Abstract
Neuroanatomic connections link the olfactory and limbic systems potentially explaining an association between olfactory dysfunction and depression. Some previous studies have demonstrated that olfactory dysfunction is associated with increased depressive symptoms. However, these studies were cross-sectional and unable to establish which develops first. We used longitudinal data to determine if impaired odor identification increased subsequent depressive symptoms or vice versa. We assessed olfaction and depression in the National Social Life, Health, and Aging Project, a nationally representative, 15-year longitudinal study of older US adults. Olfaction was measured using a validated odor identification test (Sniffin’ Sticks). Depressive symptoms were measured using a modified version of the validated Center for Epidemiological Studies Depression Scale. Multivariable logistic regression models examined the temporal relationships between developing olfactory dysfunction and depression while accounting for demographics, disease comorbidities, alcohol use, smoking, and cognition. Older adults with olfactory dysfunction had concurrent frequent depressive symptoms (odds ratio [OR] = 1.20, 95% confidence interval [CI] = 1.00–1.43). Among healthy adults at baseline, those who had olfactory dysfunction were more likely to develop frequent depressive symptoms 5 or 10 years later (OR = 2.22, 95% CI = 1.13–4.37). Conversely, those with frequent depressive symptoms at baseline were not more likely to develop olfactory dysfunction 5 or 10 years later. We show for the first time that olfactory dysfunction predicts subsequent development of depression in older US adults. These data support screening for depression in older adults with chemosensory impairment and set the stage for disentangling the relationship between olfaction and depression.
Keywords: aging, depression, mood, olfaction, smell, Sniffin’ Sticks
Introduction
Olfactory impairment is a prevalent phenomenon among older adults, with up to 25% of older US adults experiencing smell loss (Murphy et al. 2002). This sensory condition has been linked to many important adverse health outcomes, including increased 5-year mortality, injury from environmental hazards (chemicals, smoke, and spoiled foods), and malnutrition (Pinto et al. 2014; Yang and Pinto 2016). In addition to physical health outcomes, olfactory dysfunction may also affect mental and social health. Indeed, many cross-sectional studies have identified associations between concurrent olfactory dysfunction and depression (Deems et al. 1991; Negoias et al. 2010; Boesveldt et al. 2011; Gopinath et al. 2011; Joo et al. 2015; Kohli et al. 2016; Sivam et al. 2016; Hur et al. 2018), as well as psychosocial states, such as increased feelings of loneliness, worse quality of life, and schizophrenia (Moberg et al. 1999; Brämerson et al. 2007; Sivam et al. 2016).
The association between olfactory dysfunction and depression rests upon the reciprocal neuroanatomical connections between regions in the brain that are involved in these conditions (Croy and Hummel 2017). In humans, olfactory information is transmitted to the olfactory bulb, a central nervous system structure that serves as the primary hub for relaying olfactory information, and then conducted to the primary olfactory cortex, which then projects to the amygdala, hippocampus, anterior cingulate cortex, insula, and orbitofrontal cortex (Soudry et al. 2011; Croy and Hummel 2017). While all of these central structures are involved in emotional processing and modulation, autonomic regulation, and memory (Soudry et al. 2011), numerous studies have identified the amygdala, a structure responsible for processing aversive stimuli and expressing fear (Soudry et al. 2011), and the hippocampus, a neuroplastic temporal lobe structure responsible for both consolidating and providing an emotional context to memories (Soudry et al. 2011), as primary neurobiological structures implicated in the pathophysiology of depression (Mervaala et al. 2000; Nestler et al. 2002; Campbell and MacQueen 2004).
The reciprocal interplay between olfactory and limbic structures is highlighted by several human studies. One study found a small correlation between olfactory function and hippocampal volume, which is a structure implicated in the pathogenesis of depression (Smitka et al. 2012). Another study demonstrated that hyposmic patients have reduced gray matter hippocampal volume in comparison to normosmic controls (Gellrich et al. 2018). Conversely, it has also been shown that depressed patients have reduced olfactory bulb volumes (Negoias et al. 2010), a structure that has been shown to correlate with olfactory function (Buschhüter et al. 2008). Given the significant neuroplastic capacity of the hippocampus and olfactory bulb (Croy and Hummel 2017), these neuroanatomical studies highlight the possibility that either olfactory loss or depression may be the antecedent pathology that drives the other.
Most psychological studies of olfaction and depression, however, are cross-sectional and unable to identify whether olfactory loss precedes or follows depression. In addition, some cross-sectional clinical studies with relatively small samples to date demonstrate that patients with olfactory dysfunction are more likely to exhibit depressive symptoms, whereas others report the converse, namely that depressed patients are more likely to exhibit olfactory dysfunction (Kohli et al. 2016). Population-based studies are contradictory, albeit conducted in different populations; some have demonstrated a significant association between olfactory dysfunction and depression, while others have not. Among older US adults, significant associations were reported (Boesveldt et al. 2011; Gopinath et al. 2011), particularly among men (Sivam et al. 2016). Significant associations were also found among the general Korean population (Joo et al. 2015). On the other hand, no such associations were reported either for older Swedish adults (Seubert et al. 2017) or the general US population that included young and middle-aged adults (Schubert et al. 2012).
