Abstract
With the arrival of the internet of things, smart environments are becoming increasingly ubiquitous in our everyday lives. Sensor data collected from smart home environments can provide unobtrusive, longitudinal time series data that are representative of the smart home resident’s routine behavior and how this behavior changes over time. When longitudinal behavioral data are available from multiple smart home residents, differences between groups of subjects can be investigated. Group-level discrepancies may help isolate behaviors that manifest in daily routines due to a health concern or major lifestyle change. To acquire such insights, we propose an algorithmic framework based on change point detection called Behavior Change Detection for Groups (BCD-G). We hypothesize that, using BCD-G, we can quantify and characterize differences in behavior between groups of individual smart home residents. We evaluate our BCD-G framework using one month of continuous sensor data for each of fourteen smart home residents, divided into two groups. All subjects in the first group are diagnosed with cognitive impairment. The second group consists of cognitively healthy, age-matched controls. Using BCD-G, we identify differences between these two groups, such as how impairment affects patterns of performing activities of daily living and how clinically-relevant behavioral features, such as in-home walking speed, differ for cognitively-impaired individuals. With the unobtrusive monitoring of smart home environments, clinicians can use BCD-G for remote identification of behavior changes that are early indicators of health concerns.
Keywords: Activity recognition, smart environments, change point detection, unsupervised machine learning, remote health monitoring
I. Introduction
Sensor technology is becoming a commonplace aspect of everyday lives. With the internet of things, persons can wear sensors on their bodies and install sensors in their environments. Specifically, for smart environments, sensors are often installed in a residence to create a smart home, in a workplace to create a smart office, and even on and around streets to create smart cities. These sensors continuously and unobtrusively collect time series data reflecting a person’s everyday behavior, both in and out of the home. Such time series data consist of sensor state values and associated timestamps, reported when a change in state occurs. Using machine learning, time series data can be analyzed to detect changes in our everyday behavior over short or long time periods [1], [2]. Often changes in an individual’s behavior patterns are attributed to changes in health [3], [4], such as breaking a bone or catching a cold. Health changes can begin abruptly with an isolated event, such as a fall in the home, or can slowly manifest over time [5], such as the onset of dementia. For all such health changes, sensor data collected from smart environments can provide unprecedented, naturalistic insight about how different individuals and groups experience the onset of and recovery from a health concern.
In this paper, we propose an algorithmic framework for comparing the daily behavior patterns of groups of individuals living in smart environments. Our framework is called Behavior Change Detection for Groups (BCD-G). This framework represents a novel expansion of our prior work, BCD, that analyzes changes within time series collected from a single smart home resident [6], [7]. BCD and BCD-G are change point detection-based methods for analyzing changes in time series data. Change point detection algorithms compute a change score value that quantifies how much change is surrounding a candidate point in time. We propose BCD-G as a novel approach to detect and compare behavior differences between groups of individuals by treating a health or behavior trait like a candidate change point. We hypothesize that these differences can provide behavioral insights into a specific health trait that will allow earlier detection and recovery.
To perform BCD-G-based comparison, one group contains individuals with the same health/behavioral trait that is under investigation (e.g. a “trait” group). The second group contains age-matched individuals who do not exhibit the same health/behavioral trait as the first group. This second group serves as a control group (e.g. a “no-trait” group). BCD-G extends beyond simply classifying “trait” or “no-trait”. This process is different than traditional supervised learning. Applying BCD-G to these two groups provides a description and statistical significance of the differences in everyday behavior that are consistent with the trait under scrutiny.
We evaluate our BCD-G approach with two groups of seven individuals, each living in smart homes. All smart home residents in the first group have a cognitive impairment and are diagnosed with mild cognitive impairment (MCI) or mild dementia. The second group consists of cognitively-healthy smart home residents who are age-matched to the first group. Using our BCD-G framework, we are able to describe behavioral differences that manifest in cognitively-impaired individuals. More specifically, our results describe differences in terms of common activities of daily living, mobility, and sleeping patterns. This information can be leveraged by clinicians, caretakers, and the residents to detect onset of a health condition and enable earlier care.
II. Related Work
Identifying health differences between groups has been a topic of considerable research because of its many applications. Recent approaches have applied machine learning techniques to explore characteristics that can be used as predictors for group classification, such as for diagnosing cancer and predicting related outcomes. Researchers predicted persistent pain in post-mastectomy patients using a rules-based classifier that included physical and behavioral health features [8]. Support vector machine and neural network classifiers were used to determine cancer types impacted by sepsis [9]. Convolutional neural networks were also used to classify pathology images [10] and random forest, logistic regression, neural network, and support vector machines were used for diagnosing cancer [11], [12].
Also using machine learning techniques, researchers have investigated the connection between behavior and health. While in the past, such investigations have relied on observation and self-report, the recent ability to unobtrusively collect large amounts of sensor data opens the pathway to quantifying and understanding this connection in an ecologically-valid manner. Sensors provide information on a wide array of physiological and behavioral features. Wearable sensors are embedded in mobile phones, smartwatches, and even clothes, and are typically used to monitor and track movement-based activities such as sit, stand, walk, ran, and he down. Using these mobile packages, researchers have quantified the relationship between cognitive health and specific targeted behavior components such as sleep [13], [14], gait [15], [16], time out of the home [17], and amount of phone-based social interaction [18].
In other studies, researchers assessed behavior parameters in home settings using ambient sensors. While many studies were performed in controlled conditions with scripted activities, significant correlations were discovered between behavioral factors and neuropsychological test scores [19]–[21]. These behavior parameters also correlated with other health components, including fall risk, cognitive function, motor function, and dyskinesia “on” states [20]–[24]. A few recent studies monitored individuals in their homes over multiple months or years [25], [26]. In these cases, walking speed, time out of the home, time spent in specific rooms, and variation in daily routine were predictors of cognitive health [26]–[29].
One aspect that characterizes most of these studies is that scientists sought to understand the connection between a specific behavioral characteristic and a component of cognitive or physical health. In contrast, the study we present in this paper takes the opposite view. We collect sensor-based behavior data for individuals from multiple subgroups. We then introduce data mining techniques that find the behavior differences that can be used to predictively characterize the health differences between the groups. This way, we can quantify the amount of difference in overall routine that exists between individuals within the same diagnosis category and between different diagnosis categories. Furthermore, we can characterize the nature of these differences. These insights can be used to better understand the behavior impacts of cognitive impairment and to inform the design of assessment measures.
