Abstract
Background
Hyperthermia in combination with DnaJA4-knockout (KO) obviously affects the anti-viral immunity of HaCaT cells. The mechanisms of this process are not yet fully explored. However, it is known that DnaJA4 interacts with actin cytoskeleton after hyperthermia. Our aim was to investigate the effects of DnaJA4 on F-actin in HaCaT cells following hyperthermia.
Methods
Wild-type (WT) and DnaJA4-KO HaCaT cells were isolated at either 37°C (unheated) or 44°C (hyperthermia) for 30 min followed by testing under conditions of 37°C and assessing at 6, 12, and 24 h after hyperthermia. The cytoskeleton was observed with immunofluorescence. Flow cytometry and Western blotting were used to detect the expression of F-actin and relevant pathway protein.
Results
DnaJA4-KO and hyperthermia changed the cytoskeleton morphology of HaCaT cells. F-actin expression levels were elevated in DnaJA4-KO cells compared with WT cells (6364.33 ± 989.10 vs. 4272.67 ± 918.50, P < 0.05). In response to hyperthermia, F-actin expression levels of both WT and DnaJA4-KO cells showed a tendency to decrease followed by an obvious recovery after hyperthermia (WT cells: unheated vs. 6 h after hyperthermia or 24 h after hyperthermia: 0.34 ± 0.02 vs. 0.24 ± 0.01, 0.31 ± 0.01, P < 0.001, P < 0.05; DnaJA4-KO cells: unheated vs. 6 h after hyperthermia or 24 h after hyperthermia: 0.44 ± 0.01 vs. 0.30 ± 0.01, 0.51 ± 0.02, P < 0.001, P < 0.01). WT cells restored to baseline levels observed in the unheated condition, while DnaJA4-KO cells exceeded baseline levels in the recovery. As the upstream factors of F-actin, a similar profile in rho-associated serine/threonine kinase 1 (ROCK 1) and RhoA expressions was observed after hyperthermia. While E-cadherin expression was decreased in response to hyperthermia, it was increased in DnaJA4-KO cells compared with WT cells.
Conclusions
Hyperthermia affects the expression levels of F-actin in HaCaT cells. DnaJA4 knockout increases the expression of F-actin in HaCaT cells after hyperthermia. DnaJA4 regulates the expressions of F-actin and the related pathway proteins in response to hyperthermia in HaCaT cells.
Keywords: Hyperthermia, DnaJA4, F-actin, HaCaT
Introduction
Actin is a highly conserved multi-functional protein. It is present in essentially all eukaryotic cells, and has an approximate mass of 42 kDa.[1] Actin can be found as either a spherical monomer, referred to as globular actin (G-actin), or as a polymer of spherical actin, called fibros actin (F-actin) or microfilaments, which comprises the principal component of the cytoskeleton.[2] The two forms can reversibly convert to each other under certain physiologic conditions, but only F-actin possesses physiologic activity.[3] Actin participates in a number of critical cellular processes, including establishment and maintenance of cell junctions and cell shape, cell migration, and cell signaling. It can also influence cell phagocytosis, apoptosis, and proliferation.[4–6]
Heat shock proteins (HSPs) are a type of acute reaction proteins. HSPs are highly conserved with considerable diversity among species and increased expression under stressful conditions such as hyperthermia and ultraviolet B radiation.[7,8] Among these HSPs, DnaJ/HSP40s, with a molecular weight of approximately 40,000 Da, functions as co-chaperone of HSP70 to protect damaged cells.[9] As a chaperone, HSP40 can directly or indirectly affect the replication of viruses.[10] Hyperthermia, as defined by a 30- to 60-min exposure to a thermal stimulus of 40°C to 44°C has been used to treat various diseases, such as breast cancer, non-small cell lung cancer.[11,12] In our clinic we have used local hyperthermia (44°C for 30 min) for the treatment of plantar warts (verruca vulgaris), and found this method to be more effective than conventional therapies.[13] Results from studies within our laboratory have revealed that DnaJA4 expression in HaCaT cells was increased following hyperthermia, and it was confirmed that hyperthermia affected the anti-viral immunity of HaCaT cells, and DnaJA4-knockout (KO) combined with hyperthermia enhanced this response.[14] When analyzing these samples with use of mass spectrometry, it was found that DnaJA4 interacts with the cytoskeleton proteins, tubulin and actin in response to hyperthermia. Therefore, to better understand the bases for these responses, the purpose of this report was to examine the effects of DnaJA4 on F-actin in HaCaT cells in response to hyperthermia.
