Abstract
Introduction
In an effort to reduce methicillin-resistant Staphylococcus aureus (MRSA) transmission through universal screening and isolation, the Department of Veterans Affairs (VA) launched the National MRSA Prevention Initiative in October 2007. The objective of this analysis was to quantify the budget impact and cost effectiveness of this initiative.
Methods
An economic model was developed using published data on MRSA hospital-acquired infection (HAI) rates in the VA from October 2007 to September 2010, estimates of the costs of MRSA HAIs in the VA, and estimates of the intervention costs, including salaries of staff members hired to support the initiative at each VA facility. To estimate the rate of MRSA HAIs that would have occurred if the initiative had not been implemented, two different assumptions were made: no change and a downward temporal trend. Effectiveness was measured in life-years gained.
Results
The initiative resulted in an estimated 1,466–2,176 fewer MRSA HAIs. The initiative itself was estimated to cost $207 million over this 3-year period while the cost savings from prevented MRSA HAIs ranged from $27–75 million. The incremental cost-effectiveness ratios ranged from $28,048 to $56,944/life-years. The overall impact on the VA’s budget was $131–$179 million.
Conclusions
Wide-scale implementation of a national MRSA surveillance and prevention strategy in VA inpatient settings may have prevented a substantial number of MRSA HAIs. Although the savings associated with prevented infections helped offset some but not all of the cost of the initiative, this model indicated that the initiative would be considered cost effective.
Introduction
Staphylococcus aureus, a bacterium carried in the nares of up to 40% of healthy individuals, can cause a wide range of clinically significant infections.1–3 Among adults colonized with methicillin-resistant S. aureus (MRSA), a substantial proportion (18%–33%) will go on to develop infections such as pneumonia, soft tissue infections, or bloodstream infections.4–8 Though MRSA infections are a significant contributor to morbidity, mortality, and healthcare utilization in the U.S.,9 the observed incidence in hospital settings has decreased steadily since 2005.10,11 This decline may be due to increased attention to infection prevention.
In October 2007, in an effort to reduce transmission of MRSA in hospitals, the Department of Veterans Affairs (VA) implemented the MRSA Prevention Initiative.12 This initiative consisted of a bundle that included:
universal nasal surveillance for MRSA;
contact precautions for patients whose nasal test for MRSA was positive;
improved hand hygiene efforts; and
an increased emphasis on infection control being the responsibility of all healthcare workers.
Several recently published studies have shown that MRSA hospital-acquired infections (HAIs) decreased significantly after the implementation of the initiative.12,13
Cost-effectiveness analysis (CEA) is a common analytic tool used to evaluate the costs and clinical benefits of two or more strategies. Several CEAs have been published demonstrating that that the MRSA Prevention Initiative components are cost saving or cost effective, including universal nasal surveillance, contact precautions, and improved hand hygiene.14 Budget impact analyses (BIAs), on the other hand, are complementary to but slightly different from CEAs. Whereas the purpose of a CEA is to examine the trade-off between costs and benefits at a per-patient level, BIAs are designed to examine the expected expenditures a healthcare system might face after implementation of a new intervention.15 The objective of this study was to conduct both a BIA and CEA of the VA MRSA Prevention Initiative.
Methods
Budget Impact Model
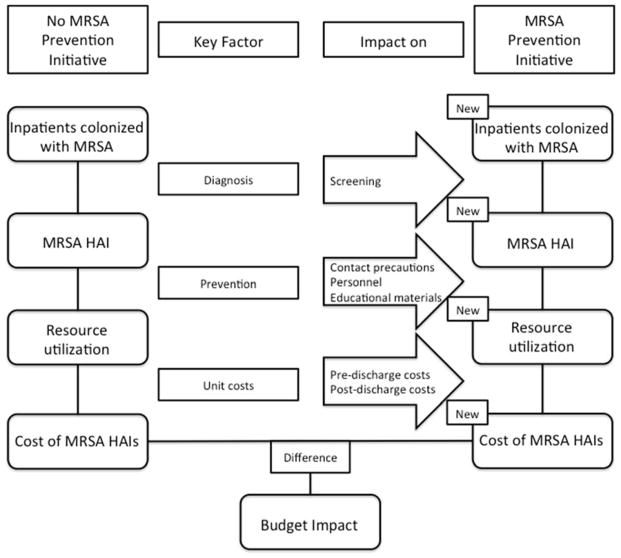
The budget impact model compared the observed rate of MRSA HAIs that occurred in the VA nationwide after the implementation of the initiative with the estimated rate of MRSA HAIs that would have occurred if the initiative had not been implemented (Appendix Figure 1). The expected rate of MRSA HAIs in the absence of the intervention was estimated under two possible scenarios. First, it was assumed that the MRSA HAI rate would have remained flat (straight line). Second, there is evidence to suggest that the rate of MRSA HAIs was decreasing across the U.S. leading up to the MRSA Prevention Initiative and that it continued to decline after its implementation.10 Therefore, it was also assumed that, without the intervention, the MRSA HAI rate in the VA would have decreased at the same rate as it did outside the VA.