III. Methods
In this paper, we analyze smart home data collected from 14 single-resident apartments instrumented with the CASAS “smart home in a box” [30]. Each CASAS smart home contains several ceiling-installed passive infrared motion sensors, combined with ambient light sensors, in each major room of the house. Additional two-piece magnetic sensors, which also monitor ambient temperature, are attached to exterior doors as well as selected kitchen and bathroom cabinets. The CASAS system logs events from each sensor when a state change occurs (e.g., from “motion” to “no motion” or door “open” to “closed”). For each of the 14 subjects, we analyzed one month (30 days) of continuously and unobtrusively-collected sensor event data. Subjects were selected to form two groups of seven residents each: the “cognitive impairment” group, denoted CI, and the “healthy control” group, denoted HC. The cognitive impairment group members were diagnosed with MCI or dementia before data collection began. The groups are matched for age and education, as summarized in Table I.
Table I.
Smart home resident participant characteristics
| Group | Participant ID | Age (years) | Education (years) | Health problems |
|---|---|---|---|---|
| Cognitive Impairment (CI) | hh101 | 87 | 16 | MCI |
| hh104 | 83 | 16 | Dementia | |
| hh116 | 79 | 20 | MCI | |
| hh118 | 83 | 16 | MCI | |
| hh119 | 81 | 18 | MCI | |
| hh122 | 82 | 18 | MCI, early dementia | |
| hh123 | 89 | 18 | Dementia | |
| Healthy Control (HC) | hh103 | 79 | 20 | N/A |
| hh105 | 83 | 16 | N/A | |
| hh106 | 73 | 16 | N/A | |
| hh108 | 80 | 16 | N/A | |
| hh109 | 90 | 16 | N/A | |
| hh111 | 81 | 20 | N/A | |
| hh114 | 93 | 12 | N/A |
MCI = mild cognitive impairment.
A. Activity Recognition
To provide context for what the resident is doing in the smart home, we use activity recognition (AR) to label each sensor event with an activity label. To do this, we employ the CASAS-AR algorithm which has demonstrated high labeling accuracy in our previous work [31]. The CASAS-AR algorithm assigns each sensor event with one of 40 activity labels, such as “Eat”, “Enter Home”, and “Sleep”. Ground truth labels are provided by external staff. Annotators label each sensor data reading with a corresponding activity label based on the home’s floor plan, sensor layout, and a resident-provided description of the common times and locations for routine activities.
Many of the activity labels represent predefined basic or instrumental activities of daily living, while other labels are based on individualized smart home resident routine behavior. There is one activity label, “Other Activity”, that is assigned when the detected resident behavior does not match a predefined behavior. Because not all participants performed all 40 activities at some point during the month of data collection, in a post-processing step we combine specific activity labels into more general activity labels to form a common activity set. To do this, we combine activities based on similarity. For example, “Cook”, “Cook Breakfast”, “Cook Dinner”, and “Cook Lunch” are all combined into the single activity label “Cook.” This reduces the initial set of activities from 40 to 18 labels. We then remove any labels from this set of 18 if a participant did not perform the activity at least once during the one month of data we are analyzing. Two activities, “Exercise” and “Housekeeping”, did not meet the criteria and were removed from analysis. Our final activity label set contains the following 16 labels (with the combined activity labels for a final label in parentheses):
Bathe
Bed to Toilet Transition
Cook (Cook, Cook Breakfast/Lunch/Dinner)
Dress
Eat (Eat, Eat Breakfast/Lunch/Dinner, Drink)
Enter Home
Leave Home (Leave Home, Step Out)
Meds (Take Medicine, Evening Meds, Morning Meds)
Other Activity
Personal Hygiene (Personal Hygiene, Groom)
Relax (Relax, Watch TV, Read)
Sleep (Sleep, Sleep Out of Bed, Go to Sleep, Nap, Wake Up)
Socialize (Entertain Guests, Phone)
Toilet
Wash Dishes (Wash Dishes, Wash Breakfast/Lunch/Dinner Dishes)
Work (Work, Work at Desk/Table/Computer)
B. Feature Extraction
Using the activity-labeled time series data, we perform several transformations of the data to prepare it for input to our BCD-G algorithm. First, we compute the probability of each activity occurring at each horn of the day. These hourly probabilities form a multivariate time series of size 24D x 16, where 24 is the number of horns in a day, D is the number of days of analyzed smart home data, and 16 is the number activity labels. For our analysis, D = 30 days because we are analyzing one month of daily behavior data. Secondly, we extract features from the smart home event and activity data in order to provide context for analyzing behavioral changes. We utilize features that quantify daily behavior and are straightforward to interpret. Within a 24-hour period, we define the day subperiod to be 7:00am to 6:59pm. We define the night subperiod to be 7:00pm to 6:59am. Specifically for nighttime sleep, we calculate the sum of sleep during two periods: the first period beginning the previous day at 7:00pm and ending at 11:59pm, and the second period beginning on the current day at 12:00am and ending at 6:59am. In total, we extract 57 features describing daily behavior. These features are summarized in Table II.
Table II.
Features extracted for change analysis
| Type | Daily Feature |
|---|---|
| Activity | Total duration of each activity |
| First occurrence of each activity (measured as seconds past midnight) | |
| Last occurrence of each activity (measured as seconds past midnight) | |
| Sleep | Total duration of sleep during the day |
| Total duration of sleep during the night | |
| Number of nighttime sleep interruptions (measured as number of non-sleep activity sequences between sleep sequences during the night) | |
| Mobility | Total movement in the home (measured as distance in meters) |
| Average walking speed (measured as meters per second during bouts of movement) | |
| Routine | Circadian rhythm strength (ratio of nighttime duration of activity (e.g. non-sleep and non-relax activities) divided by the previous day daytime duration of activity) [32] |
| Complexity of routine (measured as entropy of activity probabilities) | |
| Number of different daily activities | |
| Variability in activity durations (measured as the standard deviation of activity sequence durations) |
C. Behavior Change Detection
Given the aforementioned hourly activity probabilities and daily behavior features, we aim to describe the differences between the CI and HC groups using change point detection. In our prior work, we proposed a window-based change detection approach called Behavior Change Detection (BCD) [6], [7]. BCD processes time series data from a single participant, outputting detected changes between two time periods, or windows, within the data. BCD output consists of a list of change scores computed between equal-size time windows (e.g. between two days, weeks, months, etc.). For large scores representing a significant change between two windows, BCD also provides information to interpret the source of the detected change. To summarize, BCD is comprised of four main steps:
Segment the data into windows
Compute change scores between windows
Test the significance of the detected changes
Analyze the source of significant changes
BCD is a framework that supports alternative change detection algorithms to be “plugged in” for step #2. In our previous work, we utilized several different algorithms, including Relative Unconstrained Least-Squares Importance Fitting (RuLSIF) [33], Permutation-based Change Detection in Activity Routine (PCAR) [34], small window adaption of PCAR (sw-PCAR) [35], texture dissimilarity [36], [37], and virtual classifier (VC) [38]. In this paper, we utilize the PCAR and VC algorithms, as well as a new change detection algorithm for BCD, SEParation change point detection (SEP) [39]. We use the PCAR algorithm because it was designed for analyzing longitudinal smart home data. We use the VC algorithm because it provides a test for significance based on the binomial distribution that is applied to step #3 of BCD, as well a decision tree that is informative for step #4 of BCD. We include SEP because it is a new change point detection algorithm that has demonstrated superior performance on smart home time series data [39], [40]. SEP also supports multi-dimensional time series data, which allows us to apply SEP to daily feature matrices. In the following three sections, we briefly describe each of these three change point detection algorithms.
