously described in pomegranate [59]. Likewise, compound 26 displayed a deprotonated molecule at a m/z of 291.0146 and a deprotonated molecular formula of C13H7O8. These results enabled its identification as brevifolin carboxylic acid which has previously been detected in pomegranate peel [11]. Furthermore, compound 33 displayed a molecular formula of C12H8O6 and it was characterized as brevifolin [60]. In addition, brevifolincarboxylic acid ethyl ester was found and assigned to compound 36 which gave a m/z of 319.0459. The occurrence of this compound in this extract may be caused by conditions applied during the PLE extraction procedure.
Finally, the MS spectra acquired characterized ellagic acid and five derivatives. The first eluted ellagic acid derivative was ellagic acid hexoside (compound 31). Then, compound 32 (C13H8O7) was characterized as decarboxyellagic acid. Compounds 40 and 41 were identified as ellagic acid pentoside and elagic acid deoxyhexoside with the same elution order that has been previously reported in the literature [27]. Altogether, a deprotonated molecular formula of C14H5O8 was given by compound 43. This compound, characterized as ellagic acid, comprised more than 50% of the total phenolic compounds in pomegranate peel, the highest intensity of all the compounds studied [61]. The last ellagitannin eluted in the chromatographic run was compound 48 which gave a m/z of 329.0303, identified as ellagic acid dimethyl ether.
Ellagitannins, mainly punicalagin, are responsible for the antioxidant and antimicrobial activity of pomegranate peel extract. In addition, the anticarcinogenic effects and inhibition of inflammatory processes are attributed to punicalagin, ellagic acid and gallic acid [21].
3.3.4. AMA
The AMA of PPE-PLE was evaluated on S. aureus and E. coli by measuring the diameter of the inhibition zone of the tested bacteria. AMA was only observed on S. aureus with an inhibition diameter of 14 mm. These results could be explained by the differences between Gram-negative and Gram-positive bacteria membrane. Gram-negative bacteria (e.g., E. coli) would be more resistant to polyphenolic extracts than Gram-positive bacteria (e.g., S. aureus), because Gram-negative bacteria possess a complex outer lipopolysaccharide membrane which slows down the passage of polyphenols [62]. The antimicrobial mechanism of phenolic compounds has not yet been elucidated, however, it is known that they have many action sites at the cellular level. Indeed, the presence of OH functional groups is relevant to the antibacterial activity of many phenolic compounds, which interact with bacteria’s cellular membranes by hydrogen bonding [62]. Thus, the membrane’s functions of nutrient uptake and enzyme activity would be affected, causing microbial cell death [22].
PPE-PLE obtained under optimal conditions shows a diameter of inhibition of 14 mm on S. aureus. This value was higher than the inhibition diameters obtained in the experimental design (i.e., 2 to 8.3 mm, Table 1). This result could be associated to the higher TPC of PPE-PLE obtained under optimal conditions. Although AMA of pomegranate peel extract has been mainly associated with ellagitannins such as punicalagin and ellagic acid [63], other phenolic compounds of PPE such as phenolic acids, flavonoids, proanthocyanidins, hydrolysable tannins, among others, could also exhibit antimicrobial synergistic effects towards different microorganisms such as bacteria, yeast, and molds [64]. Of note, it has been reported that a pomegranate peel extract containing a mixture of polyphenolic compounds showed better AMA than individually isolated compounds [14]. The use of PPE-PLE therefore showed promising results in AMA against S. aureus. Further research should be performed to evaluate the efficacy of PPE-PLE on the control of S. aureus in food applications.