Methods
Cell lines and culture
Wild-type (WT) HaCaT cells were purchased from GENE (Shanghai, China). We had entrusted the company (GENE) to successfully constructed DnaJA4-KO HaCaT cells utilizing CRISPR/Cas9 technology. The cells were cultured in high glucose Dulbecco modified Eagle medium (Biological Industries (BI), Beit HaEmek, Israel) supplemented with 10% fetal bovine serum (Biological Industries) and 1% penicillin/streptomycin (Biological Industries) while being maintained in a humidified incubator at 37°C with 5% CO2.
Hyperthermia treatment
Cells were inoculated in 60 mm petri-dishes (Thermo Fisher Scientific, Wilmington, DE, USA), or in 12-well plates (Thermo Fisher Scientific) and grown to 60% to 70% cell fusion overnight. The petri dishes or 12-well plates were then immersed in a constant temperature water bath at 44°C (±0.1°C) for 30 min. After hyperthermia treatment, cells were returned to the incubator at 37°C for recovery and harvested at varying recovery time points of 6, 12, and 24 h for further analyses. Both WT and DnaJA4-KO HaCaT cells were subjected to identical treatments.
Staining of F-actin
Special cell coverslips were soaked in 70% ethanol overnight, washed three times with phosphate-buffered saline (PBS; Biological Industries) and then placed in the 12-well plates. Cells were inoculated to the cell coverslips in the 12-well plates. After cell growth to 60% fusion, the cells were subjected to hyperthermia (as described above) or remained unheated at 37°C. Cells were then rinsed with PBS and fixed in 4% paraformaldehyde for 15 min and then permeabilized with 0.1% Triton X-100 for 10 min. Staining of F-actin was performed using phalloidin conjugated to Alexa Fluor 488 (dilution 1:40; Cell Signaling Technology, Boston, MA, USA). Cells were stained for 60 min at room temperature in the dark. Nuclear DNA was stained using 4′,6-diamidino-2-phenylindole (100 ng/mL; Solarbio, Beijing, China). Coverslips were mounted in anti-fluorescent attenuation mounts and examined with use of Confocal laser scanning microscopy (Olympus, Tokyo, Japan). Three non-overlapping fields were randomly collected under high magnification (400×) of each glass slide under conditions of a fixed light source brightness and exposure.
Flow cytometry assay
WT cells and DnaJA4-KO cells were trypsinized and counted with use of a cytometer. Four hundred thousand cells were washed with PBS and then fixed in 0.5 mL/tube Fixation Buffer (Biolegend, San Diego, CA, USA) in the dark for 20 min. Cells were resuspended in Intracellular Staining Perm Wash Buffer (Biolegend) and centrifuged twice at 350 × g for 10 min. Cells were then incubated with primary antibodies consisting of mouse anti-human F-actin monoclonal antibodies (dilution 1:10, ab250; Abcam, Cambridge, UK) for 1 h and the secondary antibody being Alexa Fluor 488-conjugated goat anti-mouse immunoglobulin (Ig) M μ chain (dilution 1:2000, ab150121; Abcam) for 30 min. Finally, all cells were tested with use of a BD LSRFortessa instrument (BD Bioscience, San Diego, CA, USA) and a minimum of 10,000 cells were gated.