1). Permutation-based Change Detection in Activity Routine
The PCAR algorithm detects changes in smart home data using an activity curve model. The activity curve model represents activity probability distributions for each time interval in a day (m time intervals per day), aggregated over a window of n days. The algorithm detects change between two activity curves by first aligning the two curves using dynamic time warping (DTW). A distance function is used to quantify the dissimilarity of the DTW-aligned curves for each time interval. To produce a single change score from this vector of m distances, the two windows are concatenated to form a window of 2n total days. The activity curve extraction, DTW-alignment, and distance computation process are repeated N times for random permutations of the days in the concatenated windows. This procedure produces a N × m permutation matrix. The ratio of permutation-based distances exceeding the original distance is computed for each time interval, producing a list of ratios. These ratios represent p-values, or the probability the original distance was sampled from the same distribution produced by the permutations for a given time interval. A Benjamini-Hochberg correction is applied for α < 0.05 to account for the multiple comparisons [41]. Finally, PCAR counts the remaining p-values that are significant at the given α level. This count forms the PCAR change score representing the change between the two activity curves.
2). SEParation Change Point Detection
A group of non-parametric change point detection algorithms, called likelihood ratio estimators, compute a change score by estimating the ratio of probability distributions between two time series windows surrounding a suspected change point. The higher the ratio, the more likely the windows are different and there is a valid change point between them. One of the most common likelihood ratio estimation algorithms is called RuLSIF [33]. RuLSIF uses the Pearson divergence dissimilarity measure to estimate the probability distribution. Like RuLSIF, we introduced SEP in prior work as a non-parametric change point detection algorithm that uses a separation distance function [39]. Both RuLSIF and SEP require computing a parameter threshold value to determine if a computed change score is significant or not based on whether it is above or below the threshold. We are interested in using SEP over RuLSIF because of its increased performance in prior work and because it supports both one-dimensional and two-dimensional time series data [39], [40]. Furthermore, we found in prior work that SEP outperformed RuLSIF in detecting true behavior transitions from smart home data [40].
3). Virtual Classifier
Hido and colleagues proposed the VC algorithm as part of their change analysis framework [38]. Change analysis computes a change score, while providing context to identity the features that contributed to the change. The approach begins with two n × z feature matrices, where n is the time series window size (e.g. a number of days) and z is the number of features extracted from each day in the window. Feature vectors (rows) in the first matrix are labeled with a hypothetical “positive” class, while feature vectors in the second matrix are labeled with a hypothetical “negative” class. A decision tree is trained using k-fold cross validation to distinguish between these two classes. The mean classification accuracy is then compared to a significance threshold, which is based on the expected accuracy of a binary classifier trained on random samples. Using the binomial distribution’s inverse survival function, a probability value pcritical is computed using the two window sizes and a significance level, α (α < 0.05). If the VC accuracy is greater than or equal to pcritical, then the VC accuracy is considered to be significant [38]. For example, if the two window sizes are both five days and α < 0.05, then using the inverse survival function of the binomial distribution, pcritical = 0.8. This would mean a VC accuracy acquired by training using only 10 days would have to be greater than or equal to 0.8 in order to be determined significant. If the VC accuracy is significant, then retraining a decision tree on the entire dataset produces a tree with the features most responsible for the change between the two windows towards the top of the tree.
D. BCD for Group Analysis
In previous work, we developed BCD to find points in time with detected behavior changes that could be indicative of health events [6]. Here, we introduce a novel method to expand the BCD framework for comparing populations (e.g. groups) instead of comparing behavior for one individual at different time points. To describe BCD-G, let G1 and G2 represent time series data sampled from two different groups. Our goal is to compare behavior between the groups and characterize the differences. Let G1 contain behavior data for N participants while G2 contains behavior data for M participants. The changes between G1 and G2, denoted G1ΔG2, can be quantified using BCD-G by setting the “windows” to be “participants”. BCD-G compares two data windows from two different participants, rather than two windows from two different timepoints. Additionally, we design BCD-G to perform comparisons in two different configurations: group-to-group and pair-to-pair.
1). Group-to-Group Comparisons
Suppose G1 and G2 both contain the same number of participants. We concatenate equal-length time series for all participants, p ∈ G1, to form a new time series window, W1. This process is repeated for all participants q ∈ G2, to form W2. W1 and W2 are then concatenated to form a time series that is input to BCD-G with window size set to |W1|, where |W1| = |W2|. Each day in the W1 window is labeled as a positive class, while each day in the W2 window is labeled with a negative class. Figure 1 shows an example of these two groups, each containing seven participants. A single comparison is made between W1 and W2 using a virtual classifier trained to discriminate between positive and negative days.
Fig. 1.
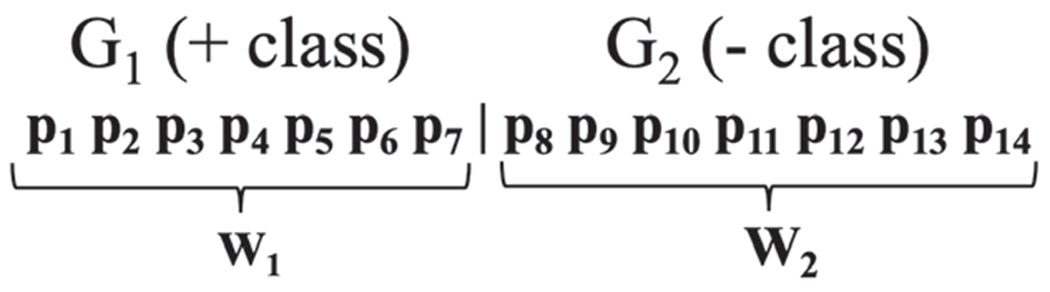
Window representation of group-to-group comparisons between two groups, G1 and G2, each containing 7 participants. Each pi is an equal-length behavior time series collected from participant i.
