Abstract
Background:
The prognostic role of the expression of metastasis-associated in colon cancer-1 (MACC1) in gynecologic cancers and breast cancer remains unclear. The aim of this systematic review and meta-analysis was to determine the prognostic significance of MACC1 expression in gynecologic cancers and breast cancer.
Materials and methods:
PubMed, Web of Science and Embase were comprehensively searched up to February 9, 2020. Studies focusing on the relationship between the expression of MACC1 and prognosis in gynecologic cancers and breast cancer were included into the analysis. Pooled hazard ratio (HR) or odd ratio with 95% confidence interval (CI) was used to estimate the prognostic value of the expression of MACC1.
Results:
A total of 1,811patients with gynecologic cancers or breast cancer were included into the analysis. Patients with high expression of MACC1 tended to suffer a shorter overall survival (HR = 2.76, 95%CI = 2.12–3.59, P < .01) and recurrence-free survival (HR = 2.37, 95%CI = 1.44–3.90, P < .01) compared to those with low expression of MACC1. High expression of MACC1 was significantly associated with worse tumor differentiation (P = .04), more advanced FIGO stage (P < .01) and earlier lymph node metastasis (P < .01) compared to low expression of MACC1.
Conclusion:
Compared to low expression of MACC1, high expression of MACC1 predicts a worse prognosis of gynecologic cancers and breast cancer. The expression of MACC1 can serve as a prognostic indicator of gynecologic cancers and breast cancer.
Keywords: breast cancer, gynecologic cancers, meta-analysis, metastasis-associated in colon cancer-1, prognosis
1. Introduction
Gynecologic cancers and breast cancer have become the leading cause of death for women worldwide.[1] Despite great development of diagnosis and treatment, patients with gynecologic cancers or breast cancer surfer from a disappointing prognosis, especially those at advanced clinical stage.[2,3] Hence, many investigators try to identify promising prognostic indicators to serve as potential therapeutic targets and improve the clinical decision-making.[4–6]
Metastasis-associated in colon cancer-1 (MACC1), located on chromosome 7 at position 7p21.1, was proved to regulate the hepatocyte growth factor/Met signaling pathway in colon cancer.[7,8] Previous researches showed the dysregulated expression of MACC1 contributed to the carcinogenesis, invasion, and migration of human tumors.[9] The dysregulated expression of MACC1 has been proved to be associated with the prognosis of several digestive cancers, including colon cancer,[10] gastric cancer[11] and hepatocellular carcinoma.[12] Recently, accumulating evidence showed MACC1 may play an important role in the progression of gynecologic cancers and breast cancer, and MACC1 may have the potential capacity to predict the prognosis of these patients with gynecologic cancers or breast cancer.[13–22]Yu et al. analyzed 207 cases diagnosed as ovarian cancer and found high expression of MACC1 was associated with shorter overall survival (OS) compared to low expression of MACC1 [hazard ratio (HR)=4.06, P < .01].[20]Zhou et al study showed, compared to low expression of MACC1, high expression of MACC1 was related to worse OS in cervical cancer (HR=2.99, P = .04).[22] Huang et al also found high expression of MACC1 might predict a worse OS compared to low MACC1 expression in breast cancer (HR = 3.19, P < .01).[15] Currently, despite diverse articles revealed the potential link between the expression of MACC1 and prognosis of gynecologic cancers and breast cancer, the prognostic value of MACC1 expression in gynecologic cancers and breast cancer remains contradictory, which may be attributed to the limited sample size and imperfect design.[13–23] Here, we performed this systematic review and meta-analysis to determine the prognostic value of MACC1 expression in gynecologic cancers and breast cancer.
2. Materials and methods
Ethical approval and informed consent were unnecessary because no data of individuals was used in the analysis. This study was conducted strictly according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses.[24]
2.1. Eligibility criteria
The included studies should meet the following criteria:
-
(1)
Participant: patients diagnosed as any gynecologic cancers or breast cancer;
-
(2)
Intervention: high or positive expression of MACC1;
-
(3)
Control: low or negative expression of MACC1;
-
(4)
Outcome: OS, progression-free survival (PFS), recurrence-free survival (RFS), disease-free survival (DFS) and clinicopathological parameters;
-
(5)
Study design: retrospective or prospective studies.
