Dear Editor,
We have recently recognized several new cases of multiple vertebral fractures in patients who temporarily discontinued their denosumab (DMAB) therapy. This phenomenon was first described in 2017 [1], but became less apparent in the last years due to increasing awareness as well as education of patients and primary care physicians. The appearance of increasing cases of rebound-associated fractures drew our attention to a possible relationship to the coronavirus disease 2019 (COVID-19) related lockdowns.
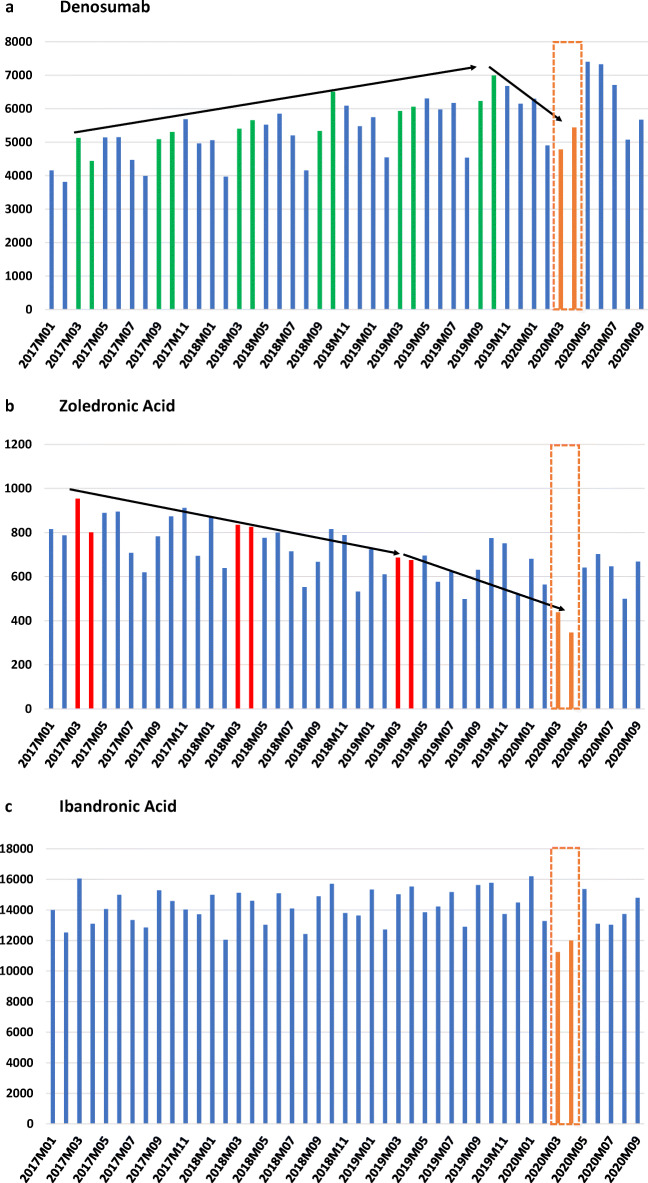
The COVID-19 pandemic was first recognized in February 2020 in Austria, followed by a first extensive lockdown in March and April 2020. In that time, there was limited access to outpatient clinics. We present the national data of the DVSV (Dachverband der Sozialversicherungsträger—umbrella association of the health insurance companies including all social security agencies in Austria). Data are based on filled prescriptions for denosumab, zoledronic acid (ZOL), and ibandronic acid (IBA) covering a period from January 2017 until September 2020. The results showed a continuous increase of DMAB prescriptions over the last 2 years with a remarkable decrease only during the first COVID-19-lockdown in March and April 2020. Thus, a 22 and 23% reduction as compared with 6 months prior for DMAB prescriptions were observed. Moreover, also the number of prescriptions for (ZOL) was significantly lower in the 2 months of the first lockdown compared with 12 months prior (−36 and −49%, respectively). The number of filled prescriptions for intravenous IBA was decreased by 23 and 18% (when compared with the mean number of filled prescriptions of the prior 12 months) (see Fig. 1).
Fig. 1.