Notably, there are 2 major deficiencies in this body of work: analyses are cross-sectional and samples are not representative. To our knowledge, there are no longitudinal population-based studies investigating the association between olfactory dysfunction and depression during aging. Here, we sought to elucidate the directionality of the association between olfactory dysfunction and depression using a longitudinal, nationally representative sample of older US adults. We hypothesized that 1) older adults with olfactory dysfunction would be more likely to also exhibit frequent depressive symptoms and 2) older adults with olfactory dysfunction at baseline would be more likely to develop frequent depressive symptoms subsequently. We hypothesized that impaired olfaction could lead to a decreased experience of pleasure, which could, in turn, lead to increased depressive symptoms.
Materials and methods
Subjects
We examined the relationships between olfaction and depression using longitudinal data from the National Social Life, Health, and Aging Project (NSHAP), a representative, population-based survey of the social, psychological, and physical health of older community-dwelling US adults. NSHAP first collected data in 2005–2006 from respondents born between 1920 and 1947. Five years later (2010–2011), NSHAP collected information from these same respondents, as well as the same information from their domestic partners. In 2015–2016, 10 years after the first data collection, NSHAP surveyed these returning respondents, thus yielding a total of 3546 unique respondents contributing at least 1 observation to the study and 1905 unique respondents contributing at least 2 observations to the analyses here.
Field interviewers from the National Opinion Research Center (NORC) conducted in-home interviews with questionnaires and biomeasures, and participants completed self-administered questionnaires afterward. Details of each measure used in these analyses are provided below. Further detail regarding the study design and methodology of NSHAP can be found elsewhere (O’Muircheartaigh et al. 2009; Smith et al. 2009; Jaszczak et al. 2014; Waite et al. 2019). The study was approved by the institutional review board of the University of Chicago and NORC, and written informed consent was obtained from all respondents.
Assessment of olfactory identification ability
Olfaction was assessed at all 3 time points using a validated, 5-item odor identification test. Respondents were asked to smell a Sniffin’ Stick (Kern et al. 2014) that contained a common odorant (either rose, leather, orange, fish, or peppermint) and were then asked to identify it from a set of 4 word/picture choices. The respondents’ answers were marked as correct/incorrect and scored from 0 to 5 based on the number of errors. Olfactory dysfunction was defined as incorrectly identifying 2 or more odors as in previous studies (Kern et al. 2014).
Assessment of depressive symptoms
At all 3 time points, depressive symptoms were measured with the NSHAP Depressive Symptoms Measure (NDSM) (Payne et al. 2014), an 11-item questionnaire derived from the validated Center for Epidemiological Studies Depression Scale (CES-D). This measure assessed the respondents’ frequency of depressive symptoms within the past week. The NDSM is scored from 0 to 22, with higher scores reflecting more depressive symptoms. For this study, an NDSM score ≥9 indicated that a respondent had frequent depressive symptoms, a validated category for clinically significant depression (Payne et al. 2014).
Statistical analysis
In total, 3546 unique participants completed both the odor identification test and the depression measure during at least 1 time point. Table 1 summarizes the participants’ demographic characteristics, as well as the values for the measures and covariates at the time point at which they first entered the study. Of these participants, 1905 (53.7%) had 2 or more time points enabling longitudinal analysis.
Table 1.
Demographics, olfactory performance, and depressive symptoms of respondents at study entry
| Number of unique respondents contributing at least 1 observation | 3546 |
| Number of unique respondents contributing at least 2 observations | 1905 |
| Age (mean ± SD, range) | 69.9 ± 7.8, 57–91 |
| Gender | |
| % Male | 48.2 |
| % Female | 51.8 |
| Race and ethnicity | |
| % White | 71.8 |
| % Black | 15.7 |
| % Hispanic, non-Black | 10.1 |
| % Other | 2.4 |
| Education, highest level achieved | |
| % <High school | 22.0 |
| % High school/equivalent | 26.0 |
| % Vocational certificate/some college | 29.1 |
| % Bachelors or more | 22.9 |
| CCI | |
| % respondents with CCI = 0 | 26.4 |
| % respondents with CCI = 1 | 31.7 |
| % respondents with CCI ≥2 | 41.9 |
| Odor identification test score (mean ± SD) | 4.0 ± 1.2 |
| % Respondents with olfactory dysfunction (2–5 errors) | 26.3 |
| NDSM score (mean ± SD) | 5.0 ± 4.4 |
| % Respondents with frequent depressive symptoms (NDSM ≥ 9) | 21.1 |
Data for both cross-sectional and longitudinal models were survey weighted, thus allowing for broader inferences about the US population. Three cross-sectional analyses (Models A–C) were performed using multivariable logistic regression to determine the association between olfactory dysfunction (≥2 errors on odor identification test) and frequent depressive symptoms (NDSM score ≥9; dependent variable). To maximize the contribution of all data collected, we utilized all observations from each respondent, with each respondent contributing both olfactory and depression measures during at least 1 and up to 3 time points. Thus, the unit of analysis for the cross-sectional analyses was the observation of olfactory and depressive measures collected at the same time point (i.e., N = 6456 participant-time point observations). These cross-sectional analyses used robust standard errors to adjust for the clustering of multiple observations per respondent (Huber 1967; White 1980).
