Abstract
Iron deficiency (ID) is especially common in pregnant women and may even persist following childbirth. This is of concern in light of reports demonstrating that ID may be sufficient to produce homeostatic dysregulation of other metals, including manganese (Mn). These results are particularly important considering the potential introduction of the Mn-containing gas additive, methyl cyclopentadienyl manganese tricarbonyl (MMT), in various countries around the world. In order to model this potentially vulnerable population, we fed female rats fed either control (35 mg Fe/kg chow; 10 mg Mn/kg chow) or low iron/high-manganese (IDMn; 3.5 mg Fe/kg chow; 100 mg Mn/kg chow) diet, and examined whether these changes had any long-term behavioral effects on the animals’ spatial abilities, as tested by the Morris water maze (MWM). We also analyzed behavioral performance on auditory sensorimotor gating utilizing prepulse inhibition (PPI), which may be related to overall cognitive performance. Furthermore, brain and blood metal levels were assessed, as well as regional brain isoprostane production. We found that treated animals were slightly ID, with statistically significant increases in both iron (Fe) and Mn in the hippocampus, but statistically significantly less Fe in the cerebellum. Additionally, isoprostane levels, markers of oxidative stress, were increased in the brain stem of IDMn animals. Although treated animals were indistinguishable from controls in the PPI experiments, they performed less well than controls in the MWM. Taken together, our data suggest that vulnerable ID populations exposed to high levels of Mn may indeed be at risk of potentially dangerous alterations in brain metal levels which could also lead to behavioral deficits.
Keywords: Iron deficiency, Manganese, Morris water maze, MMT, Prepulse inhibition (PPI), Isoprostanes
Introduction
Iron deficiency (ID) is recognized as a global problem that may affect as many as two billion people worldwide (Umbreit 2005). It is especially common in pregnant women and young children (Simopoulos 1981; Prevention CfDCa 1998; Siega-Riz et al. 2006), and the long-term consequences of ID are well-documented for infants and juveniles (Eden 2005; Iannotti et al. 2006; Zhou et al. 2006). Few studies, however, have examined the effect of chronic ID in mothers following the birth of a child.
It is known that ID and ID anemia (IDA) may lead to decreased cognitive ability and work capacity, as well as immune system dysfunction and impaired thermoregulation (Beard and Connor 2003; Umbreit 2005). Although ID is easily treated with iron (Fe) supplementation, it is often unrecognized and undiagnosed. This is particularly the case in women from minority and economically disadvantaged populations (Prevention CfDCa 1998; Skalicky et al. 2006). Thus, many groups have sought ways to educate vulnerable individuals concerning methods of increasing dietary Fe (Samadpour et al. 2004; Haider and Bhutta 2006; Iannotti et al. 2006; Mannar 2006). Additionally, ID is associated with homeostatic dysregulation of other metals (e.g., manganese (Mn), copper, zinc) (Pathak et al. 2004; Duque et al. 2007; Knovich et al. 2008). Indeed, it is known that various regions of the brain are especially vulnerable to ID-associated Mn accumulation (Erikson et al. 2002, 2004; Brain et al. 2006), as well as ID-related zinc dysregulation (Shoham and Youdim 2002).
Exposure to high levels of Mn is known to cause increased brain Mn levels and is commonly associated with various occupations, e.g., Mn mining (Garcia Avila and Penalver Ballina 1953; Rodier 1955) and potentially welding (Racette et al. 2001, 2005; Sadek et al. 2003; Josephs et al. 2005). With the possible addition of methylcyclopentadienyl manganese tricarbonyl (MMT) to gasoline, however, it is likely that additional populations, from infants to the elderly, could now be exposed to chronic low-level of Mn (Zayed et al. 2003; Bolte et al. 2004; Boudia et al. 2006). Extensive data exists related to the effects of exposure to high levels of Mn (>1–5 mg Mn/m3) and the ensuing toxicity that may ultimately result in a movement disorder, called manganism (Calne et al. 1994; Pal et al. 1999). Although removal from the Mn source usually results in reversal of the early psychological signs and symptoms of the disease, once the patient exhibits movement disturbances, manganism is often considered to be irreversible (Calne et al. 1994). If MMT is added to gasoline, however, it might be difficult to remove a person from this environmental source of inhaled and ingested Mn.
With the increased potential for wide-spread Mn exposure, new vulnerable populations emerge. For example, pregnant women who are even mildly ID may be a new at risk group. This is particularly important in light of recent data suggesting that ID alone, with no Mn exposure, leads to increased brain Mn deposition (Erikson et al. 2002, 2004), as well as changes in numerous other important dietary metals, such as copper and zinc (Garcia et al. 2007). With this in mind, our goal was to determine whether pregnant dams (rats), modeling this important human population, receiving an ID diet with high Mn concentrations (10-fold higher Mn than control levels), had increased brain Mn and oxidative stress compared to animals on control diets (normal dietary Fe and Mn).
Thus, following pregnancy and removal of their litters, dams were maintained on either control or ID-high Mn (IDMn) diets for a total of 43 weeks, sufficiently long to induce mild ID, while perhaps allowing homeostatic mechanisms to compensate for long-term ID. Since others have reported that similar dietary changes result in specific behavioral and cognitive deficits (Youdim et al. 1989; Shoham and Youdim 2002), we examined the animals’ spatial abilities and working memory as tested by the Morris water maze (MWM). As a second measure of overall cognitive performance (Geyer 2006a, b) and potential attentional abilities (Geyer et al. 2001), we also analyzed behavioral performance on auditory sensorimotor gating utilizing prepulse inhibition (PPI).
Materials and Methods
Animals
All animals were treated in accordance with approved Institutional Animal Care and Use Committee (IACUC) protocols established by Wake Forest University Health Sciences and East Tennessee State University. Timed pregnant Sprague-Dawley rats (gestational day 5–7) from Harlan (Indianapolis, IN) had free access to one of the two diets (Bio-Serv, Frenchtown, NJ), which varied only in Mn and Fe content, for a total of 43 weeks. Control rats (CN, n = 5) received a diet with 35 mg Fe/kg diet and 10 mg Mn/kg diet, while the treatment group (n = 6) received a diet that was both iron-deficient (ID) with supplemented Mn (IDMn; 3 mg Fe/kg diet; 100 mg Mn/kg diet). Diets were assayed by Bio-Serv to ensure that other metals were within normal limits, and that only Mn and Fe concentrations were altered. The dietary Fe concentration, based on previous studies (Erikson et al. 2002), is sufficiently low to induce mild ID, which is more common in human populations (WHO 2003; Umbreit 2005), rather than severe ID or IDA in these animals.
Blood and Brain Tissue Collection
To assess maternal hemoglobin (Hb) during lactation, blood was collected from dams via tail prick on PN4, PN11, and PN21. Subsequent blood was collected during weeks 21, 33, and 43 for determination of Hb, plasma Fe, plasma transferrin (Tf), and total iron binding capacity (TIBC). At the conclusion of the study (week 43), dams were euthanized with ketamine (100 mg/kg) and xylazine (12 mg/kg), and rapidly decapitated. Brains were removed and dissected into five regions: cerebellum, brain stem (pons and medulla), midbrain, hippocampus, striatum, and cortex. Regional wet brain masses were recorded, and regions were immediately frozen on dry ice and stored at −80°C until metal analysis. Trunk blood was collected in heparinized test tubes for further analysis.
Hematological Parameters
Blood collected from dams was assayed for assessment of ID status: Hb, plasma Fe, Tf, and TIBC. Hb was measured colorimetrically by a standard cyanmethemoglobin method (procedure #525, Sigma, St. Louis, MO). Whole blood was centrifuged for 30 min at 2000g to separate red blood cells from plasma. Plasma was frozen and stored at −20°C until analysis in the Clinical Chemistry Laboratory at Wake Forest University (Dr. Zak Shihabi). Plasma Fe was reacted with ferrozine as the color reagent and measured with the AVIDA 1650™ (Bayer Corp., Tarrytown, NY). Plasma Tf was measured by turbidimetric immunoassay. TIBC was measured by a colorimetric diagnostic kit (procedure #565, Sigma, St. Louis, MO).
Brain Metal Analysis by Atomic Absorption Spectroscopy
Tissue Mn and Fe concentrations were measured with graphite furnace atomic absorption spectroscopy (Varian AA240, Varian, Inc USA). Brain regions were digested in ultrapure nitric acid (1:10 w/v dilution) for 48–72 h in a sandbath (60°C). A total of 100 μl of digested tissue was brought to 1 ml total volume with 2% nitric acid and analyzed for Mn and Fe.