Protein preparation and Western blotting assay
WT cells and DnaJA4-KO cells were harvested at varying recovery time points after hyperthermia treatment. The cells were washed three times with PBS and lysed using radio-immunoprecipitation assay lysis buffer (Beyotime, Shanghai, China) with 1 mmol/L of phenylmethanesulfonyl fluoride (PMSF) (Beyotime), cOmplete, ethylene diamine tetraacetic acid (EDTA)-free protease inhibitor cocktail (Roche, Basel, Switzerland) and PHOSstop phosphatase inhibitor (Roche) prepared according to the manufacturer's protocols. Each plate was then maintained on ice for 15 min. Cell lysates were collected and centrifuged at 15,000 × g at 4°C for 15 min. The concentration of protein lysates was measured with use of the BCA protein assay kit (Beyotime) according to the manufacturer's instructions. Protein aliquots (30 μg) were loaded with sodium dodecyl sulfate (SDS) buffer (Beyotime) and boiled at 99°C for 10 min. The denatured protein samples were then electrophoresed, transferred to a membrane, blocked, and incubated with antibodies. Primary antibodies were mouse anti-human F-actin monoclonal antibodies (dilution 1:500, ab250; Abcam), rabbit anti-human RhoA monoclonal antibody (dilution 1:2000, ab187027; Abcam), rabbit anti-human rho-associated serine/threonine kinase 1 (ROCK1) monoclonal antibody (dilution 1:5000, ab45171; Abcam), rabbit anti-human E-cadherin monoclonal antibody (dilution 1:2500, ab40772; Abcam), rabbit anti-human β-catenin monoclonal antibody (dilution 1:3000, ab6302; Abcam), and rabbit anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibodies (dilution 1:10,000, ab181602; Abcam). Secondary antibodies were horseradish peroxidase-conjugated goat anti-mouse IgM mu chain (dilution 1:2000, ab97230; Abcam) and horseradish peroxidase-conjugated goat anti-rabbit IgG polyclonal antibody (dilution 1:5000, ZB-2301; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd, Beijing, China). Antibodies were all diluted according to the manufacturer's instructions.
Statistics analysis
Experimental data were expressed as means and standard deviation. The figure was generated using GraphPad software (GraphPad Software, San Diego, CA, USA). Statistical analysis was performed using the SPSS 22.0 software (IBM Corp, Armonk, NY, USA). Students’ t tests were used for comparisons between the two groups, while one-way analysis of variance was used for comparisons involving multiple groups. P < 0.05 was required for results to be considered statistically significant.
Results
DnaJA4-knockout and hyperthermia induced morphologic changes of HaCAT cells
In the unheated group, WT cells showed many filopodias, loose intercellular connections, and dispersed growth [Figure 1A]. However, fewer filopodia, tight intercellular connections, and cell fusion were observed in DnaJA4-KO cells [Figure 1E]. In response to hyperthermia, WT cells were shrunken and rounded at 6 h after hyperthermia, but essentially resumed their normal state at 24 h post-hyperthermia. In addition, filopodias in these hyperthermia treated WT cells were increased, achieving maximal numbers at 12 h after heat treatment [Figure 1 B and 1C]. Although supple intercellular connections were observed at 24 h after hyperthermia, no significant changes in actin cytoskeleton within DnaJ4-KO cells were obtained at 6 and 12 h after heating [Figure 1 F–H].
Figure 1.
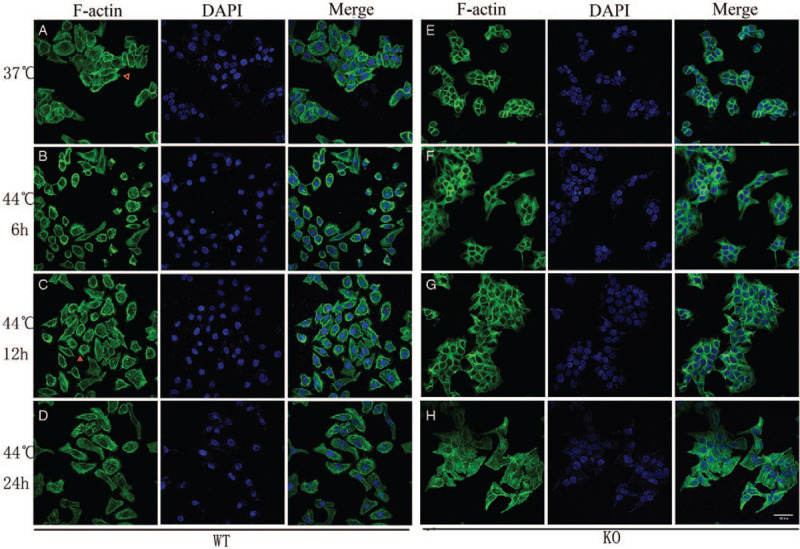
Immunofluorescence staining of F-actin (original magnification ×400). Cells were heated at 44.0°C for 30 min and allowed to recover under normal growing condition for up to 6, 12, and 24 h (original magnification ×400). At different time intervals, the cells were stained and processed for scanning confocal microscopy. (A) Unheated WT cells; (B–D) Hyperthermia treatment of WT cells, (B) 6 h, (C) 12 h, (D) 24 h after hyperthermia. (E) Unheated DnaJA4-KO cells; (F–H) hyperthermia treatment of DnaJA4-KO cells, (F) 6 h, (G)12 h, (H) 24 h after hyperthermia. F-actin (phalloidin) shown in green and nuclei (DAPI) shown in blue. WT: Wild-type; DnaJA4-KO: DnaJA4-knockout; DAPI: 4′,6-diamidino-2-phenylindole.
