Abstract
The endosymbiotic bacterium Wolbachia shows viral blocking in its mosquito host, leading to its use in arboviral disease control. Releases with Wolbachia strains wMel and wAlbB infecting Aedes aegypti have taken place in several countries. Mosquito egg survival is a key factor influencing population persistence and this trait is also important when eggs are stored prior to releases. We therefore tested the viability of mosquitoes derived from Wolbachia wMel and wAlbB-infected as well as uninfected eggs after long-term storage under diurnal temperature cycles of 11–19°C and 22–30°C. Eggs stored at 11–19°C had higher hatch proportions than those stored at 22–30°C. Adult Wolbachia density declined when they emerged from eggs stored for longer, which was associated with incomplete cytoplasmic incompatibility (CI) when wMel-infected males were crossed with uninfected females. Females from stored eggs at both temperatures continued to show perfect maternal transmission of Wolbachia, but storage reduced the fecundity of both wMel and wAlbB-infected females relative to uninfected mosquitoes. Furthermore, we found a very strong negative impact of the wAlbB infection on the fertility of females stored at 22–30°C, with almost 80% of females hatching after 11 weeks of storage being infertile. Our findings provide guidance for storing Wolbachia-infected A. aegypti eggs to ensure high fitness adult mosquitoes for release. Importantly, they also highlight the likely impact of egg quiescence on the population dynamics of Wolbachia-infected populations in the field, and the potential for Wolbachia to suppress mosquito populations through cumulative fitness costs across warm and dry periods, with expected effects on dengue transmission.
Author summary
The endosymbiont bacterium, Wolbachia, has been successfully established in natural Aedes aegypti populations to help suppress the transmission of arboviral diseases such as dengue. The fertility of infected mosquitoes experiencing a quiescent egg stage will influence the efficiency of mosquito releases when mass-reared eggs are stored and may also influence invasion. We tested stored eggs with the wMel or wAlbB infections and found that females derived from eggs stored in a warm environment had reduced fecundity. We also discovered a novel and strong fertility cost on wAlbB-infected A. aegypti, in that a high proportion of females became infertile even though they mated successfully, which was determined by the period when mosquito eggs were quiescent. This effect across life stages was not evident when eggs were stored under cooler conditions and represents the first time that Wolbachia was found to induce female infertility independent of cytoplasmic incompatibility, pointing to a likely additional phenotypic effect of Wolbachia affecting dengue transmission.
Introduction
Aedes aegypti is a major vector of dengue, Zika, chikungunya and yellow fever viruses and is distributed mainly in tropical and subtropical areas [1]. A. aegypti abundance is determined by seasonal fluctuations in temperature, humidity and precipitation [2]; temperature influences mortality and development rates while rainfall affects the availability of larval habitats and egg hatching success. Long term survival of eggs is the main factor influencing population persistence especially in areas where there is a cold, dry season [3,4]. Under ideal laboratory conditions, A. aegypti eggs can be stored for ten weeks under 27°C with more than 80% of eggs hatching when submerged [5]. In nature when eggs are exposed to harsh environments as well as predators and parasites [6], egg survival can still be around 50% after overwintering [7,8], but high temperature and low humidity conditions increase egg mortality [9].
Wolbachia, a common endosymbiont in arthropods, has antiviral effects on its host and was artificially introduced into A. aegypti to help suppress arbovirus transmission [10–12]. Wolbachia-infected populations have been established in the field including the wMel [13–15] and wAlbB strains [16]. This approach relies on the ability of Wolbachia infections to invade through cytoplasmic incompatibility (CI), when infected males cause the death of offspring when mated with uninfected females [17]. However, the Wolbachia infection can also induce fitness costs on its host to reduce the host population [18], while environmental conditions can influence infection dynamics in populations by affecting Wolbachia density, leading to other impacts like CI leakage [19] and maternal transmission failure [20,21]. Both these effects can increase the uninfected proportion of mosquitoes in a population across generations. A reduction in Wolbachia frequency in a population will reduce efficiency whereby Wolbachia invades a population [22–24] with consequences for successful disease control.