Determination of F2-Isoprostane Levels
F2-isoprostanes (IsoPs) were quantified in Folch extracts of dissected brain regions using gas chromatography with negative ion chemical ionization mass spectrometry with selective ion monitoring using a published method (Morrow and Roberts 1994; Milatovic et al. 2005). Briefly, samples were extracted by the method of Folch, a stable isotope internal standard added, and then prepared for gas chromatography (GC) through a series of thin-layer chromatography (TLC) and SepPak separations. GC was performed using a fused silica capillary column (Rtx 1701, 15 m × 0.25 mm ID) with column temperature programmed from 190 to 290°C at 20°C/min. The injector temperature was 260°C and He was used as the carrier gas at a flow rate of 1.5 ml/min. Ion source temperature was 270°C, electron energy was 70 eV, and filament current was 0.25 mA, and methane flow rate was 1 ml/min. Negative ion chemical ionization (NICI) mass spectrometry (MS) was performed using a Hewlett-Packard HP5989A GC/MS instrument interfaced with a computer system with ChemStation (NT). Total protein content was determined by BCA assay (Pierce, Rockford, IL) with bovine serum albumin as the standard (Smith et al. 1985).
Morris Water Maze (MWM)
The water tank, made of galvanized steel with a black painted interior, measured 1.45 m in diameter and 58.4 cm in height. A round platform, 12.7 cm in diameter and 38.1 cm in height, was placed centrally in the southwest quadrant of the pool. The platform, also painted black, was constructed of standard plastic polyvinyl chlorine (PVC) pipe and weighted to maintain the platform underneath the surface of the water. Water was colored opaque using a nontoxic powdered paint (Rich Art, Northvale, NJ), and was maintained at 19–21°C throughout the behavioral testing. The pool was surrounded by several extra-maze cues, including several posters, a video monitor, two waste baskets that could be seen from the surface of the pool, and the experimenter, always dressed in a white lab coat and sitting at the ‘south’ release point.
Rats were given eight training trials/day over three consecutive days, with a trial consisting of the rat being released with its nose pointing toward the wall of the pool, and all rats were given 60 s to reach the platform. On each trial, rats were released from one of four randomly assigned release points (N, W, S, and E). Within each daily training session of eight trials, the rat was released twice from each release point. Acquisition latency was defined as the measure of latency to reach platform during acquisition. If the rat failed to reach the platform within 60 s, it was placed on the platform by the experimenter. Regardless of whether the rat located the platform on each trial, it spent the last 10 s of each trial on the platform.
Immediately after the last training trial on the final training day, all rats were given a probe trial in which the platform was removed from the pool. On the probe trial, rats were released from the north release point and allowed to swim for 60 s, with swim patterns recorded by a CCD videocamera (Rockhouse products, NJ) mounted above the pool. The swim patterns were later analyzed on videotape. The dependent measures utilized on the probe trial were the mean zone difference (MZD) and mean search difference (MSD) score, which have both been described elsewhere (Brown et al. 2000, 2001; Gonzalez et al. 2000). Briefly, the amount of time spent in the quadrant that formerly contained the platform (D) was separately subtracted from time spent searching in the other three quadrants (A, B, and C), which did not contain the platform, summed, and divided by three. The MZD is similarly calculated, with the exception that the number of visits to the precise former platform location (D) are recorded and separately subtracted from the number of visits to zones of equal size in the other three quadrants (A, B, and C). The formula for both the MSD and MZD scores follow:
Both the MSD and MZD scores provide a measure of spatial bias to the former platform location relative to the three other non-target quadrants. The higher the score on the either the MSD or MZD score, the stronger the spatial bias to the former platform location. Finally, swim speed was analyzed as cm/s on the probe trial.
Prepulse Inhibition (PPI)
PPI testing was performed in SR-lab sound-attenuated chambers (San Diego Instruments, San Diego, CA, USA). Rats were placed in cylindrical Plexiglas chambers 10 cm in diameter and mounted on a platform inside this chamber located 25 cm below a high-frequency loudspeaker. A 70 dB white noise provided the background auditory stimulus. The animal was placed within the cylinder for testing, and Plexiglas walls kept the animal in the cylinder during testing. The animal was not restrained in these cylinders, but was able to move and turn around within this enclosure. The startle response of the animal was measured through a unit mounted underneath the Plexiglas cylinder that sent an analog signal to the computer that was digitized and stored in the computer. Calibrations were performed before behavioral testing commenced to maintain accurate acoustic stimuli presentations and mechanical-vibration measures.
At the beginning of each daily testing session, all animals were placed into the cylindrical animal enclosure and were exposed to a 70-dB ambient white noise for a 5 min acclimation period. The acclimation period was immediately followed by a test session consisting of the randomized presentation of 32 trials. Of these 32 trials, there were 17 pulse trials and 15 prepulse trials. A pulse trial consisted of a high intensity startle auditory stimulus that was 115 dB in auditory intensity and persisted for 40-ms. There were 15 prepulse trials that consisted of a prepulse auditory stimulus of 73, 76, or 82 dB intensity that were 20 ms in length administered 100 ms before the 40-ms 115 db pulse. The mean response after each pulse and prepulse trial was recorded for 100 ms after the stimulus was administered. The average inter-trial interval was 15 s. One 32 trial session was given each day for 10 consecutive days, and stimuli were randomly arranged on each day. Finally, on each daily testing session, the first two trials were pulse trials and were dropped from the data analysis because of the increased startle response on these trials, which were over 10 standard deviations above the mean of all other trials (Trial 1 and 2 mean = 135 ± 25.3; mean of the final 15 pulse trials = 33 ± 4.8). It has been common in the PPI literature to drop the first few pulse trials or last few pulse trials from the final analysis due to instability of the startle response (Van den Buuse and Eikelis 2001). For all prepulse trials, the percent PPI was calculated using the following equation:
A higher percent PPI implies greater inhibition of startle response due to presentation of the prepulse.
Statistical Analysis
Non-behavioral data were analyzed using Student’s t-test on GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com). Body mass, brain metal accumulation, and blood parameters are presented as mean ± SEM and considered significant at P < 0.05. Individual data points were considered outliers if they were ±2 standard deviations from the mean, and were omitted from statistical analyses. For the analysis of acquisition latency on the MWM, a 2 (dietary condition: IDMn or CN) × 6 (trial blocks) repeated measures ANOVA was utilized. For both MSD and MZD measures on the probe trial, an independent t-test was utilized to analyze the difference between the two dietary conditions. For both the mean startle response and PPI, performance was analyzed on days 1, 4, 7, and 10, identical to the data analyses utilized by Culm and colleagues (Culm et al. 2003; Culm and Hammer 2003) that also utilized a 10-day PPI testing procedure. The mean startle response was analyzed using a 2 × 4 two-way ANOVA with dietary condition (IDMn or CN), and day of testing (Within subject variable: day of testing, four levels) as the two factors. PPI was analyzed separately at each auditory intensity using a 2 × 4 two-way ANOVA with dietary condition (IDMn or CN) and day of testing (Days 1, 4, 7, and 10) as the two factors. Any significant interactions were analyzed using Fisher’s Least Significant Difference (LSD) test (P < 0.05).
Results
Body Mass Analysis
At the beginning of the study, the initial body mass of pregnant dams, prior to assignment to either cohort, was determined to be similar (Fig. 1). Following implementation of the respective diets and body mass, a gross measure of general health, continued to remain comparable between groups up to the birth of pups (week 2). At week 4, however, dams on the IDMn diet were statistically significantly lighter than the control group (P = 0.01), suggesting that they were unable to recover body mass appropriately following birth. By week 6, the IDMn group began gaining body mass that was similar to that of the control animals. Overall, during the course of the study following pregnancy, IDMn dams were statistically significantly lighter than the controls (P < 0.001) as determined linear regression analysis (data not shown) of the respective growth curves. Indeed, at the conclusion of the study, the body mass for the control group was 425 g, compared to 320 g for IDMn animals (***P = 0.001). Food intake was monitored throughout the study and was determined to be similar between groups (data not shown), suggesting that the lack of increase in body mass was due to treatment rather than decreased food consumption by the IDMn animals.
Fig. 1.