3.3.5. Cytotoxicity
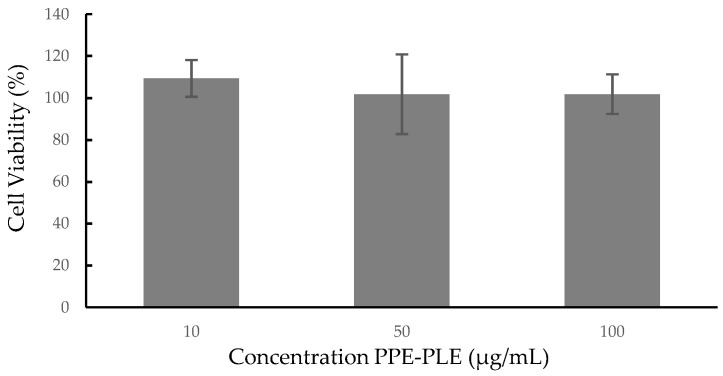
Cellular cytotoxicity studies are an initial and essential step in determining the potential toxicity of a substance, including plant extracts or biologically active compounds isolated from them. In this study, the cytotoxic effects of PPE-PLE were investigated on the human colorectal cancer cell line Caco-2. As shown in Figure 3, the extract proved to be nontoxic to the cells at the three concentrations studied (10, 50 and 100 µg/mL), retaining 100% viability. A lower cell viability (50%) was reported on normal colon cells (CCD112) treated with 250 µg/mL of aqueous pomegranate peel extract [65]. The effects of hydroalcoholic pomegranate peel extract on different human cancer cell lines (HTB140, HTB177, MCF7, HCT116) and on MRC-5 normal fibroblasts, showed that the extract expressed selective cytotoxicity for cancer cells compared to a normal cell line [66]. According to these results, the concentration of extract and the type of cell line are factors that influence cell viability, therefore, it is necessary to carry out new studies using higher concentrations of PPE-PLE and other cell lines.
Figure 3.
Cytotoxicity of PPE-PLE on human colorectal cancer cell line Caco-2.
4. Conclusions
This research provides new insights into the efficacy of PLE on the recovery of bioactive compounds from pomegranate peel as material recycling. Of note, pomegranate peel represents 10.5% of the pomegranate fruit fresh weight. A combination of pressurized water and ethanol results in a PPE-PLE with a TPC and punicalagin content of 164.3 ± 10.7 mg GAE/g DW and 17 ± 3.6 mg/g DW obtained under optimal conditions. The combination of pressurized water and ethanol was not efficient for punicalagin recovery. Nevertheless, AMA and cytotoxicity findings showed promising results of PPE-PLE. Indeed, either polyphenol composition and other extracted bioactive compounds influenced the AMA. Further research in synergy or additive effects should be evaluated. The efficacy of PPE-PLE in food applications must continue to be studied in order to achieve adequate information on its potential for developing new food additives.
Acknowledgments
We thank Luis López for his valuable feedback on the manuscript.
Author Contributions
Conceptualization, P.G. and C.F.; methodology, P.G., P.R. and I.C.; formal analysis, P.G., I.C., C.F., J.L.-S., F.J.L.-J., C.V. and P.J.; investigation, P.G., I.C., J.L.-S., F.J.L.-J., P.J. and P.R. resources, P.G.; data curation, P.G. and C.F.; writing—original draft preparation, P.G. and C.F.; writing—review and editing, P.G. and C.F.; project administration, P.G.; funding acquisition, P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT, grant number 11160541.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vázquez-Rowe I., Kahhat R., Santillán-Saldívar J., Quispe I., Bentín M. Carbon Footprint of Pomegranate (Punica granatum) Cultivation in a Hyper-Arid Region in Coastal Peru. Int. J. Life Cycle Assess. 2017;22:601–617. doi: 10.1007/s11367-016-1046-4. [DOI] [Google Scholar]
- 2.Andrade M.A., Lima V., Sanches Silva A., Vilarinho F., Castilho M.C., Khwaldia K., Ramos F. Pomegranate and Grape By-Products and Their Active Compounds: Are They a Valuable Source for Food Applications? Trends Food Sci. Technol. 2019;86:68–84. doi: 10.1016/j.tifs.2019.02.010. [DOI] [Google Scholar]
- 3.Kaderides K., Goula A.M., Adamopoulos K.G. A Process for Turning Pomegranate Peels into a Valuable Food Ingredient Using Ultrasound-Assisted Extraction and Encapsulation. Innov. Food Sci. Emerg. Technol. 2015;31:204–215. doi: 10.1016/j.ifset.2015.08.006. [DOI] [Google Scholar]
- 4.Papargyropoulou E., Lozano R., Steinberger J.K., Wright N., Bin Ujang Z. The Food Waste Hierarchy as a Framework for the Management of Food Surplus and Food Waste. J. Clean. Prod. 2014;76:106–115. doi: 10.1016/j.jclepro.2014.04.020. [DOI] [Google Scholar]