Given that Wolbachia can play an important role in arboviral disease control [13,16], it is crucial to determine environmental conditions when invasion dynamics are more likely to favor Wolbachia to persist at a high level. In areas where A. aegypti occurs, factors such as temperature and rainfall variability will impact population abundance, through influencing egg quiescence [25,26]. Egg quiescence can be affected by high temperatures and Wolbachia infection status under laboratory conditions. The effect of wMelPop on A. aegypti eggs is particularly strong [27,28], whereas wMel- and wAlbB-infected eggs can survive much longer [29,30], although fitness costs under some conditions are apparent. For example, wMel-infected eggs are more sensitive than uninfected eggs to high temperatures, with lower hatch proportions when eggs are stored at 26–36°C [19]. The impacts of rainfall variability, which is an important factor in the seasonal outbreak of mosquitoes [31], has received less attention. Moreover, environmental and Wolbachia effects on egg quiescence have so far been characterized within the egg life stage, whereas Wolbachia invasion depends on effects on host phenotypes across life stages.
In this study, we tested the viability of wMel-infected, wAlbB-infected and uninfected eggs after storage under 11–19°C and 22–30°C cycling temperatures for different durations. We then tested Wolbachia infection density and fitness of the adults emerging from these eggs as well as on CI and maternal transmission. These experiments led to the surprising finding that many wAlbB-infected females became infertile when emerging from warm-stored eggs, and females with either infection showed a reduction in fecundity. Such effects could influence the local population dynamics of Wolbachia, and the seasonal size of outbreaks of A. aegypti populations in areas once Wolbachia has established, with secondary effects on disease transmission. Our results also provide guidance on efficient accumulation of Wolbachia-infected eggs prior to release which can help release programs [16,32] and they point to novel options for suppressing mosquito populations by Wolbachia strains in some contexts.
Materials and methods
Ethics statement
Female mosquitoes in this experiment were blood fed by a volunteer as described by the University of Melbourne Human Ethics committee (approval 0723847). All participants provided written informed consent.
Mosquito strains and maintenance
Uninfected, wMel-infected and wAlbB-infected A. aegypti were used in this work. The wMel-infected and uninfected populations were derived from eggs collected from Cairns, Queensland, Australia [30] while the wAlbB-infected population was generated by Xi et al [33] and then crossed to an Australian background [30]. Mosquitoes were maintained at 26 ± 1°C in a controlled temperature room [34] until experiments commenced. Wolbachia-infected populations were backcrossed to an uninfected population at least three times a year to maintain a similar genetic background in all the stocks [27]. Another three generations of backcrossing were undertaken directly before this experiment. After a week, egg batches were collected on sandpaper strips, rolled up in paper towels and sealed in a plastic bag for three days before the viability of eggs was tested.
Egg viability across storage time
We cut egg batches into small 1 cm × 3 cm pieces. Each piece contained 50 to 150 eggs which were hatched to calculate the initial hatch rate (week 0) or stored egg hatch rate under cycling temperatures of 11–19 ± 1°C or 22–30 ± 1°C in two incubators with a 12:12 h photoperiod. The eggs were labelled and sealed in a plastic box with a cup of saturated sodium chloride solution to balance the humidity at approximately 75% [35]. Temperature and humidity in the box were checked by data loggers (Thermochron; 1-Wire, iButton.com, Dallas Semiconductors, Sunnyvale, CA, USA) (S1 Fig). After storage for 0, 1, 4, 8, 11, 14 or 16 weeks, four to six batches of eggs per population and temperature cycle were submerged in reverse osmosis (RO) water with fish food tablets (Tetra, Melle, Germany) ad libitum and yeast (to stimulate the hatching) and a second immersion was carried out with a one-day drying interval to hatch any viable eggs that had failed to hatch in the first immersion [36]. Intact and hatched eggs (with a detached egg cap) were counted to indicate dead or surviving larvae. After hatching, larvae were reared until the adult stage for further testing.
Wolbachia density across storage time
Wolbachia-infected eggs before (0 weeks) and after cycling temperature treatments (8, 11, 14, or 16 weeks) were hatched. Two-day-old larvae were density controlled to a maximum of 30 larvae in trays of 300 mL RO water in each treatment and reared to adulthood. Eleven 2-day-old females per treatment were stored in 100% ethanol for Wolbachia screening. Individual DNA was extracted in 200 μL 5% Chelex 100 Resin (Bio-Rad Laboratories, Hercules, CA) [37], and diluted ten times for quantitative PCR analyses. Mosquito-specific (mRpS6) primers, A. aegypti-specific (aRpS6) primers and Wolbachia-specific primers (w1 primers for wMel-infected or wAlbB primers for wAlbB-infected) were used in the same run for each sample [30,38,39]. Three technical replicates were completed for each sample with two requirements: first, the difference of the crossing point (ΔCp) between mRpS6 and aRpS6 primers, which was used to indicate the quality of each run, had to be less than 1; and second, the variance of ΔCp between the three runs was expected to be less than 0.1. Relative density was calculated by power 2 transformation of ΔCp differences between aRpS6 and Wolbachia primers and technical replicates were averaged before testing treatment differences (storage time, Wolbachia infection type, temperature).