Exclusion criteria were as follows: not gynecologic cancers or breast cancer, cell or animal experiments, reviews, case reports, letters, insufficient data or duplicated patients.
2.2. Literature search and study selection
PubMed, Web of Science and Embase were comprehensively research on February 9th, 2020 using the following strategy: (“MACC1” OR “Metastasis-associated in colon cancer-1”) AND (“carcinoma” OR “cancer” OR “tumor” OR “neoplasm”) AND (“survival” OR “prognosis”). The references of retrieved studies were also checked to avoid missing relevant studies. Then, study selection was performed according to the eligibility by 2 investigators independently, and any disagreement would be solved by group discussion.
2.3. Data extraction
Data extraction was conducted independently by 2 investigators using a prepared template, and any disagreement was resolved by reaching a consensus on all contents. Following items were extracted from included studies: first author, publication year, retrospective design, sample size, patients with high or low expression of MACC1, detection method of the expression of MACC1, source of sample, cancer type, outcomes, and analysis model of OS. As for prognostic variables (eg, OS, RFS, PFS and DFS), HR with 95% confidence interval (CI) was directly extracted from articles published or indirectly extracted as described by Tierney et al.[25] Besides, clinicopathological parameters were also extracted, including tumor differentiation, tumor size, FIGO stage, ascites, and lymph node metastasis.
2.4. Risk of bias of included studies
Risk of bias of included studies was evaluated using the Newcastle-Ottawa Scale score, in which the scores varied from 0 to 9, and a score greater than 5 was regarded as low risk of bias.[26]
2.5. Statistical analysis
All analyses in this study were conducted by Review Manager 5.3 software (Cochrane Collaboration, London, UK) and Stata 12.0 (StataCorp, College Station, TX). Pooled HR with corresponding 95% CI was utilized to explore the association between MACC1 expression and prognosis in gynecologic cancers and breast cancer. The association between the expression of MACC1 and clinicopathological parameters was described using odds ratio (OR) with corresponding 95% CI. Cochran Q and Higgins I2 tests were applied to assess the heterogeneity among included studies. Heterogeneity was considered obvious as I2 > 50% and P < .10, and a random-effect model was used; otherwise, a fixed-effect model was applied. To comprehensively evaluate the association between the expression of MACC1 and OS, subgroup analysis was conducted. Begg and Egger tests were performed to evaluate the publication bias, and sensitivity analysis was employed to confirm the reliability of the results using Stata 12.0. A 2-side P value less than .05 indicated that the association between the expression of MACC1 and prognostic outcomes was statistically significant.
3. Results
3.1. Literature search and study selection
As shown in Figure 1, a total of 550 articles were retrieved from 3 common databases, and 241 articles remained for further examination after the removal of duplications. Among 241 articles, 207 studies were directly excluded by scanning the titles or abstracts. For the rest articles, full-text of each study was evaluated and 23 studies were excluded for following reasons: review type (n = 4), not gynecologic cancers or breast cancer (n = 7), insufficient data (n = 2), irrelevant to this topic (n = 6) and experiment studies (n = 4). Finally, a total of 11 studies were included into this systematic review and meta-analysis.[13–23]
Figure 1.
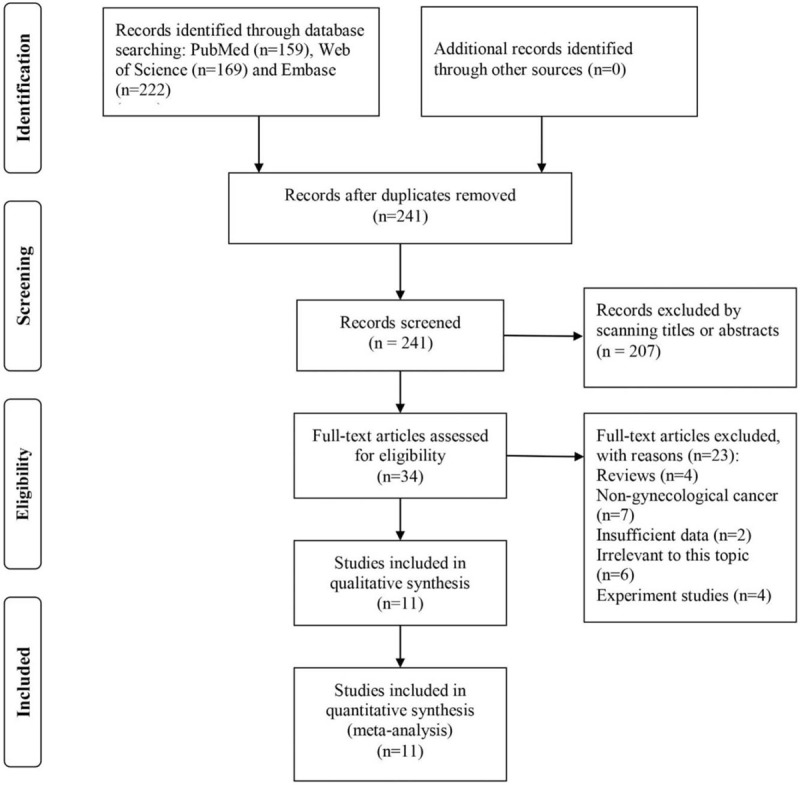
Flow chart of literature search and study selection.