Appendix Figure 1.
Schematic representation of budget impact analysis.
Both the observed rate of MRSA HAIs and the hypothetical, counterfactual rates were applied to the 1,746,690 admissions that occurred in the 153 VA hospitals between October 2007 and September 2010 to generate estimates of the total number of MRSA HAIs under each scenario. The difference between the counterfactual number of MRSA HAIs and the observed number of MRSA HAIs was the estimated number of infections prevented because of the initiative. Estimates of the cost of MRSA HAIs in the VA were then applied to the counts of prevented infections to generate aggregate cost savings due to prevented infections. The final budget impact calculations consisted of comparing the cost savings from the initiative with the estimated costs of implementing the initiative.
Cost-Effectiveness Analysis
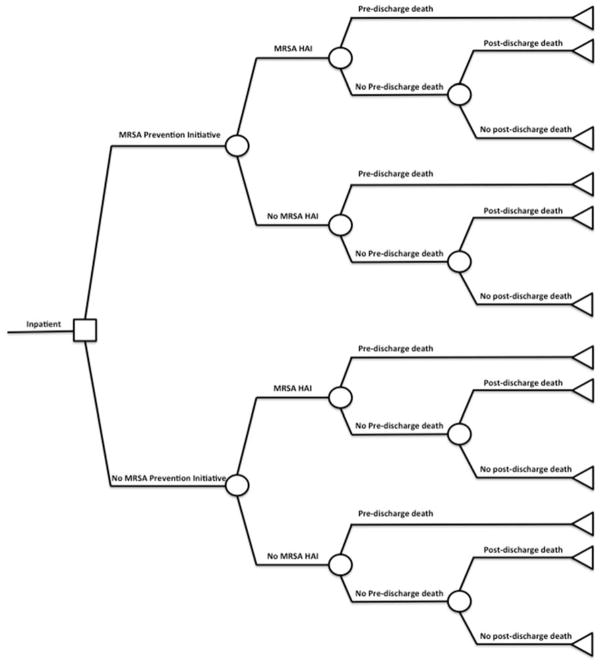
A decision analytic model (Appendix Figure 2) was constructed using TreeAgePro 2013. In addition to the cost estimates generated from the budget impact model, the effectiveness outcome in the CEA was life-years (LYs) gained. The costs and LYs gained were combined to construct incremental cost-effectiveness ratios (ICERs), a commonly used metric in CEAs, which was calculated by taking the ratio of the difference in costs and the difference in LYs between the initiative and the no initiative scenarios. In this analysis, the ICER measures the amount of money spent by the VA for the MRSA Prevention Initiative for each additional year of patient life that resulted from the initiative. Finally, a probabilistic sensitivity analysis was conducted by performing 10,000 Monte Carlo simulations. Cost parameters were assumed to have gamma distributions and probability parameters were assumed to have beta distributions.
Appendix Figure 2.
Diagram of decision analytic model used for cost-effectiveness analysis.
Data
The source for observable rates of MRSA HAIs in the VA was a 2011 paper by Jain et al.12 That study documented the decline in MRSA HAI rates using data on infections entered into an electronic database maintained by the VA Inpatient Evaluation Center by the MRSA prevention coordinator at each VA facility. In that study, HAIs were reported separately by intensive care unit (ICU) or non-ICU. In the current study, it was assumed that 10.9% of patients admitted to a VA hospital are admitted to the ICU.16
In two recent studies, the authors estimated the attributable cost of MRSA HAIs from the perspective of the VA (Table 1). The first paper estimated the excess cost incurred prior to discharge from the hospital17 and the second estimated the readmission and pharmacy costs attributable to an MRSA HAI during the 1-year period following discharge from the hospital.18 In both instances, inpatient costs were separated into fixed and variable costs, a distinction that is important when attempting to estimate the expenses that could be saved by preventing HAIs.19 Fixed costs are those that are associated with long-term obligations and are difficult to change in the short run. Variable costs can be avoided in the short run and therefore represent expenditures that could be saved if an HAI is prevented.
Table 1.
