Abstract
Spinal cord injury (SCI) results in perturbations to the immune system leading to increased infection susceptibility. In parallel, the consumption of high-fat diets (HFD) leads to a chronic inflammation in circulation and body tissues. We investigated the impact of an HFD on chronic SCI. SCI rats under both chow and HFD had chronic peripheral leukocyte changes that include reduced percentages of total, helper and cytotoxic T, and natural killer cells. Expression of immune-related genes in the spleen and thymus reflected impact of both chronic injury and diet. Changes to the immune system following SCI are adversely impacted by HFD consumption.
Keywords: spinal cord injury, immune system, thymus, spleen, obesity, high-fat diet
1. INTRODUCTION
To date, 285,000 Americans live with spinal cord injuries (SCI), with ~17,500 new cases per year (2017, Devivo, 2012). SCI persons suffer from a variety of long-term, debilitating sequelae, which may include partial or complete paralysis of locomotor function, diverse deficits to the somatosensory system and damage to autonomic control of organs. Specifically, damage to the innervation of the immune organs potentially leads to immune depression syndrome (IDS), a compilation of symptoms in which natural and adaptive immune health following SCI are adversely affected for an extended duration (Cruse et al., 1992). IDS is marked by a reduced number and function of lymphocytes, specifically impaired T- and natural killer (NK) cell activity (Cruse et al., 1992, Monahan et al., 2015). Furthermore, increased circulating markers of stress reactivity (i.e. cortisol and urinary epinephrine) persist with IDS but appear to improve with appropriate rehabilitative methods (Cruse et al., 1992). Similar to the immune system changes observed with traumatic brain injury and stroke, IDS elevates susceptibility to bacterial infections, thus, with an overall worsening of outcomes when pathogens are introduced (Meisel et al., 2005). Furthermore, peripheral blood lymphocytes obtained from individuals having a spinal injury have reduced in vitro mitogen-activated responses, suggesting a dampened immune responsivity (Monahan et al., 2015). Beyond the increased susceptibility to infection, SCI persons who experience cord injury in tandem with a post-operative wound or respiratory infections have reduced upward improvement in ASIA scores in the 1-year period following injury (Failli et al., 2012). Both motor and sensory gain are reduced if early injury is coupled with bacterial infections, thus implicating immune health during injury recovery in long-term rehabilitative potential (Failli et al., 2012). The taxing of the immune system is substantial following cord injury.
Chronic consumption of diets rich in fat and carbohydrates results in increased stored adipose tissue leading to obesity and its accompanying metabolic disorders. Obesity is also characterized as a disease of chronic low-grade inflammation (Donath and Shoelson, 2011, Shoelson et al., 2006) and is associated with elevations in circulating blood lymphocytes and neutrophils (Dixon and O’ Brien, 2006, Farhangi et al., 2013, Veronelli et al., 2004). Leptin, an adipokine that is secreted in proportion to the level of adipose tissue mass, is elevated in obesity. Leptin, in concert with other obesity factors, acts as a significant driver of hematopoietic proliferation in obesity (Bennett et al., 1996) and, in particular, T-cell expansion. Though the increased numbers of immune cells observed in obesity in circulation and the periphery are the obvious sources of pro-inflammatory cytokines, the adipocytes themselves also produce a host of chemokines that add to the inflammatory milieu (Trayhurn and Wood, 2004a). Further, as the adipose tissue expands in obesity, immune cells infiltrate the adipose tissue and in turn produce cytokines that are released into circulation (Esser et al., 2014, Lumeng et al., 2007, Neels and Olefsky, 2006, Trayhurn and Wood, 2004b). Taken together, the consumption of diets high in fats increases the inflammatory milieu of the organism.
Given that two-thirds of the U.S. population is currently overweight or obese (Flegal et al., 2016), SCI persons are likely to consume a diet that is high in saturated fats prior to and following injury. With an eye to understand the impact of diet on immune sequelae following cord injury, we placed naïve or T10 injured male Long Evans rats on either chow or high-fat diet (HFD) for 16 weeks and interrogated the impact of diet on the immune system. We obtained counts of peripheral circulating immune cells by flow cytometry and key gene changes by real-time PCR in the immune tissues such as thymus and spleen. Chronic consumption of HFD following SCI results in a significant negative interaction between injury and the immune system.
2. MATERIALS AND METHODS
2.1. Animals.
2.1.1. Regulatory Oversight.
All procedures for animal use complied with the Guidelines for the Care and Use of Laboratory Animals by the National Research Council of the National Academies. Procedures were reviewed and approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee (IACUC #1469) and the US Army’s Animal Care and Use Review Office. In conducting research using animals, the investigators adhered to the laws of the United States of America and regulations of the Department of Agriculture.
2.1.2. Rats.
Long Evans strain (RRID: RGD_5508398, Envigo, Indianapolis, IN) males (300–350g, approximately 14 weeks) (N=32) were maintained in standard plexiglass cages, in a room with a 12 h light/12 h dark cycle at 23.0 ± 2 °C temperature, and 50–60% humidity with ad libitum access to water and standard chow (#8640, Envigo, 3.0 kCal/g; 17% fat, 54% carbohydrate, 29% protein). Rats were acclimated to the vivarium for one week before the injury. Rats were assigned to either thoracic 10 (T10) spinal cord injury (SCI) (N=14) or remained Naïve to surgery in a counterbalanced fashion based on body weight on the day prior to the start of surgery. Following injury, animals either remained on standard chow or switched to HFD (#D03082706, Research Diets, New Brunswick, NJ, 4.54 kCal/g; 40% fat, 45% carbohydrate, 15% protein). Our N sizes at the end of the study were Naïve-Chow (N=7), SCI-Chow (N=7), Naïve-HFD (N=7), and SCI-HFD (N=7). Four SCI rats, two Chow and two HFD died early in the experiment due to bladder rupture during early post-operative care regimen. The number of animals necessary for this study was determined by power analyses using variance from our historical data with peripheral blood lymphocyte numbers. N size of 7/group was determined by providing a 90% chance of finding a significant effect of 25% or greater for the between-subject variable. Due to attrition, we started with N=9 for SCI/ N=7 naive.
2.1.3. Surgical procedures.
All surgical procedures were performed on animals that were deeply anesthetized using 5% isoflurane with a gradual decrease to 2.5%. SCI surgeries were performed as previously described (Harris et al., 2019, Spann et al., 2017). Briefly, animals were placed on a heating pad set to 41° C. Incisions were made on the animals’ dorsal skin and overlying muscles, and the vertebral column was exposed. A laminectomy was performed at T10, and the vertebral column was stabilized using Anderson Forceps that grasp the ventral surface of the lateral spinous processes at vertebral levels T9 and T11. Using an Infinite Horizon Spinal Impactor Device (Precision Systems and Instrumentation, LLC, Fairfax Station, VA), a moderate contusion injury was delivered to the T10 spinal cord using 150 kdynes of force with a 1-sec dwell. The area was inspected for bruising, and the digital trace of the impact was inspected to ensure no bone obstruction during injury. The dura mater remained closed for the entire duration of SCI surgeries. Immediately following SCI, the overlying muscles were sutured, and the skin was securely closed using stainless steel wound clips.