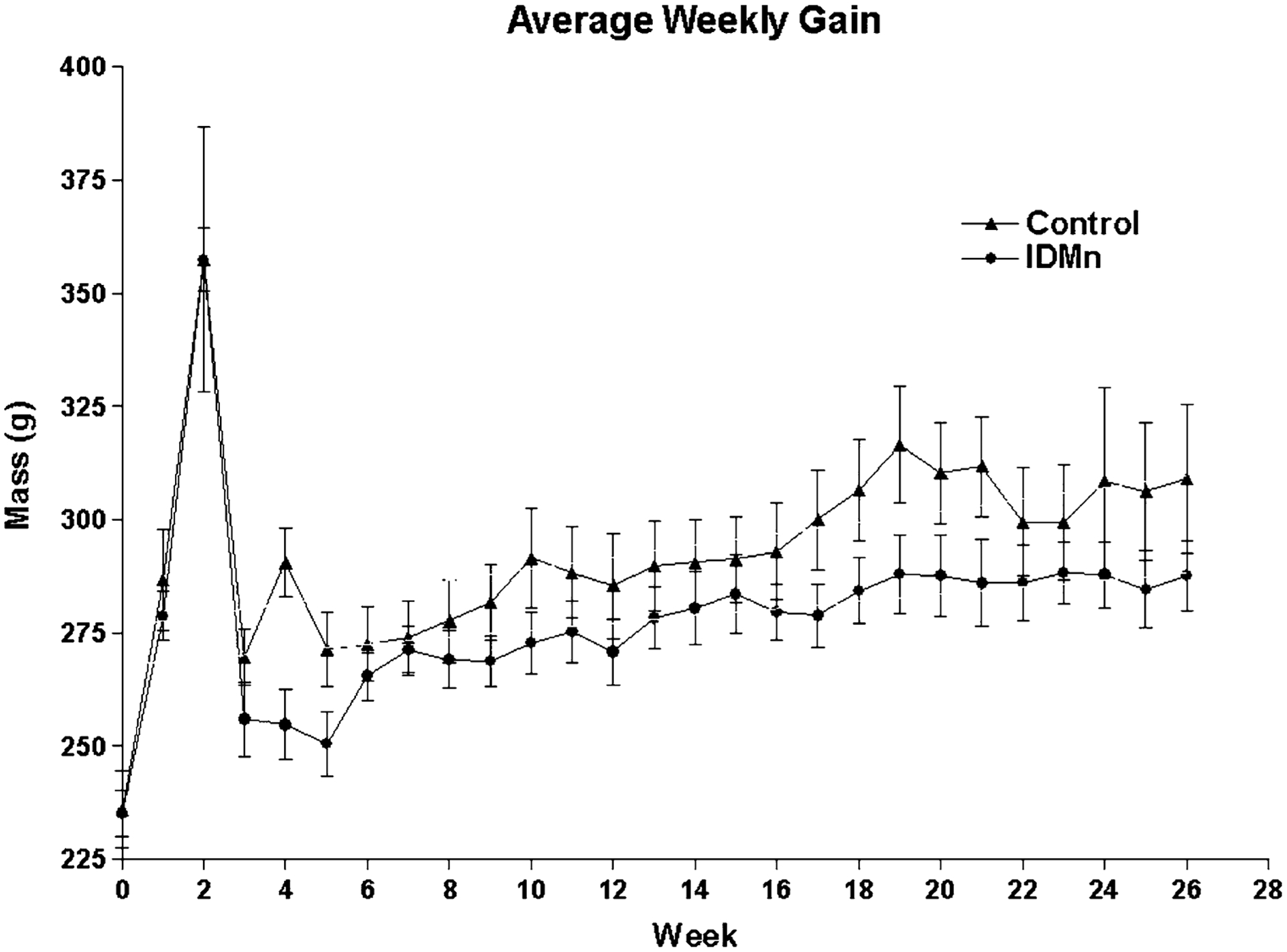
Average weekly gain. Pregnant dams were weighed weekly as a measure of general health. Loss of apparent body mass at week 2 indicates birth of pups. In general, dams on an iron-deficient/high Mn (IDMn) diet were statistically significantly lighter (P < 0.0001) than controls as determined by linear regression
Hematological Parameters
During the initial part of the study, pregnant dams receiving the IDMn diet had decreased Hb levels, although statistical significance was achieved only at PN21 for the IDMn group (Garcia et al. 2007). After PN21 when the pups were weaned but dams remained on their respective diets, Hb levels remained decreased, but not statistically different from CN rats. In general, plasma Fe levels in treated dams were lower than CNs, reaching statistical significant at week 33 (P = 0.0017), but approaching CN levels by week 43 (Table 1). At week 21, both Tf levels and TIBC were statistically significantly higher in IDMn animals compared to CN (P = 0.0038 for Tf and TIBC). As with plasma Fe levels, these two clinical measures of ID began to approach those of CNs by week 33, with the trend continuing through week 43. Taken together, these data suggest that rats on an IDMn diet were marginally ID throughout the study, appropriately modeling to what is often observed in human populations (Looker et al. 1997).
Table 1.
Clinical blood data
| Blood parameters | Week 3 | Week 21 | Week 33 | Week 43 | ||||
|---|---|---|---|---|---|---|---|---|
| Control | IDMn | Control | IDMn | Control | IDMn | Control | IDMn | |
| Hb (mg/ml) | 12.89 ± 2.10 | 11.50 ± 2.10 | 11.53 ± 0.59 | 11.07 ± 0.34 | N/A | N/A | 19.24 ± 1.04 | 22.00 ± 1.58 |
| Fe (μg/dl) | N/A | N/A | 196.50 ± 24.74 | 134.20 ± 15.46 | 215.00 ± 6.35 | 135.33 ± 3.71* | 218.67 ± 56.69 | 142.00 ± 41.13 |
| Tf (mg/dl) | N/A | N/A | 88.67 ± 5.81 | 142.2 ± 8.20 | 114.67 ± 2.91 | 131.50 ± 11.53 | 136.50 ± 36.02 | 96.33 ± 9.82 |
| TIBC (μg/dl) | N/A | N/A | 126.77 ± 8.31 | 203.36 ± 11.72 | 163.97 ± 7.95 | 188.05 ± 16.50* | 195.20 ± 51.50 | 137.77 ± 14.05 |
Student’s t-test determined a statistically significant difference in blood-iron levels between control and treated animals during week 33. Other parameters showed strong trends in differences between control and treated dams. The lack of statistical significance is likely due to the small sample size in this pilot study
P < 0.05 compared to control
Brain Masses
Following completion of the study, brains were removed from the treated animals during week 33. Other parameters showed strong trends in differences between control and treated dams. The lack of statistical significance is likely due to the small sample size in this pilot study (*P < 0.05) compared to control.
Metal Accumulation
Following completion of the study, brains were removed from the animals and dissected into six discrete regions: cerebellum, brain stem (pons and medulla), midbrain (including thalamus), hippocampus, striatum, and cortex. Although decreased brain mass is often associated with severe ID, there was no statistically significant difference in regional brain masses between the two groups (Fig. 2). This is consistent with the hematological parameters, suggesting that treated animals in this study were only mildly ID.
Fig. 2.