- 5.De Laurentiis V., Corrado S., Sala S. Quantifying Household Waste of Fresh Fruit and Vegetables in the EU. Waste Manag. 2018;77:238–251. doi: 10.1016/j.wasman.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Adhikari B.K., Barrington S., Martinez J. Predicted Growth of World Urban Food Waste and Methane Production. Waste Manag. Res. 2006;24:421–433. doi: 10.1177/0734242X06067767. [DOI] [PubMed] [Google Scholar]
- 7.Gustavsson J., Cederberg C., Sonesson U., Otterdijk V., Meybeck A. Global Food Losses and Food Waste. FAO; Rome, Italy: 2011. [Google Scholar]
- 8.FAO . The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction. FAO; Rome, Italy: 2019. [Google Scholar]
- 9.United Nations Sustainable Development Goals. [(accessed on 20 September 2020)]; Available online: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/
- 10.Brito T.B.N., Ferreira M.S.L., Fai A.E.C. Utilization of Agricultural By-Products: Bioactive Properties and Technological Applications. Food Rev. Int. 2020:1–25. doi: 10.1080/87559129.2020.1804930. [DOI] [Google Scholar]
- 11.Fischer U.A., Carle R., Kammerer D.R. Identification and Quantification of Phenolic Compounds from Pomegranate (Punica granatum, L.) Peel, Mesocarp, Aril and Differently Produced Juices by HPLC-DAD–ESI/MSn. Food Chem. 2011;127:807–821. doi: 10.1016/j.foodchem.2010.12.156. [DOI] [PubMed] [Google Scholar]
- 12.Saad H., Charrier-El Bouhtoury F., Pizzi A., Rode K., Charrier B., Ayed N. Characterization of Pomegranate Peels Tannin Extractives. Ind. Crop. Prod. 2012;40:239–246. doi: 10.1016/j.indcrop.2012.02.038. [DOI] [Google Scholar]
- 13.Al-Rawahi A. Phenolic Constituents of Pomegranate Peels (Punica granatum L.) Cultivated in Oman. European J. Med. Plants. 2014;4:315–331. doi: 10.9734/EJMP/2014/6417. [DOI] [Google Scholar]
- 14.Akhtar S., Ismail T., Fraternale D., Sestili P. Pomegranate Peel and Peel Extracts: Chemistry and Food Features. Food Chem. 2015;174:417–425. doi: 10.1016/j.foodchem.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Mushtaq M., Sultana B., Anwar F., Adnan A., Rizvi S.S.H. Enzyme-Assisted Supercritical Fluid Extraction of Phenolic Antioxidants from Pomegranate Peel. J. Supercrit. Fluids. 2015;104:122–131. doi: 10.1016/j.supflu.2015.05.020. [DOI] [Google Scholar]
- 16.Çam M., Hişil Y. Pressurised Water Extraction of Polyphenols from Pomegranate Peels. Food Chem. 2010;123:878–885. doi: 10.1016/j.foodchem.2010.05.011. [DOI] [Google Scholar]
- 17.Negi P.S. Plant Extracts for the Control of Bacterial Growth: Efficacy, Stability and Safety Issues for Food Application. Int. J. Food Microbiol. 2012;156:7–17. doi: 10.1016/j.ijfoodmicro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Smaoui S., Hlima H.B., Mtibaa A.C., Fourati M., Sellem I., Elhadef K., Ennouri K., Mellouli L. Pomegranate Peel as Phenolic Compounds Source: Advanced Analytical Strategies and Practical Use in Meat Products. Meat Sci. 2019;158:107914. doi: 10.1016/j.meatsci.2019.107914. [DOI] [PubMed] [Google Scholar]
- 19.Trigo J.P., Alexandre E.M.C., Saraiva J.A., Pintado M.E. High Value-Added Compounds from Fruit and Vegetable by-Products—Characterization, Bioactivities, and Application in the Development of Novel Food Products. Crit. Rev. Food Sci. Nutr. 2020;60:1388–1416. doi: 10.1080/10408398.2019.1572588. [DOI] [PubMed] [Google Scholar]
- 20.Alexandre E.M.C., Silva S., Santos S.A.O., Silvestre A.J.D., Duarte M.F., Saraiva J.A., Pintado M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019;115:167–176. doi: 10.1016/j.foodres.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Gullón P., Astray G., Gullón B., Tomasevic I., Lorenzo J.M. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules. 2020;25:2859. doi: 10.3390/molecules25122859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh B., Singh J.P., Kaur A., Singh N. Antimicrobial Potential of Pomegranate Peel: A Review. Int. J. Food Sci. Technol. 2019;54:959–965. doi: 10.1111/ijfs.13964. [DOI] [Google Scholar]