Cytoplasmic incompatibility
The wMel- and wAlbB-infected eggs that not been stored (preserved under constant 26°C for less than three weeks, regarded as untreated) or had been stored (treated) for 14 weeks at 11–19°C and 10 (wAlbB) or 11 (wMel) weeks at 22–30°C were hatched and larvae were reared to adulthood for crossing experiments. Wing lengths were measured for seven to ten randomly selected females from each treatment as a measure of mosquito size. Eight replicate cages, each consisting of five uninfected untreated females crossed with five Wolbachia-infected treated males, were set up to test cytoplasmic incompatibility (CI), while four replicates involving crosses with Wolbachia-infected untreated males were set up as controls. Eight replicates, each with five Wolbachia-infected treated females crossed with five Wolbachia-infected untreated males, were set up to test for CI rescue, while four replicates of Wolbachia-infected untreated females crossed with untreated males were set up as controls. After adults emerged, they were left with a 10% sucrose cup and water cup for four or five days to ensure that females had matured and mated. Females were blood fed once and allowed to oviposit on sandpaper (3.8 × 18 cm; Saint-Gobain Abrasives Pty. Ltd., Thomastown, Victoria, Australia) which was half submerged into water which had been used to rear larvae. These egg strips were collected at the fifth and seventh day and hatched on the ninth day after blood feeding.
Maternal transmission
To test for maternal transmission, 20 Wolbachia-infected female mosquitoes that had been stored for 14 weeks at 11–19°C and 10 (wAlbB) or 11 (wMel) weeks at 22–30°C were crossed to uninfected males that had not been stored before blood feeding. Four to five days old females were blood fed once, engorged individuals were isolated in a 70 mL cup and allowed to lay eggs for one week. After laying eggs, mothers were stored in ethanol for Wolbachia screening. Mothers who died before being stored were excluded from the experiment. We selected six to seven mothers per treatment which each had more than 30 offspring alive, and we screened the mother and eight to ten offspring for Wolbachia infection. wAlbB-infected females from the 22–30°C treatment that failed to lay any eggs were dissected after being stored in ethanol to look for the presence of mature eggs. Since no wAlbB-infected females stored for 10 weeks at 22–30°C laid eggs in this experiment, we instead tested maternal transmission from wAlbB-infected females stored for 11 weeks at 22–30°C in below fertility experiment.
Female fertility
We further investigated the loss of fertility in Wolbachia-infected females derived from eggs stored at 22–30°C by hatching batches of wAlbB-infected (stored for 0, 6, 9 or 11 weeks), wMel-infected (0, 9 or 11 weeks) and uninfected eggs (0, 6, 9 or 11 weeks). To understand the role males played, females hatched at each time point were (1) crossed to males from the same treatment, (2) crossed to males with the same Wolbachia infection type that were not stored, and (3) crossed to uninfected untreated males. Between 30 to 42 females from each cross were blood fed once, isolated in a 70 mL cup and provided with a strip of sandpaper to lay eggs. Eggs were collected once at the sixth day and hatched at the ninth day after blood feeding to determine fecundity and egg hatch proportions. Females that died before egg collection were excluded from analyses.
We determined the prevalence of female infertility by scoring the proportion of females in each cross that did not lay eggs on the collection day. We dissected female mosquitoes that failed to produce eggs from each cross at 9 weeks, and at 11 weeks we randomly selected and dissected up to ten female mosquitoes from each cross. We identified female insemination status by looking at their spermathecae under a compound light microscope (Motic B1 series, Australian Instrument Services Pty. Ltd., Australia). Each female has three spermathecae, one larger one and two smaller ones. The spermathecae containing sperm were distinguished from those that did not base on their texture and transparency, with spermathecae containing sperm having a dark and solid appearance with visible sperm swirling around the perimeter (S2 Fig).