Input Parameters for Budget Impact Analysis Model
| Input | Value | Source |
|---|---|---|
| Admission-related parameters | ||
| Number of admissions/year in VA | 582,230 | Jain (2011) |
| Proportion of VA inpatients admitted to ICU | 0.109 | Chen (2012) |
| ICU patient-days per month | 39,783 | Jain (2011) |
| Non-ICU patient-days per month | 212,298 | Jain (2011) |
|
| ||
| MRSA screening tests | ||
| Number performed on admission | ||
| FY2008 | 585,200 | Jain (2011) |
| FY2009 | 637,500 | Jain (2011) |
| FY2010 | 644,500 | Jain (2011) |
| Number performed on transfer or discharge | ||
| FY2008 | 447,500 | Jain (2011) |
| FY2009 | 506,500 | Jain (2011) |
| FY2010 | 507,500 | Jain (2011) |
|
| ||
| HAI MRSA rates (per 1,000 patient days) | ||
| ICU, baseline | 1.64 | Jain (2011) |
| Non-ICU, baseline | 0.46 | Jain (2011) |
| Monthly change if no initiative, downward trend assumption | -0.8% | Dantes (2013) |
|
| ||
| Costs | ||
| MRSA HAI | ||
| Pre-discharge variable | $12,272 | Nelson (2015) |
| Pre-discharge total | $24,015 | Nelson (2015) |
| Post-discharge inpatient variable | $5,826 | Nelson (2015) |
| Post-discharge inpatient total | $11,044 | Nelson (2015) |
| Post-discharge pharmacy | $710 | Nelson (2015) |
| Cost of initiative - variable | ||
| Screening test | $25 | Clancy (2006), McKinnell (2015) |
| Gloves | 0.07 | Nelson (2010) |
| Gown | 0.80 | Nelson (2010) |
| Time to don gloves and gown (min) | 2 | Kang (2012) |
| Number of visits by nurse per day – non-ICU | 20 | Morgan (2013), Cohen (2012), McArdle (2006) |
| Number of visits by doctor per day – non-ICU | 4 | Morgan (2013), Cohen (2012), McArdle (2006) |
| Cost of initiative - fixed (per facility) | ||
| MRSA Prevention Coordinator | ||
| Salary + benefits | $89,679 | BLS |
| Lab tech | ||
| Salary + benefits | $65,503 | BLS |
| Proportion used | 0.5 | VA MRSA Initiative |
| Educational materials (per facility) | ||
| FY 2007 | $5,618 | VA MRSA Initiative |
| FY 2008 | $1,082 | VA MRSA Initiative |
| FY 2009 | $1,086 | VA MRSA Initiative |
| FY 2010 | $1,068 | VA MRSA Initiative |
|
| ||
| Effectiveness outcome (life years gained) | ||
| Average age of patients | 50 | Assumption |
| Average life expectancy | 78.8 | CDC FastStats |
| Mortality | ||
| Probability of pre-discharge death attributable to MRSA HAI | 0.101 | Internal VA data |
| Probability of post-discharge death | 0.0042 | U.S. Social Security Administration |
| Post-discharge hazard ratio for death attributable to MRSA HAI | 1.46 | Nelson (2015) |
VA, Department of Veterans Affairs; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; HAI, healthcare-associated infection
Estimates of the costs of the initiative included those associated with screening all admitted patients, the use of gloves and gowns for patients placed on contact precautions, and salaries of an MRSA prevention coordinator and 50% of a laboratory technician for each facility. The role of the MRSA prevention coordinator was to manage the implementation of the initiative, collect data, assist healthcare providers, and develop strategies for overcoming any challenges that arose. It was assumed that this position was filled by a registered nurse and the estimated salary was obtained from the U.S. Bureau of Labor Statistics. Polymerase chain reaction tests for MRSA were assumed to cost $25 per test.20,21 Gloves and gowns cost $0.07 and $0.80 each,22 respectively, and patients on contact precautions were visited by nurses 20 times and by doctors four times per day.23–25 In addition to the cost of the supplies, the cost of the time required to don the gloves and gowns on each use was also included (2 minutes).26 Finally, the model included the cost of educational materials such as literature on the importance of hand hygiene and contact precautions in reducing transmission of MRSA in the hospital.
For the CEA, the average age of a patient admitted to a VA hospital was assumed to be 50 years and the life expectancy of these individuals was assumed to be 78.8 years.27 Outcomes occurring in future time periods but related to the initial hospital stay were discounted at a rate of 3%. Therefore, patients who did not die from MRSA HAIs were assumed to gain 20.2 discounted LYs. The absolute risk of pre-discharge mortality in patients with MRSA HAI was assumed to be 10.1%. From a previously published study, the authors found a hazard ratio of 1.46 associated with mortality in patients with MRSA compared with those without MRSA HAI during the 1-year post-discharge period.28 The 1-year probability of death for individuals aged 50 years (0.0042) was obtained from the actuarial tables from the U.S. Social Security Administration. This probability was converted into a rate, multiplied by the death hazard ratio for patients with MRSA HAI compared with patients without MRSA HAI, then converted back to a probability to obtain the 1-year post-discharge probability of death for patients with MRSA HAI. Because all costs associated with the initial hospital stay were assumed to occur in the first year, costs were not discounted. Costs were converted to 2013 U.S. dollars.