2). Pair-to-Pair Comparisons
We compute change scores for all possible pairs of participants within each group and between groups. To elaborate, there are three distributions of pairwise change scores for two groups G1 and G2, intragroup pairwise change scores and intergroup pairwise changes scores. Intragroup pairwise change scores are computed between all possible pairs of participants within a group (see Figure 2a for a diagram of pairs in a general group). If there are multiple groups, as is the case in this paper with the two groups CI and HC, intragroup pairwise change scores are computed separately for each group. Intergroup pairwise change scores are computed between all possible pairs of participants in G1 and G2; one participant in the pair from the G1 group and the other participant in the pair from the G2 group (see Figure 2b for a diagram of pairs).
Fig. 2.
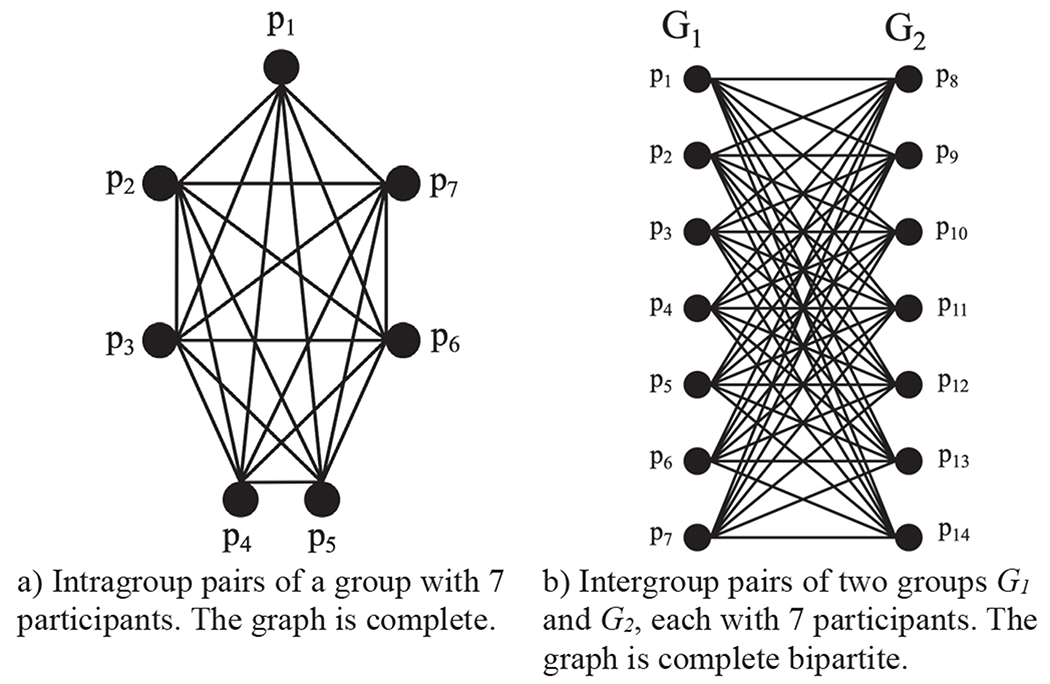
Graphical representation of pair-to-pair comparisons.
IV. Results
In this paper, we examine the differences between two groups of participants, which are labeled as follows:
G1: CI (Cognitive Impairment group): 7 participants with mild cognitive impairment or mild dementia
G2: HC (Healthy Control group): 7 healthy, age-matched participants
Additionally, we denote the intergroup change scores between CI and HC with the label IN. We use BCD-G to compute the changes CIΔHC in two different configurations: group-to-group VC change scores and pair-to-pair participant change scores. The results can be further categorized into comparisons using all features and comparisons using individual activities.
A. Group-to-Group VC Comparisons
First, BCD-G compares behavior for cognitively impaired and control participants using all of the activity time series data and using all of the extracted features. We perform a group-to-group comparison of CI to HC using BCD-G with VC. All 30-day time series in the CI group are concatenated to form window W1 and all 30-day time series in the HC group are concatenated to form window W2. The window size was set to 210 days (30 days multiplied by 7 participants in each group). The group-to-group CIΔHC VC change score is 0.826, which is the mean accuracy of leave-one-out-cross-validation. This result is significant because the score exceeds the significance threshold of pcritical = 0.541 (calculated with α < 0.05). The resulting VC decision tree is shown in Figure 3. A decision tree is an ideal choice for VC because it provides accurate classification and human-interpretable models. However, we also performed leave-one-out-cross-validation using three other types of classifiers. These classifiers and their mean accuracies include K-nearest-neighbors with K = 5 (0.855), naïve Bayes (0.781), and a neural network (0.824). We tested the K-nearest-neighbors accuracy compared to the VC decision tree accuracy and found the improvement was not significant α < 0.05).
Fig. 3.
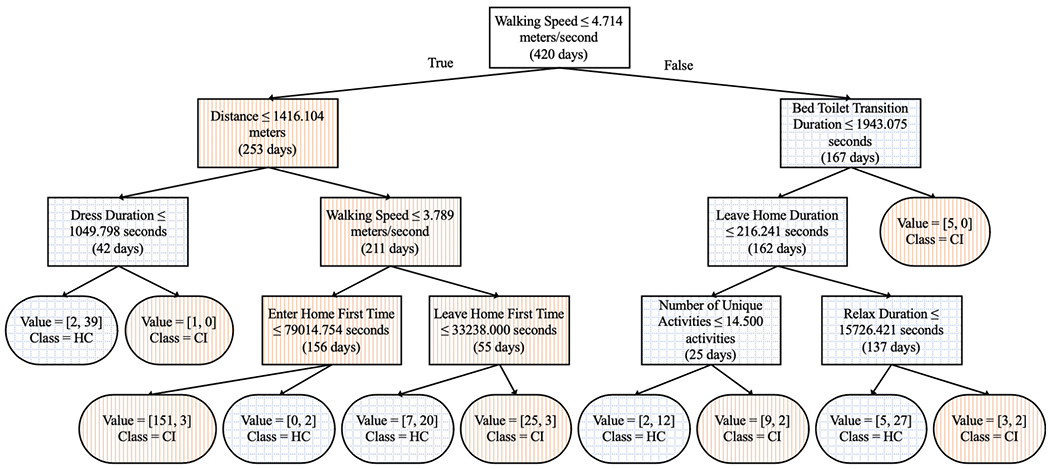
Top virtual classifier decision tree rules for group-to-group comparisons using all activities. Rectangles are decision nodes and rounded rectangles are leaf nodes. Left branches are true and right branches are false. Orange vertical hatch represents participant subgroups containing mostly individuals from the cognitive impairment (CI) group and blue cross hatch represents participant subgroups containing mostly individuals from the healthy control group (HC).