Statistical analysis
All data analysis and visualization were undertaken with R v.3.6.0. We performed Kaplan-Meier survival tests and log rank tests [40] to compare treatment effects on changes in egg viability across time, and used the Benjamini p-value adjustment method for pairwise comparisons. We used ANOVAs to compare treatment effects for normally distributed data including fecundity and log transformed Wolbachia density, and used logistic regression to analyze treatment and storage time effects on proportional data such as egg hatch and female infertility. We ran Kruskal-Wallis rank tests to analyze single factor effects of hatch proportions in the maternal transmission experiment.
Results
Quiescent egg viability depends on Wolbachia infection type and egg storage temperature
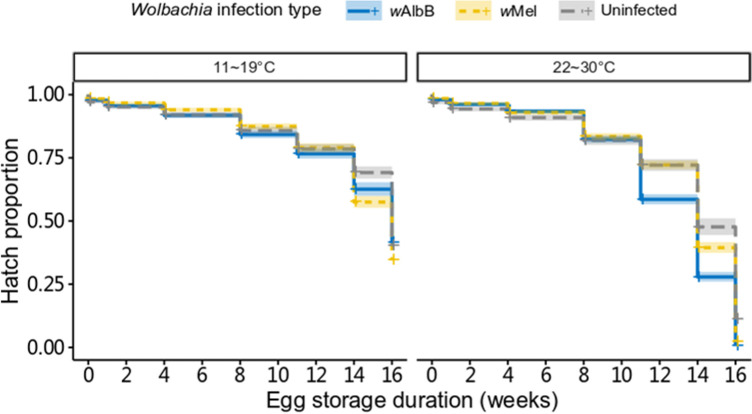
We compared the viability of uninfected, wMel-infected and wAlbB-infected eggs when stored at 11–19°C or 22–30°C for up to 16 weeks. There was a significant effect of Wolbachia infection type (log rank test, χ2 = 310, df = 2, p < 0.001) when eggs were stored at 22–30°C (Fig 1), with wAlbB-infected eggs dying faster than uninfected and wMel-infected eggs (pairwise comparisons, wAlbB-infected: uninfected, p < 0.001; wAlbB-infected: wMel-infected, p < 0.001; wMel-infected: uninfected, p = 0.004). Eggs were viable for longer under 11–19°C (all colonies, p < 0.001), and there were only marginally significant differences between Wolbachia infection type at this temperature (log rank test, χ2 = 6.4, df = 2, p = 0.04); wMel-infected eggs died faster than uninfected eggs (pairwise comparisons, wAlbB-infected: uninfected, p = 0.287; wAlbB-infected: wMel-infected, p = 0.626; wMel-infected: uninfected, p = 0.027).
Fig 1. Quiescent egg viability depends on Wolbachia infection type and storage temperature.
Survival of eggs based on hatch proportions of uninfected, wMel-infected and wAlbB-infected Aedes aegypti eggs stored under cycling temperatures of 11–19°C or 22–30°C for 0, 1, 4, 8, 11, 14 or 16 weeks. Shaded areas represent 95% confidence intervals.
Wolbachia density decreases across storage time
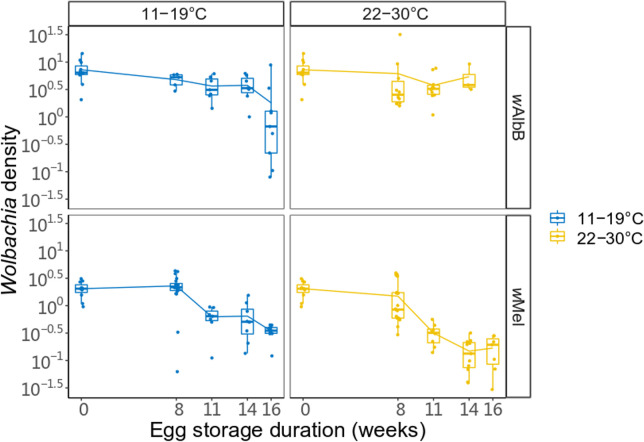
We found that Wolbachia density (log transformed) in female adults hatching from quiescent eggs was significantly affected by storage time and Wolbachia infection type, but not by temperature (three-way ANOVA, storage time: F1,176 = 26.869, p < 0.001; Wolbachia infection type: F1,176 = 10.363, p = 0.002; temperature: F1,176 = 0.618, p = 0.433). Density tended to decrease with egg storage time (Fig 2). There was a significant interaction among the three variables (three-way interaction, F1,176 = 8.609, p = 0.004) but two-way interactions were not significant (p > 0.05). When different mosquito populations were considered separately, both the density of wMel and wAlbB-infected mosquitoes were related to storage time but not storage temperature (storage time: both p < 0.001; storage temperature: wMel-infected: F1,107 < 0.001, p = 0.996; wAlbB-infected: F1,69 = 0.578, p = 0.450).