DnaJA4-knockout up-regulated F-actin expression in HaCaT cells
To accurately compare F-actin expression levels in WT cells vs. DnaJA4-KO cells, flow cytometry was performed following F-actin staining. DnaJA4-KO cells showed increased degrees of mean fluorescent intensity (6364.33 ± 989.10 vs. 4272.67 ± 918.50, t = 8.375, P = 0.014) as compared with that observed in WT cells [Figure 2], indicating that greater levels of F-actin expression were present in these DnaJA4-KO cells.
Figure 2.
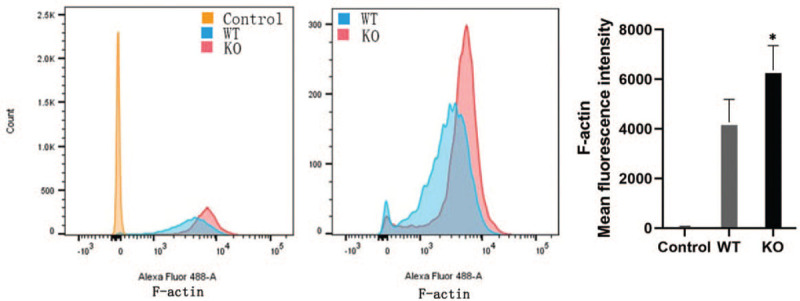
DnaJA4-KO up-regulated F-actin expression in HaCaT cells. Cells were stained with mouse anti-human F-actin monoclonal antibodies and Alexa Fluor 488-conjugated goat anti-mouse immunoglobulin M mu chain secondary antibody. Then cells were tested with use of flow cytometry assay. WT cells vs. DnaJA4-KO cells, ∗P < 0.05. Yellow: Unstained control group; Blue: Stained WT cells; Red: Stained DnaJA4-KO cells; WT: Wild-type; DnaJA4-KO: DnaJA4-knockout.
DnaJA4-knockout up-regulated the expression of F-actin and related pathway proteins
To determine the underlying mechanism by which DnaJA4 participates in hyperthermia, we analyzed the expression of F-actin and related pathway proteins following hyperthermia by Western blotting. In WT cells, compared with the unheated group, F-actin expression decreased at 6 h after hyperthermia and then increased to the initial unheated treatment baseline levels at 12 h after hyperthermia and remained slightly lower than baseline levels at 24 h after hyperthermia (37°C vs. 44°C-6 h or 44°C-12 h or 44°C-24 h: 0.34 ± 0.02 vs. 0.24 ± 0.01, 0.35 ± 0.02, 0.31 ± 0.01, P = 0.000, P = 0.537, P = 0.034). In DnaJA4-KO cells, compared with the unheated group, expression of F-actin also decreased at 6 h after hyperthermia and gradually recovered at 12 h after hyperthermia but exceeded those of the baseline unheated condition at 24 h after hyperthermia (37°C vs. 44°C-6 h or 44°C-12h or 44°C-24 h: 0.44 ± 0.01 vs. 0.30 ± 0.01, 0.38 ± 0.03, 0.51 ± 0.02, P < 0.001, P = 0.007, P = 0.001) [Figure 3]. In response to hyperthermia, compared with the unheated group, F-actin expression within both WT and DnaJA4-KO cells showed an initial tendency to decrease followed by an increase after hyperthermia. Furthermore, DnaJA4-KO cells showed an overall greater level of F-actin expression as compared with that of WT cells throughout the post-hyperthermia sampling periods.