While change scores using all activities provides an overview of changes between groups, zooming in to investigate the changes in each individual activity provides a closer look at the source of the changes. For each of the 16 activities, we apply BCD-G to investigate which activities exhibit the greatest differences between the two groups. We utilize BCD-G with the VC approach to compare participants in each group, but for this comparison we use only features related to each activity. For example, for the Cook activity, the features included are Cook duration, time of Cook first occurrence, and time of Cook last occurrence. Table III lists each activity’s VC change score and whether the score is significant or not. Only the VC change score for the Other Activity label does not exceed the significance threshold (pcritical = 0.541).
TABLE III.
GROUP-TO-GROUP ACTIVITY CIΔHC VC CHANGE SCORES
| Activity | Virtual Classifier Score |
|---|---|
| Bathe | 0.595* |
| Bed to Toilet Transition | 0.600* |
| Cook | 0.581* |
| Dress | 0.710* |
| Eat | 0.610* |
| Enter Home | 0.695* |
| Leave Home | 0.698* |
| Meds | 0.695* |
| Other Activity | 0.529 |
| Personal Hygiene | 0.652* |
| Relax | 0.645* |
| Sleep | 0.633* |
| Socialize | 0.571* |
| Toilet | 0.650* |
| Wash Dishes | 0.643* |
| Work | 0.581* |
denotes a significant change score with significance threshold pcritical = 0.541, α < 0.05.
B. Pair-to-Pair Comparisons
Next, we quantify the CIΔHC change within groups and the change between groups at an individual subject level. To do this, we compute change scores for all possible pairs of participants within the CI and HC groups and between these two groups. To elaborate, there are three distributions of pairwise change scores (91 total pairs):
Intragroup pair-to-pair change scores (CI and HC, 42 pairs): computed as all possible combinations of two participants sampled from a group of seven participants (2 * 7C2 = 42 pairs).
Intergroup pairwise change scores (IN, 49 pairs): computed between pairs of 14 participants, one participant in the pair from the CI group and the other participant from the HC group (7 * 7 = 49 pairs).
Initially, PCAR processed all hourly probability time series data for all activities, while SEP and VC processed all features. The BCD-G results for all features are shown in Figure 4. Figures 4a–c show the distribution of change scores for each group (within CI, within HC, and within both groups) as box plots. Figures 4d–f show the individual mean pairwise change scores for each participant in the CI group as bars with standard deviations highlighted as error lines. For reference, horizontal lines show percentile-based control group scores. Lastly, we perform pairwise comparisons again, but this time utilizing only data related to each individual activity. Of the 48 algorithm configurations (16 activities for each of the three algorithms, respectively), we include results for the three activities with the most distinct observed changes for each algorithm. These are the Bed to Toilet Transition PCAR change scores, Bathe SEP change scores, and Enter Home VC change scores. These results are shown as box plots and bar charts in Figure 5.
Fig. 4.
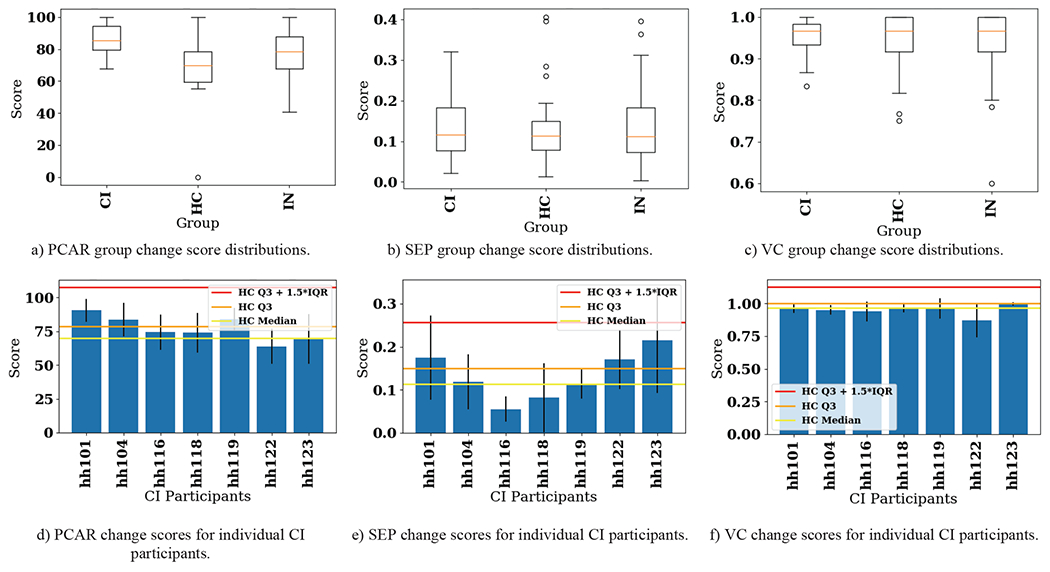
CIΔHC pairwise change score results for PCAR, SEP, and VC. Top: Distribution of change scores shown as box plots. Bottom: Pairwise changes for each participant in the CI group shown as bar plots. Bars show mean pairwise change scores while error lines on the bars show +/− one standard deviation of pairwise change scores within the CI group. Three different values of the control group scores (using only HC participants) are shown to provide context for significant changes. CI = cognitive impairment group, HC = healthy control group, IN = intergroup, Q3 = third quartile (75th percentile), IQR = interquartile range.
Fig. 5.