Fig 2. Wolbachia density declines with egg storage.
Box plots of log relative Wolbachia density of wMel-infected or wAlbB-infected 2-day-old female mosquitoes hatched from eggs stored under temperature cycles of 11–19°C or 22–30°C for 0, 8, 11, 14 or 16 weeks. Points represent individuals.
Cytoplasmic incompatibility leakage and rescue after egg quiescence
The wAlbB-infected males caused complete CI with uninfected females (no eggs hatched) regardless of egg storage duration. For wMel-infected A. aegypti, incomplete CI (CI leakage) occurred when males were reared from eggs stored at either 11–19°C or 22–30°C. No eggs hatched from the control cross where wMel-infected males were hatched from unstored eggs. However, averaged hatch percentages in CI crosses were 0.35% and 0.26% when wMel-infected fathers were stored at 11–19°C and 22–30°C respectively (S1 Table), suggesting a minor CI leakage.
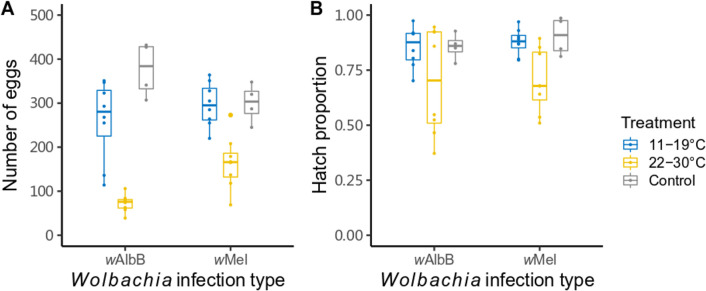
We performed crosses between Wolbachia-infected treated females (hatched from eggs stored at 11–19°C or 22–30°C) and Wolbachia-infected untreated males to test for CI rescue. The number of eggs laid by females was impacted by their egg storage treatment (ANOVA, F2,34 = 39.593, p < 0.001), but not by their Wolbachia infection type (F1,34 = 1.423, p = 0.241). Wolbachia-infected females derived from eggs stored under 22–30°C for 11/10 weeks laid approximately 45% and 70% fewer eggs for wMel-infected and wAlbB-infected respectively than when stored under 11–19°C (Fig 3A). Egg hatch proportions from these crosses were unaffected by the egg storage treatment of the female parents (Fig 3B, Logistic regression: F2,34 = 2.669, p = 0.084), or their Wolbachia infection type (F1,34 = 0.130, p = 0.721), suggesting that Wolbachia-infected females are able to restore compatibility with Wolbachia-infected males regardless of storage conditions.
Fig 3. Reduced fertility of Wolbachia-infected warm-stored females crossed to Wolbachia-infected unstored males.
(A) Number of eggs laid by females and (B) their hatch proportions. Wolbachia-infected females were derived from eggs stored under 26°C for less than three weeks (control) or a cycling temperature of 11–19°C for 14 weeks or 22–30°C for 11 (wMel-infected) or 10 (wAlbB-infected) weeks. Each data point represents the total number of eggs laid and their hatch proportion from a replicate cage of five females and five males.
We measured the wing length of female mosquitoes to estimate their body size. No significant differences were found for wMel or wAlbB between the two temperature treatments and untreated samples (ANOVA, wMel: F2,23 = 0.578, p = 0.569; wAlbB: F2,23 = 3.153, p = 0.062). There were weakly significant differences in wing length between untreated wMel-infected, wAlbB-infected and uninfected females (F2,19 = 4.62, p = 0.023), where wAlbB-infected females were somewhat larger than females from the other two populations (S2 Table).