- 23.Amyrgialaki E., Makris D.P., Mauromoustakos A., Kefalas P. Optimisation of the Extraction of Pomegranate (Punica granatum) Husk Phenolics Using Water/Ethanol Solvent Systems and Response Surface Methodology. Ind. Crops Prod. 2014;59:216–222. doi: 10.1016/j.indcrop.2014.05.011. [DOI] [Google Scholar]
- 24.Elfalleh W., Tlili N., Nasri N., Yahia Y., Hannachi H., Chaira N., Ying M., Ferchichi A. Antioxidant Capacities of Phenolic Compounds and Tocopherols from Tunisian Pomegranate (Punica granatum) Fruits. J. Food Sci. 2011;76:C707–C713. doi: 10.1111/j.1750-3841.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 25.Khalil A.A. In Vitro Antioxidant Activity and Punicalagin Content Quantification of Pomegranate Peel Obtained as Agro-Waste after Juice Extraction. Pakistan J. Agric. Sci. 2018;55:197–201. doi: 10.21162/PAKJAS/18.5663. [DOI] [Google Scholar]
- 26.Abid M., Yaich H., Cheikhrouhou S., Khemakhem I., Bouaziz M., Attia H., Ayadi M.A. Antioxidant Properties and Phenolic Profile Characterization by LC–MS/MS of Selected Tunisian Pomegranate Peels. J. Food Sci. Technol. 2017;54:2890–2901. doi: 10.1007/s13197-017-2727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumere B.R., de Souza M.C., dos Santos M.P., Bezerra R.M.N., da Cunha D.T., Martinez J., Rostagno M.A. Combining Pressurized Liquids with Ultrasound to Improve the Extraction of Phenolic Compounds from Pomegranate Peel (Punica granatum L.) Ultrason. Sonochem. 2018;48:151–162. doi: 10.1016/j.ultsonch.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Fierascu R.C., Fierascu I., Avramescu S.M., Sieniawska E. Recovery of Natural Antioxidants from Agro-Industrial Side Streams through Advanced Extraction Techniques. Molecules. 2019;24:4212. doi: 10.3390/molecules24234212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AOAC . Official Methods of Analysis. 17th ed. The Association of Official Analytical Chemists; Gaithersburg, MD, USA: 2000. [Google Scholar]
- 30.AOAC . Official Methods of Analysis. The Association of Official Analytical Chemists; Gaithersburg, MD, USA: 2005. [Google Scholar]
- 31.Singleton V.L., Rossi J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 32.Zhang Y., Wang D., Lee R., Henning S.M., Heber D. Absence of Pomegranate Ellagitannins in the Majority of Commercial Pomegranate Extracts: Implications for Standardization and Quality Control. J. Agric. Food Chem. 2009;57:7395–7400. doi: 10.1021/jf9010017. [DOI] [PubMed] [Google Scholar]
- 33.Bezerra M.A., Santelli R.E., Oliveira E.P., Villar L.S., Escaleira L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Benzie I.F., Strain J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 35.Dávalos A., Gómez-Cordovés C., Bartolomé B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC−Fluorescein) Assay. J. Agric. Food Chem. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 36.Teigiserova D.A., Hamelin L., Thomsen M. Review of High-Value Food Waste and Food Residues Biorefineries with Focus on Unavoidable Wastes from Processing. Resour. Conserv. Recycl. 2019;149:413–426. doi: 10.1016/j.resconrec.2019.05.003. [DOI] [Google Scholar]
- 37.USDA Food Data Central. [(accessed on 9 September 2020)]; Available online: https://fdc.nal.usda.gov/
- 38.Institute of Medicine (U.S.) Panel on the Definition of Dietary Fiber and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes . Dietary Reference Intakes: Proposed Definition of Dietary Fiber: A Report of the Panel on the Definition of Dietary Fiber and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. National Academy Press; Washington, DC, USA: 2001. [Google Scholar]