3.2. Characteristics of included studies
The characteristics of included studies were listed in Table 1. A total of 11 retrospective studies containing 1,811 patients with gynecologic cancers or breast cancer were included into this research.[13–23] Especially, Huang et al study contained 2 independent clinical cohorts.[15] Nine studies were conducted in China,[13–16,18–22] 1 study was conducted in Germany[17] and 1 study was conducted in Bosnia and Herzegovina.[19] The expression of MACC1 was detected using immunohistonchemistry in 6 studies,[13–16,20,22] real-time quantitative polymerase chain reaction in 3 studies[17,18,21] and other methods in 2 studies.[23] The sample was obtained from tumor tissue in 9 studies[13–16,18–22] and preoperative serum in 2 studies.[17,23] Four types of cancer were investigated, including ovarian cancer,[16,17,20,21] breast cancer,[15,19,23] cervical cancer[14,18,22] and endometrial cancer.[13] The FIGO stage was reported in all included studies.[13–23] For outcomes, 10 studies reported the clinicopathological parameters,[13–16,18–23] 7 studies reported the OS[13–17,19,20] and 6 studies reported the RFS, PFS, or DFS.[13–15,17,19,23] The association between the expression of MACC1 and OS was evaluated using the multivariate analysis in 4 studies[14,15,20,22] and using the univariate analysis in 2 studies.[13,17] The Newcastle-Ottawa Scale score of all included studies was larger than 5, indicating no obvious risk of bias among studies.[13–23]
Table 1.
Characteristics of included studies.
| Study | Country | Study design | Detection method | Source | MACC1 expression (n) (total/high/low) | Cancer type | FIGO stage (I+II/III+IV) | Outcome | Analysis model | NOS |
| Huang et al 2013[1,15] | China | R | IHC | Tumor tissue | 245/136/109 | Breast cancer | 172/73 | CP, RFS, OS | M | 8 |
| Huang et al 2013[2,15] | China | R | IHC | Tumor tissue | 185/111/74 | Breast cancer | 101/84 | CP, OS | M | 8 |
| Guo et al 2014[14] | China | R | IHC | Tumor tissue | 104/51/53 | Cervical cancer | 71/33 | CP, RFS, OS | M | 8 |
| Li et al al 2014[16] | China | R | IHC | Tumor tissue | 47/33/14 | Ovarian cancer | 10/37 | CP | NA | 6 |
| Zhou et al 2015[22] | China | R | IHC | Tumor tissue | 181/96/85 | Cervical cancer | 130/51 | CP, OS | M | 7 |
| Chen et al 2016[13] | China | R | IHC | Tumor tissue | 158/68/90 | Endometrial cancer | 136/22 | CP, RFS, OS | U | 8 |
| Tang et al 2016[23] | China | R | ELISA | Serum | 378/253/125 | Breast cancer | 270/108 | CP, DFS | NA | 7 |
| Yu et al 2017[20] | China | R | IHC | Tumor tissue | 207/126/81 | Ovarian cancer | 106/101 | CP, OS | M | 8 |
| Prguda-Mujic et al 2018[19] | Bosnia and Herzegovina | R | WB | Tumor tissue | 105/30/75 | Breast cancer | T1/T2/T3–4, 23/69/11; N0/N1,67/38 | CP, OS, DFS | NA | 8 |
| Link et al 2019[17] | Germany | R | RT–qPCR | Serum | 79/NA/NA | Ovarian cancer | 16/63 | PFS, OS | U | 7 |
| Meng et al 2019[18] | China | R | RT–qPCR | Tumor tissue | 57/29/28 | Cervical cancer | 27/30 | CP | NA | 6 |
| Zhang et al 2019[21] | China | R | RT–qPCR | Tumor tissue | 65/33/32 | Ovarian cancer | 12/53 | CP | NA | 6 |
CP = clinicopathological parameters, DFS = disease-free survival, ELISA = enzyme linked immunosorbent assay, FIGO = International Federation of Gynecology and Obstetrics, IHC = immunohistonchemistry, M = multivariate, MACC1 = metastasis-associated in colon cancer-1, NA = not available, NOS = Newcastle-Ottawa Scale, OS = overall survival, PFS = progression-free survival, R = retrospective, RFS = recurrence-free survival, RT–qPCR = real-time quantitative polymerase chain reaction, U = univariate, WB = western blot.