Results
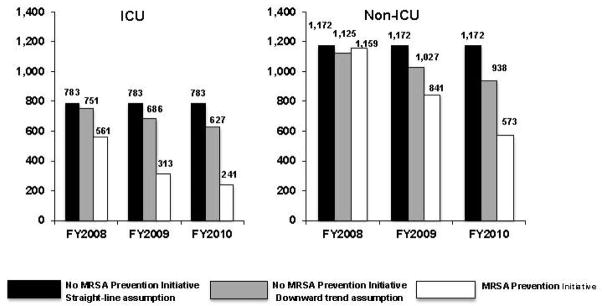
Figure 1 depicts the number of MRSA HAIs calculated based on rates reported in 2011 by Jain and colleagues12 during fiscal year 2008–2010 as well as the hypothetical number of HAIs that would have occurred in non-ICU and ICU settings had the MRSA Prevention Initiative not been implemented. Under the straight-line assumption of the rate of MRSA HAIs, the initiative resulted in an estimated 943 fewer non-ICU MRSA HAIs and 1,234 fewer MRSA HAIs in the ICU over the 3-year time period. This equates to an absolute risk reduction of 0.12 and 0.86 MRSA HAIs per 1,000 patient-days, respectively. If the rate of MRSA HAIs had taken a downward trend as seen elsewhere in the U.S., the initiative would have led to approximately 517 fewer non-ICU MRSA HAIs (absolute risk reduction, 0.07 per 1,000 patient-days) and 949 fewer MRSA HAIs in the ICU (absolute risk reduction, 0.66 per 1,000 patient-days).
Figure 1.
Number of MRSA HAIs with and without the VA MRSA Prevention Initiative.
ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; VA, Department of Veterans Affairs
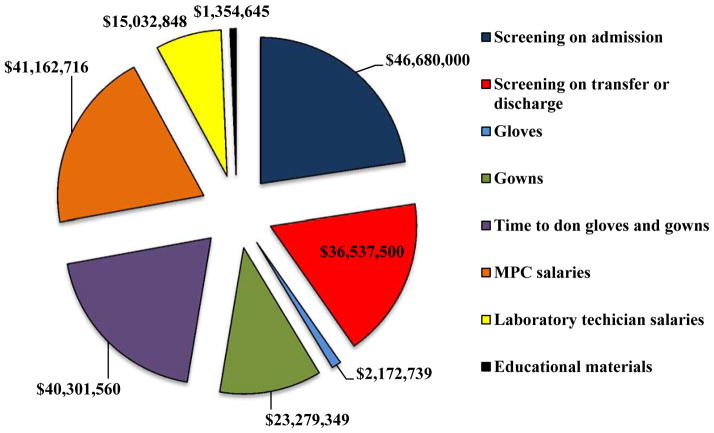
The estimated total costs of the initiative are depicted in Appendix Figure 3. More than one third of the $206.5 million costs of the initiative ($83.2 million) were due to screening of patients on hospital admission, ward transfer, or discharge from the facility. Salaries for the MRSA prevention coordinators assigned to each hospital accounted for 20% ($41.2 million) and laboratory technicians accounted for 7% ($15.0 million) of the costs of the initiative. Including the MRSA prevention coordinator and laboratory technician salaries into the cost calculations is one of the unique aspects of this study.
Appendix Figure 3.
Pie chart of VA MRSA Prevention Initiative expenses – FY2008–FY2010.
The cost savings due to MRSA HAIs prevented as a result of the initiative depended on the assumptions of the number of MRSA HAIs that would have occurred without the initiative. The overall cost savings were $75.3 million and $50.7 million for total costs and $40.1 million and $27.0 million for variable costs under the assumption of a straight-line and downward trend in MRSA HAIs, respectively (Table 2).
Table 2.