2.1.4. Post-operative care.
Animals received one dose of buprenorphine SR (Sustained Release) (1.0 to 1.2 mg/kg SQ (ZooPharm, Laramie, WY) and 72 h later, single-dose buprenorphine for post-surgical pain management (0.025 mg/kg, twice daily for a period of 2 d, then as needed). Animals also receive: 1) antibiotic, Naxcel (5 mg/kg SQ, Zoetis, NJ) once daily for a period of 5 d, 2) 3–5 mL of 0.9% saline, twice daily for a period of 3 d to ensure hydration. Beginning the day of spinal injury, each rat’s urinary bladder was manually expressed 2–3 times daily until the animal recovered the ability to void its bladder. The T10 contusion disrupts the supraspinal pathways that are responsible for bladder voiding. In our hands, control of neurogenic bladder function returns in approximately 14 days. As a rule, bladder care was discontinued for an animal when it exhibited an already-voided bladder on two consecutive bladder care sessions.
2.1.5. Hindlimb locomotor function assessment.
Hindlimb locomotor function was assessed using the Basso, Beattie, and Bresnahan (BBB) open-field locomotor scale (Basso et al., 1995). BBB scores were initially assessed on day one following injury and 16 weeks post-injury. Briefly, the rat was placed into the open field and allowed to move freely for approximately 4 min. Movement and articulation of the joints of each hindlimb were scored using a scoring sheet. A 21-point BBB Open-Field Rating Scale was used for the determination of intact locomotor behavior. For each animal, the locomotor scores for both hindlimbs were averaged to produce one score per test session.
2.1.6. Body weight and fat composition.
Terminal body weight was measured. Fat mass composition was analyzed using Echo Magnetic Resonance Imaging (echoMRI) (EchoMedical Systems, Houston, TX) one day before euthanasia.
2.1.7. Tissue harvest.
During week 16 post-injury, rats were overnight fasted and euthanized by conscious decapitation starting at 2.5 h following the onset of the light cycle. Tissues excised include terminal plasma, spleen, and thymus. Tissue was microdissected into two portions. Half of each tissue was flash frozen with methylbutane on dry ice and then stored in −80 °C until further processing. The other portion was post-fixed in paraformaldehyde (PFA) for histologic assessment.
2.2. Cell Isolation Protocol.
Total blood was collected for cell sorting following decapitation in EDTA collection tubes and kept on wet ice. All samples were run in duplicate and operator was blinded to the group until all analyses were complete. Erythrocytes were lysed using 1X PharmLyse (BD Biosciences, Franklin Lakes, NJ) according to the manufacturer’s instructions. Peripheral blood leukocytes (PBL) were washed and resuspended in PBS (pH 7.4) containing 2% FCS and 0.09% sodium azide (stain buffer). Cells were first incubated with Fc receptor blocker (anti-CD32, #550271, BD Biosciences) for 5 min. Cells were then stained with immune cell-specific antibodies (BD Biosciences) as follows: PE-CD3 (#554833), FITC-CD3 (#554832), Alexa Fluor 647-CD8a (#561611), FITC-CD4 (#554837), PE-CD161a (#555009), PE-CD45RA (#554881), FITC-HIS48 (#554907), or PE-CD43 (#202812, BioLegend, San Diego, CA) at a concentration of 1:100 diluted in stain buffer for 30 min on ice. Based on the granularity of neutrophils and the high intensity of their CD43 staining, they were distinguished from CD43Lo/His48Hi and CD43Hi/His48Lo monocyte populations in order to determine the relative percentages of these cell types. Cells were then washed two times with 2 mL stain buffer and centrifuged at 350×g for 5 min at 4°C. Cells were resuspended in 400 μL of stain buffer and immediately analyzed using a Beckman Coulter Gallios analyzer at the UMMC Cancer Institute Flow Cytometry Core Facility. Data were analyzed using Kaluza software.
2.3. RNA processing and real-time PCR.
RNA from spleen and thymus was extracted using a QIAGEN miniprep RNA kit (QIAGEN, Inc, Valencia, CA), and concentration was determined using NanoDrop with purity levels of 2.0 or greater. Complementary DNA was transcribed using an iScript complementary DNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Quantitative polymerase chain reaction was performed on a Step-One Plus Real-Time PCR machine coupled with StepOne Software (v2.3) (Applied Biosystems) using TaqMan inventoried gene expression assays (Life Technologies, Foster City, CA). Reference gene, Rpl32 (ribosomal protein L32, Rn00820748_g1), was used to normalize all genes of interest using the 2-ΔΔ CT method. The following genes of interest were also probed: Casp3 (Caspase-3, Rn00563902_m1), Ccl5 (chemokine (C-C motif)) ligand 5 (Rn00579590_m1), Ccl25 (chemokine (C-C motif) ligand 25, Rn01403352_m1), Cd3g (cluster of differentiation (cd) g; Rn01417940_m1), Cd4 (Rn00562286_m1), Cd8a (Rn00580577_m1), Cd14 (Rn00572656_g1), Cd68 (Rn01495634_g1), Cxcl12 (chemokine (C-X-C motif) ligand 12; Rn00573260_m1), Foxp3 (forkhead box P3; aka, RGD1562112, Rn01525092_m1), IL4 (interleukin 4; Rn01456866_m1), IL7 (interleukin 7; Rn00681900_m1), MPO (myeloperoxidase, Rn01460205_m1), NFKB1 (nuclear factor of kappa light polypeptide gene enhancer in B-cells 1, Rn01399572_m1) and PTPRC (protein tyrosine phosphatase, receptor type, C; aka, CD45, Rn00709901), complement component 1q (C1q, Rn01495634_g1). All samples were run in duplicate and blinded to group.
2.4. Paraffin embedding, standard stains, and TUNEL assay.
PFA post-fixed spleen and thymus were subjected to standard paraffin-embedding, and then sectioned at 5 μm on to glass slides for staining with hematoxylin and eosin (H&E), and additional slides were processed for TUNEL staining with Click-iT Plus TUNEL Assay for In Situ Apoptosis Detection, Alexa Fluor® 488 dye (#C10617, Molecular Probes, Inc., Eugene, OR), according to the manufacturer’s specifications. Bright field and fluorescent microscopy photographs were obtained with an Olympus BX60 F5 light microscope with a Leica DFC310 FX camera and Leica Application Suite software version 4.6 (Leica Microsystems, Buffalo Grove, IL) at 20X magnification. Images were obtained with operator blinded to group.
2.5. Histology of the thymus and spleen
Slides were submitted to the UMMC Pathology Department for digital scanning (PHILIPS Digital Pathology Solutions Image Management System, Philips, Amsterdam, Netherlands). Tracing tool was used to determine the area of total thymic lobule and medullary region. Ratio was determine using measurements. Tracing tool was also used to determine total area of spleen and raw counts obtained of lymphoid nodules (identified by presence of germinal center) per unit area of splenic tissue. All images were obtained with the operator blinded to the group of the samples.
2.6. Statistics.
All statistical analyses were performed using GraphPad Prism version 8.1.2 (GraphPad Software, San Diego, California). Statistical significance was determined with a two-way analysis of variance for variables of injury and diet followed by Tukey’s post hoc test for individual comparisons. All results are given as means ± SEM. Results were considered statistically significant when p < 0.05.
