ABSTRACT
Pancreatic metastases from ovarian carcinoma are rare. We present a case of a patient with pancreatic metastasis from primary ovarian carcinoma diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Our case illustrates a unique presentation of a patient with ovarian carcinoma presenting with symptoms secondary to common bile duct dilatation from a pancreatic head mass confirmed through a much less invasive approach. This diagnosis was essential in determining management and prognosis for the patient.
INTRODUCTION
Pancreatic lesions are most often primary pancreatic neoplasms. However, not all masses in the pancreas are of pancreatic origin. Some can represent metastatic disease, most commonly secondary to renal cell carcinoma or lung neoplasms.1–3 Pancreatic metastases from ovarian carcinoma are rare, with only 17 cases cited in the literature.4,5 Unlike most other cancers, ovarian carcinoma is primarily locally invasive, with spread to other reproductive organs such as the uterus and fallopian tubes, liver, spleen, and sigmoid colon. It is important to correctly determine the origin of pancreatic lesions since there are different treatments and prognoses in patients with primary pancreatic cancer compared with metastatic cancer to the pancreas from other primary malignancies, such as ovarian carcinoma. We present a case of a patient with pancreatic metastasis from primary ovarian carcinoma diagnosed by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA).
CASE REPORT
A 62-year-old woman with a medical history of BRCA1 mutation and recurrent serous ovarian carcinoma previously on paclitaxel and bevacizumab infusions presented with 4-day history of epigastric and right upper quadrant pain radiating to right flank, exacerbated by inspiration. The patient's relevant family history was uncertain but included uterine or ovarian cancer in both mother and maternal grandmother. Also of note, the patient had first been diagnosed 7 years before current admission and had been on chemotherapy since diagnosis with 4-month breaks between chemotherapy. Most recently, the patient had her last chemotherapy session 2 months before admission.
On presentation, the patient was afebrile, normotensive, and saturating well on room air. Physical examination was pertinent for abdominal tenderness. Laboratory test results were notable for no leukocytosis, alkaline phosphate 180 U/L, and lactic acid 1.0 mmol/L. Right upper quadrant ultrasound showed mild gallbladder wall thickening, pericholecystic edema, and gallbladder sludge. General surgery was consulted for cholecystectomy and recommended medical management only with an oral trial. The patient was started on intravenous ceftriaxone and metronidazole for presumptive acute cholecystitis.
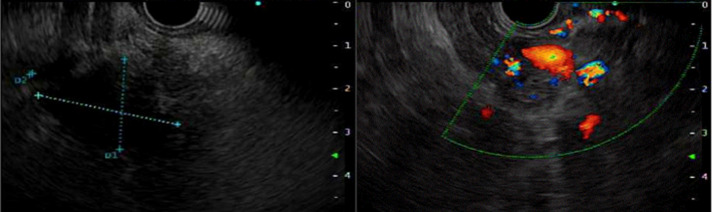
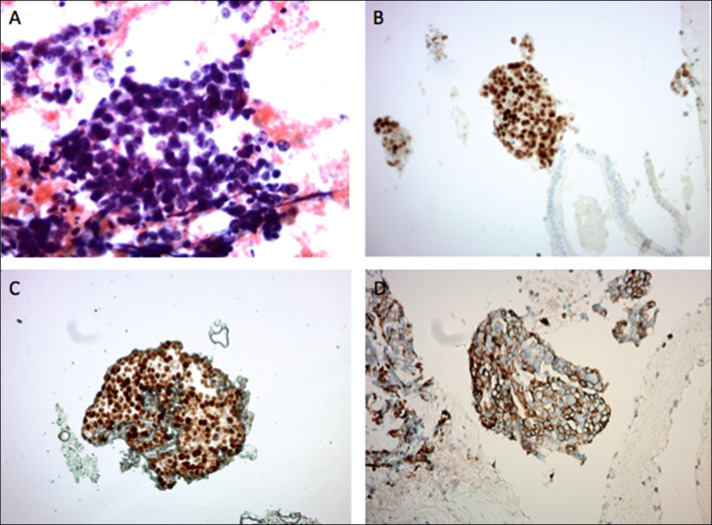
On hospital day 2, her bilirubin increased from 1.6 to 4.1 mg/dL. Magnetic resonance cholangiopancreatography revealed a 1.6-cm hypoenhancing lesion in the uncinate process of the pancreas suspicious for pancreatic neoplasms (Figure 1). Endoscopic ultrasound was performed, and fine-needle aspiration of the lesion in the head of the pancreas was obtained (Figure 2). Four passes were made with a 25-gauge needle using a transduodenal approach with a good visible core of tissue obtained. Rapid onsite evaluation was used. Because of biliary obstruction from the lesion, an endoscopic retrograde cholangiopancreatography was performed with the placement of 2 stents to treat the obstruction of the mass. Since no diagnosis of malignancy was confirmed at the time, a plastic stent was placed in the ventral pancreatic duct and a metal stent was placed in the common bile duct to allow for drainage. A preliminary report was definitive of primary ovarian carcinoma. Final pathology results showed high-grade carcinoma, positive for paired box gene 8 immunostain, diffusely positive for p53 mutant phenotype, positive for CK7, and negative for TTF-1 which supports the diagnosis of recurrence of the patient's diagnosis of metastatic serous carcinoma (Figure 3).