Aggregate Cost Savings Due to VA MRSA Prevention Initiative
| Pre-discharge costs | Post-discharge costs | Overall costs | |||||
|---|---|---|---|---|---|---|---|
| Year | Total | Variable | Inpatient total | Inpatient variable | Pharmacy | Total | Variable |
| Straight line assumption | |||||||
| FY2008 | $5,636,973 | $2,947,939 | $2,330,502 | $1,229,401 | $149,824 | $8,117,300 | $4,327,164 |
| FY2009 | $19,227,002 | $10,055,045 | $7,949,047 | $4,193,331 | $511,031 | $27,687,080 | $14,759,407 |
| FY2010 | $27,399,269 | $14,328,854 | $11,327,719 | $5,975,669 | $728,240 | $39,455,229 | $21,032,763 |
| Total | $52,263,245 | $27,331,838 | $21,607,268 | $11,398,401 | $1,389,095 | $75,259,608 | $40,119,334 |
| Downward trend assumption | |||||||
| FY2008 | $3,741,700 | $1,956,777 | $1,546,936 | $816,050 | $99,450 | $5,388,086 | $2,872,277 |
| FY2009 | $13,427,428 | $7,022,072 | $5,551,321 | $2,928,467 | $356,885 | $19,335,633 | $10,307,425 |
| FY2010 | $18,033,768 | $9,431,026 | $7,455,727 | $3,933,092 | $479,316 | $25,968,810 | $13,843,434 |
| Total | $35,202,895 | $18,409,876 | $14,553,984 | $7,677,609 | $935,651 | $50,692,530 | $27,023,135 |
VA, Department of Veterans Affairs; MRSA, methicillin-resistant Staphylococcus aureus; FY, fiscal year
The overall budget impact of the VA’s MRSA Prevention Initiative is shown in Table 3. When focusing on variable costs, the model indicated that the initiative cost the VA $166.4 million over the 3-year period if the rate of MRSA HAIs had remained flat without the initiative and $179.5 million if the rate of MRSA HAIs had shown a downward trend over time. For total costs, the cost was $131.3 million and $155.8 million under these assumptions.
Table 3.
Budget Impact and Cost-Effectiveness Analysis Results of VA MRSA Prevention Initiative
| Budget Impact Analysis | Cost-Effectiveness Analysis | ||||
|---|---|---|---|---|---|
|
| |||||
| Year | Difference
|
Incremental LYs gained | ICER
|
||
| Total cost | Variable cost | Total Cost | Variable Cost | ||
| Straight line assumption | |||||
|
| |||||
| FY2008 | −$57,847,528 | −$61,637,664 | 504.8 | $114,605 | $122,114 |
| FY2009 | −$42,286,440 | −$55,214,112 | 1,721.7 | $24,561 | $32,070 |
| FY2010 | −$31,127,782 | −$49,550,248 | 2,453.4 | $12,687 | $20,196 |
| Total | −$131,261,750 | −$166,402,024 | 4,679.8 | $28,048 | $35,557 |
| Downward trend assumption | |||||
|
| |||||
| FY2008 | −$60,576,742 | −$63,092,551 | 335.0 | $180,801 | $188,310 |
| FY2009 | −$50,637,886 | −$59,666,094 | 1,202.3 | $42,116 | $49,625 |
| FY2010 | −$44,614,200 | −$56,739,577 | 1,614.8 | $27,628 | $35,137 |
| Total | −$155,828,828 | −$179,498,223 | 3,152.2 | $49,435 | $56,944 |
VA, Department of Veterans Affairs; MRSA, methicillin-resistant Staphylococcus aureus
Note: LY, life-year; ICER, incremental cost-effectiveness ratio, interpreted as the expenses toward the VA MRSA Prevention Initiative required to yield an additional year of patient life
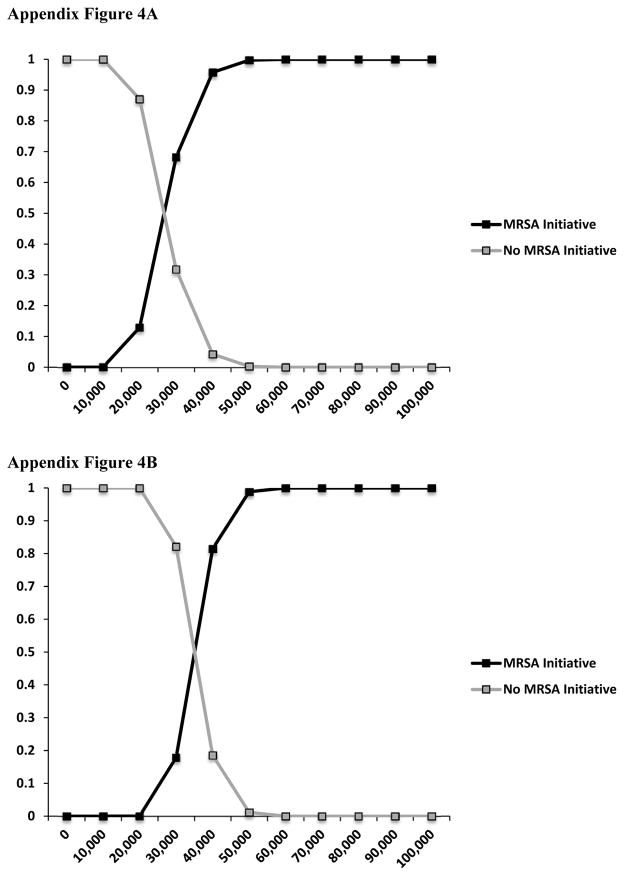
Table 3 also shows the results from the CEA. The ICER ranged from $28,048/LY to $49,435/LY when considering total costs and $35,557/LY to $56,944/LY when considering variable costs only across all 3 years. Probabilistic sensitivity analyses are presented as cost-effectiveness acceptability curves in Appendix Figures 4A and 4B for the straight-line and downward trend assumptions, respectively.