3. RESULTS
3.1. Body mass, fat mass, organ weight and BBB 16 weeks post-injury.
HFD-fed male rats 16 weeks post-injury had elevated body weight in comparison to Chow-fed rats irrespective of injury, main effect of diet, p < 0.01 (TABLE 1). Also, the HFD-fed rats have increased levels of fat mass in comparison to Chow-fed rats, main effect of diet, p < 0.01 (TABLE 1). HFD-fed rats had increased spleen weight in comparison to Chow-fed rats, main effect of diet, p < 0.05 (TABLE 1). Thymic weight was reduced in SCI rats in comparison to Naïve rats, main effect of injury, p < 0.05 (TABLE 1). Further, following 16 weeks of injury the BBB score, which assesses locomotor function of the thoracic injured rats, was approximately 11 in comparison to the score of 21 in the Naïve rats p<0.0001 (TABLE 1).
Table 1.
Body mass, fat mass and lymphoid tissue weight. Data are presented as mean ± SEM. Two-way ANOVA by injury and diet. N=7/group.
| Naïve | SCI | Naïve | SCI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Output measure | SEM | SEM | SEM | SEM | Statistics | ||||
| Body weight (g) | 14.64 | 33.30 | 14.54 | 33.73 | p(diet) < 0.01 | ||||
| Body fat (g) | 5.38 | 11.84 | 13.76 | 28.79 | p(diet) < 0.01 | ||||
| Spleen weight (g) | 0.02 | 0.08 | 0.06 | 0.12 | p(diet) < 0.05 | ||||
| Spleen weight as % | 0.00 | 0.01 | 0.01 | 0.01 | p(diet) = 0.080 | ||||
| Thymus weight (g) | 0.02 | 0.01 | 0.02 | 0.02 | p(injury) < 0.05 | ||||
| Thymus weight as % | 0.01 | 0.00 | 0.00 | 0.00 | p(injury) = 0.0572 | ||||
| BBB score | 0.00 | 1.33 | 0.00 | 1.02 | p(injury) < 0.0001 | ||||
3.2. Peripheral blood lymphocytes.
Total T cells (CD3+) were reduced in SCI rats 16 weeks post-injury irrespective of diet in comparison to Naïve rats, main effect of injury, p < 0.001, (Fig. 1A). Helper T cells (CD3+/CD4+) and cytotoxic T cells (CD3+/CD8+) were also reduced in SCI rats in comparison to Naïve, main effect of injury, p < 0.001 (Fig. 1B) and p < 0.01, (Fig. 1C) respectively. Myeloid cells (HIS48+/CD43+), were subcategorized into monocytes and neutrophils ((Fig. 1D and E). Whereas there was no difference in the percentage of monocytes among groups (Fig. 1D), the percentage of neutrophils were elevated in SCI rats in comparison to Naïve, main effect of injury, p < 0.01 (Fig. 1E). Natural killer cells (CD3-/CD161+) were reduced in SCI rats in comparison to Naïve, main effect of injury, p < 0.01 (Fig. 1F). No differences were measured in B cell (CD45RA+) numbers (Fig. 1G).
Figure 1. Peripheral blood lymphocytes at 16 weeks in Naïve/Chow, Naïve/HFD, SCI/Chow and SCI HFD.
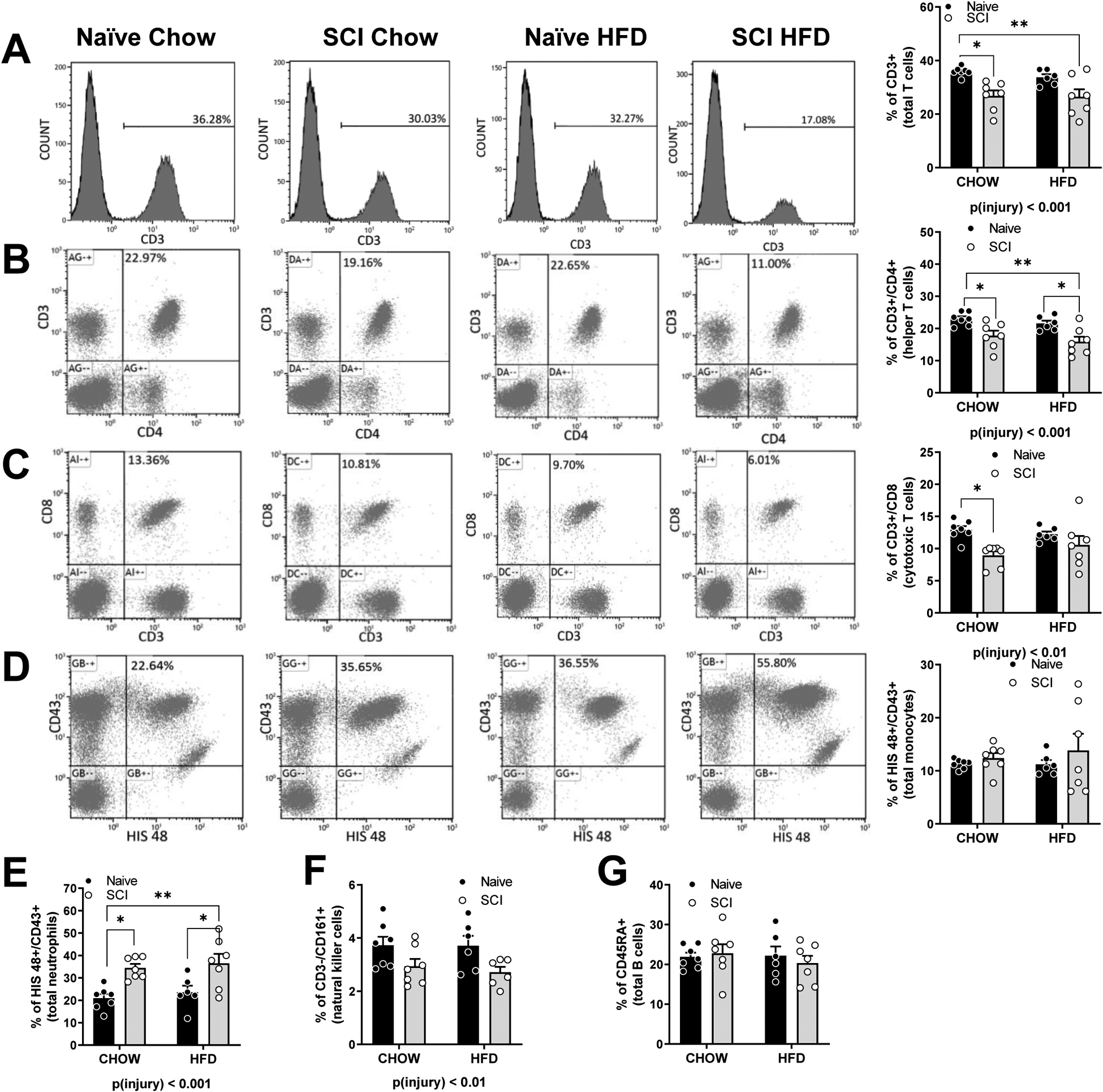
Representative histograms and percentage of counts of (A) CD3+ Total cells. (B) CD3/CD4+ helper T cells. (C) CD8/CD3+ cytotoxic T cells. (D) HIS48/CD43+ neutrophils and monocytes. (E) CD161 high/ CD3 low natural killer cells. (F) CD45RA+ B cells. Data are presented as mean ± SEM. Statistical comparison of injury and diet performed by two-way ANOVA reported below each graph with post hoc Tukey’s. Relevant statistical comparison reported by *p < 0.05, ** p< 0.01, (N=6–7/group).