Figure 1.
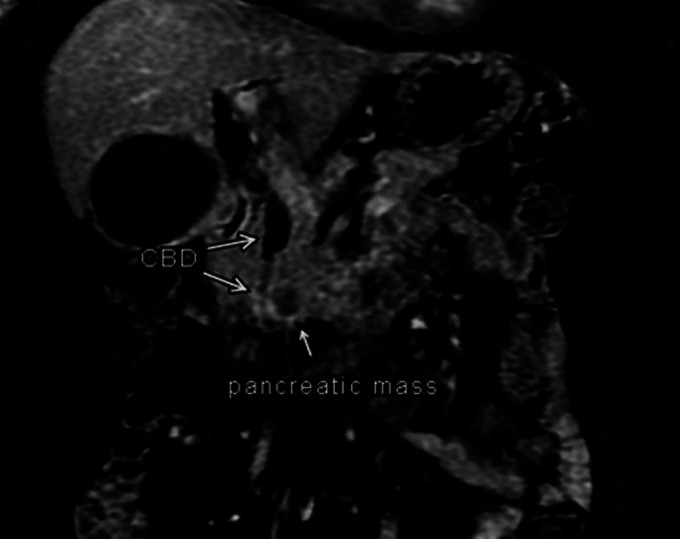
Magnetic resonance cholangiopancreatography revealed a 1.6-cm hypoenhancing lesion in the uncinate process in the head of the pancreas with dilated CBD (11–12 mm at maximum diameter) proximal to mass and compressed CBD distal to pancreas. CBD, common bile duct.
Figure 2.
Endoscopic-ultrasound endoscopy depicting lesion in the pancreas.
Figure 3.
Fine-needle aspiration of pancreatic uncinate process mass. (A) Pap smear showing collections of malignant cells with hyperchromatic nuclei, high N/C ratio, nuclear pleomorphism, and occasional mitotic figures. (B) PAX-8 immunostaining showing diffuse nuclear positivity. (C) P53 immunostaining showing mutant-type diffuse nuclear positivity. (D) CK7 immunostaining showing diffuse membranous positivity. The immunostaining profile supports the diagnosis of metastatic serous carcinoma. Original magnification: A: ×400 magnification; B–D: ×200 magnification.
DISCUSSION
Pancreatic metastases from ovarian carcinoma are rare, but their diagnosis should be considered and confirmed by EUS-FNA in all patients with ovarian cancer who present with new pancreatic masses. Studies of EUS-FNA of pancreatic lesions have found that the majority (60%) of pancreatic lesions are malignant with the primary pancreatic origin. Metastasis to the pancreas ranges from 4.2% to 10.7% of lesions with origins of the neoplasm including kidney, colon, lung, breast, and rarely ovarian.3,6,7 Previous methods of diagnosis in the literature have been invasive. They have included percutaneous biopsy which has been associated with increased risk of seeding, and surgical exploration/resection, which can be associated with significant morbidity.4,8 Percutaneous biopsies have an increased risk of iatrogenic seeding of cancerous cells when compared with EUS-guided FNA.9 EUS-FNA is becoming an increasingly superior technique for biopsy because of its diagnostic efficacy and safety profile.10
Genetic risk factors for ovarian cancer include a family history of BRCA1 and BRCA2 or hereditary nonpolyposis colorectal cancer syndrome.11 Other risk factors include delayed childbearing, early menarche, endometriosis, or estrogen replacement therapy >5 years.11 Our patient has BRCA1 mutation, and it is not documented whether she had other risk factors.