Appendix Figure 4.
Appendix Figure 4A Cost-effectiveness acceptability curve – straight-line assumption.
Appendix Figure 4B. Cost-effectiveness acceptability curve – downward trend assumption.
Discussion
Using published estimates of the MRSA HAIs rates after the VA’s MRSA Prevention Initiative, per-patient cost of MRSA HAIs in the VA, cost of the initiative, and a range of estimates of what the rate of MRSA HAIs would have been had the initiative not been implemented, this analysis presents the impact of the initiative on the VA’s budget as well as the cost effectiveness of this intervention. Using several different assumptions of the number of MRSA HAIs that occurred and would have occurred over this 3-year period without the initiative, the initiative resulted in 1,466–2,176 fewer MRSA HAIs.
Historically, the threshold for cost effectiveness has been considered $50,000 per quality-adjusted LY, a metric similar to the LY metric used in this study but that reflects morbidity as well as mortality. Incorporating the initiative’s cost as well as the costs saved by preventing MRSA HAIs, the model yielded ICERs within or close to this threshold. Of course, it is important to note that this analysis was done from the perspective of the VA healthcare system and does not incorporate other perspectives, including the patient perspective. Future studies that extend this work by examining the patient or societal perspectives would be valuable.
The results from this model can be useful to VA decision makers as a way to evaluate a program that was implemented nationwide in the largest integrated healthcare system in the U.S. BIAs are particularly useful in integrated healthcare systems like the VA where short-term financial consequences can be weighed against long-term clinical outcomes among both providers and payers of health care. In addition, despite the use of mostly VA-specific input parameters, these results can be useful to decision makers in other healthcare systems who are considering adopting a similar strategy of universal surveillance for MRSA in an inpatient setting.
Although the purpose of the current analysis was to evaluate the universal surveillance strategy that was implemented in the VA healthcare system, alternative strategies in which only a subset of the patients admitted to a hospital are screened may be more efficient in terms of healthcare resources.29 Examples of these strategies include screening only ICU patients26 or those with other risk factors such as a high number of previous healthcare encounters, prior MRSA colonization, and previous antibiotic therapy.30 Future economic evaluations should compare universal and targeted surveillance strategies.
The distinction between total and variable costs is important. Total costs are made up of fixed costs and variable costs. Fixed costs in health care are those that must be paid regardless of how many patients are treated or in what manner they are treated. Variable costs, on the other hand, are those that could be avoided if infections are prevented. In this paper, both the total and variable costs of the MRSA HAIs that were prevented by the VA MRSA Prevention Initiative are reported. The variable cost results are certainly relevant because they represent the true cost savings for the VA for HAIs prevented in the short run. The total costs results are reported because all costs are variable over a long enough time horizon. Therefore, the results that include the total cost of MRSA HAIs are relevant for long-term decision making because they include all costs that could be saved in the long run due to reduction of MRSA HAIs. Though the variable cost estimates from the budget impact model are positive, ranging from $166.4 million to $179.5 million per year, they represent a small fraction of the VA’s annual budget for medical care, which was $47.4 billion in 2010.
This analysis did not include the opportunity cost of lost bed-days, an important measure of the economic impact of HAIs.19 This opportunity cost essentially amounts to the value of alternative uses of the hospital beds that are not possible when they are occupied by patients with HAIs. Although a recent study used contingent valuation methods to generate estimates of the value of these bed-days from the perspective of administrators of European hospitals, no such estimates exist for the VA.31
Though this is the first BIA of a universal surveillance strategy to detect and isolate patients with MRSA colonization to reduce MRSA transmission, several previous studies have examined the cost effectiveness of this intervention. Using a decision analytic model, the authors previously compared the cost effectiveness of universal surveillance and universal surveillance plus decolonization with a topical antibiotic with no surveillance.22 Universal surveillance was found to be both more effective and less costly than no surveillance. This is a slightly different result from what was found in the current analysis (i.e., that universal surveillance results in an overall increase in costs). The reason for this is that the previous analysis did not include the cost of MRSA prevention coordinators and did not include the cost of testing on transfer and discharge. A subsequent analysis reported by Kang et al.26 in 2012 found that universal surveillance was more costly than no surveillance but that this increased cost resulted in sufficiently fewer MRSA HAIs to result in cost effectiveness.