3.3. Association between the expression of MACC1 and OS
A total of 6 studies containing 7 cohorts were included into the analysis[13–15,17,20,22] (Fig. 2). There was no heterogeneity among studies, as a result, a fixed-effect model was used (I2 = 0%, Pheterogeneity = .45), and patients with high expression of MACC1 tended to have a shorter OS compared to those with low expression of MACC1 (HR = 2.76, 95%CI = 2.12–3.59, P < .01). The subgroup analysis also showed high expression of MACC1 was an unfavorable prognostic factor of gynecologic cancers and breast cancer (P < .05) (Table 2).
Figure 2.
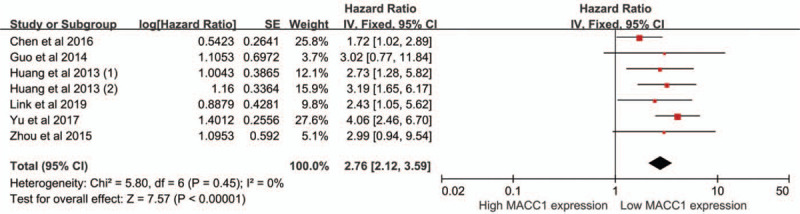
Meta-analysis of association between MACC1 expression and OS.
Table 2.
Subgroup analysis of association between the MACC1 expression and OS.
| Variables | Included cohort (n) | HR 95%CI | P | I2 (%) | Pheterogeneity | Model |
| Country | ||||||
| China | 6 | 2.80 (2.12, 3.70) | <.01¶ | 12 | .34 | Fixed |
| Others | 1 | 2.43 (1.05, 5.62) | .04¶ | NA | NA | Fixed |
| Sample size (n) | ||||||
| ≤158 | 3 | 1.98 (1.30, 3.01) | <.01¶ | 0 | .64 | Fixed |
| >158 | 4 | 3.43 (2.45, 4.81) | <.01¶ | 0 | .83 | Fixed |
| Cancer type | ||||||
| Ovarian cancer | 2 | 3.55 (2.31, 5.45) | <.01¶ | 6 | .30 | Fixed |
| Endometrial cancer | 1 | 1.72 (1.02, 2.89) | .04¶ | NA | NA | Fixed |
| Cervical cancer | 2 | 3.00 (1.24, 7.27) | .01¶ | 0 | .99 | Fixed |
| Breast cancer | 2 | 2.98 (1.81, 4.90) | .01¶ | 0 | .76 | Fixed |
| Detection Method | ||||||
| IHC | 6 | 2.80 (2.12, 3.70) | <.01¶ | 12 | .34 | Fixed |
| Others | 1 | 2.43 (1.05, 5.62) | .04¶ | NA | NA | Fixed |
| Analysis model | ||||||
| Univariate | 2 | 1.89 (1.22, 2.94) | <.01¶ | 0 | .49 | Fixed |
| Multivariate | 5 | 3.41 (2.45, 4.73) | <.01¶ | 0 | .92 | Fixed |
CI = confidence interval, HR = hazard ratio, IHC = immunohistonchemistry, MACC1 = metastasis-associated in colon cancer-1, NA = not available, OS = overall survival.
P < .05 indicating the significant association between MACC1 expression and OS.