Model A (N = 6456 observations) adjusted for self-reported sociodemographic variables at the time of observation: age (based on birth date), gender, race/ethnicity, and education. Education was defined as the highest degree achieved and treated as a categorical variable. Model B (N = 6393 observations) further adjusted for known health covariates, related to either olfaction or depression, at the time of observation: comorbid diseases (Charlson Comorbidity Index [CCI], modified for survey use; range 0–16; Charlson et al. 1987), heavy alcohol use (≥4 drinks per day; Hur et al. 2018), and smoking status (current, former, or never; Litvack et al. 2008; Hur et al. 2018). Model C (N = 6393 observations) further adjusted for cognition at the time of observation to control for its known association with both olfaction and depression (Cole and Dendukuri 2003; Schubert et al. 2011; Rock et al. 2014). Because our assessment of olfaction measured odor identification performance, which is a cognitive process, it was imperative to adjust for cognitive function in the pursuit of determining the effect of olfactory dysfunction on depression. Cognitive function scores were standardized (z-score) because different scales were used at different time points: the Short Portable Mental Status Questionnaire (2005–2006; Pfeiffer 1975) and the survey adaptation of the Montreal Cognitive Assessment (2010–2011 and 2015–2016; Nasreddine et al. 2005; Shega et al. 2014).
The sequence in which olfactory dysfunction and depression developed was tested with 2 different longitudinal analyses utilizing multivariable logistic regression: 1) the effect of olfactory dysfunction at baseline (2005–2006) on subsequently developing frequent depressive symptoms 5 or 10 years later and, conversely, 2) the effect of frequent depressive symptoms at baseline on subsequently developing olfactory dysfunction 5 or 10 years later. These 2 longitudinal analyses only included participants with at least 2 or more observations. The first approach, Model D, selected participants (N = 1793) without frequent depressive symptoms at baseline and then compared those with and without olfactory dysfunction at baseline, measuring their likelihood of developing frequent depressive symptoms 5 or 10 years later. Conversely, the second approach, Model E, selected participants (N = 1176) without olfactory dysfunction at baseline and then compared those with and without frequent depressive symptoms at baseline, measuring their likelihood of developing olfactory dysfunction 5 or 10 years later. The covariates in Models D and E were identical to those in Model C (age, gender, race/ethnicity, education, comorbid diseases, heavy alcohol use, smoking status, and cognition). The comorbidity interaction was tested because prior literature indicates that this might be important (Weinstock et al. 1993; Landis et al. 2004; Chang-Quan et al. 2010). This was significant in Model D and so included in the reported model but was not significant and, therefore, excluded in Model E for parsimony and ease of interpretation.
All analyses were conducted using Stata 15 (StataCorp, College Station, TX). Stata code is available from the authors upon request. NSHAP’s data are publicly available (https://www.icpsr.umich.edu/web/pages/NACDA/nshap.html).
Results
In cross-sectional analyses, older adults with olfactory dysfunction were 32% more likely to also have frequent depressive symptoms, controlling for demographic variables (odds ratio [OR] = 1.32, 95% confidence interval [CI] = 1.11–1.57; Table 2, Model A). Moreover, controlling for the number of comorbid diseases, heavy alcohol use, and smoking status did not change the OR, indicating that the association was not mediated by physical health (OR = 1.30, 95% CI = 1.09–1.55; Table 2, Model B). Further adjusting for cognitive ability slightly attenuated this association, but it remained significant (OR = 1.20, 95% CI = 1.00–1.43; Table 2, Model C). We also replicated the established correlates of depression among older adults: being a woman, absence of a college degree, low cognitive function, a high burden of comorbid diseases, heavy alcohol use, and smoking (Table 2, Model C). Frequent depressive symptoms were not significantly associated with age or race/ethnicity.
Table 2.
Cross-sectional associations between olfactory dysfunction and odds of having depression at the same time point
| Model A (N = 6456 observations) | Model B (N = 6393 observations) | Model C (N = 6393 observations) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Olfactory dysfunction | 1.32 (1.11–1.57) | 0.002 | 1.30 (1.09–1.55) | 0.004 | 1.20 (1.00–1.43) | 0.049 |
| Women (vs. men) | 1.40 (1.17–1.67) | <0.001 | 1.46 (1.22–1.75) | <0.001 | 1.47 (1.23–1.75) | <0.001 |
| Age (per decade) | 1.10 (0.97–1.23) | 0.126 | 1.12 (0.99–1.27) | 0.070 | 1.06 (0.94–1.20) | 0.328 |
| Race (reference = White) | ||||||
| Black | 1.21 (0.94–1.56) | 0.140 | 1.17 (0.90–1.51) | 0.241 | 1.04 (0.79–1.35) | 0.790 |
| Hispanic, non-Black | 1.08 (0.82–1.42) | 0.573 | 1.13 (0.86–1.49) | 0.393 | 1.00 (0.75–1.33) | 0.988 |
| Other | 1.07 (0.59–1.93) | 0.833 | 0.94 (0.51–1.71) | 0.833 | 0.87 (0.48–1.56) | 0.631 |
| Education (reference = no HS) | ||||||
| HS/equivalent | 0.71 (0.55–0.92) | 0.010 | 0.76 (0.59–0.98) | 0.038 | 0.83 (0.64–1.08) | 0.159 |
| Some college/associates | 0.58 (0.45–0.74) | <0.001 | 0.62 (0.48–0.80) | <0.001 | 0.71 (0.54–0.91) | 0.008 |
| Bachelors or more | 0.34 (0.25–0.46) | <0.001 | 0.39 (0.29–0.53) | <0.001 | 0.46 (0.34–0.63) | <0.001 |
| Comorbid diseases (CCI ≥ 1) | 2.05 (1.66–2.52) | <0.001 | 2.02 (1.64–2.49) | <0.001 | ||
| Heavy alcohol use (≥4 drinks daily) | 1.65 (1.06–2.56) | 0.027 | 1.69 (1.09–2.61) | 0.019 | ||
| Current smoker | 1.46 (1.15–1.85) | 0.002 | 1.43 (1.12–1.81) | 0.004 | ||
| Cognition (z-score) | 0.79 (0.73–0.86) | <0.001 | ||||
| McFadden’s pseudo-R2 | 0.034 | 0.052 | 0.059 | |||
Bolded values are statistically significant (Ps ≤ 0.05).