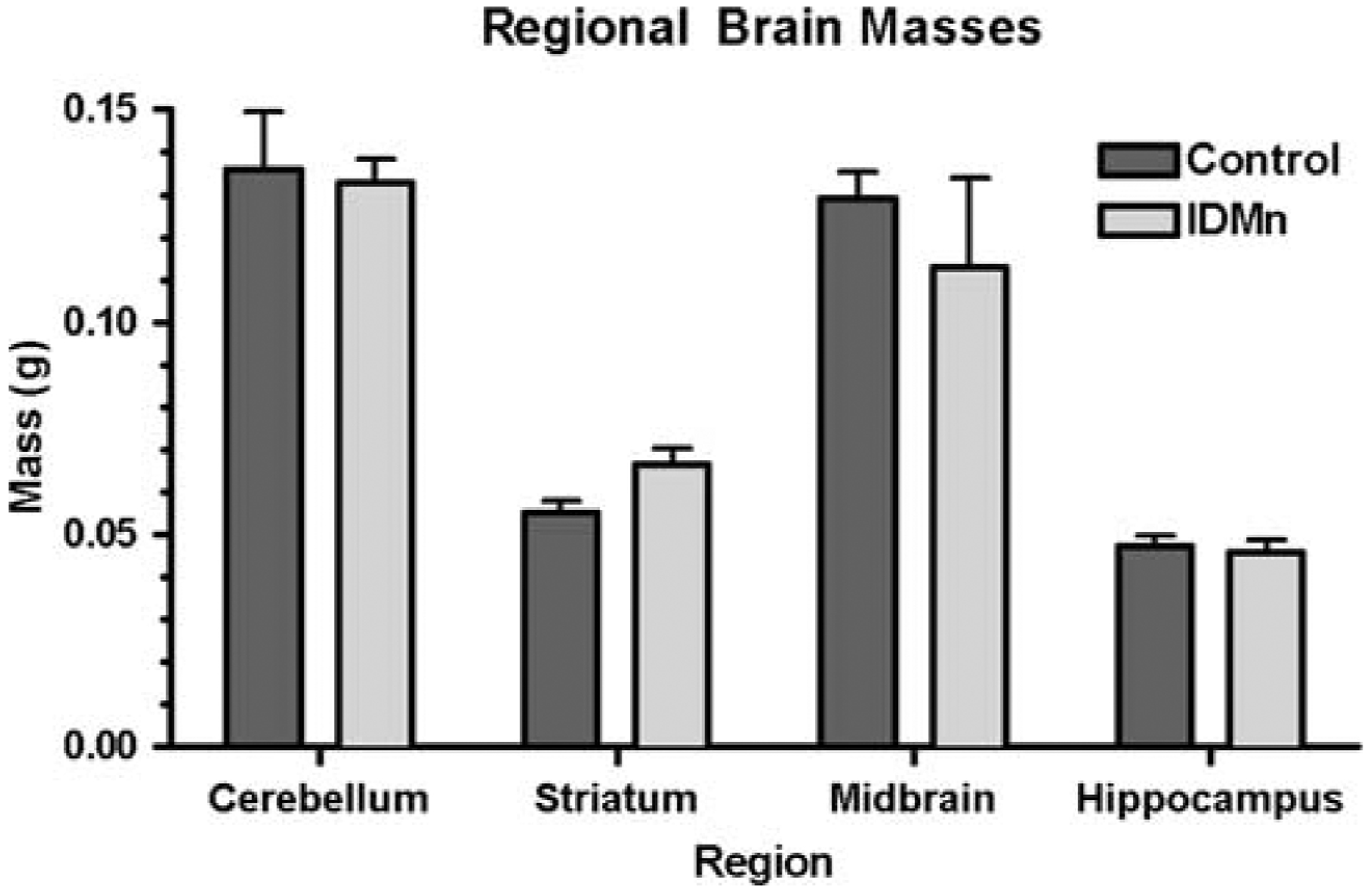
Regional brain masses. Brains were removed and dissected into various regions for metal and isoprostane analysis. No statistically significant difference in regional wet mass was determined for each region
Brain metal accumulation was also determined (Fig. 3) in discrete regions from the right hemispheres from both groups. Student’s t-test determined that there was significant Mn accumulation (P = 0.05) in the hippocampus in the treated dams. A trend toward increased Mn levels was also observed in the cerebellum (P = 0.062). Interestingly, this change in Mn concentration was accompanied by a concomitant increase in hippocampal Fe (P = 0.0062), but a decease in cerebellar Fe (P = 0.035). Both metals were similar to controls in the other regions assayed.
Fig. 3.
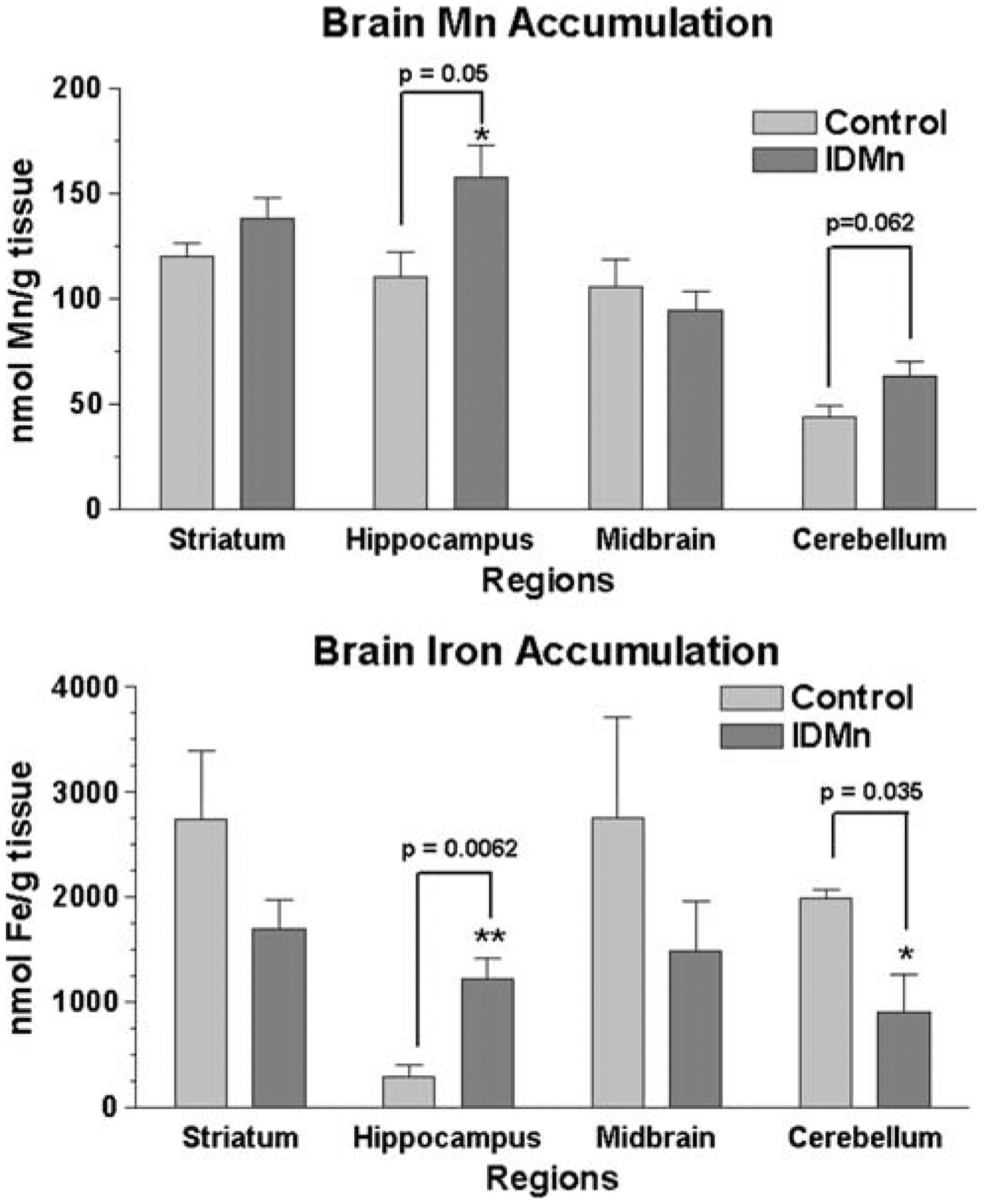
Brain metal accumulation. Metal amount was determined by atomic absorption spectroscopy (AAS). Student’s t-test indicated significant regional accumulation of, a Mn or b iron in treated animals compared to controls. Mn (P = 0.05) and iron (P = 0.0062) were higher in the hippocampus of treated rats. In the cerebellum, iron levels were reduced (P = 0.035), while Mn content approached statistical significance (P = 0.062) compared to cerebella from animals on a control diet
Regional Brain Oxidative Stress Endpoints
In order to assess whether alteration in brain Fe and Mn accumulation led to increased oxidative stress, regions from the left hemispheres were used for F2-isoprostane (IsoP) analysis (Fig. 4). IsoP formation was statistically significantly decreased in the striatum (P = 0.0064), but significantly increased in the brain stem (P = 0.0487) in IDMn animals compared to CN. It should be noted that IsoP levels approached significance (P = 0.1410) in the midbrain. However, tissue storage and preparation was not optimized for this type of analysis and may have obscured the results.
Fig. 4.