Complete maternal transmission of Wolbachia after egg quiescence
Wolbachia-infected females stored at 11–19°C or 22–30°C transmitted the infection to the next generation at a frequency of 100% regardless of Wolbachia infection type and egg storage temperature. In our first test of wAlbB-infected females stored at 22–30°C, all twelve isolated females did not lay eggs, and no mature eggs were observed in their body upon dissection. We then counted the number of eggs laid by wAlbB-infected females stored at 11–19°C and wMel-infected females from both treatments and their hatch proportions (S3 Table). No significant differences in hatch proportion were found (Kruskal-Wallis rank tests, comparison between wMel-infected and wAlbB-infected mosquitoes under 11–19°C: χ2 = 0.776, df = 1, p = 0.379; comparison between 11–19°C and 22–30°C for wAlbB-infected mosquitoes: χ2 = 3.564, df = 1, p = 0.059). However, wMel-infected females produced fewer eggs when stored at 22–30°C compared to 11–19°C (ANOVA, F1,27 = 4.875, p = 0.036).
Wolbachia-infected females show fertility loss after egg quiescence
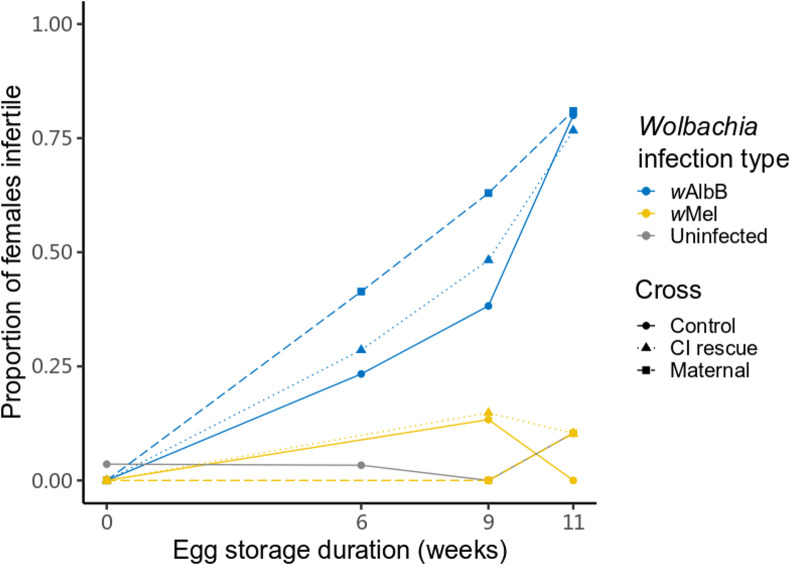
The results in the previous experiments suggest fertility effects from storage in that Wolbachia-infected females produced low egg numbers (or did not lay eggs at all) when stored as eggs under 22–30°C for a period. To further investigate the apparent loss of female fertility under egg storage, we determined the proportion of Wolbachia-infected and uninfected females that did not lay eggs when female parents were derived from eggs stored under 22–30°C. When Wolbachia infection type was considered separately, there were differences for the wAlbB-infected strain for both egg storage duration (F3,6 = 185.138, p < 0.001) and cross (F2,6 = 8.985, p = 0.016), whereas wMel-infected and uninfected strains showed no significant effects of these factors (all p > 0.05). The proportion of wAlbB-infected females not laying eggs increased dramatically with egg storage duration, with almost 80% of wAlbB-infected females not producing eggs by the final assessment time (Fig 4). All dissected females were inseminated (S2 Fig) but lacked visible eggs in their ovaries, indicating that infertility was not due to a lack of mating.
Fig 4. Female fertility loss when wAlbB-infected females derived from stored eggs.
Females hatched at each time point were crossed to males from the same treatment (Control), crossed to males with the same Wolbachia infection type that stored under 26°C for less than three weeks (CI rescue) and crossed to uninfected males stored under 26°C for less than three weeks (Maternal). Each data point is based on the proportion of 22–42 tested females.
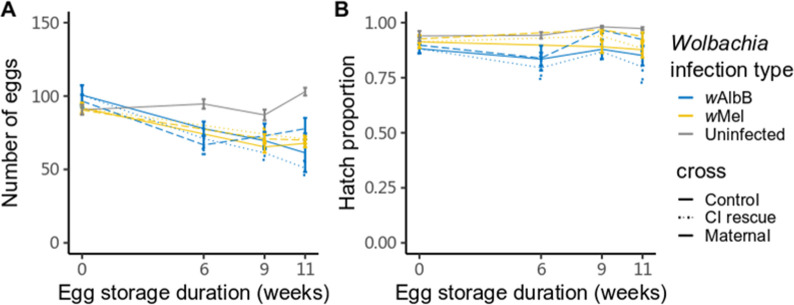
For those females that did lay eggs, there were effects of Wolbachia infection type (ANOVA, F2.537 = 18.361, p < 0.001) and egg storage duration (F1.537 = 90.650, p < 0.001) on the number of eggs per female laid but no effect of cross (F2.537 = 0.038, p = 0.962). Both wMel- and wAlbB-infected females laid fewer eggs with increasing egg storage duration in all crosses (Fig 5A). In contrast, uninfected female fecundity did not decline. For the hatch proportion of these eggs, effects were induced by Wolbachia infection type (Fig 5B, logistic regression, F2.537 = 17.100, p < 0.001), cross (F2.537 = 5.734, p = 0.003) but not egg storage duration (F1.537 = 0.523, p = 0.470).