HS, high school.
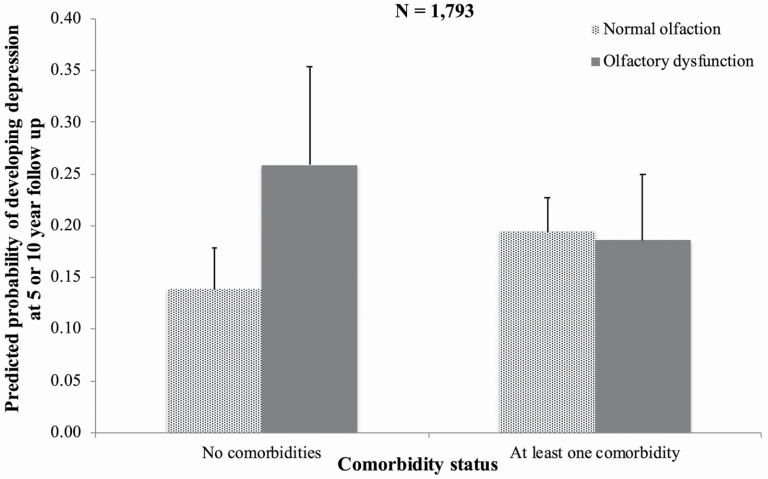
We then asked which came first, olfactory dysfunction or depression, by using the longitudinal data. Of the 1793 respondents included in Model D, 336 respondents developed depression at 5- or 10-year follow-up. Among otherwise healthy older adults (CCI = 0; N = 552), those with olfactory dysfunction at baseline were over twice as likely as those without it to develop frequent depressive symptoms during the next 5–10 years (Figure 1; OR = 2.22, 95% CI = 1.13–4.37; Table 3, Model D, controlling for age, gender, race/ethnicity, education, heavy alcohol use, smoking status, and cognition). In addition, having at least one chronic disease (CCI ≥ 1; N = 1241) at baseline also predicted subsequent frequent depressive symptoms among those with normal olfactory function (OR = 1.51, 95% CI = 1.08–2.11; Table 3, Model D) but did not among those with olfactory dysfunction (OR = 0.65, 95% CI = 0.33–1.25, Model D). The presence of these chronic diseases masked the detection of olfactory impairment’s overall effect on developing frequent depressive symptoms (interaction OR = 0.43, 95% CI = 0.20–0.90, Figure 1; Table 3). Indeed, among people with at least one chronic disease (CCI ≥ 1), olfactory dysfunction at baseline did not predict the development of frequent depressive symptoms (OR = 0.95, 95% CI = 0.60–1.49; Figure 1). Women were more likely than men to develop frequent depressive symptoms over time (OR = 1.39, 95% CI = 1.02–1.88; Table 3, Model D) as did current smokers compared to those who had previously or never smoked (OR = 1.77, 95% CI = 1.23–2.56; Table 3, Model D).
Figure 1.
Predicted probabilities displayed in the figure are based on Model D, Table 3. Error bars indicate 95% confidence intervals. Among healthy older adults without any chronic diseases (CCI = 0), baseline olfactory performance significantly predicted subsequent development of depression. It did not among those with at least one chronic disease (CCI ≥ 1).
Table 3.
The effect of olfactory dysfunction at baseline on the incidence of depression 5 or 10 years later
| Model D (N = 1793 respondents) | ||
|---|---|---|
| OR (95% CI) | P | |
| Olfactory dysfunction × comorbid diseases | 0.43 (0.20–0.90) | 0.026 |
| Olfactory dysfunction | 2.22 (1.13–4.37) | 0.022 |
| Comorbid diseases (CCI ≥ 1) | 1.51 (1.08–2.11) | 0.016 |
| Women (vs. men) | 1.39 (1.02–1.88) | 0.038 |
| Age (per decade) | 1.35 (1.15–1.58) | <0.001 |
| Race (reference = white) | ||
| Black | 0.93 (0.59–1.47) | 0.760 |
| Hispanic, non-Black | 0.96 (0.48–1.91) | 0.912 |
| Other | 1.05 (0.49–2.26) | 0.894 |
| Education (reference = no HS) | ||
| HS/equivalent | 0.97 (0.55–1.70) | 0.905 |
| Some college/associates | 0.84 (0.51–1.37) | 0.468 |
| Bachelors or more | 0.68 (0.37–1.27) | 0.221 |
| Heavy alcohol use (≥4 drinks daily) | 1.25 (0.64–2.44) | 0.508 |
| Current smoker | 1.77 (1.23–2.56) | 0.003 |
| Cognition (z-score) | 0.94 (0.79–1.12) | 0.496 |
At baseline, none of these older adults had frequent depressive symptoms (all ≤8 on the modified CES-D). Bolded values are statistically significant (Ps ≤ 0.05).
HS, high school.
We sought to verify that this pattern of predictive relationships held under a broader definition of “healthy” that included respondents with either one chronic disease or none. Indeed, olfactory dysfunction remained a robust predictor of frequent depressive symptoms among those with CCI ≤ 1 (N = 1179; OR = 1.70, 95% CI = 1.09–2.66) but did not among those with CCI ≥ 2 (N = 614; OR = 0.62, 95% CI = 0.33–1.17).