3.4. Association between the expression of MACC1 and RFS/PFS/DFS
Three studies reported the RFS,[13–15] 2 studies reported the DFS[19,23] and 1 study reported the PFS,[17] and all of them were included into the analysis of RFS. A random-effect model was applied for the obvious heterogeneity (I2 = 57%, Pheterogeneity = .04). High expression of MACC1 was significantly associated with shorter RFS/PFS/DFS in gynecologic cancers and breast cancer (HR = 2.37, 95%CI = 1.44–3.90, P < .01) (Fig. 3).
Figure 3.
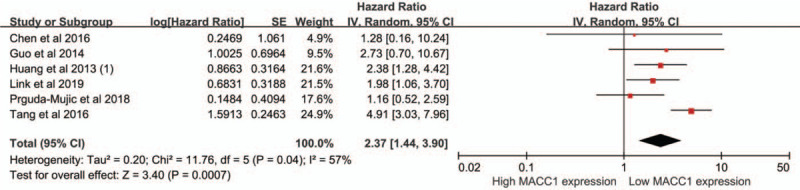
Meta-analysis of association between MACC1 expression and RFS.
3.5. Association between the expression of MACC1 and clinicopathological parameters
As listed in Table 3, high expression of MACC1 was significantly associated with worse tumor differentiation (OR = 2.23, 95%CI = 1.05–4.74, P = .04), more advanced FIGO stage (OR = 3.53, 95%CI = 2.71–4.60, P < .01) and earlier lymph node metastasis (OR = 2.87, 95%CI = 2.19–3.77, P < .01) when compared to low expression of MACC1. No obvious association between the expression of MACC1 and clinicopathological parameters was observed in terms of age (P = .84), tumor size (P = .56) or ascites (P = .79).
Table 3.
Association between the MACC1 expression and clinicopathological parameters.
| Variables | Cohort (n) | Patients (n) | OR 95%CI | P | I2 (%) | Pheterogeneity | Model | Begg test | Egger test |
| Age (old/young) | 9 | 1249 | 1.02 (0.81, 1.29) | 0.84 | 0 | .6 | Fixed | 0.47 | 0.05 |
| Differentiation (poor/ well or moderate) | 6 | 762 | 2.23 (1.05, 4.74) | 0.04¶ | 76 | <.01 | Random | 0.13 | 0.15 |
| Tumor size (large/small) | 2 | 264 | 1.16 (0.70, 1.92) | 0.56 | 50 | .16 | Fixed | NA | NA |
| FIGO stage (III+IV/I+II) | 9 | 1249 | 3.53 (2.71, 4.60) | <0.01¶ | 0 | .65 | Fixed | 0.18 | 0.05 |
| Ascites (yes/no) | 2 | 272 | 1.23 (0.27, 5.58) | 0.79 | 81 | .02 | Random | NA | NA |
| LNM (yes/no) | 8 | 1202 | 2.87 (2.19, 3.77) | <0.01¶ | 36 | .14 | Fixed | 0.11 | 0.06 |
CI = confidence interval, FIGO = International Federation of Gynecology and Obstetrics, LNM = lymph node metastasis, MACC1 = metastasis-associated in colon cancer-1, NA = not available, OR = odd ratio.
P < .05 indicating the significant association between MACC1 expression and clinicopathological parameters.
3.6. Sensitivity analysis
The reliability of the association between the expression of MACC1 and OS (Fig. 4A) or RFS (Fig. 4B) was checked by sensitivity analysis.
Figure 4.
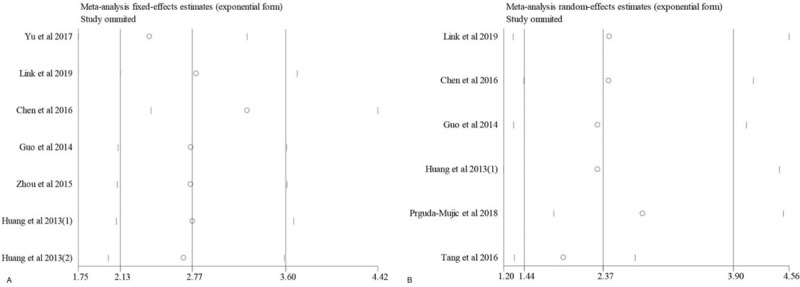
Sensitivity analysis of association between MACC1 expression and OS or RFS (A, OS; B, RFS).