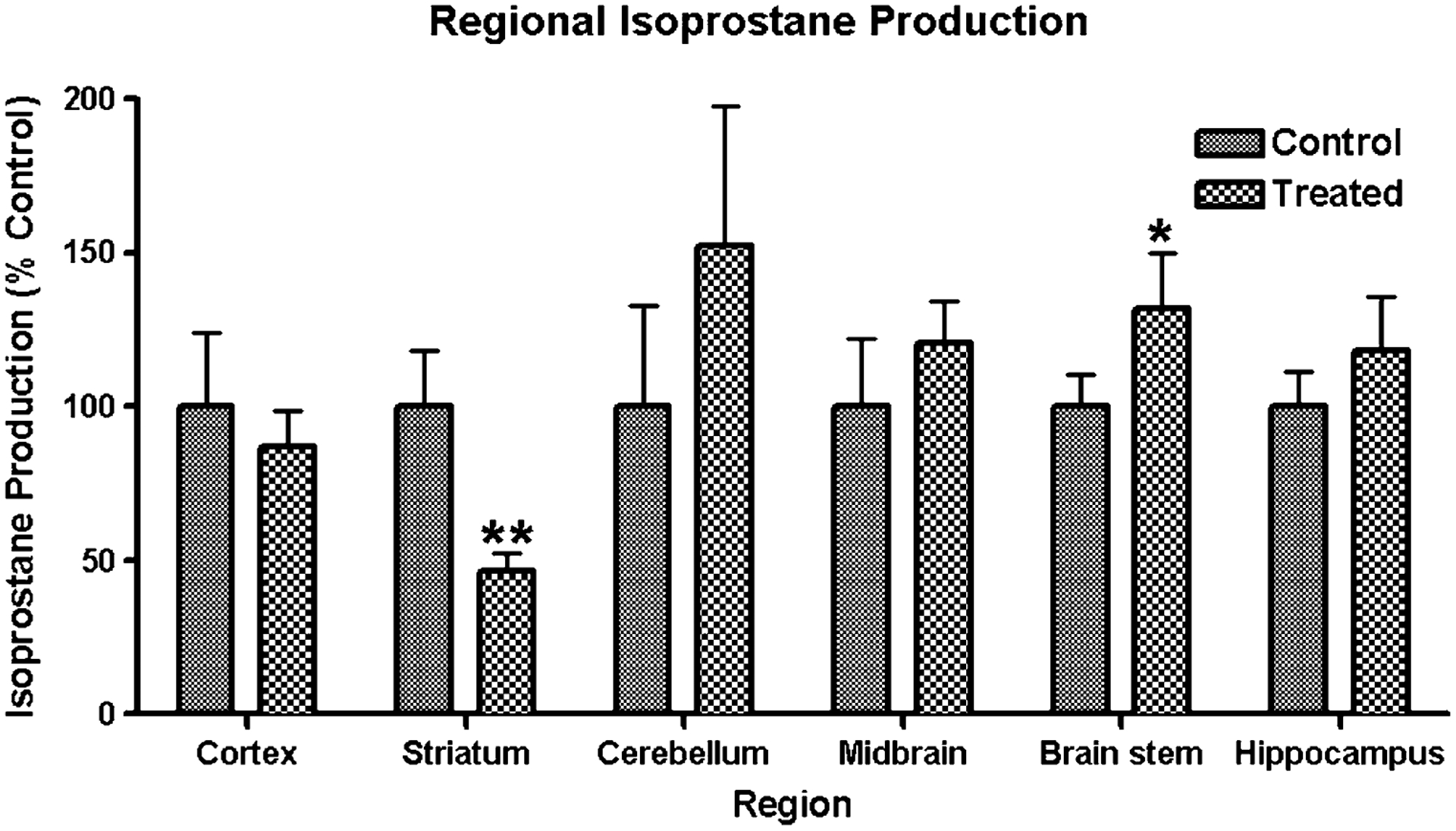
Regional isoprostane (IsoP) formation. IsoP levels were determined by mass spectrometery as described in the Sect. Material and Methods. Data is presented as percent control IsoP production, mean ± SEM. *P = 0.0487, **P = 0.0064
Morris Water Maze (MWM)
Acquisition latency is presented in Fig. 5a, and the probe trial measures MSD and MZD are presented in Fig. 5b and c, respectively. Observation of acquisition latency results did not appear to show any significant differences between the two dietary conditions. Indeed, a 2 × 6 repeated measure ANOVA revealed no significant main effects of dietary condition or a significant interaction of Dietary condition × Trial block, although there was a significant main effect of trial block F(5,35) = 16.02, P < 0.001. Therefore, the dietary metal status did not produce any significant effects on acquisition performance, although both groups appear to significantly improve the latency to locate the platform over trials. Observation of the probe trial results appeared to show a cognitive deficit in animals administered the IDMn diet. An independent t-test on each of the probe trial measures support this claim, and revealed a significant main effect of dietary condition for both the MSD score [t(7) = 15.85, P < 0.005] and the MZD score [t(7) = 5.78, P < 0.04]. Rats administered with the IDMn diet demonstrated a significant deficit on both probe trial measures as compared to controls. This result demonstrates that a long-term ID diet fortified with Mn can result in impairment in spatial performance. A similar analysis of swim speed revealed no significant differences between the groups. Thus, a decrease in swim speed cannot account for the results observed on the MWM.
Fig. 5.
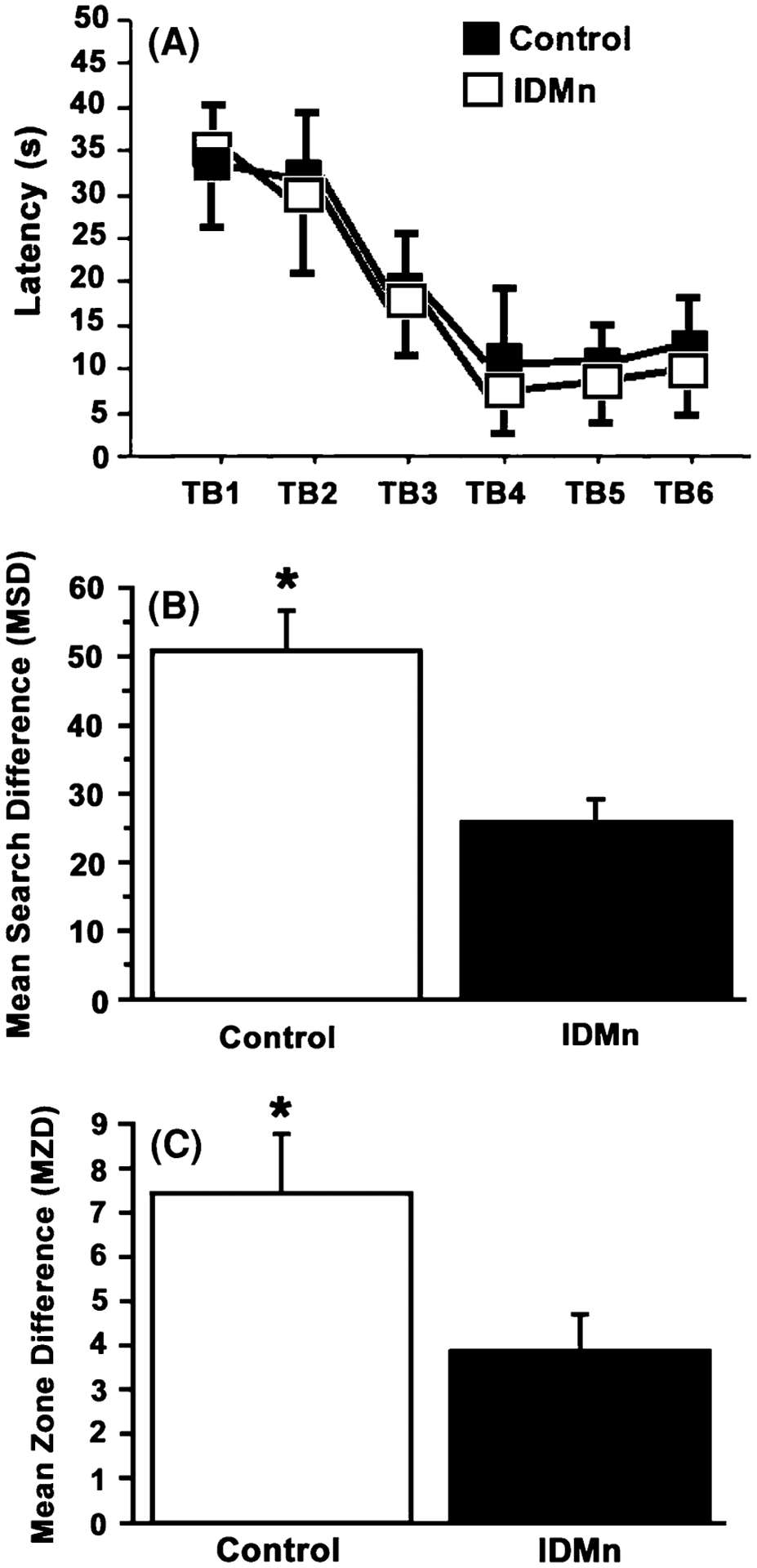
Morris water maze. a Acquisition latency is represented as a function of trial block for both control and IDMn groups. There were no significant differences between groups; b Mean search difference (MSD) score is presented as a function of group. The IDMn group demonstrated a significantly lower MSD score on the probe trial as compared to controls, demonstrating these animals searched less frequently in the quadrant formerly containing the platform as compared to controls; c Mean zone difference (MZD) score is presented as a function of group. Similar to the MSD score, the IDMn group demonstrated a significantly lower MZD score on the probe trial as compared to controls, demonstrating they made less visits to the former platform location as compared to controls. *P < 0.05
Prepulse Inhibition (PPI)
The results for the 73-, 76-, and 82-dB prepulse trials are presented in Fig. 6a, b and c, respectively. Through observation of these figures, it appears that CN animals demonstrated improved PPI performance compared to IDMn animals, especially at the 73-and 76-dB prepulse auditory intensities. However, separate ANOVA on each of the three auditory intensities did not reveal any significant main effects of dietary condition, nor any significant interactions of dietary condition with the repeated measure. However, there was a significant main effect of day of testing at each auditory intensity 73-dB: F(1,8) = 4.19, P < 0.01; 76-dB F(1,8) = 13.74, P < 0.001, and 82-dB F(1,8) = 3.32, P < 0.04, demonstrating that there was indeed a change in PPI responding over days of testing. Additionally, a 2 × 4 ANOVA on mean startle response (results not shown) did not reveal any significant main effects of dietary condition. Therefore, it does not appear that IDMn diet produced any significant deficits in PPI performance regardless of the auditory intensity.