Limitations
This study had several limitations. First, assumptions were made regarding the number MRSA HAIs that would have occurred if the intervention had not been implemented. There is no way of knowing what the rate of HAIs would have been in the absence of the initiative, but several different assumptions were explored in order to present a range of budget impact and cost-effectiveness estimates. Second, most of the inputs to the model were generated using VA data. Therefore, these results may not be as applicable in other healthcare systems. For example, unlike private and non-profit healthcare systems, which are financed through care provided and paid for by patients or by third-party payers, the VA is funded through annual congressional appropriations. The costs saved from prevention of HAIs have different ramifications based on these different financial models. In the case of the VA, this means less expenditure on care provided now which impacts budget requests in the future. For non–government funded hospitals, fewer HAIs can affect reimbursements and, in turn, profits. These different financial incentives may lead to different input parameters in each step of the economic models developed here from the effect of the intervention to the cost saved by each HAI prevented. However, the extensive use of VA data to parameterize the model, thus using context-specific costs and consequences in order to evaluate an initiative that was implemented within the VA system, is also a strength of this study. Third, the model focused solely on MRSA HAI prevention and costs whereas the infection control interventions that comprised the MRSA Prevention Initiative may have reduced transmission of many other pathogens that lead to HAIs, such as vancomycin-resistant enterococcus and Clostridium difficile.12 However, further studies are necessary to examine the economic impact of the VA MRSA Initiative on other pathogens.
Conclusions
Preventing MRSA HAI can improve survival and reduce costs among hospitalized patients. However, these prevention efforts come at a cost and the overall budget impact and cost effectiveness of the intervention depends on how many infections can be expected to be prevented. Using a model that explored several different assumptions for the rate of MRSA HAIs that would have occurred in the VA if the MRSA Prevention Initiative had not been implemented, the initiative was found to be cost effective.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs, CDC, or the U.S. government. The contents of this article were presented at the Third International Conference on Prevention & Infection Control in June 2015 in Geneva, Switzerland and the Department of Veterans Affairs Health Services Research and Development Conference in Philadelphia, Pennsylvania in July 2015. The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development Service (CDA 11-210) with Dr. Nelson as the principal investigator. REN, MJ, MHS, MEE, MLS, ELP, and MAR are employees of the Department of Veterans Affairs.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.Choi CS, Yin CS, Bakar AA, et al. Nasal carriage of Staphylococcus aureus among healthy adults. J Microbiol Immunol Infect. 2006 Dec;39(6):458–464. [PubMed] [Google Scholar]
- 2.Kluytmans JA, Mouton JW, Ijzerman EP, et al. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis. 1995 Jan;171(1):216–219. doi: 10.1093/infdis/171.1.216. [DOI] [PubMed] [Google Scholar]
- 3.Williams RE. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963 Mar;27:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datta R, Huang SS. Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis. 2008 Jul 15;47(2):176–181. doi: 10.1086/589241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freitas EA, Harris RM, Blake RK, Salgado CD. Prevalence of USA300 strain type of methicillin-resistant Staphylococcus aureus among patients with nasal colonization identified with active surveillance. Infect Control Hosp Epidemiol. 2010 May;31(5):469–475. doi: 10.1086/651672. [DOI] [PubMed] [Google Scholar]
- 6.Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol. 2001 Nov;22(11):687–692. doi: 10.1086/501846. [DOI] [PubMed] [Google Scholar]
- 7.Huang SS, Hinrichsen VL, Datta R, et al. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PloS one. 2011;6(9):e24340. doi: 10.1371/journal.pone.0024340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003 Feb 1;36(3):281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 9.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013 Dec 9–23;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 10.Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013 Nov 25;173(21):1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA. 2012 Jul 4;308(1):50–59. doi: 10.1001/jama.2012.7139. [DOI] [PubMed] [Google Scholar]