3.7. Publication bias
There was no obvious publication bias across studies with respect to the association of the expression of MACC1 with OS (Begg test, P = .76; Egger test, P = .95) (Fig. 5A), RFS (Begg's test, P = 0.45; Egger test, P = .30) (Fig. 5B) or clinicopathological parameters (Table 3).
Figure 5.
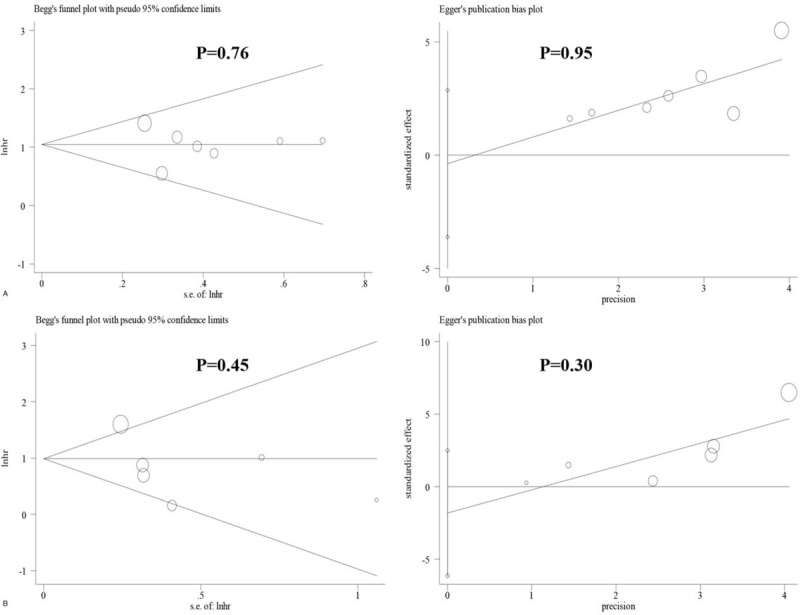
Publication bias of association between MACC1 expression and OS or RFS (A, OS; B, RFS).
4. Discussion
Plenty of studies have showed the dysregulated expression of MACC1 contributed to the tumor progression and could predict the prognosis of gynecologic cancers and breast cancer, otherwise, definite conclusion has not been obtained for the small sample size and contradictory results among previous studies.[13–23] In the current study, we performed a systematic review and meta-analysis to determine the prognostic role of MACC1 expression in gynecologic cancers and breast cancer, and our results showed high expression of MACC1 was significantly associated with shorter OS and RFS compared to low expression of MACC1. The subgroup analysis stratified by the country, sample size, cancer type, detection method and analysis model of OS confirmed the significant association between the expression of MACC1 and OS. Moreover, patients with high expression of MACC1 tended to suffer from worse tumor differentiation, more advanced clinical stage and earlier lymph node metastasis compared to those with low expression of MACC1. In a word, our results showed high MACC1 expression is an unfavorable prognostic factor of patients with gynecologic cancers or breast cancer, and the MACC1 may be a potential therapeutic target of gynecologic cancers and breast cancer.
Although a great number of studies have reported the prognostic role of MACC1 expression in gynecologic cancers and breast cancer, the underlying mechanism remains indistinct.[13–23] Chen et al study showed the overexpression of miR-23b can reduce the expression of MACC1, induce the G1 phrase arrest, and suppress the cell proliferation in endometrial cancer.[13] Li et al found that MACC1 may promote the tumor progression via the signal pathway of hepatocyte growth factor/c-Met in ovarian cancer.[16] Zhang et al study showed the expression of miR-338–3p is negatively correlated to the expression of MACC1 in ovarian cancer.[21] Zhang et al study showed MACC1 can improve the chemosensitivity of cisplatin in ovarian cancer cells through the ERK1/2 signaling pathway on glycoprotein and its downstream apoptosis proteins.[27] Meng et al study indicated that the expression of MACC1 may be regulated by the miR-877, and MACC1 can restore the miR-877 overexpression-mediated the suppression of proliferation and invasion of cervical cancer cells.[18] Yu et al found that long non-coding RNA HCP5 can promote the progression of cervical cancer by regulating MACC1 via the suppression of microRNA-15a.[28] Wang et al drew the conclusion that miR-485 can suppress the tumor progression via targeting MACC1 and inhibiting the Met/AKT signaling pathway.[29]
There were several highlights in the current study. First, to our knowledge, we are first to evaluate the prognostic value of MACC1 expression in gynecologic cancers and breast cancer in the form of systematic review and meta-analysis. Second, we explore the association of MACC1 expression with OS, RFS and clinicopathological parameters, therefore, the analysis of the prognostic value of MACC1 expression in our study is comprehensive and convincing. Third, a large population of 1,811 patients are included into the current study, which can provide the convincing conclusions.