3.3. Thymus gene expression.
Semi-quantitative PCR was used to probe for gene expression of markers in the thymus. There was no difference in T cell marker CD3 (Fig. 2A) or CD8 (Fig. 2C). However, helper T cell marker CD4 was elevated in SCI, main effect of injury, p < 0.05 (Fig. 2B). Regulatory T cell marker (FOXP3) was also elevated, main effect of injury, p < 0.05 (Fig. 2D) with no difference in pan hematopoietic marker, PTPRC (Fig. 2E). We observed increased complement marker, C1q in SCI rat thymus, main effect of injury, p < 0.05 with a trend towards an effect of diet, p = 0.0573 (Fig. 2F). There was no difference in CCL5 (RANTES) in the thymus (Fig. 2G). CCL25 (TEC, thymus-expressed chemokine) was increased in HFD-fed rats, main effect of diet, p < 0.05 (Fig. 2H). CD68, a macrophage marker , and CXC12 also known as monocyte chemotactic protein 5 (MCP-5), and important for T cell lineage commitment in the thymus, were both increased in the thymus, main effect of injury, p < 0.05 with a trend towards a main effect of diet p = 0.0672 and p=0.0573 respectively (Fig. 2I, J). IL4 and IL7 are both important in the differentiation of naive helper T cells to Th2 cells. IL7 but not IL4 was increased in the thymus in SCI rats, main effect of diet, p < 0.05 (Fig. 2K, L). There were no differences in the master regulator of inflammation, NFKB1 by mRNA (not shown).
Figure 2. Gene expression by rtPCR in the thymus of Naïve/Chow, Naïve/HFD, SCI/Chow and SCI HFD normalized to ribosomal gene L32.
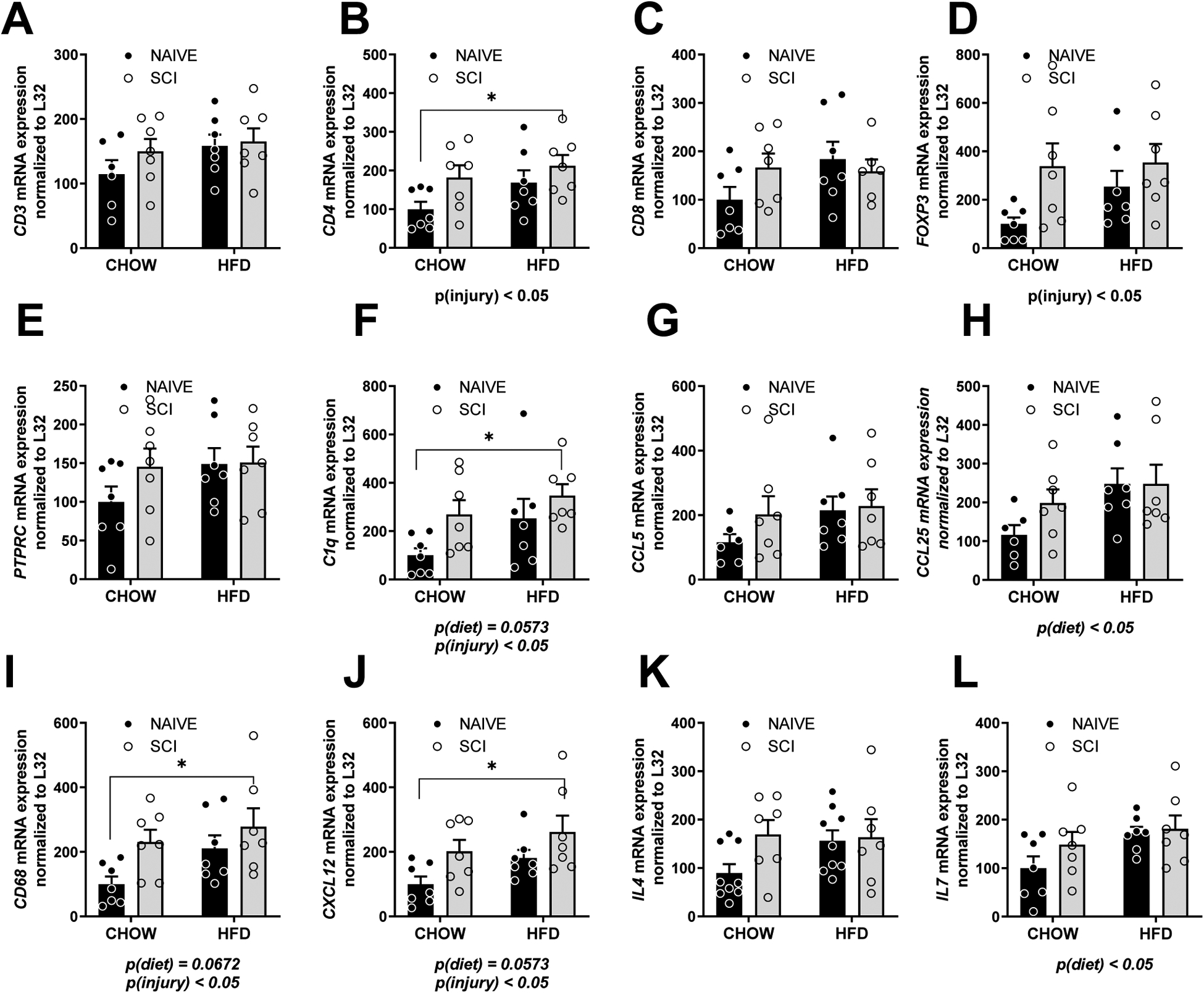
(A) CD3. (B) CD4. (C) CD8. (D) FOXP3. (E) PTPRC. (F) C1Q. (G) CCL5. (H) CCL25. (I) CD68. (J) CXCL12. (K) IL4. (L) IL7. (M). Data are presented as mean ± SEM. Statistical comparison of injury and diet performed by two-way ANOVA reported below each graph with post hoc Tukey’s. Relevant statistical comparison reported by *p < 0.05, ** p< 0.01, (N=6–7/group).
3.4. Spleen gene expression.
Semi-quantitative PCR was also used to probe for gene expression of markers in the spleen. There was a reduction in T cell marker CD3, main effect of injury, p < 0.05 (Fig. 3A) but not CD4 or CD8 (Fig. 3B, C). Regulatory T cell marker (FOXP3) and pan hematopoietic marker, PTPRC were also not changed in the spleen (Fig. 3D, E). We observed increased marker of apoptosis, caspase 3 (CASP3), with HFD and SCI, main effect of diet, p < 0.05 with a trend towards a main effect of injury, p = 0.054 (Fig. 2F). CD14, a lipopolysaccharide-binding protein, which functions as an endotoxin receptor, and macrophage marker CD68 were both increased in the spleen in HFD-fed rats, main effect of diet, p < 0.05 (Fig. 3G, H). We observed increased complement marker, C1q in SCI rat spleen, main effect of diet, p < 0.01 (Fig. 3I). CXCL12 was increased in the spleen, main effect of diet, p < 0.05 (Fig. 3J). Myeloperoxidase (MPO), abundantly expressed in neutrophil granulocytes and marker for neutrophils, was elevated in HFD-fed rats and SCI rats, main effect of injury, p < 0.05, main effect of diet, p < 0.05 (Fig. 3K). TLR4, co-receptor with CD14 for LPS was increased in the spleen in HFD-fed rats, main effect of diet, p < 0.05 (Fig. 3L). There was no difference in gene expression of the master inflammatory regulator, NFKB1 by mRNA (data not shown).