We then sought to test the converse relationship, whether depression predicted the development of olfactory dysfunction. Older adults with frequent depressive symptoms at baseline were not more likely to develop olfactory dysfunction 5 or 10 years later, controlling for gender, age, race/ethnicity, education, comorbid diseases, heavy alcohol use, smoking status, and cognition (OR = 1.21, 95% CI = 0.81–1.82; Table 4, Model E). Moreover, there was no significant effect of having baseline comorbidities on subsequent olfactory dysfunction (OR = 1.14, 95% CI = 0.77–1.68; Table 4, Model E), nor was there an interaction between baseline comorbidities and frequent depressive symptoms (OR = 1.04, 95% CI = 0.20–5.40). As expected, participants were twice as likely to develop olfactory dysfunction after 10 years of aging (OR = 2.22, 95% CI = 1.70–2.90; Table 4, Model E). Women were less likely than men to develop olfactory dysfunction 5–10 years later (OR = 0.71, 95% CI = 0.50–0.99; Table 4, Model E). Black respondents were 3 times more likely than White respondents to lose olfactory function over 5–10 years (OR = 3.00, 95% CI = 1.46–6.15; Table 4, Model E) and Hispanic respondents were twice as likely to do so (OR = 2.11, 95% CI = 1.17–3.80; Table 4, Model E).
Table 4.
Depression at baseline and its potential effect on developing olfactory dysfunction 5 or 10 years later
| Model E (N = 1176 respondents) | ||
|---|---|---|
| OR (95% CI) | P | |
| Depression (frequent depressive symptoms) | 1.21 (0.81–1.82) | 0.340 |
| Comorbid diseases (CCI ≥ 1) | 1.14 (0.77–1.68) | 0.511 |
| Women (vs. men) | 0.71 (0.50–0.99) | 0.047 |
| Age (per decade) | 2.22 (1.70–2.90) | <0.001 |
| Race (reference = white) | ||
| Black | 3.00 (1.46–6.15) | 0.004 |
| Hispanic, non-Black | 2.11 (1.68–3.80) | 0.014 |
| Other | 2.56 (0.62–10.49) | 0.188 |
| Education (reference = no HS) | ||
| HS/equivalent | 1.27 (0.70–2.34) | 0.425 |
| Some college/associates | 0.68 (0.40–1.15) | 0.145 |
| Bachelors or more | 1.01 (0.59–1.71) | 0.982 |
| Heavy alcohol use (≥4 drinks daily) | 1.50 (0.75–3.00) | 0.249 |
| Current smoker | 0.70 (0.41–1.18) | 0.175 |
| Cognition (z-score) | 0.93 (0.80–1.09) | 0.382 |
At baseline, all participants were normosmic (only 0–1 odor identification errors). Bolded values are statistically significant (Ps ≤ 0.05).
HS, high school.
Discussion
Using a nationally representative sample of community-dwelling older US adults, this study demonstrated that older adults with olfactory dysfunction were significantly more likely to have frequent depressive symptoms, an indicator of clinical depression (Payne et al. 2014). These cross-sectional findings clarify the mixed literature regarding olfactory dysfunction and depression. Our data are consistent with some previous population-based studies that also demonstrated a significant cross-sectional association between impaired odor identification and depressive symptoms among older adults (Boesveldt et al. 2011; Gopinath et al. 2011; Sivam et al. 2016). These past studies, as well as the current study, all measured olfaction and depression in similar ways; olfaction was measured via an odor identification test and depression was measured by tabulating depressive symptoms from a questionnaire. Thus, the current study not only replicates these findings but also demonstrates robustness by utilizing different analytical methods and additional covariates. Another population-based study found a similar positive association between olfactory dysfunction and depression; however, the study assessed olfactory impairment by simply asking whether or not respondents experienced problems with their smell within the past 3 months (Joo et al. 2015). It is important to note that the current study assessed only odor identification and, thus, could not address the relationship between other olfactory modalities (e.g., threshold) and depression. Furthermore, we recognize that odor identification has a cognitive component to the task; although we adjusted for cognitive performance in our model, this may not have completely accounted for the influence of cognition on the findings.
Our results differ from some other population-based studies that failed to find a significant association between olfactory dysfunction and depression (Schubert et al. 2012; Seubert et al. 2017). The discrepancy from the Schubert et al. (2012) study may have resulted from their smaller sample size of older adults in a comparable age range. Thus, future research should compare the association between olfaction and depression in younger and older adults in order to determine whether the association is a function of an aging nervous system or if it holds in all age groups. The discrepancy from the Seubert et al. (2017) study may have resulted from a slight variation in their methodology of olfaction assessment. They assessed olfaction via the Sniffin’ test of odor memory, a measure that tests olfactory memory after 17 days, while we assessed odor identification. Hence, further population-based research should investigate whether the association is unique to odor identification or if it extends to other modalities of olfaction such as olfactory threshold and memory.