Figure 3.
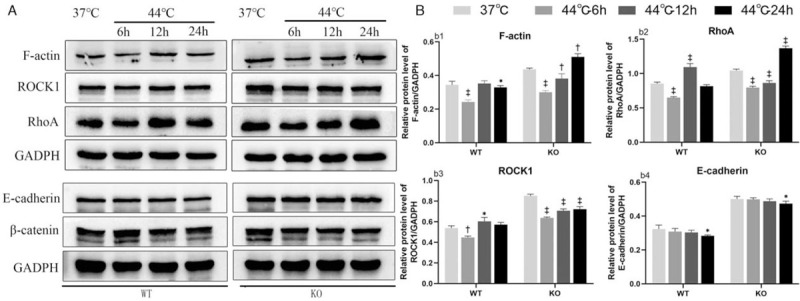
DnaJA4-KO up-regulated the expression of F-actin and related pathway proteins. (A) Western blotting analysis was used to evaluate F-actin, RhoA, ROCK1 E-cadherin, β-catenin expression. (B) Unheated (37°C) vs. hyperthermia (44.0°C) treated WT cells or DanJa4-KO cells as determined at 6, 12, and 24 h following treatment, respectively. ∗P < 0.05, †P < 0.01, ‡P < 0.001. WT: Wild-type cells; DnaJA4-KO: DnaJA4-knockout cells; GADPH: Glyceraldehyde-3-phosphate dehydrogenase; ROCK1: rho-associated serine/threonine kinase 1.
When evaluating responses of the upstream factors of F-actin, a similar profile was observed in ROCK1 (WT cell: 37°C vs. 44°C-6 h or 44°C-12 h or 44°C-24 h: 0.54 ± 0.02, 0.45 ± 0.01, 0.60 ± 0.04, 0.57 ± 0.02, P = 0.003, P = 0.013, P = 0.139; DnaJA4-KO cells: 37°C vs. 44°C-6 h or 44°C-12 h or 44°C-24 h: 0.85 ± 0.02, 0.64 ± 0.01, 0.71 ± 0.02, 0.72 ± 0.02, P < 0.001, P < 0.001, P < 0.001) and RhoA (WT cell: 37°C vs. 44°C-6 h or 44°C-12 h or 44°C-24 h: 0.85 ± 0.02, 0.65 ± 0.01, 1.09 ± 0.05, 0.82 ± 0.02, P < 0.001, P < 0.001, P = 0.163. DnaJA4-KO cells: 37°C vs. 44°C-6 h or 44°C-12 h or 44°C-24 h: 1.04 ± 0.02, 0.80 ± 0.02, 0.86 ± 0.03, 1.36 ± 0.03, P < 0.001, P < 0.001, P < 0.001) expressions as determined at 6, 12, and 24 h after heating [Figure 3]. In response to hyperthermia, compared with the unheated group, their expression within both WT and DnaJA4-KO cells all showed an initial tendency to decrease followed by an increase after hyperthermia. In addition, their overall expressions were also significantly greater in DnaJA4-KO vs. WT cells.
In contrast, the expression of E-cadherin in both WT cells and DnaJA4-KO cells was decreased at 24 h after hyperthermia (WT cells: 37°C vs. 44°C-24 h: 0.32 ± 0.02, 0.28 ± 0.01, P = 0.020; DnaJA4-KO cells: 37°C vs. 44°C-24 h: 0.50 ± 0.02, 0.47 ± 0.01, P = 0.036) [Figure 3]. However, the overall expression of E-cadherin in DnaJA4-KO cells remained significantly greater than that in WT cells. The expression of β-catenin was not significantly changed in response to hyperthermia.