Figure 3. Gene expression by rtPCR in the spleen of Naïve/Chow, Naïve/HFD, SCI/Chow and SCI HFD normalized to ribosomal gene L32.
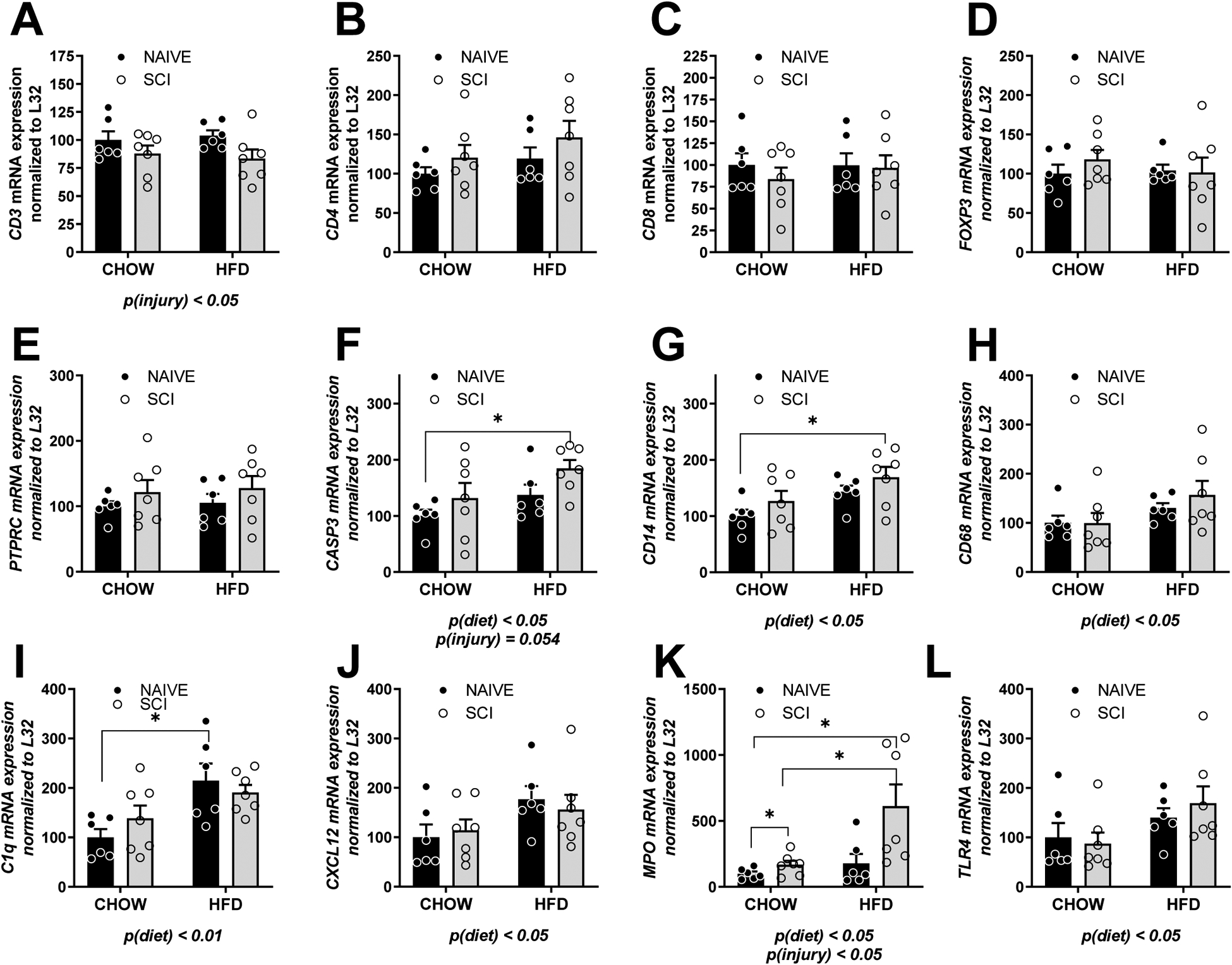
(A) CD3. (B) CD4. (C) CD8. (D) FOXP3. (E) PTPRC. (F) CASP3. (G) CD14. (H) CD68. (I) C1Q. (J) CXCL12. (K) MPO. (L) TLR4. Data are presented as mean ± SEM. Statistical comparison of injury and diet performed by two-way ANOVA reported below each graph with post hoc Tukey’s. Relevant statistical comparison reported by *p < 0.05, (N=6–7/group).
3.5. Histology of thymus and spleen.
Representative images of the thymus stained for H&E for each of the groups show thymic organization typical for each group of animals (Fig. 4A). Using slides that were digitally scanned, we measured the ratio of the cortical area to medullary area of a subset of thymic lobes in each rat. There was no difference between (Fig. 4D). Representative images of the spleen stained for H&E for each of the groups show follicular organization (Fig. 4B). H&E suggested potential differences in the white pulp so we counted lymphoid nodules per unit area and we measured no difference in average number of nodules per mm2in between groups, (Fig. 4E). However, intra-lobular areas of SCI-HFD have some increased marginal zone areas as illustrated in the representative image (Fig. 4B). Staining by TUNEL in the spleen (Fig. 4C) showed an increased number of apoptotic cells in the spleen in SCI in comparison to Naïve, main effect of injury, p < 0.05 (Fig. 4E).
Figure 4. Histology of immune tissues of Naïve/Chow, Naïve/HFD, SCI/Chow and SCI HFD spleen 16 weeks after time of initial injury.