- 12.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011 Apr 14;364(15):1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 13.Jones M, Ying J, Huttner B, et al. Relationships between the importation, transmission, and nosocomial infections of methicillin-resistant Staphylococcus aureus: an observational study of 112 Veterans Affairs Medical Centers. Clin Infect Dis. 2014 Jan;58(1):32–39. doi: 10.1093/cid/cit668. [DOI] [PubMed] [Google Scholar]
- 14.Farbman L, Avni T, Rubinovitch B, Leibovici L, Paul M. Cost-benefit of infection control interventions targeting methicillin-resistant Staphylococcus aureus in hospitals: systematic review. Clin Microbiol Infect. 2013 Dec;19(12):E582–593. doi: 10.1111/1469-0691.12280. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014 Jan-Feb;17(1):5–14. doi: 10.1016/j.jval.2013.08.2291. [DOI] [PubMed] [Google Scholar]
- 16.Chen LM, Render M, Sales A, Kennedy EH, Wiitala W, Hofer TP. Intensive care unit admitting patterns in the Veterans Affairs health care system. Arch Intern Med. 2012 Sep 10;172(16):1220–1226. doi: 10.1001/archinternmed.2012.2606. [DOI] [PubMed] [Google Scholar]
- 17.Nelson RE, Samore MH, Jones M, et al. Reducing time-dependent bias in estimates of the attributable cost of healthcare-associated methicillin-resistant Staphylococcus aureus infections: a comparison of three estimation strategies. Med Care. 2015 doi: 10.1097/MLR.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 18.Nelson RE, Jones M, Liu CF, et al. The impact of healthcare-associated methicillin-resistant Staphylococcus aureus infections on post-discharge healthcare costs and utilization. Infect Control Hosp Epidemiol. 2015 May;36(5):534–542. doi: 10.1017/ice.2015.22. [DOI] [PubMed] [Google Scholar]
- 19.Graves N, Harbarth S, Beyersmann J, Barnett A, Halton K, Cooper B. Estimating the cost of health care-associated infections: mind your p’s and q’s. Clin Infect Dis. 2010 Apr 1;50(7):1017–1021. doi: 10.1086/651110. [DOI] [PubMed] [Google Scholar]
- 20.Clancy M, Graepler A, Wilson M, Douglas I, Johnson J, Price CS. Active screening in high-risk units is an effective and cost-avoidant method to reduce the rate of methicillin-resistant Staphylococcus aureus infection in the hospital. Infect Control Hosp Epidemiol. 2006 Oct;27(10):1009–1017. doi: 10.1086/507915. [DOI] [PubMed] [Google Scholar]
- 21.McKinnell JA, Bartsch SM, Lee BY, Huang SS, Miller LG. Cost-benefit analysis from the hospital perspective of universal active screening followed by contact precautions for methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2015 Jan;36(1):2–13. doi: 10.1017/ice.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson RE, Samore MH, Smith KJ, Harbarth S, Rubin MA. Cost-effectiveness of adding decolonization to a surveillance strategy of screening and isolation for methicillin-resistant Staphylococcus aureus carriers. Clin Microbiol Infect. 2010 Dec;16(12):1740–1746. doi: 10.1111/j.1469-0691.2010.03324.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen B, Hyman S, Rosenberg L, Larson E. Frequency of patient contact with health care personnel and visitors: implications for infection prevention. Jt Comm J Qual Patient Saf. 2012 Dec;38(12):560–565. doi: 10.1016/s1553-7250(12)38073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McArdle FI, Lee RJ, Gibb AP, Walsh TS. How much time is needed for hand hygiene in intensive care? A prospective trained observer study of rates of contact between healthcare workers and intensive care patients. J Hosp Infect. 2006 Mar;62(3):304–310. doi: 10.1016/j.jhin.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Morgan DJ, Pineles L, Shardell M, et al. The effect of contact precautions on healthcare worker activity in acute care hospitals. Infect Control Hosp Epidemiol. 2013 Jan;34(1):69–73. doi: 10.1086/668775. [DOI] [PubMed] [Google Scholar]
- 26.Kang J, Mandsager P, Biddle AK, Weber DJ. Cost-effectiveness analysis of active surveillance screening for methicillin-resistant Staphylococcus aureus in an academic hospital setting. Infect Control Hosp Epidemiol. 2012 May;33(5):477–486. doi: 10.1086/665315. [DOI] [PubMed] [Google Scholar]
- 27.Life Expectancy. [Accessed April 20, 2015];Centers for Disease Control and Prevention, FastStats. http://www.cdc.gov/nchs/fastats/life-expectancy.htm.
- 28.Nelson RE, Stevens VW, Jones M, Samore MH, Rubin MA. Health care-associated methicillin-resistant Staphylococcus aureus infections increases the risk of postdischarge mortality. Am J Infect Control. 2015 Jan;43(1):38–43. doi: 10.1016/j.ajic.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med. 2006 Mar 13;166(5):580–585. doi: 10.1001/archinte.166.5.580. [DOI] [PubMed] [Google Scholar]
- 30.Pasricha J, Harbarth S, Koessler T, et al. Methicillin-resistant Staphylococcus aureus risk profiling: who are we missing? Antimicrobial resistance and infection control. 2013;2(1):17. doi: 10.1186/2047-2994-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewardson AJ, Harbarth S, Graves N, Group TS. Valuation of hospital bed-days released by infection control programs: a comparison of methods. Infect Control Hosp Epidemiol. 2014 Oct;35(10):1294–1297. doi: 10.1086/678063. [DOI] [PubMed] [Google Scholar]