In addition to identifying a cross-sectional association between concurrent olfactory dysfunction and depression, this study was the first to investigate this phenomenon longitudinally. Among healthy older adults, those with olfactory dysfunction at baseline were more likely to develop frequent depressive symptoms at 5- or 10-year follow-up (Model D). On the other hand, frequent depressive symptoms at baseline, regardless of health status, did not lead to a higher likelihood of developing olfactory dysfunction at 5- or 10-year follow-up (Model E). Even in a well-powered study, a statistically significant result that is close to the threshold warrants further cautious interpretation, especially regarding longitudinal effects. These results demonstrated a wide CI indicating that there may be other factors affecting the relationship between olfactory dysfunction on the development of depression. Of course, we may not be adequately controlling for all pertinent covariates. Finally, our goal here was to determine if those with olfactory dysfunction develop depression subsequently rather than to develop a comprehensive model that incorporates all factors that contribute to the development of depression. Indeed, olfaction appears to play a relatively small role in the larger process of the development of depression overall (low McFadden’s pseudo-R2, Table 2). Nevertheless, our results strongly support the concept that olfactory dysfunction precedes the development of depression, which can be replicated in future work.
These results help disentangle the directionality of the relationship between olfactory dysfunction and depression and are consistent with animal studies that have investigated the consequences of damaging olfactory structures. Prior studies in rats have demonstrated that the removal of both olfactory bulbs leads to increased depressive-like symptoms, as well as changes in neurotransmitter, endocrine, and immune systems that are similar to those seen in clinical depression (Kelly et al. 1997; Song and Leonard 2005). As a result, bulbectomized rats have been used as a model of depression (Kelly et al. 1997; Song and Leonard 2005).
The results of this study are also consistent with human studies investigating the relationship between olfactory structures and depression. Studies have demonstrated that reduced olfactory bulb volume is associated with not only depression but also with worse severity of depression and poorer therapeutic response to psychotherapy (Negoias et al. 2010, 2016). Given this evidence, Croy and Hummel (2017) suggest that olfactory bulb volume may serve as a significant marker of increased susceptibility to depression. Croy and Hummel (2017) propose that reduced olfactory bulb volumes could lead to reduced signaling in the amygdala, hippocampus, and orbitofrontal cortex, thus disrupting normal emotional processes and potentially increasing susceptibility to depressive symptoms. The behavioral consequences of altered signaling in these brain regions have been substantiated by multiple studies. Many studies have demonstrated that, in comparison to healthy controls, depressed patients have altered activity in their amygdala in response to negative stimuli (Drevets et al. 2008). Furthermore, numerous studies have demonstrated that depressed patients have reduced hippocampal volumes (Sheline et al. 1996; Videbech and Ravnkilde 2004), a consequence that aligns with the neuroplastic capacity of the hippocampus. Given that olfactory bulb volumes have been shown to be positively correlated with olfactory identification ability in humans (Buschhüter et al. 2008), such a mechanism appears to plausibly underlie the predictive power of olfactory dysfunction for depression as seen in the current study.
Croy et al. (2014) also propose a psychosocial mechanism that can help explain the relationship between olfactory dysfunction and depression. Olfactory dysfunction can potentially hinder many daily activities and functions that depend on an intact olfactory system, including food enjoyment, detection of environmental hazards, and maintenance of personal hygiene (Croy et al. 2014). Patients with olfactory impairment have decreased enjoyment of food and decreased appetites (Croy et al. 2014). In addition, patients with olfactory impairment demonstrate significant worry in regards to not being able to detect environmental hazards and or their own body odor (Croy et al. 2014). Croy et al. (2014) suggest that the disruption of these essential functions due to olfactory impairment could cause significant distress, which could possibly lead to depression.
Increased comorbidity also led to worse depressive symptoms, which obscured detecting the effect of olfactory function on depression among individuals with higher comorbidities because they were already more depressed. Given that increased comorbidity is both positively correlated with depressive symptoms (Sutor et al. 1998) and a risk factor for developing depression in older adults (Chang-Quan et al. 2010), this could explain why the effect of baseline olfactory dysfunction on an increased incidence of frequent depressive symptoms is only seen in healthy individuals. Nevertheless, more rigorous investigations regarding the mechanisms underlying comorbidity differences in olfaction and depression are warranted.
The clinical implications of this study’s findings are multifold. Given the potential effect of olfactory dysfunction on the development of depressive symptoms over time, it could be worthwhile to incorporate assessments of olfactory performance into depression screenings for older adults. Assessments of olfaction can potentially help identify older patients who are at risk for developing depression. Early identification of patients at risk would allow clinicians to better prevent and track depressive symptoms. An additional potential clinical application of our findings relates to the treatment of depressive symptoms. Olfactory training, a technique that can help restore olfactory function, including odor identification ability (Sorokowska et al. 2017), has been an intriguing potential target for therapy for those with depressive symptoms. The current literature regarding the efficacy of olfactory training in treating depression is limited. One recent study investigating this phenomenon found that older adults who underwent 5 months of olfactory training had significantly reduced depressive symptoms in comparison to a control group (Birte-Antina et al. 2018). These results, combined with the idea that olfactory training can possibly increase olfactory bulb volume (Negoias et al. 2017), are consistent with the neurobiological link between olfaction and depression. Theoretically, olfactory training could potentially increase olfactory bulb volume and subsequently strengthen the signaling between the olfactory bulb and limbic system, thus modulating depressive symptoms. On the other hand, a recent exploratory randomized controlled trial found that olfactory training did not significantly improve depressive symptoms in a cohort of depressed outpatients (Pabel et al. 2020). Given these conflicting data, caution must remain in interpreting the validity of olfactory training in the treatment of depression. Furthermore, the clinical applications of our findings must be considered in the context of the aforementioned study limitations.