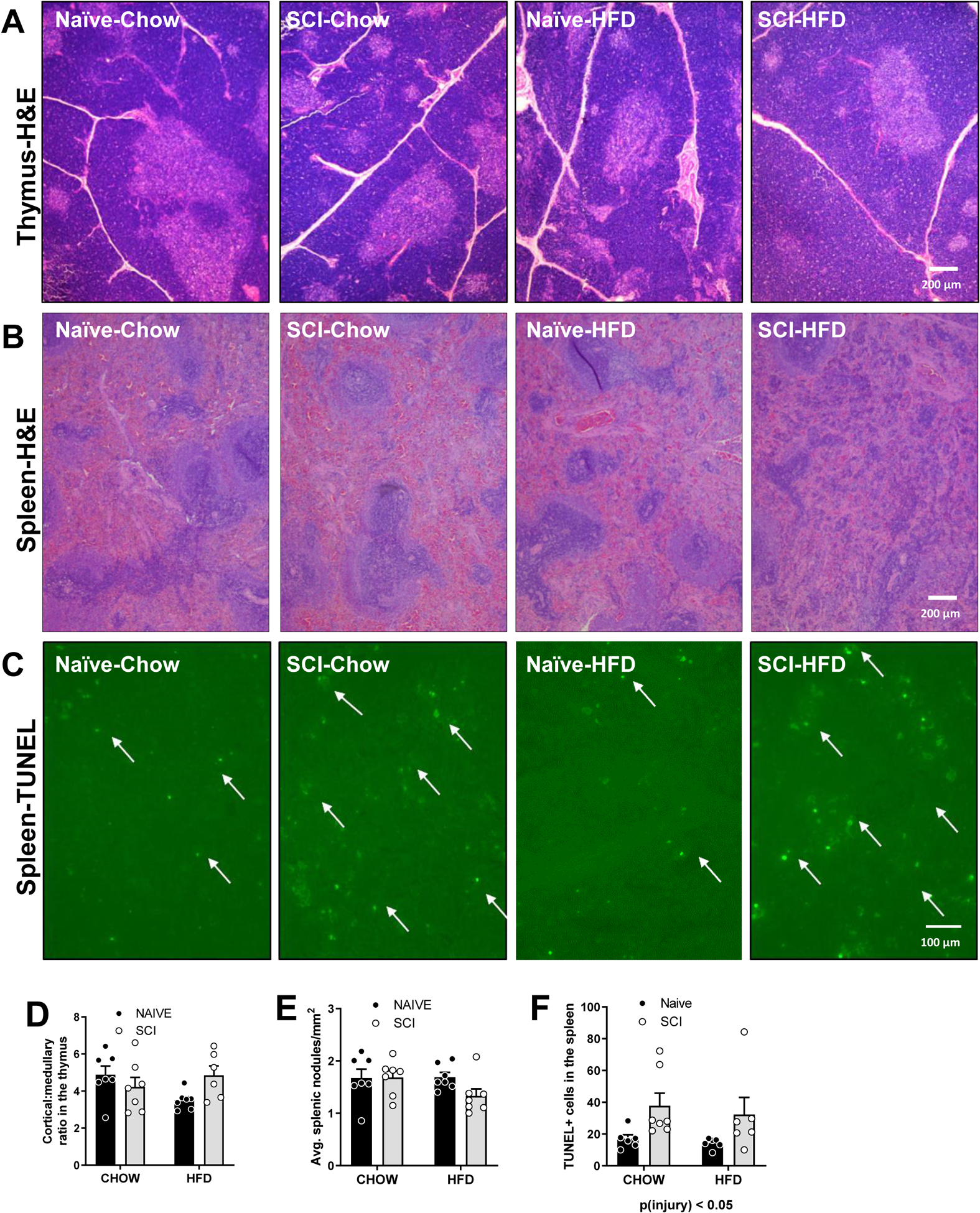
(A) Representative thymus H&E histology (B) Representative spleen histology. (C) Fluorescent TUNEL staining in the spleen. (D) Thymus cortex: medulla ratio. (E) Average number of nodules/mm2 (F) TUNEL+ cells in spleen. Data are presented as mean ± SEM. Statistical comparison of injury and diet performed by two-way ANOVA reported below each graph with post hoc Tukey’s. Relevant statistical comparison reported by *p < 0.05, (N=6–7/group).
4. DISCUSSION.
SCI results in trauma to the cord influencing sensory, motor, and visceral innervation of tissues and organs; in parallel, there is a profound negative impact on the immune system. The development of immune depression syndrome (IDS) has long-reaching effects on the trajectory of post-injury rehabilitation. After 16 weeks of recovery post-SCI, continued immuno-hematologic trauma was observed in the injured rats, such as suppressed circulating lymphocytes and NK cells, elevated numbers of neutrophils, and altered gene expression in the spleen and thymus. The current work is the first demonstration in a rat model of T10 spinal contusion of chronic immune insult beyond the original trauma by consumption of an obesogenic high-fat diet.
4.1. Immune cells in the cord following spinal injury
Damage to the surrounding neural, connective, and musculoskeletal tissues after SCI profoundly changes the organization of the cells within the region of injury. In the cord, there is high recruitment of diverse immune cell phenotypes, e.g., T lymphocytes, microglia, macrophages, and neutrophils (Popovich et al., 1997), which exacerbate damage to the cord and retard recovery (Popovich and Jones, 2003). In the acute phase of injury, these immune cells are greatly increased in the injured cord within hours and lasting days after the insult (Popovich et al., 1997), resulting in up-regulation of the master transcription activator for inflammation, NFκB (Bethea et al., 1998). Even 180 days after the injury, cord-specific T cells, macrophages/microglia, and neutrophils remained detectably elevated (Beck et al., 2010). Following SCI macrophages, in particular, undergo maladaptive transitioning from the pro-inflammatory M1 macrophages to activated M2 macrophages that have been identified in samples of chronic, non-healing wounds (Gensel and Zhang, 2015). Thus, the immune system after SCI generally inhibits rehabilitative recovery.
4.2. Peripheral immune cells in the post-SCI phase.
In humans following SCI, there is a rapid decrease in circulating monocytes, CD3+ T lymphocytes and B lymphocytes within 24 h of injury, reaching a minimum within the first week of injury (Riegger et al., 2009). These diminished immune cell counts are further worsened with methylprednisolone treatment, a commonly used corticosteroid to reduce inflammation directly following SCI (Riegger et al., 2009). Similar changes have been shown in rodents where decreased CD8+ lymphocytes were identified during the first week (Popovich et al., 2001) in addition to increased sensitization of lymphocytes by myelin basic protein that is spiked in circulation due to the trauma (Popovich et al., 2001). Further, circulating corticosterone is elevated within 24 h of injury and remains elevated for up to a month (Popovich et al., 2001). The release of glucocorticoids and norepinephrine as a result of injury adversely sensitizes lymphocytes to β-adrenergic stimulation, thus, increasing their propensity for apoptosis (Lucin et al., 2009). Even one month after injury, exhaustion of CD8+ cells remains impaired, reducing T-cell cytokine production and increased expression of programmed cell death 1 (PD-1) marker (Zha et al., 2014). In vitro blockade of PD-1 was able to restore T-cell function in a mouse model of T9 injury (Zha et al., 2014).
In the current study, we report reduced percentages of total T cells which include helper and cytotoxic, even 16 weeks post-injury. NK cells were also reduced. Neutrophils were increased. This profile suggests the immune system continues to remain taxed after SCI and may result in reduced responsivity. Monocytes and B cell percentages, however, were not different from Naïve in the current study. Though B cell counts in the circulation of a mouse model of T3 SCI was transiently depressed immediately after injury, the injury appeared not to affect maturation per se or pre-existing B cell memory (Oropallo et al., 2012). Chronic, high-thoracic SCI impairs the ability to mount optimal antibody responses to new antigenic challenges but spares established humoral immunity (Oropallo et al., 2012). However, it was clearly shown using transgenic mice lacking B cells have obtained SCI, that improved locomotor recovery and lesion pathology suggest an important role for B cell in SCI to produce increased (auto)antibodies and also the accumulation of complement component 1q in the region of cord trauma (Ankeny et al., 2009). Taken together, the B cells exert their influence that is long-lasting through effector molecules but not necessarily altered counts post-SCI.
We predicted that obesity induced by high-fat feeding in both the naïve and SCI male rats would increase lymphocyte counts. However, in this experiment using male rats, it was not the case. We previously reported in other studies that HFD-feeding specifically in female Long Evans increased peripheral blood counts (Himel et al., 2019), but when this same work was performed in male Long Evans rats, no peripheral blood lymphocyte increases were seen (unpublished observation) as occurred in the current study. Thus, this outcome is congruent with our other studies in male Long Evans.