Discussion
Hyperthermia destroys cell membranes by disrupting their stability and increasing their permeability. It can also inhibit Na+ and Ca2+ pumps, leading to decreases in intracellular K+ and increases in Ca2+ concentrations.[15] Under such conditions, F-actin tends to be depolymerized into G-actin.[16] Thermotherapy can down-regulate integrin levels resulting in inhibition of integrin-mediated adhesion kinase activity, which further dephosphorylates adhesion plaque components leading to disintegration and disappearance. As actin is in contact with the adhesive plaque, disruption of adhesive plaques tends to depolymerize microfilaments, thus weakening cell adhesion ability and producing rounded and buoyant cell bodies.[17] Simultaneously, expressions of HSPs are increased after hyperthermia which serves to protect these damaged cells. In specific, this stress induces rapid phosphorylation of Hsp27 and promotes F-actin polymerization, thus stabilizing the actin cytoskeleton.[18] In general, following hyperthermia, the actin cytoskeleton is initially depolymerized and shortened, followed by reassembly, polymerization and some degree of extension, which is the results of the unique assembly dynamics of the actin cytoskeleton.[19] These events are consistent with the profiles of F-actin expression as revealed in Western blots as well as cell morphologic changes observed in immunofluorescent assays. Our findings that DnaJA4-KO increased the aggregation of F-actin in HaCaT cells following heating, suggest that DnaJA4 might down-regulate the expression of F-actin in response to hyperthermia.
The Rho family of small GTPases is key regulatory molecules linking cell membrane surface receptors with the actin cytoskeleton.[20] Within this family, RhoA, Rac1, and Cdc42 are the best studied. RhoA induces stress fibers and enhances focal adhesions, while Cdc42 and Rac1 contribute to the formation of filopodia and lamellipodia, respectively.[21,22] In the Rho/ROCK signaling pathway, RhoA can induce ROCK activation. Activation of the downstream LIM domain kinase, phosphorylates cofilin which then inhibits the depolymerization of F-actin or further activates downstream myosin light chain phosphorylation to promote the formation of stress fibers.[23,24] In this experiment, ROCK1 and RhoA as the upstream factors of F-actin, their expression is consistent with F-actin. It suggests that DnaJA4 affects the expression of F-actin-related RhoA/ROCK1 pathway proteins [Figure 4].
Figure 4.
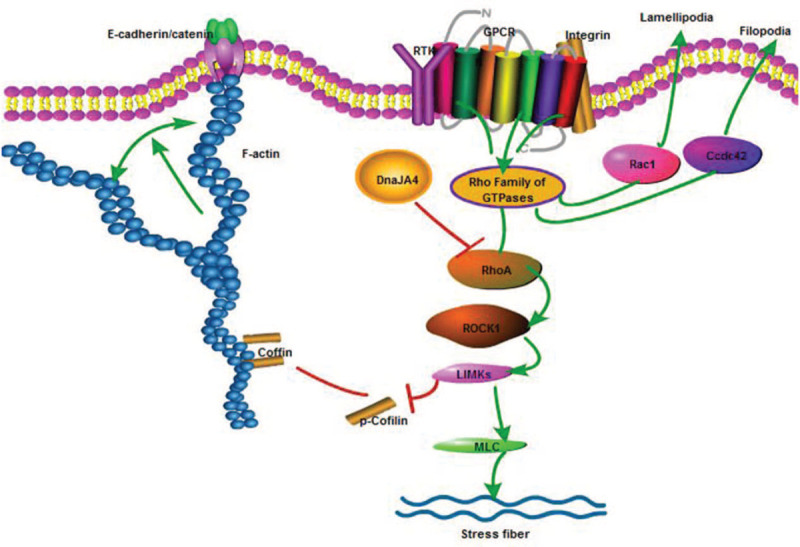
F-actin correlation pathway diagram. RhoA and ROCK1 were regulatory factors upstream of F-actin. The expression of F-actin was regulated by RhoA/ROCK1/LIMK/coffin or MLC pathway. DnaJA4 may inhibit the RhoA/ROCK1 pathway protein expression. GPCR: G-protein-coupled receptor; MLC: Myosin light chain; ROCK: rho-associated serine/threonine kinase.