Fig. 6.
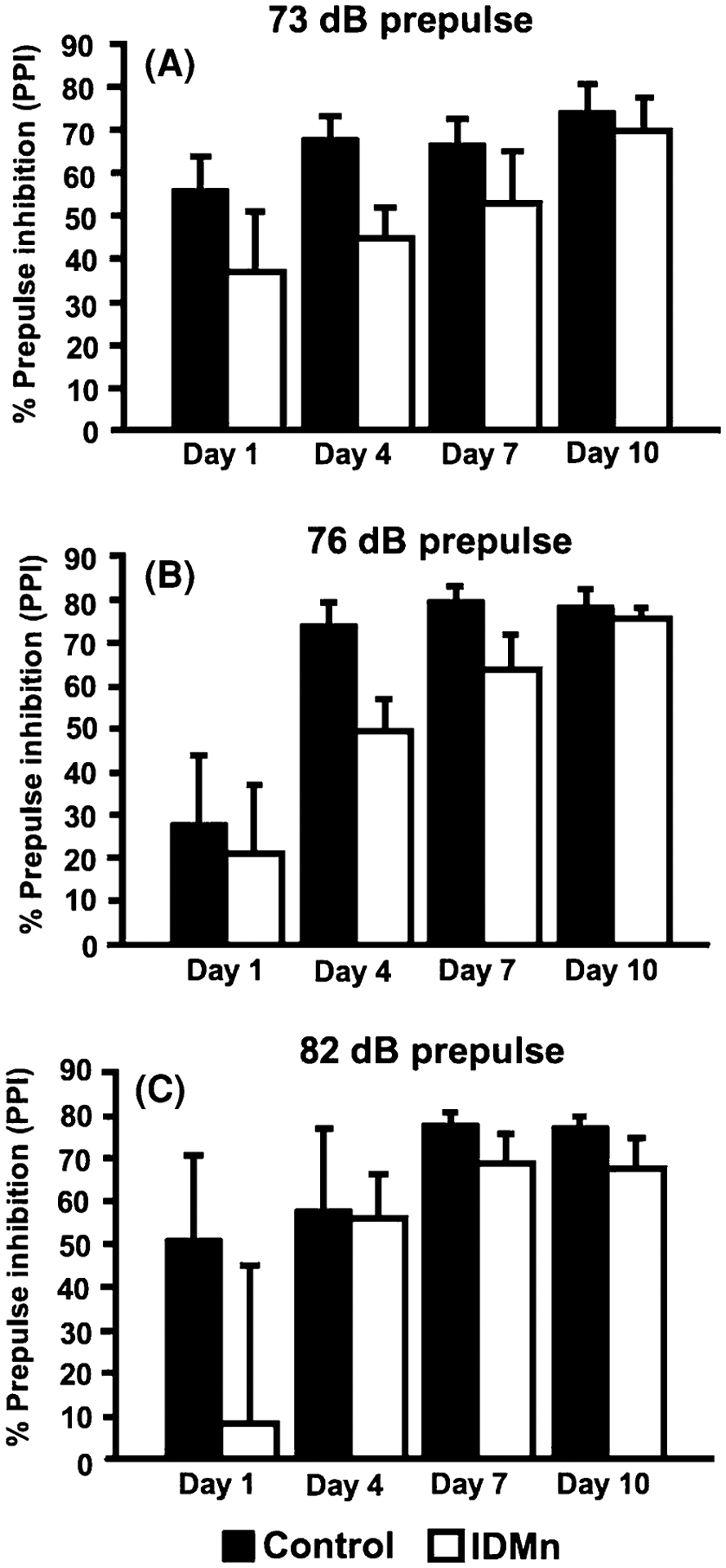
Prepulse inhibition. a 73 dB prepulse trials; b 76 dB prepulse trials; c 82 dB prepulse trials. There were no statistically significant differences between groups regardless of the prepulse auditory intensity
Discussion
Numerous reports have indicated that ID in infants and young children can lead to long-term decreases in cognitive functioning (Eden 2005; Iannotti et al. 2006; Zhou et al. 2006). It is also known that ID alone in rodents may lead to abnormal brain Mn regulation without altering Mn intake (Erikson et al. 2002, 2004; Beard and Connor 2003). While numerous studies have examined the individual effects of ID or high levels of Mn, to our knowledge no groups have studied the combination of the two treatments in the female rats. In the current study, we set out to determine whether dietary ID coupled with increased Mn levels (IDMn diet) would lead to behavioral changes often associated with ID. Generally, women, especially pregnant women, as well as children from economically disadvantaged areas are more prone to ID or anemia than other groups (Prevention CfDCa 1998; Siega-Riz et al. 2006; Skalicky et al. 2006). Additionally, there is a renewed concern of potential increase in chronic Mn exposure due to the proposed addition of MMT to gasoline as an anti-knocking agent. It is thus possible that ID populations will also be exposed to potentially high levels of Mn.
As pregnant women are more likely to suffer from ID than other groups, we utilized pregnant Sprague–Dawley dams, following birth of viable litters, fed an IDMn diet both during pregnancy and for 42 weeks beyond giving birth. General body mass, clinical blood parameters, brain metal levels, and two behavioral tests valid to cognitive function were conducted to assess the effects of this treatment in the IDMn adults compared to CN animals. The results from this work are also significant in light of the fact that previous studies have looked at developing male rats. Females are known to respond differently than males to both ID and Mn exposure in terms of neurobiological outcomes (Anderson et al. 2007). Thus, our model may shed more light on unique biological outcomes relevant to pregnant females and women following child-birth than other studies in the literature.
The treatment protocol was devised in order to produce only mild ID, and the blood data demonstrate that IDMn animals tended to have reduced Hb and Fe, with higher Tf and TIBC, without inducing full-blown anemia (Fig. 3). As most people who suffer from anemia demonstrate only mild changes in blood parameters, this model appears to be an accurate representation of what is observed in humans. Brain Fe levels, however, were generally decreased in the entire brain, consistent with consumption of an Fe-deficient diet. Interestingly, by the final week of treatment, blood parameters appeared to be approaching more normal levels. This likely indicates that homeostatic mechanisms in these dams were returning to a healthy, more CN-like phenotype.
Isoprostanes (IsoPs) are formed when arachodonic acid in the cellular membrane undergoes free radical attack. Thus, excess production is often used as an indicator of oxidative stress (Cai et al. 2007; Franco et al. 2007; Milatovic et al. 2007). Although we were able to detect regional variations in brain IsoP levels, it is difficult to interpret this data. This could be due to the fact that the tissue preparation was not optimized for this type of analysis. However, when regional brain metal data is compared with IsoP data, it is interesting to note that decreased striatal Fe levels mirrored decreased production of IsoPs, although the same trend was not observed for the cerebellum. Thus, it appears that, while increased oxidative stress may be occurring in the striatum, midbrain, and brain stem of treated animals, it may not directly correlate to brain metal levels. It is known that dietary changes in Mn and Fe levels not only perturb these metals, but many others as well (Garcia et al. 2007). It is likely that the relationship between Fe and/or Mn and IsoP levels is not as straightforward as originally anticipated.
While it is known that severe ID in humans leads to decreased cognitive functioning in infants and children, we wanted to determine whether similar phenomena are also observed in adult female animals. Consistent with data from younger animals (Burhans et al. 2006; Felt et al. 2006), our adult females (IDMn) also demonstrated impaired cognitive performance on the MWM as compared to CN rats. Regional brain metal analysis indicated that both Mn and Fe deposition were significantly altered in treated rats, particularly in the hippocampus. Conversely, hippocampal levels of IsoPs, used as a marker for oxidative stress, were not statistically significantly different in IDMn dams compared to CN rats. Interestingly, there were no statistically significant effects on auditory sensorimotor gating as measured by PPI, which has also been shown to be affected by loss of function in the hippocampus or hippocampal formation (Caine et al. 1991, 1992), although PPI function appears to be mediated by a complex circuit involving basal forebrain structures, the frontal cortex, as well as areas of the brain stem (Swerdlow et al. 2000).