4.3. Autonomic damage to the innervation of the lymphoid system following SCI.
Because the innervation of the primary and secondary lymphoid tissues such as the spleen, thymus, bone marrow and lymph nodes is accomplished by the autonomic nerve fibers of the sympathetic nervous system neurons found throughout the thoracolumbar spinal cord (Allison and Ditor, 2015), the level of the spinal injury influences the severity by which the immune system is affected. In particular, with high-level SCI, autonomic dysreflexia is a significantly dangerous complication resulting in exaggerated activation of spinal sympathetic responses, which includes lymphoid glands (as well as adrenal). High-thoracic injury is associated with atrophy of the spleen, splenic leucopenia, increased apoptotic marker, caspase-3, and elevated corticosterone and norepinephrine (Lucin et al., 2009); these were less salient in mid-level (T9) injuries (Lucin et al., 2007). Within lymphoid organs, postganglionic noradrenergic fibers release catecholamines, which can directly influence the activity of the immune cells within the specific lymphoid organ (Allison and Ditor, 2015). Through the use of in vivo telemetry, autonomic dysreflexia (AD) has been shown to cause a secondary immune depression following the initial immune depression caused directly by trauma (Zhang et al., 2013). The AD-induced immune depression can be prevented through systemic pharmacologic inhibition of noradrenaline and glucocorticoid receptors (Zhang et al., 2013). Though adrenalectomy directly following SCI prevents the negative effect of excess glucocorticoids that produce leucopenia, only restoration of the adrenal gland, be it denervated, actually restores the ability to ward off infections such as pneumonia following SCI (Pruss et al., 2017). These data support the presence of a powerful link between the HPA axis and immune system to repress immune health following SCI (Pruss et al., 2017). Studies from another group support that circulating cytokine, soluble TNFα, is an active mediator of AD following SCI (Mironets et al., 2018).
4.4. Peripheral lymphoid organs following SCI.
Within the first week of injury, immune cells isolated from spleen and lymph nodes sorted by flow cytometry show an elevation in CD4+ lymphocytes and reduction in CD8+ lymphocytes (Popovich et al., 2001). Further work suggests that incomplete thoracic but not cervical injury results in increased expression of splenic caspase, corticosterone and norepinephrine during the first week of injury (Hong et al., 2019) suggesting that there is a level dependence of IDS and further, it responsive even to partial disruption of sympathetic tone.
Our studies suggest that 16 weeks following SCI, the thymus exhibits increased gene expression of markers for CD4+ T cells and regulatory T cells (marked by FOXP3). The increased gene expression of IL7, which is important in thymic T cell maturation may be upregulated to compensate for the low percentages of T cells in circulation. The reduced size of the thymus in SCI rats coupled with the increased presence of the complement marker, C1q, commonly observed in various forms of neurodegeneration (Cho, 2019), supports chronic stress of SCI (Yan et al., 2017). This is further supported by the evidence of higher levels of macrophage marker CD68 in SCI rats whose expression may be higher in the spleen because in order to clear the debris of apoptotic cells.
Within the spleen, the CD3, marker for T cells was reduced in SCI and mirrored the reduction that was observed in circulation. In particular, SCI rats fed HFD appear to have altered structure in the interlobular regions, perhaps affected by marked infiltration of plasma cells, T and B cells and dendritic cells that would be supported by the mRNA markers. Because of the poor immunohistochemical reagents for immune cells in the rat, we chose specifically to hone in on the potential categories of changes using mRNA as a proxy for these immune targets. The pan marker for apoptosis, CASP3, gene expression was congruent with TUNEL staining, indicating an increase in dead or dying cells within the spleen as a result of injury. This dovetails with increased gene expression in SCI for a neutrophil marker, MPO, in the spleen, which parallels the increase in neutrophils in circulation with injury.
4.5. Diet alters the immune system in both Naïve and SCI rats.
Following 16 weeks of HFD, obesity results in the elevated size of the spleen independent of injury in the current study. Gene markers within the spleen and thymus exhibit continued collateral damage as a result of spinal cord insult. For example, CD3, a marker for total T cells, is suppressed in the spleen in SCI in comparison to Naïve. Both injury and diet elevated MPO, a marker of neutrophils which is also elevated in circulation, but macrophage marker, CD68, is elevated in the spleen with HFD-feeding. HFD-feeding also increases endotoxin receptors TLR4 and CD14 suggesting that the potential for toxic LPS is upregulated with diet. Altogether these data suggest that concurrent obesity and chronic injury may propagate immune dysfunction in the spleen. Though the mRNA marker for apoptosis is affected by both diet and injury, TUNEL staining is elevated in the spleen only by injury. The interplay between diet and injury keenly drive further immune dysfunction.
Similarly, the thymus also experiences the dual insult of injury and diet. Concerning thymic weight, a significant level of thymic involution is produced 16 weeks post-injury and may be the result of chronic stress in the SCI rats. T cell markers, CD4, and FOXP3 were both elevated in the thymus of SCI, suggesting a compensatory response to mature T cells in response to leukopenia. This is further supported by increased IL7 mRNA within the thymus, which is important in the maturation of the T cells. Similar to the spleen, injury increases the presence of macrophages within the thymus which has previously not been reported.
4.6. Immune modulators for SCI rehabilitation.
Some immune-modulatory treatments performed in rodents targeting the immune system appear promising to overall rehabilitation after SCI. For example, blockade of chemoattractant C5a-induced inflammation 14 days after injury reduced the time of locomotor recovery suggesting a potential rehabilitative influence of inflammatory mediators after initial injury (Beck et al., 2010). Short term (7 days) treatment with calcitriol, an FDA approved a biologically-active form of Vitamin D, showed increased numbers of motor neurons in the ventral horn 12 weeks after SCI (Khajoueinejad et al., 2019). Proliferation test performed on lymphocytes from the spleen and lymph nodes showed improvements, including reduced interferon-γ and IL17A (Khajoueinejad et al., 2019). Dual pharmacologic treatment following SCI with liposomal-encapsulated clodronate (to deplete macrophages), and rolipram, a type 4 phosphodiesterase (PDE4) inhibitor, provided neuroprotection and enhanced locomotor recovery through myelin and axon tissue sparing (Iannotti et al., 2011) suggesting that the elevated macrophages contribute substantively to reduced recovery. Another study using high-doses of glatiramer acetate shows that suppresses division of autoreactive lymphocytes against myelin basic protein may protect against the deleterious responses of adaptive immune system against self-antigens (Askarifirouzjaei et al., 2019).
4.7. Caveats and alternate interpretations
In the current study, we did not test the reactivity and production of cytokines of the various immune cells in circulation, but simply their relative percentage. Further, we did not dissociate the immune cells in the spleen and thymus to perform a characterization with advanced flow cytometry, but simply measured gene expression for the genes, thus using mRNA as a surrogate. Further, the chow diet we used does have some differences in the source of calories, and thus, the chow and HFD are not matched for micronutrients. Further, in the current study, we did not study females, which may have somewhat different absolute numbers of cells and altered gene expression. Also, we focused on chronic SCI and therefore using these particular diets, we do not have shorter-time frame comparisons with these diets and cannot speak to the impact of an abbreviated stint on these diets.