- 39.Robert P., Gorena T., Romero N., Sepulveda E., Chavez J., Saenz C. Encapsulation of Polyphenols and Anthocyanins from Pomegranate (Punica granatum) by Spray Drying. Int. J. Food Sci. Technol. 2010;45:1386–1394. doi: 10.1111/j.1365-2621.2010.02270.x. [DOI] [Google Scholar]
- 40.Zafrilla P., Ferreres F., Tomás-Barberán F.A. Effect of Processing and Storage on the Antioxidant Ellagic Acid Derivatives and Flavonoids of Red Raspberry (Rubus idaeus) Jams. J. Agric. Food Chem. 2001;49:3651–3655. doi: 10.1021/jf010192x. [DOI] [PubMed] [Google Scholar]
- 41.Cea Pavez I., Lozano-Sánchez J., Borrás-Linares I., Nuñez H., Robert P., Segura-Carretero A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules. 2019;24:3108. doi: 10.3390/molecules24173108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambigaipalan P., de Camargo A.C., Shahidi F. Phenolic Compounds of Pomegranate Byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities. J. Agric. Food Chem. 2016;64:6584–6604. doi: 10.1021/acs.jafc.6b02950. [DOI] [PubMed] [Google Scholar]
- 43.Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of Antioxidant Properties of Pomegranate Peel Extract in Comparison with Pomegranate Pulp Extract. Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- 44.Rongai D., Pulcini P., Di Lernia G., Nota P., Preka P., Milano F. Punicalagin Content and Antifungal Activity of Different Pomegranate (Punica ganatum L.) Genotypes. Horticulturae. 2019;5:52. doi: 10.3390/horticulturae5030052. [DOI] [Google Scholar]
- 45.Bustamante A., Hinojosa A., Robert P., Escalona V. Extraction and Microencapsulation of Bioactive Compounds from Pomegranate (Punica granatum Var. Wonderful) Residues. Int. J. Food Sci. Technol. 2017;52:1452–1462. doi: 10.1111/ijfs.13422. [DOI] [Google Scholar]
- 46.Legua P., Forner-Giner M.Á., Nuncio-Jáuregui N., Hernández F. Polyphenolic Compounds, Anthocyanins and Antioxidant Activity of Nineteen Pomegranate Fruits: A Rich Source of Bioactive Compounds. J. Funct. Foods. 2016;23:628–636. doi: 10.1016/j.jff.2016.01.043. [DOI] [Google Scholar]
- 47.Conidi C., Cassano A., Caiazzo F., Drioli E. Separation and Purification of Phenolic Compounds from Pomegranate Juice by Ultrafiltration and Nanofiltration Membranes. J. Food Eng. 2017;195:1–13. doi: 10.1016/j.jfoodeng.2016.09.017. [DOI] [Google Scholar]
- 48.Hmid I., Elothmani D., Hanine H., Oukabli A., Mehinagic E. Comparative Study of Phenolic Compounds and Their Antioxidant Attributes of Eighteen Pomegranate (Punica granatum L.) Cultivars Grown in Morocco. Arab. J. Chem. 2017;10:S2675–S2684. doi: 10.1016/j.arabjc.2013.10.011. [DOI] [Google Scholar]
- 49.Türkyılmaz M., Özkan M. Effects of Condensed Tannins on Anthocyanins and Colour of Authentic Pomegranate (Punica granatum L.) Juices. Food Chem. 2014;164:324–331. doi: 10.1016/j.foodchem.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 50.Abdulla R., Mansur S., Lai H., Ubul A., Sun G., Huang G., Aisa H.A. Qualitative Analysis of Polyphenols in Macroporous Resin Pretreated Pomegranate Husk Extract by HPLC-QTOF-MS. Phytochem. Anal. 2017;28:465–473. doi: 10.1002/pca.2695. [DOI] [PubMed] [Google Scholar]
- 51.Arun K.B., Jayamurthy P., Anusha C.V., Mahesh S.K., Nisha P. Studies on Activity Guided Fractionation of Pomegranate Peel Extracts and Its Effect on Antidiabetic and Cardiovascular Protection Properties. J. Food Process. Preserv. 2017;41:e13108. doi: 10.1111/jfpp.13108. [DOI] [Google Scholar]