The strengths of this study include its power and generalizability, which was accomplished by using data from a large nationally representative sample of older US adults. Additionally, this study was a prospective study that was able to control for many possible confounders by collecting data on a comprehensive list of variables assessing physical, mental, and social well-being. This study was limited in that it only included surviving subjects in the longitudinal analyses; the fact that the analyses excluded subjects that died prior to the 5- or 10-year follow-up could have diluted our effect size. This study was also limited because data were only collected every 5 years. More frequent data collection could allow for a more precise temporal assessment of the predictive power of olfactory dysfunction on the development of depression. Additionally, this study only examined age-related olfactory loss and, thus, was unable to comment on whether the development of depression was related to specific causes of olfactory dysfunction, such as rhinitis, head trauma, or neurodegenerative disorders. Finally, although the study utilized a validated questionnaire-based measure of clinically significant depression (Payne et al. 2014), the study was not able to ascertain whether or not respondents had a clinical diagnosis of depression. Future population-based studies should investigate whether the effect of olfactory dysfunction on depression holds even when analyzing incident cases of clinically diagnosed depression.
This study contributes to existing literature by showing that olfactory dysfunction is associated with depressive symptoms both cross-sectionally and longitudinally. In addition, it is the first to tease out which comes first. Among healthy older adults without depression, baseline olfactory dysfunction predicts the development of depression, but there is no evidence to substantiate the converse. These findings could have clinical applications in the prevention, assessment, and treatment of depression in healthy older adults.
Acknowledgments
David Glick, MD, Vernon Leo Towle, PhD, and Kevin Hellman, PhD, from the Pritzker School of Medicine Summer Research Program provided critical support and feedback. Jay Shah, BS, at the Pritzker School of Medicine provided useful comments and feedback. We thank NSHAP respondents for their generous participation in this study.
Funding
This research was supported by the National Institutes of Health (NIA T35AG029795, R01AG021487, R37AG030481, R01AG033903, R01AG043538, R01AG048511, R37AG030481, and R01AG043538) and the Pritzker School of Medicine.
Conflict of interest
All authors report no conflict of interests.
References
- Birte-Antina W, Ilona C, Antje H, Thomas H. 2018. Olfactory training with older people. Int J Geriatr Psychiatry. 33:212–220. [DOI] [PubMed] [Google Scholar]
- Boesveldt S, Lindau ST, McClintock MK, Hummel T, Lundstrom JN, Lindstrom JN. 2011. Gustatory and olfactory dysfunction in older adults: a national probability study. Rhinology. 49(3):324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brämerson A, Nordin S, Bende M. 2007. Clinical experience with patients with olfactory complaints, and their quality of life. Acta Otolaryngol. 127(2):167–174. [DOI] [PubMed] [Google Scholar]
- Buschhüter D, Smitka M, Puschmann S, Gerber JC, Witt M, Abolmaali ND, Hummel T. 2008. Correlation between olfactory bulb volume and olfactory function. NeuroImage. 42:498–502. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. 2004. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 29(6):417–426. [PMC free article] [PubMed] [Google Scholar]
- Chang-Quan H, Xue-Mei Z, Bi-Rong D, Zhen-Chan L, Ji-Rong Y, Qing-Xiu L. 2010. Health status and risk for depression among the elderly: a meta-analysis of published literature. Age Ageing. 39:23–30. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 40(5):373–383. [DOI] [PubMed] [Google Scholar]
- Cole MG, Dendukuri N. 2003. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. AJP. 160:1147–1156. [DOI] [PubMed] [Google Scholar]
- Croy I, Hummel T. 2017. Olfaction as a marker for depression. J Neurol. 264(4):631–638. [DOI] [PubMed] [Google Scholar]
- Croy I, Nordin S, Hummel T. 2014. Olfactory disorders and quality of life—an updated review. Chem Senses. 39(3):185–194. [DOI] [PubMed] [Google Scholar]
- Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB Jr. 1991. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 117(5):519–528. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. 2008. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 213(1-2):93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellrich J, Han P, Manesse C, Betz A, Junghanns A, Raue C, Schriever VA, Hummel T. 2018. Brain volume changes in hyposmic patients before and after olfactory training. Laryngoscope. 128(7):1531–1536. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Anstey KJ, Sue CM, Kifley A, Mitchell P. 2011. Olfactory impairment in older adults is associated with depressive symptoms and poorer quality of life scores. Am J Geriatr Psychiatry. 19(9):830–834. [DOI] [PubMed] [Google Scholar]
- Huber PJ. 1967. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the 5th Berkeley Symposium on Mathematical Statistics and Probability. Berkeley (CA): University of California Press. Vol. 1. p. 221–223. [Google Scholar]
- Hur K, Choi JS, Zheng M, Shen J, Wrobel B. 2018. Association of alterations in smell and taste with depression in older adults: Association of Smell and Taste with Depression. Laryngoscope Investig Otolaryngol. 3:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaszczak A, O’Doherty K, Colicchia M, Satorius J, McPhillips J, Czaplewski M, Imhof L, Smith S. 2014. Continuity and innovation in the data collection protocols of the second Wave of the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 69(Suppl 2):S4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo YH, Hwang SH, Han KD, Seo JH, Kang JM. 2015. Relationship between olfactory dysfunction and suicidal ideation: the Korea National Health and Nutrition Examination Survey. Am J Rhinol Allergy. 29(4):268–272. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. 1997. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 74(3):299–316. [DOI] [PubMed] [Google Scholar]