Specific to the hippocampus, studies have shown that PPI function appears to be primarily affected by ablation of the ventral hippocampus, but only if the lesion is given during development, (Lipska et al. 1995a, b; Le Pen et al. 2002; Le Pen and Moreau 2002; Daenen et al. 2003), whereas MWM performance has been shown to be more susceptible to the damage of the dorsal, but not ventral hippocampus (Moser et al. 1993, 1995). These behavioral data may indicate that the dorsal hippocampus is more vulnerable to ID. Thus, cognitive impairments may be more directed at tasks involving complex cognitive associations related to the dorsal hippocampus versus a disruption in development of the ventral hippocampus and related structures, which appears to be important in sensorimotor gating. This hypothesis is speculative at this point, and different regions of the hippocampus must be analyzed to determine whether ID is more disruptive to the dorsal hippocampus versus other subregions of this structure.
Regarding MWM performance, there were no significant effects on acquisition of the platform location, but there were significant deficits in IDMn rats on the probe trial as compared to controls. Studies have shown that rats with a brain insult, if given the appropriate training procedures, may not demonstrate deficits on acquisition latency, but rather show poor performance on the probe trial at the end of training. Although this may seem contradictory, several studies have demonstrated a dissociation between acquisition latency and probe trial performance in rats tested on the MWM (Scheff et al. 1997; Gerlai 2001; Brown et al. 2002). Whishaw et al. (1995) have distinguished between the ability to reach the platform during acquisition and probe trial performance as “getting there” and “knowing where”. In essence, rats can learn a strategy to navigate to the platform during acquisition, such as a motor or landmark strategy, but may not necessarily have a cognitive representation of where the platform is located. Hypothetically, if an animal has memorized a strategy to locate the platform during acquisition and the probe trial is administered, the strategy of memorizing a set of movements to reach the platform location will fail, once the animal reaches the former platform site. Conversely, a rat with knowledge of the former platform location on the probe trial can revert to several different strategies, including extra-maze cue associations, path integration, allothetic or kinesthethic cues, as well as other previously successful search strategies.
In this experiment, it appears that IDMn rats were able to learn a specific strategy to locate the platform during acquisition, but did not have the ability to integrate these strategies on the probe trial. This suggests that iron deficiency compounded by high Mn levels may result in an inability to integrate information on behavioral tasks that are more demanding on the cognitive system. It is possible that the changes we observed were due to alterations in glutamate metabolism or transport, since glutamate plays an indispensable role in learning and memory (Richter-Levin et al. 1995; Riedel and Reymann 1996). Additionally, other data suggest abnormal brain Mn accumulation affects various aspects of glutamatergic systems (Fitsanakis et al. 2006; Crooks et al. 2007; Erikson et al. 2007). Interestingly, chronic liver failure, which can lead to increased brain Mn accumulation, has recently been shown to impair glutamate transmission in vivo (Monfort et al. 2007).
In conclusion, our data suggest that an IDMn diet is useful in producing mild ID in adult female rats, without severe anemia, in dams during and following pregnancy. Additionally, this treatment leads to altered regional brain deposition of both Mn and Fe, which resulted in spatial memory deficits in a behavioral task requiring complex cognitive associations. These data suggest that, while blood Fe parameters in IDMn animals may approach CN levels after almost 1 year of treatment, lingering cognitive changes may persist in adult females long after pregnancy. More studies need to be completed to demonstrate that reversal of the dietary changes also lead to a reversal of cognitive impairments. However, our data suggest that vulnerable ID populations exposed to high levels of Mn are indeed at risk of potentially dangerous alterations in brain metal levels.
Contributor Information
Vanessa A. Fitsanakis, Department of Biology, King College, Bristol, TN 37620, USA
Kimberly N. Thompson, Department of Psychology, East Tennessee State University, Johnson City, TN 37614, USA
Dejan Milatovic, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN 37232, USA.
Zak K. Shihabi, Department of Pathology, Wake Forest University School of Medicine, Winston-Salem, NC 27157, USA
Keith M. Erikson, Department of Nutrition, University of North Carolina Greensboro, Greensboro, NC 27402-6170, USA
Russell W. Brown, Department of Psychology, East Tennessee State University, Johnson City, TN 37614, USA Department of Anatomy and Cell Biology, Quillen College of Medicine, East Tennessee State University, Johnson City, TN 37614, USA.
Michael Aschner, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN 37232, USA; Department of Pharmacology, Center for Molecular Neuroscience, Vanderbilt University Medical Center, 6110 MRB-III, 1161 21st Avenue South, Nashville, TN 37232-2495, USA.
References
- Anderson JG, Cooney PT, Erikson KM (2007) Brain manganese accumulation is inversely related to gamma-amino butyric acid uptake in male and female rats. Toxicol Sci 95:188–195 [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR (2003) Iron status and neural functioning. Annu Rev Nutr 23:41–58 [DOI] [PubMed] [Google Scholar]
- Bolte S, Normandin L, Kennedy G, Zayed J (2004) Human exposure to respirable manganese in outdoor and indoor air in urban and rural areas. J Toxicol Environ Health A 67:459–467 [DOI] [PubMed] [Google Scholar]
- Boudia N, Halley R, Kennedy G, Lambert J, Gareau L, Zayed J (2006) Manganese concentrations in the air of the Montreal (Canada) subway in relation to surface automobile traffic density. Sci Total Environ 366:143–147 [DOI] [PubMed] [Google Scholar]
- Brain JD, Heilig E, Donaghey TC, Knutson MD, Wessling-Resnick M, Molina RM (2006) Effects of iron status on transpulmonary transport and tissue distribution of Mn and Fe. Am J Respir Cell Mol Biol 34:330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Bardo MT, Mace DD, Phillips SB, Kraemer PJ (2000) d-amphetamine facilitation of Morris water task performance is blocked by eticlopride and correlated with increased dopamine synthesis in the prefrontal cortex. Behav Brain Res 114:135. [DOI] [PubMed] [Google Scholar]
- Brown RW, Gonzalez CL, Whishaw IQ, Kolb B (2001) Nicotine improvement of Morris water task performance after fimbria–fornix lesion is blocked by mecamylamine. Behav Brain Res 119:185–192 [DOI] [PubMed] [Google Scholar]
- Brown R, Gass J, Kostrzewa R (2002) Ontogenetic quinpirole treatments produce spatial memory deficits and enhance skilled reaching in adult rats. Pharmacol Biochem Behav 72:591–600 [DOI] [PubMed] [Google Scholar]
- Burhans MS, Dailey C, Wiesinger J, Murray-Kolb LE, Jones BC, Beard JL (2006) Iron deficiency affects acoustic startle response and latency, but not prepulse inhibition in young adult rats. Physiol Behav 87:917. [DOI] [PubMed] [Google Scholar]
- Cai W, He JC, Zhu L, Chen X, Wallenstein S, Striker GE, Vlassara H (2007) Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression. Am J Pathol 170:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR (1991) Carbachol infusion into the dentate gyrus disrupts sensorimotor gating of startle in the rat. Psychopharmacology (Berl) 105:347–354 [DOI] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR (1992) Hippocampal modulation of acoustic startle and prepulse inhibition in the rat. Pharmacol Biochem Behav 43:1201–1208 [DOI] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W (1994) Manganism and idiopathic parkinsonism: similarities and differences. Neurology 44:1583–1586 [DOI] [PubMed] [Google Scholar]
- Crooks DR, Welch N, Smith DR (2007) Low-level manganese exposure alters glutamate metabolism in GABAergic AF5 cells. Neurotoxicology 28:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culm KE, Hammer RP Jr (2003) Recovery of sensorimotor gating without G protein adaptation after repeated D2-like dopamine receptor agonist treatment in rats. J Pharmacol Exper Ther 308:487–494 [DOI] [PubMed] [Google Scholar]
- Culm K, Lim A, Onton J, Hammer R Jr (2003) Reduced G(i) and G(o) protein function in the rat nucleus accumbens attenuates sensorimotor gating deficits. Brain Res 982:12–18 [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Van Der Heyden JA, Kruse CG, Van Ree JM (2003) Neonatal lesions in the amygdala or ventral hippocampus disrupt prepulse inhibition of the acoustic startle response; implications for an animal model of neurodevelopmental disorders like schizophrenia. Eur Neuropsychopharmacol 13:187–197 [DOI] [PubMed] [Google Scholar]
- Duque X, Flores-Hernandez S, Flores-Huerta S, Mendez-Ramirez I, Munoz S, Turnbull B, Martinez-Andrade G, Ramos RI, Gonzalez-Unzaga M, Mendoza ME, Martinez H (2007) Prevalence of anemia and deficiency of iron, folic acid, and zinc in children younger than 2 years of age who use the health services provided by the Mexican Social Security Institute. BMC Public Health 7:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden AN (2005) Iron deficiency and impaired cognition in toddlers: an underestimated and undertreated problem. Paediatr Drugs 7:347–352 [DOI] [PubMed] [Google Scholar]