- 52.Tapias V., Cannon J.R., Greenamyre J.T. Pomegranate Juice Exacerbates Oxidative Stress and Nigrostriatal Degeneration in Parkinson’s Disease. Neurobiol. Aging. 2014;35:1162–1176. doi: 10.1016/j.neurobiolaging.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viuda-Martos M., Fernández-López J., Pérez-Álvarez J.A. Pomegranate and Its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010;9:635–654. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- 54.Singh J.P., Kaur A., Shevkani K., Singh N. Composition, Bioactive Compounds and Antioxidant Activity of Common Indian Fruits and Vegetables. J. Food Sci. Technol. 2016;53:4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fellah B., Bannour M., Rocchetti G., Lucini L., Ferchichi A. Phenolic Profiling and Antioxidant Capacity in Flowers, Leaves and Peels of Tunisian Cultivars of Punica granatum L. J. Food Sci. Technol. 2018;55:3606–3615. doi: 10.1007/s13197-018-3286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khanavi M., Moghaddam G., Oveisi M.R., Sadeghi N., Jannat B., Rostami M., Saadat M.A., Hajimahmoo M. Hyperoside and Anthocyanin Content of Ten Different Pomegranate Cultivars. Pakistan J. Biol. Sci. 2013;16:636–641. doi: 10.3923/pjbs.2013.636.641. [DOI] [PubMed] [Google Scholar]
- 57.Parashar A., Gupta C., Gupta S.K., Kumar A. Antimicrobial Ellagitannin From Pomegranate (Punica granatum) Fruits. Int. J. Fruit Sci. 2009;9:226–231. doi: 10.1080/15538360903241286. [DOI] [Google Scholar]
- 58.Aguilar-Zárate P., Wong-Paz J.E., Michel M., Buenrostro-Figueroa J., Díaz H.R., Ascacio J.A., Contreras-Esquivel J.C., Gutiérrez-Sánchez G., Aguilar C.N. Characterisation of Pomegranate-Husk Polyphenols and Semi-Preparative Fractionation of Punicalagin. Phytochem. Anal. 2017;28:433–438. doi: 10.1002/pca.2691. [DOI] [PubMed] [Google Scholar]
- 59.Zahin M., Aqil F., Ahmad I. Broad Spectrum Antimutagenic Activity of Antioxidant Active Fraction of Punica granatum L. Peel Extracts. Mutat. Res. Toxicol. Environ. Mutagen. 2010;703:99–107. doi: 10.1016/j.mrgentox.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Hussein S.A.M., Barakat H.H., Merfort I., Nawwar M.A.M. Tannins from the Leaves of Punica granatum. Phytochemistry. 1997;45:819–823. doi: 10.1016/S0031-9422(96)00888-6. [DOI] [Google Scholar]
- 61.Fawole O.A., Makunga N.P., Opara U.L. Antibacterial, Antioxidant and Tyrosinase-Inhibition Activities of Pomegranate Fruit Peel Methanolic Extract. BMC Complement. Altern. Med. 2012;12:1178. doi: 10.1186/1472-6882-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouarab Chibane L., Degraeve P., Ferhout H., Bouajila J., Oulahal N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2019;99:1457–1474. doi: 10.1002/jsfa.9357. [DOI] [PubMed] [Google Scholar]
- 63.Dey D., Debnath S., Hazra S., Ghosh S., Ray R., Hazra B. Pomegranate Pericarp Extract Enhances the Antibacterial Activity of Ciprofloxacin against Extended-Spectrum β-Lactamase (ESBL) and Metallo-β-Lactamase (MBL) Producing Gram-Negative Bacilli. Food Chem. Toxicol. 2012;50:4302–4309. doi: 10.1016/j.fct.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Kharchoufi S., Licciardello F., Siracusa L., Muratore G., Hamdi M., Restuccia C. Antimicrobial and Antioxidant Features of ‘Gabsi’ Pomegranate Peel Extracts. Ind. Crops Prod. 2018;111:345–352. doi: 10.1016/j.indcrop.2017.10.037. [DOI] [Google Scholar]
- 65.Mohamad Sukri S.N.A., Shameli K., Mei-Theng Wong M., Teow S.-Y., Chew J., Ismail N.A. Cytotoxicity and Antibacterial Activities of Plant-Mediated Synthesized Zinc Oxide (ZnO) Nanoparticles Using Punica granatum (Pomegranate) Fruit Peels Extract. J. Mol. Struct. 2019;1189:57–65. doi: 10.1016/j.molstruc.2019.04.026. [DOI] [Google Scholar]
- 66.Keta O., Deljanin M., Petkovic V., Zdunić G., Janković T., Živković J., Fira A.R., Petrović I., Šavikin K. Pomegranate (Punica granatum L.) Peel Extract: Potential Cytotoxic Agent Against Different Cancer Cell Lines. Rec. Nat. Prod. 2020;14:326–339. doi: 10.25135/rnp.170.19.11.1477. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.