Our current results support the previous findings of Sun et al who reported that hyperthermia reduced HaCaT-cell proliferation-induced cell senescence and promoted cytokine expressions responsible for anti-viral activity through a nuclear factor kappa-B-dependent pathway. DNAJA4-deficiency enhanced the activation of nuclear factor kappa-B by hyperthermia in HaCaT cells.[14] Yin et al[25] proposed that an over-expression of CRYAB (a subtype of small HSPs) may significantly increase the heat resistance of H9C2 cardiomyocytes by reducing F-actin aggregation, regulating the cell cycle and inhibiting caspase-mediated apoptosis. In this study, DnaJA4-KO increased the aggregation of F-actin in HaCaT cells following hyperthermia. Therefore, DnaJA4 may also involve in hyperthermia response by regulating the expression of F-actin to affect the apoptosis or proliferation of HaCaT cells. We also found that filamentopodia was significantly reduced in DnaJA4-KO cells, while the virus could be transmitted to the cell body along the filamentopodia, leading to cell infection.[26] We wondered whether the reduced filamentopodia caused by DnaJA4 gene deletion might affect the transmission rate of the virus in vivo.
E-cadherin is an essential adhesion molecule on cell surfaces. It participates in intercellular adhesion and signal transduction through the binding of the cadherin/catenin adhesion complex and actin filaments to the cytoskeleton.[27] In this study, although E-cadherin expression was decreased in both WT and DnaJA4-KO cells, their expression were greater in DnaJA4-KO cells. These findings were consistent with results obtained in the immunofluorescent experiment. It has been reported that excessive expression of E-cadherin in epithelial cells inhibits cell migration and proliferation and induces apoptosis, while down-regulation of this adhesion molecule weakens cell connections, stimulates cell regeneration and migration, and facilitates tissue repair, which then contributes to the maintenance of normal functioning within epithelial cells.[28,29] Based upon these findings, we speculated that DnaJA4 may affect the proliferation or apoptosis of HaCaT cells by regulating E-cadherin expression in response to hyperthermia. This hypothesis will require verification with future experiments.
Conclusion
In summary, DnaJA4 was involved with modulating responses to hyperthermia in HaCaT cells by regulating the expression of F-actin and the related pathway proteins. Future work including the establishment of a cell model of DnaJA4 over-expression will be critical for a better understanding of these mechanisms and to address some of the limitations in our current study.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 81673070).
Conflicts of interest
None.
Footnotes
How to cite this article: Liu RJ, Niu XL, Yuan JP, Chen HD, Gao XH, Qi RQ. DnaJA4 is involved in responses to hyperthermia by regulating the expression of F-actin in HaCaT cells. Chin Med J 2021;134:456–462. doi: 10.1097/CM9.0000000000001064
References
- 1.Kuhn S, Mannherz HG. Actin: structure, function, dynamics, and interactions with bacterial toxins. Curr Top Microbiol Immunol 2017; 399:1–34. doi: 10.1007/82_2016_45. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter CL. Actin cytoskeleton and cell signaling. Crit Care Med 2000; 28: 4 Suppl: N94–N99. doi: 10.1097/00003246-200004001-00011. [DOI] [PubMed] [Google Scholar]
- 3.Scipion CPM, Ghoshdastider U, Ferrer FJ, Yuen TY, Wongsantichon J, Robinson RC. Structural evidence for the roles of divalent cations in actin polymerization and activation of ATP hydrolysis. Proc Natl Acad Sci U S A 2018; 115:10345–10350. doi: 10.1073/pnas.1806394115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman SA, Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev 2014; 262:193–215. doi: 10.1111/imr.12212. [DOI] [PubMed] [Google Scholar]
- 5.Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, et al. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol 2006; 36:1782–1793. doi: 10.1002/eji.200535601. [DOI] [PubMed] [Google Scholar]