Funding Acknowledgements:
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs to B. E. G., through the Spinal Cord Injury Research Program under Award No. W81XWH-16-1-0387. B.E.G. is also supported by Award No. W81XWH-16-1-0349. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Research reported in this publication was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM104357 and the National Heart, Lung and Blood Institute under Award Number P01HL51971. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Flow cytometry experiments were performed at the UMMC Cancer Center and Research Institute Flow Cytometry Core Facility, which is supported in part through the UMMC Mississippi Center of Excellence in Perinatal Research (MS-CEPR)-COBRE (P20GM121334).
Footnotes
Competing interests statement: authors have no competing interests to declare
REFERENCES
- National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham. 2017. [Google Scholar]
- Allison DJ, Ditor DS. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015;53:14–8. [DOI] [PubMed] [Google Scholar]
- Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. The Journal of clinical investigation. 2009;119:2990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askarifirouzjaei H, Khajoueinejad L, Salek Farrokhi A, Tahoori MT, Fazeli M, Tiraihi T, et al. Implications of immunotherapy with high-dose glatiramer acetate in acute phase of spinal cord injury in rats. Immunopharmacology and immunotoxicology. 2019;41:150–62. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of neurotrauma. 1995;12:1–21. [DOI] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain : a journal of neurology. 2010;133:433–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Current Biology. 1996;6:1170–80. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic Spinal Cord Injury Induces Nuclear Factor-κB Activation. The Journal of Neuroscience. 1998;18:3251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K Emerging Roles of Complement Protein C1q in Neurodegeneration. Aging Dis. 2019;10:652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse JM, Lewis RE, Bishop GR, Kliesch WF, Gaitan E. Neuroendocrine-immune interactions associated with loss and restoration of immune system function in spinal cord injury and stroke patients. Immunologic research. 1992;11:104–16. [DOI] [PubMed] [Google Scholar]
- Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–72. [DOI] [PubMed] [Google Scholar]
- Dixon JB, O’ Brien PE. Obesity and the White Blood Cell Count: Changes with Sustained Weight Loss. Obesity surgery. 2006;16:251–7. [DOI] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature reviews Immunology. 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes research and clinical practice. 2014;105:141–50. [DOI] [PubMed] [Google Scholar]
- Failli V, Kopp MA, Gericke C, Martus P, Klingbeil S, Brommer B, et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain : a journal of neurology. 2012;135:3238–50. [DOI] [PubMed] [Google Scholar]
- Farhangi MA, Keshavarz SA, Eshraghian M, Ostadrahimi A, Saboor-Yaraghi AA. White blood cell count in women: relation to inflammatory biomarkers, haematological profiles, visceral adiposity, and other cardiovascular risk factors. Journal of health, population, and nutrition. 2013;31:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014Trends in Obesity Among Adults in the United States, 2005 to 2014Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama. 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain research. 2015;1619:1–11. [DOI] [PubMed] [Google Scholar]
- Harris KK, Himel AR, Duncan BC, Grill RJ, Grayson BE. Energy balance following diets of varying fat content: metabolic dysregulation in a rodent model of spinal cord contusion. Physiological reports. 2019;7:e14207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himel AR, Taylor EB, Phillips CL, Welch BA, Spann RA, Bandyopadhyay S, et al. Feature article: Splenectomy fails to attenuate immuno-hematologic changes after rodent vertical sleeve gastrectomy. Experimental biology and medicine (Maywood, NJ). 2019;244:1125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Chang A, Liu Y, Wang J, Fehlings MG. Incomplete Spinal Cord Injury Reverses the Level-Dependence of Spinal Cord Injury Immune Deficiency Syndrome. International journal of molecular sciences. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti CA, Clark M, Horn KP, van Rooijen N, Silver J, Steinmetz MP. A combination immunomodulatory treatment promotes neuroprotection and locomotor recovery after contusion SCI. Experimental neurology. 2011;230:3–15. [DOI] [PubMed] [Google Scholar]
- Khajoueinejad L, Askarifirouzjaei H, Namazi F, Mohammadi A, Pourfathollah AA, Rajaian H, et al. Immunomodulatory effects of Calcitriol in acute spinal cord injury in rats. International immunopharmacology. 2019;74:105726. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Experimental neurology. 2007;207:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Sanders VM, Popovich PG. Stress hormones collaborate to induce lymphocyte apoptosis after high level spinal cord injury. Journal of neurochemistry. 2009;110:1409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nature Reviews Neuroscience. 2005;6:775–86. [DOI] [PubMed] [Google Scholar]
- Mironets E, Osei-Owusu P, Bracchi-Ricard V, Fischer R, Owens EA, Ricard J, et al. Soluble TNFalpha Signaling within the Spinal Cord Contributes to the Development of Autonomic Dysreflexia and Ensuing Vascular and Immune Dysfunction after Spinal Cord Injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018;38:4146–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan R, Stein A, Gibbs K, Bank M, Bloom O. Circulating T cell subsets are altered in individuals with chronic spinal cord injury. Immunologic research. 2015;63:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neels JG, Olefsky JM. Inflamed fat: what starts the fire? The Journal of clinical investigation. 2006;116:33–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropallo MA, Held KS, Goenka R, Ahmad SA, O’Neill PJ, Steward O, et al. Chronic spinal cord injury impairs primary antibody responses but spares existing humoral immunity in mice. Journal of immunology (Baltimore, Md : 1950). 2012;188:5257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Jones TB. Manipulating neuroinflammatory reactions in the injured spinal cord: back to basics. Trends in pharmacological sciences. 2003;24:13–7. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Stuckman S, Gienapp IE, Whitacre CC. Alterations in immune cell phenotype and function after experimental spinal cord injury. Journal of neurotrauma. 2001;18:957–66. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. The Journal of comparative neurology. 1997;377:443–64. [DOI] [PubMed] [Google Scholar]
- Pruss H, Tedeschi A, Thiriot A, Lynch L, Loughhead SM, Stutte S, et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nature neuroscience. 2017;20:1549–59. [DOI] [PubMed] [Google Scholar]
- Riegger T, Conrad S, Schluesener HJ, Kaps HP, Badke A, Baron C, et al. Immune depression syndrome following human spinal cord injury (SCI): A pilot study. Neuroscience. 2009;158:1194–9. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. The Journal of clinical investigation. 2006;116:1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann RA, Lawson WJ, Grill RJ, Garrett MR, Grayson BE. Chronic spinal cord changes in a high-fat diet fed male rat model of thoracic spinal contusion. Physiol Genomics. 2017:physiolgenomics.00078.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. British Journal of Nutrition. 2004a;92:347–55. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. The British journal of nutrition. 2004b;92:347–55. [DOI] [PubMed] [Google Scholar]
- Veronelli A, Laneri M, Ranieri R, Koprivec D, Vardaro D, Paganelli M, et al. White Blood Cells in Obesity and Diabetes. Effects of weight loss and normalization of glucose metabolism. 2004;27:2501–2. [DOI] [PubMed] [Google Scholar]
- Yan F, Mo X, Liu J, Ye S, Zeng X, Chen D. Thymic function in the regulation of T cells, and molecular mechanisms underlying the modulation of cytokines and stress signaling (Review). Molecular medicine reports. 2017;16:7175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha J, Smith A, Andreansky S, Bracchi-Ricard V, Bethea JR. Chronic thoracic spinal cord injury impairs CD8+ T-cell function by up-regulating programmed cell death-1 expression. J Neuroinflammation. 2014;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guan Z, Reader B, Shawler T, Mandrekar-Colucci S, Huang K, et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:12970–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