- Kern DW, Wroblewski KE, Schumm LP, Pinto JM, McClintock MK. 2014. Field survey measures of olfaction: the olfactory function field exam (OFFE). Field Methods. 26(4):421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli P, Soler ZM, Nguyen SA, Muus JS, Schlosser RJ. 2016. The association between olfaction and depression: a systematic review. Chem Senses. 41(6):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis BN, Konnerth CG, Hummel T. 2004. A study on the frequency of olfactory dysfunction. Laryngoscope. 114(10):1764–1769. [DOI] [PubMed] [Google Scholar]
- Litvack JR, Fong K, Mace J, James KE, Smith TL. 2008. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope. 118(12):2225–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervaala E, Föhr J, Könönen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamäki H, Karjalainen AK, et al. . 2000. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 30(1):117–125. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. 1999. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 21(3):325–340. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. 2002. Prevalence of olfactory impairment in older adults. JAMA. 288(18):2307–2312. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53(4):695–699. [DOI] [PubMed] [Google Scholar]
- Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T. 2010. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 169(1):415–421. [DOI] [PubMed] [Google Scholar]
- Negoias S, Hummel T, Symmank A, Schellong J, Joraschky P, Croy I. 2016. Olfactory bulb volume predicts therapeutic outcome in major depression disorder. Brain Imaging Behav. 10(2):367–372. [DOI] [PubMed] [Google Scholar]
- Negoias S, Pietsch K, Hummel T. 2017. Changes in olfactory bulb volume following lateralized olfactory training. Brain Imaging Behav. 11(4):998–1005. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. 2002. Neurobiology of depression. Neuron. 34(1):13–25. [DOI] [PubMed] [Google Scholar]
- O’Muircheartaigh C, Eckman S, Smith S. 2009. Statistical design and estimation for the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 64(Suppl 1):i12–i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabel LD, Murr J, Weidner K, Hummel T, Croy I. 2020. Null effect of olfactory training with patients suffering from depressive disorders—an exploratory randomized controlled clinical trial. Front Psychiatry. 11:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Hedberg EC, Kozloski M, Dale W, McClintock MK. 2014. Using and interpreting mental health measures in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 69(Suppl 2):S99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E. 1975. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 23:433–441. [DOI] [PubMed] [Google Scholar]
- Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. 2014. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 9(10):e107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD. 2014. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 44(10):2029–2040. [DOI] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, Pankow JS, Nondahl DM. 2012. Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chem Senses. 37(4):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. 2011. Olfactory impairment in older adults: five-year incidence and risk factors. Laryngoscope. 121(4):873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert J, Laukka EJ, Rizzuto D, Hummel T, Fratiglioni L, Bäckman L, Larsson M. 2017. Prevalence and correlates of olfactory dysfunction in old age: a population-based study. J Gerontol A Biol Sci Med Sci. 72(8):1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shega JW, Sunkara PD, Kotwal A, Kern DW, Henning SL, McClintock MK, Schumm P, Waite LJ, Dale W. 2014. Measuring cognition: the Chicago Cognitive Function Measure in the National Social Life, Health and Aging Project, Wave 2. J Gerontol B Psychol Sci Soc Sci. 69(Suppl 2):S166–S176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. 1996. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 93(9):3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivam A, Wroblewski KE, Alkorta-Aranburu G, Barnes LL, Wilson RS, Bennett DA, Pinto JM. 2016. Olfactory dysfunction in older adults is associated with feelings of depression and loneliness. Chem Senses. 41(4):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Jaszczak A, Graber J, Lundeen K, Leitsch S, Wargo E, O’Muircheartaigh C. 2009. Instrument development, study design implementation, and survey conduct for the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 64(Suppl 1):i20–i29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitka M, Puschmann S, Buschhueter D, Gerber JC, Witt M, Honeycutt N, Abolmaali N, Hummel T. 2012. Is there a correlation between hippocampus and amygdala volume and olfactory function in healthy subjects? Neuroimage. 59(2):1052–1057. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. 2005. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 29(4–5):627–647. [DOI] [PubMed] [Google Scholar]
- Sorokowska A, Drechsler E, Karwowski M, Hummel T. 2017. Effects of olfactory training: a meta-analysis. Rhinology. 55(1):17–26. [DOI] [PubMed] [Google Scholar]
- Soudry Y, Lemogne C, Malinvaud D, Consoli SM, Bonfils P. 2011. Olfactory system and emotion: common substrates. Eur Ann Otorhinolaryngol Head Neck Dis. 128(1):18–23. [DOI] [PubMed] [Google Scholar]
- Sutor B, Rummans TA, Jowsey SG, Krahn LE, Martin MJ, O’Connor MK, Philbrick KL, Richardson JW. 1998. Major depression in medically III patients. Mayo Clin Proc. 73(4):329–337. [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. 2004. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 161(11):1957–1966. [DOI] [PubMed] [Google Scholar]
- Waite L, Cagney K, Dale W, Hawkley L, Huang E, Lauderdale D, Laumann EO, McClintock M, O’Muircheartaigh C, Schumm LP. 2019. National Social Life, Health and Aging Project (NSHAP): Wave 3, [United States], 2015–2016. Ann Arbor, MI: Inter-university Consortium for Political and Social Research.doi: 10.3886/ICPSR36873.v4. [DOI]
- Weinstock RS, Wright HN, Smith DU. 1993. Olfactory dysfunction in diabetes mellitus. Physiol Behav. 53(1):17–21. [DOI] [PubMed] [Google Scholar]
- White H. 1980. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 48:817–838. [Google Scholar]
- Yang J, Pinto JM. 2016. The epidemiology of olfactory disorders. Curr Otorhinolaryngol Rep. 4(2):130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]