- 6.Yang M, Fan Z, Wang F, Tian ZH, Ma B, Dong B, et al. BMP-2 enhances the migration and proliferation of hypoxia-induced VSMCs via actin cytoskeleton, CD44 and matrix metalloproteinase linkage. Exp Cell Res 2018; 368:248–257. doi: 10.1016/j.yexcr.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Wong JW, Shi B, Farboud B, McClaren M, Shibamoto T, Cross CE, et al. Ultraviolet B-mediated phosphorylation of the small heat shock protein HSP27 in human keratinocytes. J Invest Dermatol 2000; 115:427–434. doi: 10.1046/j.1523-1747.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 8.Leung AM, Redlak MJ, Miller TA. Role of heat shock proteins in oxygen radical-induced gastric apoptosis. J Surg Res 2015; 193:135–144. doi: 10.1016/j.jss.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Alderson TR, Kim JH, Markley JL. Dynamical structures of Hsp70 and Hsp70-Hsp40 complexes. Structure 2016; 24:1014–1030. doi: 10.1016/j.str.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knox C, Luke GA, Blatch GL, Pesce ER. Heat shock protein 40 (Hsp40) plays a key role in the virus life cycle. Virus Res 2011; 160:15–24. doi: 10.1016/j.virusres.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Maluta S, Kolff MW. Role of hyperthermia in breast cancer locoregional recurrence: a review. Breast Care (Basel) 2015; 10:408–412. doi: 10.1159/000440792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang WH, Xie J, Lai ZY, Yang MD, Zhang GH, Li Y, et al. Radiofrequency deep hyperthermia combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin Med J 2019; 132:922–927. doi: 10.1097/CM9.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo W, Gao XH, Sun XP, Qi RQ, Hong Y, McHepange UO, et al. Local hyperthermia at 44 degrees C for the treatment of plantar warts: a randomized, patient-blinded, placebo-controlled trial. J Infect Dis 2010; 201:1169–1172. doi: 10.1086/651506. [DOI] [PubMed] [Google Scholar]
- 14.Sun YZ, Ren Y, Zhang YJ, Han Y, Yang Y, Gao YL, et al. DNAJA4 deficiency enhances NF-kappa B-related growth arrest induced by hyperthermia in human keratinocytes. J Dermatol Sci 2018; 91:256–267. doi: 10.1016/j.jdermsci.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Jóźwiak Z, Leyko W. Role of membrane components in thermal injury of cells and development of thermotolerance. Int J Radiat Biol 2009; 62:743–756. doi: 10.1080/09553009214552701. [DOI] [PubMed] [Google Scholar]
- 16.Carlier MF, Valentin-Ranc C, Combeau C, Fievez S, Pantoloni D. Actin polymerization: regulation by divalent metal ion and nucleotide binding, ATP hydrolysis and binding of myosin. Adv Exp Med Biol 1994; 358:71–81. doi: 10.1007/978-1-4615-2578-3_7. [DOI] [PubMed] [Google Scholar]
- 17.Luchetti F, Mannello F, Canonico B, Battistelli M, Burattini S, Falcieri E, et al. Integrin and cytoskeleton behaviour in human neuroblastoma cells during hyperthermia-related apoptosis. Apoptosis 2004; 9:635–648. doi: 10.1023/B:APPT.0000038043.03799.6f. [DOI] [PubMed] [Google Scholar]
- 18.Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci 1997; 110:357–368. PMID: 9057088. [DOI] [PubMed] [Google Scholar]
- 19.Janmey PA, Bucki R, Radhakrishnan R. Regulation of actin assembly by PI (4,5)P2 and other inositol phospholipids: an update on possible mechanisms. Biochem Biophys Res Commun 2018; 506:307–314. doi: 10.1016/j.bbrc.2018.07.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci 2011; 124:679–683. doi: 10.1242/jcs.064964. [DOI] [PubMed] [Google Scholar]
- 21.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008; 9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 22.Hoon JL, Tan MH, Koh CG. The regulation of cellular responses to mechanical cues by rho GTPases. Cells 2016; 5:17.doi: 10.3390/cells5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CY, Lou J, Wen KK, McKane M, Eskin SG, Rubenstein PA, et al. Regulation of actin catch-slip bonds with a RhoA-formin module. Sci Rep 2016; 6:35058.doi: 10.1038/srep35058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010; 67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin B, Tang S, Xu J, Sun J, Zhang X, Li Y, et al. CRYAB protects cardiomyocytes against heat stress by preventing caspase-mediated apoptosis and reducing F-actin aggregation. Cell Stress Chaperones 2019; 24:59–68. doi: 10.1007/s12192-018-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang K, Baginski J, Hassan SF, Volin M, Shukla D, Tiwari V. Filopodia and viruses: an analysis of membrane processes in entry mechanisms. Front Microbiol 2016; 7:300.doi: 10.3389/fmicb.2016.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol 2003; 19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 28.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A 2011; 108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008; 68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]