Filled drug prescriptions for parenteral osteoporosis therapies during COVID-19 pandemic
Bisphosphonates persist in the body for many years [2] and a delay of treatment for 1 or 2 months does not seem to be unfavorable. In contrast, vertebral fractures were reported as early as 8 months since the last DMAB dose [1, 3], which can be explained by a rebound increase of bone resorption. A higher number of prescriptions after the first lockdown indicates that numerous patients have received their missed DMAB dose to a later timepoint. However, even a delay of 2 months could increase the risk for rebound-associated vertebral fractures. Therefore, and according to the position statement of the ECTS, patients should continue DMAB treatment or should be switched to an alternative agent [4].
In general, the persistence to DMAB was reported to be consistently high and better compared with other anti-osteoporotic agents [5, 6]. However, physicians are required to continue anti-osteoporotic treatment even in times of COVID-19. Especially patients on Denosumab should receive their treatment on time. That also applies to patients in nursing homes and patients on higher risk for COVID-19 infections. As for rheumatological treatments, patients could be instructed about denosumab self-administration [7]. Telemedicine including telephone and video consultation may increase the adherence to osteoporosis treatments [8]. It is currently unclear if the lower rate of DMAB prescriptions is also associated with a higher rate of rebound-associated vertebral fractures. Future studies should investigate the impact of repeated lockdowns on DMAB treatment adherence and clinical outcomes.
Orange boxes indicate first COVID-19 lockdown. Green bars indicate the regular interval (6 months) for Denosumab, red bars indicate the regular interval (12 months) for zoledronic acid. Total number of filled prescriptions are shown on Y-axis, time period on X-axis.
Funding
No funding sources.
Data availability
National Data; DVSV, Dachverband der Sozialversicherungsträger
Declarations
Ethical approval
Not applicable
Conflict of interest
Roland Kocijan, Martina Behanova, Berthold Reichardt, Judith Haschka, Annemarie Kocijan, and Jochen Zwerina declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O. Clinical Features of 24 Patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. 2017;32(6):1291–1296. doi: 10.1002/jbmr.3110. [DOI] [PubMed] [Google Scholar]
- 2.Papapoulos SE, Cremers SC. Prolonged bisphosphonate release after treatment in children. N Engl J Med. 2007;356(10):1075–1076. doi: 10.1056/NEJMc062792. [DOI] [PubMed] [Google Scholar]
- 3.Anastasilakis AD, Evangelatos G, Makras P, Iliopoulos A. Rebound-associated vertebral fractures may occur in sequential time points following denosumab discontinuation: need for prompt treatment re-initiation. Bone Rep. 2020;12:100267. doi: 10.1016/j.bonr.2020.100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC. Discontinuation of Denosumab therapy for osteoporosis: A systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Koller G, Goetz V, Vandermeer B, Homik J, McAlister FA, Kendler D, Ye C. Persistence and adherence to parenteral osteoporosis therapies: a systematic review. Osteoporos Int. 2020;31(11):2093–2102. doi: 10.1007/s00198-020-05507-9. [DOI] [PubMed] [Google Scholar]
- 6.Fahrleitner-Pammer A, Papaioannou N, Gielen E, Feudjo Tepie M, Toffis C, Frieling I, Geusens P, Makras P, Boschitsch E, Callens J, Anastasilakis AD, Niedhart C, Resch H, Kalouche-Khalil L, Hadji P. Factors associated with high 24-month persistence with denosumab: results of a real-world, non-interventional study of women with postmenopausal osteoporosis in Germany, Austria, Greece, and Belgium. Arch Osteoporos. 2017;12(1):58. doi: 10.1007/s11657-017-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal R, Bhadada SK. Managing common endocrine disorders amid COVID-19 pandemic. Diabetes Metab Syndr. 2020;14(5):767–771. doi: 10.1016/j.dsx.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paskins Z, Crawford-Manning F, Bullock L, Jinks C. Identifying and managing osteoporosis before and after COVID-19: rise of the remote consultation? Osteoporos Int. 2020;31(9):1629–1632. doi: 10.1007/s00198-020-05465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
National Data; DVSV, Dachverband der Sozialversicherungsträger