- Erikson KM, Shihabi ZK, Aschner JL, Aschner M (2002) Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res 87:143–156 [DOI] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Steinnes E, Aschner M (2004) Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. J Nutr Biochem 15:335–341 [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dorman DC, Lash LH, Aschner M (2007) Manganese inhalation by rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Toxicol Sci 97:459–466 [DOI] [PubMed] [Google Scholar]
- Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff MK, Lozoff B (2006) Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res 171:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsanakis VA, Au C, Erikson KM, Aschner M (2006) The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation. Neurochem Int 48:426–433 [DOI] [PubMed] [Google Scholar]
- Franco MC, Kawamoto EM, Gorjao R, Rastelli VM, Curi R, Scavone C, Sawaya AL, Fortes ZB, Sesso R (2007) Biomarkers of oxidative stress and antioxidant status in children born small for gestational age: evidence of lipid peroxidation. Pediatr Res 62(2):204–208 [DOI] [PubMed] [Google Scholar]
- Garcia Avila M, Penalver Ballina R (1953) Manganese poisoning in the mines of Cuba. Ind Med Surg 22:220–221 [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M (2007) Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol Sci 95:205–214 [DOI] [PubMed] [Google Scholar]
- Gerlai R (2001) Behavioral tests of hippocampal function: simple paradigms complex problems. Behav Brain Res 125:269–277 [DOI] [PubMed] [Google Scholar]
- Geyer MA (2006a) Are cross-species measures of sensorimotor gating useful for the discovery of procognitive cotreatments for schizophrenia? Dialogues Clin Neurosci 8:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA (2006b) The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res 10:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 156:117–154 [DOI] [PubMed] [Google Scholar]
- Gonzalez CLR, Miranda MI, Gutierrez H, Ormsby C, Bermudez-Rattoni F (2000) Differential participation of the NBM in the acquisition and retrieval of conditioned taste aversion and Morris water maze. Behav Brain Res 116:89. [DOI] [PubMed] [Google Scholar]
- Haider BA, Bhutta ZA (2006) Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev:CD004905. [DOI] [PubMed] [Google Scholar]
- Iannotti LL, Tielsch JM, Black MM, Black RE (2006) Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr 84:1261–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT (2005) Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology 64:2033–2039 [DOI] [PubMed] [Google Scholar]
- Knovich MA, Il’yasova D, Ivanova A, Molnar I (2008) The association between serum copper and anaemia in the adult Second National Health and Nutrition Examination Survey (NHANES II) population. Br J Nutr 99:1226–1229 [DOI] [PubMed] [Google Scholar]
- Le Pen G, Moreau JL (2002) Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology 27:1–11 [DOI] [PubMed] [Google Scholar]
- Le Pen G, Gaudet L, Mortas P, Mory R, Moreau JL (2002) Deficits in reward sensitivity in a neurodevelopmental rat model of schizophrenia. Psychopharmacology (Berl) 161:434–441 [DOI] [PubMed] [Google Scholar]
- Lipska BK, Chrapusta SJ, Egan MF, Weinberger DR (1995a) Neonatal excitotoxic ventral hippocampal damage alters dopamine response to mild repeated stress and to chronic haloperidol. Synapse 20:125–130 [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR (1995b) Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 122:35–43 [DOI] [PubMed] [Google Scholar]
- Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL (1997) Prevalence of iron deficiency in the United States. J Am Med Assoc 227:973–976 [DOI] [PubMed] [Google Scholar]
- Mannar MV (2006) Successful food-based programmes, supplementation and fortification. J Pediatr Gastroenterol Nutr 43(suppl 3):S47–S53 [DOI] [PubMed] [Google Scholar]
- Milatovic D, VanRollins M, Li K, Montine K, Montine T (2005) Suppression of murine cerebral F2-isoprostanes and F4-neuroprostanes from excitotoxicity and innate immune response in vivo by alpha- or gamma-tocopherol. J Chromatogr B Analyt Technol Biomed Life Sci 827:88–93 [DOI] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M (2007) Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol Sci 98:198–205 [DOI] [PubMed] [Google Scholar]
- Monfort P, Erceg S, Piedrafita B, Llansola M, Felipo V (2007) Chronic liver failure in rats impairs glutamatergic synaptic transmission and long-term potentiation in hippocampus and learning ability. Eur J NeuroSci 25:2103–2111 [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ II (1994) Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical-catalyzed mechanism. Methods Enzymol 233:163–174 [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P (1993) Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 13:3916–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG (1995) Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA 92:9697–9701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P, Samii A, Calne D (1999) Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology 20:227–238 [PubMed] [Google Scholar]
- Pathak P, Kapil U, Kapoor SK, Saxena R, Kumar A, Gupta N, Dwivedi SN, Singh R, Singh P (2004) Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr 71:1007–1014 [DOI] [PubMed] [Google Scholar]
- Prevention CfDCa (1998) Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 47:1–29 [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS (2001) Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology 56:8–13 [DOI] [PubMed] [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B (2005) Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology 64:230–235 [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Canevari L, Bliss TV (1995) Long-term potentiation and glutamate release in the dentate gyrus: links to spatial learning. Behav Brain Res 66:37–40 [DOI] [PubMed] [Google Scholar]
- Riedel G, Reymann KG (1996) Metabotropic glutamate receptors in hippocampal long-term potentiation and learning and memory. Acta Physiol Scand 157:1–19 [DOI] [PubMed] [Google Scholar]
- Rodier J (1955) Manganese poisoning in Moroccan miners. Br J Ind Med 12:21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek AH, Rauch R, Schulz PE (2003) Parkinsonism due to manganism in a welder. Int J Toxicol 22:393–401 [DOI] [PubMed] [Google Scholar]
- Samadpour K, Sheikholeslam R, Abdollahi Z, Salehi FM (2004) The effect of weekly dose of iron supplementation for 16 and 20 week on the iron status of adolescent girls in Iran. Asia Pac J Clin Nutr 13:S135 [Google Scholar]
- Scheff S, Baldwin S, Brown R, Kraemer P (1997) Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma 14:615–627 [DOI] [PubMed] [Google Scholar]
- Shoham S, Youdim MB (2002) The effects of iron deficiency and iron and zinc supplementation on rat hippocampus ferritin. J Neural Transm 109:1241–1256 [DOI] [PubMed] [Google Scholar]
- Siega-Riz AM, Hartzema AG, Turnbull C, Thorp J, McDonald T, Cogswell ME (2006) The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. Am J Obstet Gynecol 194:512–519 [DOI] [PubMed] [Google Scholar]
- Simopoulos AP (1981) Overview of nutritional status in the United States. Prog Clin Biol Res 67:237–247 [PubMed] [Google Scholar]
- Skalicky A, Meyers AF, Adams WG, Yang Z, Cook JT, Frank DA (2006) Child food insecurity and iron deficiency anemia in low-income infants and toddlers in the United States. Matern Child Health J 10:177–185 [DOI] [PubMed] [Google Scholar]
- Smith P, Krohn R, Hermanson G, Mallia A, Gartner F, Provenzano M, Fujimoto E, Goeke N, Olson B, Klenk D (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85 [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Taaid N, Halim N, Randolph E, Kim Y, Auerbach P (2000) Hippocampal lesions enhance startle gating-disruptive effects of apomorphine in rats: a parametric assessment. Neuroscience 96:523–536 [DOI] [PubMed] [Google Scholar]
- Umbreit J (2005) Iron deficiency: a concise review. Am J Hematol 78:225–231 [DOI] [PubMed] [Google Scholar]
- Van den Buuse M, Eikelis N (2001) Estrogen increases prepulse inhibition of acoustic startle in rats. Eur J Pharmacol 425:33–41 [DOI] [PubMed] [Google Scholar]
- Whishaw I, Cassel J, Jarrad L (1995) Rats with fimbria-fornix lesions display a place response in a swimming pool: a dissociation between getting there and knowing where. J Neurosci 15:5779–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2003) Micronutrient deficiencies: Battling iron deficiency anemia: the challenge. http://www.who.int/nut/ida.htm
- Youdim MB, Ben-Shachar D, Yehuda S (1989) Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am J Clin Nutr 50:607–615 (discussion 615–607) [DOI] [PubMed] [Google Scholar]
- Zayed J, Guessous A, Lambert J, Carrier G, Philippe S (2003) Estimation of annual Mn emissions from MMT source in the Canadian environment and the Mn pollution index in each province. Sci Total Environ 312:147–154 [DOI] [PubMed] [Google Scholar]
- Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M (2006) Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 years of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr 83:1112–1117 [DOI] [PubMed] [Google Scholar]