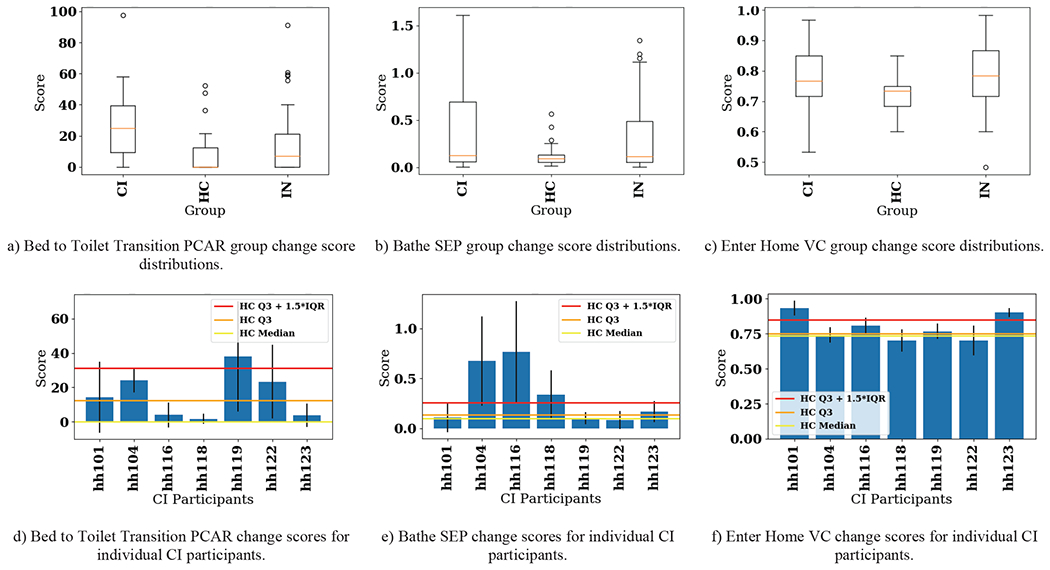
CIΔHC pairwise change score results for the activities with distinct differences observed between the two groups. Top: Distribution of change scores shown as box plots. Bottom: Pairwise changes for each participant in the CI group shown as bar plots. Bars show mean pairwise change scores while error lines on the bars show +/− one standard deviation of pairwise change scores within the CI group. Three different values of the control group scores (using only HC participants) are shown to provide context for significant changes. CI = cognitive impairment group, HC = healthy control group, IN = intergroup, Q3 = third quartile (75th percentile), IQR = interquartile range.
V. Discussion
In this paper, we introduce a novel method to compare behavior differences between two subject groups, called BCD-G, based on the BCD change point detection framework. We apply BCD-G to two groups, a group of smart home residents with MCI and/or dementia (CI) and a healthy, age-matched control group of smart home residents (HC). To explain the source of detected change, we analyze the activity and feature differences between the two groups.
A. Feature-based Change Analysis
The tree in Figure 3 shows the most discriminating features VC used to compute group-to-group CIΔHC. At the top of the tree is walking speed, which is commonly listed in the literature as a strong predictor of mortality [42]. Walking speed for the CI group averaged over all participants’ days is 2.344 ± 1.903 m/s, while walking speed for the HC group is 5.182 ± 2.997 m/s. This large disparity explains why the first rule of the tree queries whether walking speed is less than or equal to 4.714 m/s. Activities that are near the top of the tree include Bed to Toilet Transition, Dress, and Leave/Enter Home, implying healthy smart home residents tend to dress quicker, transition from the bed to the toilet quicker, and leave the home later in the day (see Figure 3 for the decision tree).
There are additional features that produce large differences between the two groups but are not necessarily discriminatory enough to be included near the top of the decision tree. These include activity duration variance, activity entropy, daytime sleep duration, and nighttime sleep duration. The cognitively impaired group has greater variance in the duration of the activities they perform (CI 9642.894 seconds; HC 8985.282 seconds) and in the entropy of those activities (CI 1.745 ± 0.291; HC 1.579 ± 0.473). The CI participants sleep more than the HC participants, with a higher daytime sleep duration (CI 1.348 ± 1.357 hours; HC 1.001 ± 1.350 hours) and nighttime sleep duration (CI 4.777 ± 2.125 hours; HC 4.129 hours ± 2.450 hours). Features that do not seem to differ much between the two groups include the number of unique activities (CI 13.724 activities per day; HC 13.657 activities per day), the number of nighttime sleep interruptions (CI 2.629 per night; HC 2.524 per night), and circadian rhythm strength, or CRS (CI 0.745; HC 0.717). This last result is particularly interesting because of how informative the CRS values have been found to be in similar research comparing cognitively healthy and cognitively impaired groups. Paavilainen el al. found a CRS of ~0.3 was common for nondemented nursing home residents, while a CRS of ~0.5 was common for demented nursing home residents [43]. The CI and HC groups both have CRS values above 0.5, indicating a high variability of activity from night to previous day. However, our results are not directly comparable to Paavilainen et al. because while they use wearable actigraphs to quantify activity counts, in this paper we use activity labels from smart home sensors.
B. Activity-based Change Analysis
Combining BCD-G with VC, we can investigate the strength of the behavior differences between the CI and HC groups for each activity. Of the 16 activities, all but one activity yields statistically-significant changes between CI and HC. The Other Activity is a catch-all label for behavior in the home that does not fit one of the original 40 activities. Considering this, it seems logical that behavior classified as Other Activity is likely somewhat random, regardless of which group a participant belongs to. The activities with the largest VC-detected change scores include Dress (0.710), Leave Home (0.698), Enter Home (0.695), and Meds (0.695). With the exception of Meds (which is not included in the decision tree at all), these features all appear near the top of the decision tree (see Figure 3) that is generated using all activities.
Unlike the group-to-group comparison results which provide single change score values, the pair-to-pair comparison results provide a distribution of change scores for three different groups, CI, HC, and IN (CI and HC combined). These distributions are visualized in Figure 4 (all activities included in BCD-G) and Figure 5 (three samples of single activity BCD-G results). Figure 4 shows that using PCAR for change detection captures change between the two groups, with the CI group exhibiting a higher median change score and lower spread (see Figure 4a). Of the three algorithms, PCAR detects the most CI individual change compared to the distribution of the HC group (see Figure 4b). This is likely due to PCAR analyzing the hourly activity probability time series directly, instead of using summary feature values as input, like SEP and VC. The boxplot distributions for SEP (see Figure 4c) and VC (Figure 4d) do not show as much difference as PCAR (see Figure 4a) between the groups. Comparing the bar charts across all three algorithms does not show the algorithms agree about which participants exhibit the most change. Because of this ambiguity, we next look at each individual activity to narrow in on the individual participant’s greatest source of behavior change.
Of the 16 activities, we included box plots and bar plots in Figure 5 for Bed to Toilet Transition (PCAR scores), Bathe (SEP scores), and Enter Home (VC scores). The CI group exhibits a large amount of variability in their routines for these three activities compared to the HC group (see Figures 5a–c). At the individual participant level, 4/7 CI smart home residents exceeded the 75th percentile of HC group change for these three activities; however, this is not a consistent set of 4 participants across all three activities. Furthermore, a CI participant with change scores outside the HC 75th percentile for all three activities does not exist. To investigate this further, we found that on average participants demonstrated behavior changes greater than the HC 75th percentile for about three activities (4.286, 3.143, and 3.000 activities for PCAR, SEP, and VC-detected changes, respectively). This suggests the CI participants exhibit radically different change from the healthy population on only a handful of activities at a time.