Some limitations should be considered when interpreting our findings. First, all studies have a retrospective design, as a result, selection bias may exist. Second, different methods are used to detect the expression of MACC1, which may reduce the reliability of results. Third, on account of the limited population, we explored the relationship between the expression of MACC1 and prognosis of all types of gynecologic cancers or breast cancer, instead of 1 specific cancer (eg, ovarian cancer, breast cancer or cervical cancer), which may limit the application of our conclusion into clinical practice. Forth, most studies are conducted in China, therefore, it is hard to popularize our findings into other countries.
5. Conclusion
High expression of MACC1 predicts shorter OS and RFS compared to low MACC1 expression in gynecologic cancers and breast cancer. High expression of MACC1 is associated with worse tumor differentiation, more advanced FIGO stage and earlier lymph node metastasis in gynecologic cancers and breast cancer. Therefore, MACC1 expression can predict the prognosis of gynecologic cancers and breast cancer. Multicenter prospective trials with large sample size and long follow-up period should be carried out to determine the prognostic value of MACC1 expression in gynecologic cancers and breast cancer in future.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Author contributions
Study concepts and design: Haiyuan Jiang; Literature search: Lijun Wang and Liying Fan; Data extraction: Lijun Wang and Hongyan Xu; Data analysis: Lijun Wang and Haiyuan Jiang; Manuscript preparation and revision: Lijun Wang and Haiyuan Jiang. All authors have participated sufficiently in the study and approved the final version.
Conceptualization: Haiyuan Jiang.
Data curation: Lijun Wang, Liying Fan.
Formal analysis: Haiyuan Jiang.
Investigation: Lijun Wang.
Methodology: Lijun Wang, Hongyan Xu, Haiyuan Jiang.
Resources: Hongyan Xu.
Software: Liying Fan.
Supervision: Lijun Wang, Liying Fan, Hongyan Xu, Haiyuan Jiang.
Validation: Lijun Wang, Liying Fan, Hongyan Xu.
Writing – original draft: Lijun Wang, Liying Fan, Hongyan Xu.
Writing – review & editing: Lijun Wang, Liying Fan, Hongyan Xu, Haiyuan Jiang.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, HR = hazard ratio, MACC1 = metastasis-associated in colon cancer-1, OR = odds ratio, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival.
How to cite this article: Wang L, Fan L, Xu H, Jiang H. Prognostic significance of the expression of metastasis-associated in colon cancer-1 in gynecologic cancers and breast cancer: a protocol for systematic review and meta-analysis. Medicine. 2021;100:8(e24255).
Ethics approval and consent to participate was not applicable.
Consent for publication was not applicable.