Differentiating primary from secondary malignancy is essential for the management and prognosis of the disease. Preferred management of ovarian cancer involves surgical debulking followed by intravenous platinum/taxane-based chemotherapy, typically with agents such as paclitaxel.12,13 Advanced ovarian carcinoma is defined as an extension to the peritoneum outside of the pelvis with positive retroperitoneal or inguinal lymph nodes and associated 5-year mortality of 20% (stage III) or with distant metastases and associated 5-year mortality of less than 10% (stage IV). Patients with advanced ovarian carcinoma require evaluation with gynecology-oncology to assess the likelihood of ability for cytoreduction to <1 cm. If cytoreduction to <1 cm is unlikely, patients typically require neoadjuvant chemotherapy before surgery. Patients with progressive disease resistant to platinum-based chemotherapy have median progression-free survival of 3–4 months. They are typically not recommended to have surgery with emphasis on end-of-life care.12,13 On the other hand, treatment of BRCA-mutant pancreatic cancer typically starts with chemotherapy. The chemotherapeutic regimen includes gemcitabine plus oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) with surgical resection providing the only chance for cure. The 5-year overall survival rate in patients with pancreatic cancer is 2%–9%.14 Patients with locally advanced pancreatic cancer with inability for surgical resection have 5-year survival rate of 6–11 months, and patients with metastatic pancreatic cancer have 5-year survival rate of 2–6 months.14
Our case illustrates a unique presentation of a patient with ovarian carcinoma presenting with symptoms secondary to common bile duct dilatation from a pancreatic head mass confirmed through a much less invasive approach: EUS-FNA. This diagnosis was essential in determining management and prognosis for the patient.
DISCLOSURES
Author contributions: S. Chan wrote the manuscript. W. Wassef revised the manuscript and is the article guarantor.
Acknowledgments: The authors acknowledge Mohammad Al-Attar and Michelle Yang for providing images for cytology. The authors acknowledge Vivek Pargaonkar and Gabriela Santos-Nunez for providing the radiological images.
Financial disclosure: None to report.
Informed consent could not be obtained from the family of the deceased patient despite several attempts. All identifying information has been removed from this case report to protect patient privacy.
REFERENCES
- 1.Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: An analysis of a surgical and autopsy database and review of the literature. Virchows Arch 2004;444:527–35. [DOI] [PubMed] [Google Scholar]
- 2.Carson HJ, Green LK, Castelli MJ, Reyes CV, Prinz RA, Gattuso P. Utilization of fine-needle biopsy in the diagnosis of metastatic tumors to the pancreas. Diagn Cytopathology 1995;12:8–13. [DOI] [PubMed] [Google Scholar]
- 3.Smith AL, Odronic SI, Springer BS, Reynolds JP. Solid tumor metastases to the pancreas diagnosed by FNA: A single institution experience and review of the literature. Cancer Cytopathology 2015;123(6):347–55. [DOI] [PubMed] [Google Scholar]
- 4.Gunay Y, Demiralay E, Demirag A. Pancreatic metastasis of high-grade papillary serous ovarian carcinoma mimicking primary pancreas cancer: A case report. Case Rep Med 2012;2012:943280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura K, Nakayama K, Ishikawa M, et al. Utility of ovarian biopsy in pancreatic metastasis of high-grade serous ovarian carcinoma: A case report. Mol Clin Oncol 2016;5:41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekulic M, Amin K, Mettler T, Miller LK, Mallery S, Stewart J. Pancreatic involvement by metastasizing neoplasms as determined by endoscopic ultrasound-guided fine needle aspiration: A clinicopathologic characterization. Diagn Cytopathology 2017;45(5):418–25. [DOI] [PubMed] [Google Scholar]
- 7.Fritscher-Ravens A, Sriram PVJ, Krause C, et al. Detection of pancreatic metastasis by EUS-guided fine needle aspiration. Gastrointest Endosc 2001;53(1):65–70. [DOI] [PubMed] [Google Scholar]
- 8.Sparks DA, Chase DM, Forsyth M, Bogen G, Arnott J. Late presentation of a mucinous ovarian adenocarcinoma which was initially diagnosed as a primary pancreatic carcinoma: A case report and review of the literature. J Med Case Rep 2010;4:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs percutaneous FNA. Gastrointest Endosc 2003;58(5):690–3. [DOI] [PubMed] [Google Scholar]
- 10.Mohan BP, Shakhatreh M, Garg R, Ponnada S, Adler DG. Efficacy and safety of EUS-guided liver biopsy: A systematic review of meta-analysis. Gastrointest Endosc 2019;89(2):238–46. [DOI] [PubMed] [Google Scholar]
- 11.Roett MA, Evans P. Ovarian cancer: An overview. Am Fam Physician 2009;80(6):609–16. [PubMed] [Google Scholar]
- 12.Naros S. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol 2016;13:255–61. [DOI] [PubMed] [Google Scholar]
- 13.Orr B, Edwards RP. Diagnosis and treatment of ovarian cancer. Hematol Oncol Clin N Am 2018;32:943–64. [DOI] [PubMed] [Google Scholar]
- 14.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24(43):4846–61. [DOI] [PMC free article] [PubMed] [Google Scholar]