For commercial products focusing on extending independence in persons with cognitive impairment, our research suggests that change point detection for the clinically-relevant activities of walking speed (from bed to toilet), bathing routines, and enter/exit home activities for CI persons should be included in smart home product design. Activities representing the milieu of symptoms associated with cognitive decline may require varied algorithmic approaches. Products detecting these changes can facilitate a type of “clinical triage” to support clinicians, caregivers, and patients. Early interventions are key to reducing premature transition to residential memory care and reducing health impairment-associated healthcare costs [44].
VI. Conclusion
Our Behavior Change Detection for Groups provides a unique algorithmic framework to compare behavior for two or more groups. Unlike standard supervised learning approaches that can predict group membership based on behavior parameters, BCD-G identifies key behavior differences between groups by leveraging state-of-the-art change point detection and machine learning algorithms. Using BCD-G and activity-labeled smart home data, differences are detected that include changes in duration, complexity, and pattern of common activities of daily living. We also analyze changes in clinically-relevant behavior features, such as quantifying how much slower cognitively-impaired subjects walk in their home as compared to age-matched healthy subjects.
Limitations of tins work include the small sample size of seven cognitively impaired smart home participants and seven age-matched smart home participants. Also, the expansion to BCD-G that performs pairwise comparisons does not scale due to its exponential time complexity. The approach is best for comparing small groups in an offline setting; however, for large groups and/or an online deployment of the algorithm, we can overcome this limitation by using a K-nearest-neighbors approach to sampling which participants within a group are most important to compare with. In the future, we plan to move BCD-G to an online setting to support clinician-in-the-loop and preventative healthcare. With BCD-G, we are able to compare the in-home behavior of a single participant to a cognitively impaired subgroup and a healthy control subgroup to identify similarities and differences. With this information, clinicians can remotely determine at-risk behavior and provide timelier and more precise medical and social interventions.
Acknowledgment
The authors would like to thank Maureen Schmitter-Edgecombe and Beiyu Lin for their assistance with this study.
This work was supported in part by the National Institutes of Health under Grants R01EB009675, R01NR016732, R25EB024327, and R25AG046114 and by the National Science Foundation under Grant 1734558.
Contributor Information
Gina Sprint, Gonzaga University, Spokane, WA 99258 USA.
Diane J. Cook, Washington State University, Pullman, WA 99164 USA.
Roschelle Fritz, Washington State University Vancouver, Vancouver, WA 98686 USA.
References
- [1].Aminikhanghahi S and Cook DJ, “A survey of methods for time series change point detection,” Knowl. Inf. Syst, vol. 51, no. 2, pp. 339–367, May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Galambos C, Skubic M, Wang S, and Rantz M, “Management of dementia and depression utilizing in-home passive sensor data,” Gerontechnology, vol. 11, no. 3, pp. 457–468, Jan. 2013,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fadem B, Behavioral Science in Medicine. Lippincott Williams & Wilkins, 2012. [Google Scholar]
- [4].Roberts AR, Adams KB, and Warner CB, “Effects of chronic illness on daily life and barriers to self-care for older women: A mixed-methods exploration,” J. Women Aging, vol. 29, no. 2, pp. 126–136, Mar. 2017. [DOI] [PubMed] [Google Scholar]
- [5].Orient JM and Sapira JD, Sapira’s Art & Science of Bedside Diagnosis. Lippincott Williams & Wilkins, 2010. [Google Scholar]
- [6].Sprint G, Cook DJ, Fritz R, and Schmitter-Edgecombe M, “Using Smart Homes to Detect and Analyze Health Events,” Computer, vol. 49, no. 11, pp. 29–37, Nov. 2016. [Google Scholar]
- [7].Sprint G, Cook D, Fritz R, and Schmitter-Edgecombe M, “Detecting Health and Behavior Change by Analyzing Smart Home Sensor Data,” in 2016 IEEE International Conference on Smart Computing (SMARTCOMP), May 2016, pp. 1–3. [Google Scholar]
- [8].Lötsch J et al. , “Machine-learning-derived classifier predicts absence of persistent pain after breast cancer surgery with high accuracy,” Breast Cancer Res. Treat, vol. 171, no. 2, pp. 399–411, Sep. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tripathi H, Mukhopadhyay S, and Mohapatra SK, “Sepsis-associated pathways segregate cancer groups,” BMC Cancer, vol. 20, no. 1, p. 309, Dec. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kumar N, Verma R, Sharma S, Bhargava S, Vahadane A, and Sethi A, “A Dataset and a Technique for Generalized Nuclear Segmentation for Computational Pathology,” IEEE Trans. Med. Imaging, vol. 36, no. 7, pp. 1550–1560, Jul. 2017. [DOI] [PubMed] [Google Scholar]
- [11].Cheng J et al. , “Computational analysis of pathological images enables a better diagnosis of TFE3 Xp11.2 translocation renal cell carcinoma,” Nat. Commun, vol. 11, no. 1, p. 1778, Dec. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kiani A et al. , “Impact of a deep learning assistant on the histopathologic classification of liver cancer,” Npj Digit. Med, vol. 3, no. 1, p. 23, Dec. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mohr DC, Zhang M, and Schueller SM, “Personal Sensing: Understanding Mental Health Using Ubiquitous Sensors and Machine Learning,” Annu. Rev. Clin. Psychol, vol. 13, no. 1, pp. 23–47, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vitiello MV, “Sleep in Normal Aging,” Sleep Med. Clin, vol. 1, no. 2, pp. 171–176, Jun. 2006. [Google Scholar]
- [15].Ayena JC, Chapwouo LD M T. Otis J-D, and Menelas B-AJ, “An efficient home-based risk of falling assessment test based on Smartphone and instrumented insole,” in 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings, May 2015, pp. 416–421. [Google Scholar]
- [16].Mancini M et al. , “Continuous Monitoring of Turning Mobility and Its Association to Falls and Cognitive Function: A Pilot Study,” J. Gerontol. Ser. A, vol. 71, no. 8, pp. 1102–1108, Aug. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O’Brien M, Mummidisetty C, Bo X, and Poellabauer C, “Quantifying community mobility after stroke using mobile phone technology,” in Proceedings of the 2017 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2017 ACM International Symposium on Wearable Computers, Sep. 2017, pp. 161–164. [Google Scholar]