Please contact author for data requests.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- [2].SIL, OAC, FD. Evaluation of gynecologic cancer with MR imaging, 18F-FDG PET/CT, and PET/MR imaging. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 2015;56:436–43. [DOI] [PubMed] [Google Scholar]
- [3].JMH, BP. The role of palliative surgery in gynecologic cancer cases. Oncologist 2013;18:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Luo Y, Kim HS, Kim M, et al. Elevated plasma fibrinogen levels and prognosis of epithelial ovarian cancer: a cohort study and meta-analysis. J Gynecol Oncol 2017;28:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ye Q, Cheng J, Ye M, et al. Association of pretreatment thrombocytosis with prognosis in ovarian cancer: a systematic review and meta-analysis. J Gynecol Oncol 2019;30:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yuan X, Zhang J, Li D, et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: a meta-analysis. Gynecol Oncol 2017;147:181–7. [DOI] [PubMed] [Google Scholar]
- [7].Galimi F, Torti D, Sassi F, et al. Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer: response to met inhibition in patient xenografts and pathologic correlations. Clin Cancer Res 2011;17:3146–56. [DOI] [PubMed] [Google Scholar]
- [8].Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med 2009;15:59–67. [DOI] [PubMed] [Google Scholar]
- [9].Kopczynska EK. The potential therapeutic applications and prognostic significance of metastasis-associated in colon cancer-1 (MACC1) in cancers. Contemporary oncology (Poznan, Poland) 2016;20:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao Y, Dai C, Wang M, et al. Clinicopathological and prognostic significance of metastasis-associated in colon cancer-1 (MACC1) overexpression in colorectal cancer: a meta-analysis. Oncotarget 2016;7:62966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jin Y, Zhou K, Zhao W, et al. Clinicopathological and prognostic significance of metastasis-associated in colon cancer-1 in gastric cancer: A meta-analysis. The International journal of biological markers 2019;34:27–32. [DOI] [PubMed] [Google Scholar]
- [12].Sun DW, Zhang YY, Qi Y, et al. Prognostic and clinicopathological significance of MACC1 expression in hepatocellular carcinoma patients: a meta-analysis. Int J Clin Exp Med 2015;8:4769–77. [PMC free article] [PubMed] [Google Scholar]
- [13].Chen S, Zong ZH, Wu DD, et al. The role of metastasis-associated in colon cancer 1 (MACC1) in endometrial carcinoma tumorigenesis and progression. Molecular carcinogenesis 2017;56:1361–71. [DOI] [PubMed] [Google Scholar]
- [14].Guo L, Lu W, Zhang X, et al. Metastasis-associated colon cancer-1 is a novel prognostic marker for cervical cancer. International journal of clinical and experimental pathology 2014;7:4150–5. [PMC free article] [PubMed] [Google Scholar]
- [15].Huang Y, Zhang H, Cai J, et al. Overexpression of MACC1 and its significance in human breast cancer progression. Cell Biosci 2013;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li H, Zhang H, Zhao S, et al. Overexpression of MACC1 and the association with hepatocyte growth factor/c-Met in epithelial ovarian cancer. Oncol Lett 2015;9:1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Link T, Kuhlmann JD, Kobelt D, et al. Clinical relevance of circulating MACC1 and S100A4 transcripts for ovarian cancer. Mol Oncol 2019;13:1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meng F, Ou J, Liu J, et al. MicroRNA-877 is downregulated in cervical cancer and directly targets MACC1 to inhibit cell proliferation and invasion. Exp Ther Med 2019;18:3650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Prguda-Mujic J, Milde-Langosch K, Mueller V, et al. The predictive significance of metastasis-associated in colon cancer-1 (MACC1) in primary breast cancer. Ann Clin Lab Sci 2018;48:191–6. [PubMed] [Google Scholar]
- [20].Yu L, Zhu B, Wu S, et al. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KiSS-1, and MACC1 in the prediction of metastasis and prognosis in ovarian carcinoma. Diagn Pathol 2017;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang R, Shi H, Ren F, et al. Down-regulation of miR-338-3p and Up-regulation of MACC1 indicated poor prognosis of epithelial ovarian cancer patients. J Cancer 2019;10:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou X, Xu CJ, Wang JX, et al. Metastasis-Associated in Colon Cancer-1 Associates With Poor Prognosis and Promotes Cell Invasion and Angiogenesis in Human Cervical Cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society 2015;25:1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tan W, Xie X, Li L, et al. Diagnostic and prognostic value of serum MACC1 in breast cancer patients. Oncotarget 2016;7:84408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. XXX 2000. [Google Scholar]
- [27].Zhang R, Shi H, Ren F, et al. Knockdown of MACC1 expression increases cisplatin sensitivity in cisplatin-resistant epithelial ovarian cancer cells. Oncol Rep 2016;35:2466–72. [DOI] [PubMed] [Google Scholar]
- [28].Yu Y, Shen HM, Fang DM, et al. LncRNA HCP5 promotes the development of cervical cancer by regulating MACC1 via suppression of microRNA-15a. Eur Rev Med Pharmacol Sci 2018;22:4812–9. [DOI] [PubMed] [Google Scholar]
- [29].Wang S, Zhang Y, Yuan S, et al. MicroRNA485 targets MACC1 and inhibits cervical cancer cell proliferation and invasion. Mol Med Rep 2018;18:2407–16. [DOI] [PubMed] [Google Scholar]