- [18].Boukhechba M, Huang Y, Chow P, Fua K, Teachman B, and Barnes L, “Monitoring social anxiety from mobility and communication patterns,” in Proceedings of the 2017 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2017 ACM International Symposium on Wearable Computers, Sep. 2017, pp. 749–753. [Google Scholar]
- [19].Alam MAU, Roy N, Gangopadhyay A, and Galik E, “A smart segmentation technique towards improved infrequent non-speech gestural activity recognition model,” Pervasive Mob. Comput, vol. 34, pp. 25–45, Jan. 2017. [Google Scholar]
- [20].Cook DJ, Schmitter-Edgecombe M, and Dawadi P, “Analyzing Activity Behavior and Movement in a Naturalistic Environment Using Smart Home Techniques,” IEEE J. Biomed. Health Inform, vol. 19, no. 6, pp. 1882–1892, Nov. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dawadi PN, Cook DJ, and Schmitter-Edgecombe M, “Automated Cognitive Health Assessment Using Smart Home Monitoring of Complex Tasks,” IEEE Trans. Syst. Man Cybern. Syst, vol. 43, no. 6, pp. 1302–1313, Nov. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nait Aicha A, Englebienne G, and Kröse B, “Continuous Gait Velocity Analysis Using Ambient Sensors in a Smart Home,” in Ambient Intelligence, Cham, 2015, pp. 219–235. [Google Scholar]
- [23].Austin D, Hayes TL, Kaye J, Mattek N, and Pavel M, “Unobtrusive monitoring of the longitudinal evolution of in-home gait velocity data with applications to elder care,” in Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE, 2011, pp. 6495–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Darnall ND et al. , “Application of machine learning and numerical analysis to classify tremor in patients affected with essential tremor or Parkinson’s disease,” Gerontechnology, vol. 10, no. 4, pp. 208–219, Mar. 2012. [Google Scholar]
- [25].Alberdi A et al. , “Smart Home-Based Prediction of Multidomain Symptoms Related to Alzheimer’s Disease,” IEEE J. Biomed. Health Inform, vol. 22, no. 6, pp. 1720–1731, Nov. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hellmers S et al. , “Towards a minimized unsupervised technical assessment of physical performance in domestic environments,” in Proceedings of the 11th EAI International Conference on Pervasive Computing Technologies for Healthcare, May 2017, pp. 207–216. [Google Scholar]
- [27].AM A, Snoek J, and Mihailidis A, “Unobtrusive Detection of Mild Cognitive Impairment in Older Adults Through Home Monitoring,” IEEE J. Biomed. Health Inform, vol. 21, no. 2, pp. 339–348, Mar. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Petersen J, Thielke S, Austin D, and Kaye J, “Phone behaviour and its relationship to loneliness in older adults,” Aging Ment. Health, vol. 20, no. 10, pp. 1084–1091, Oct. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Robben S, Englebienne G, and Kröse B, “Delta Features From Ambient Sensor Data are Good Predictors of Change in Functional Health,” IEEE J. Biomed. Health Inform, vol. 21, no. 4, pp. 986–993, Jul. 2017. [DOI] [PubMed] [Google Scholar]
- [30].Cook DJ, Crandall AS, Thomas BL, and Krishnan NC, “CASAS: A Smart Home in a Box,” Computer, vol. 46, no. 7, pp. 62–69, Jul. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krishnan NC and Cook DJ, “Activity Recognition on Streaming Sensor Data,” Pervasive Mob. Comput, vol. 10, pp. 138–154, Feb. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Merilahti J, Viramo P, and Korhonen I, “Wearable Monitoring of Physical Functioning and Disability Changes, Circadian Rhythms and Sleep Patterns in Nursing Home Residents,” IEEE J. Biomed. Health Inform, vol. PP, no. 99, pp. 1–1, 2015. [DOI] [PubMed] [Google Scholar]
- [33].Liu S, Yamada M, Collier N, and Sugiyama M, “Change-Point Detection in Time-Series Data by Relative Density-Ratio Estimation,” Neural Netw, vol. 43, pp. 72–83, Jul. 2013. [DOI] [PubMed] [Google Scholar]
- [34].Dawadi PN, Cook DJ, and Schmitter-Edgecombe M, “Modeling Patterns of Activities Using Activity Curves,” Pervasive Mob. Comput, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sprint G, Cook DJ, and Schmitter-Edgecombe M, “Unsupervised detection and analysis of changes in everyday physical activity data,” J. Biomed. Inform, vol. 63, pp. 54–65, Oct. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang S, Skubic M, and Zhu Y, “Activity Density Map Visualization and Dissimilarity Comparison for Elder care Monitoring,” IEEE Trans. Inf. Technol. Biomed, vol. 16, no. 4, pp. 607–614, Jul. 2012. [DOI] [PubMed] [Google Scholar]
- [37].Tan T-H et al. , “Indoor Activity Monitoring System for Elderly Using RFID and Fitbit Flex Wristband,” in 2014 IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Jun. 2014, pp. 41–44. [Google Scholar]
- [38].Hido S, Ide T, Kashima H, Kubo H, and Matsuzawa H, “Unsupervised Change Analysis Using Supervised Learning,” in Advances in Knowledge Discovery and Data Mining, Springer Berlin; Heidelberg, 2008, pp. 148–159. [Google Scholar]
- [39].Aminikhanghahi S, Wang T, and Cook DJ, “Real-Time Change Point Detection with Application to Smart Home Time Series Data,” IEEE Trans. Knowl. Data Eng, vol. 31, no. 5, pp. 1010–1023, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Aminikhanghahi S and Cook DJ, “Enhancing activity recognition using CPD-based activity segmentation,” Pervasive Mob. Comput, vol. 53, pp. 75–89, Feb. 2019. [Google Scholar]
- [41].Benjamini Y and Hochberg Y, “Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing,” J. R. Stat. Soc, vol. 57, no. 1, pp. 289–300, 1995. [Google Scholar]
- [42].Studenski S et al. , “Gait Speed and Survival in Older Adults,” JAMA, vol. 305, no. 1, pp. 50–58, Jan. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Paavilainen P et al. , “Circadian Activity Rhythm in Demented and Non-Demented Nursing-Home Residents Measured by Telemetric Actigraphy,” J. Sleep Res, vol. 14, no. 1, pp. 61–68, Mar. 2005. [DOI] [PubMed] [Google Scholar]
- [44].Shapiro A and Taylor M, “Effects of a Community-Based Early Intervention Program on the Subjective Well-Being, Institutionalization, and Mortality of Low-Income Elders | The Gerontologist | Oxford Academic,” The Gerontologist, vol. 42, no. 3, pp. 334–341, Jun. 2002. [DOI] [PubMed] [Google Scholar]