Fig 5. Fecundity and egg hatch of fertile females derived from stored eggs crossed to different male lines.
(A) Fecundity and (B) egg hatch proportions of eggs from female mosquitoes that were derived from eggs stored under a cycling temperature of 22–30°C for 0, 6, 9 or 11 weeks. Females hatched at each time point were crossed to males from the same treatment (Control), crossed to males with the same Wolbachia infection type that stored under 26°C for less than three weeks (CI rescue) and crossed to uninfected males stored under 26°C for less than three weeks (Maternal). The data exclude females that did not lay eggs (see Fig 4). Error bars represent means ± standard errors.
Discussion
Aedes aegypti mosquitoes carrying the wMel or wAlbB strains of Wolbachia have the potential to reduce dengue transmission through decreased mosquito vector competence [16,41], and there is already good evidence that both strains are having such impacts in Wolbachia-invaded release areas [13,16]. These releases can involve adults or eggs [15]. Releases involving eggs, where containers of eggs are hatched directly in the field, are desirable for Wolbachia release programs because they do not require local mass-rearing facilities [13,16,42] and allow eggs to be accumulated across several weeks before being transported for release. Although most releases to date have involved the wMel strain, wAlbB was used for releases in Malaysia [16] based on its desirable characteristic of being stable in its density and phenotypic effects under high temperatures [43].
In this study, we investigated the effects of long-term storage on A. aegypti eggs infected with Wolbachia strains wMel or wAlbB. Eggs survived longer under a cooler environment; more than 50% of eggs from all strains hatched after storage for 14 weeks at 11–19°C while hatch proportions were below 50% when stored at 22–30°C for the same amount of time. Although the Wolbachia density of both wMel and wAlbB-infected adults decreased with storage time, density was not affected by storage temperature, suggesting that density did not contribute to the decrease in egg viability under warmer temperatures. Nevertheless, we suspect that the decreased density contributed to incomplete CI for wMel-infected males stored under both temperature treatments given the strong relationship between CI and Wolbachia density previously reported for wMel [19]. Despite decreased density, the maternal transmission of Wolbachia remained stable after eggs had passed through a quiescent phase, regardless of storage temperature.
However, Wolbachia infections induced substantial costs to fertility when adults were reared from eggs stored under 22–30°C for 10 or 11 weeks. Both wMel and wAlbB-infected females that were stored as eggs at 22–30°C produced fewer eggs. In a second experiment where we tested individual females, a high proportion of wAlbB-infected females suffered infertility when stored as eggs at 22–30°C, regardless of the male used in crosses. In contrast, wMel-infected and uninfected females retained high rates of fertility throughout the experiment. Dissection of wAlbB-infected females that failed to lay eggs showed that they were inseminated but lacked mature eggs in their abdomens despite being blood fed. Previous studies suggest that wAlbB has only a minor negative impact on egg viability during the first two months of storage [44]. However, these costs may be underestimated because a high proportion of females hatching from stored eggs are infertile. Furthermore, both wMel and wAlbB-infected females laid fewer eggs when hatching from stored eggs though the hatch rate remain high.
The fertility loss may not be wAlbB-specific. In other work we found around 30% of wMel females suffered infertility when adults were stored as eggs under 22–30°C for 18 weeks (S4 Table). Compared to wMel, the ability of wAlbB to maintain a relatively high density in quiescing eggs may result in the accumulation of damage in such eggs through nutrition competition [45], which leads to fitness effects at a later life stage, producing female infertility and a reduction in fecundity. This effect may be exacerbated when female parents are stored as eggs. Egg provisioning or hormone modulation may also be impacted by Wolbachia under stress [44,46], but this requires further investigation. We provided same amount of fish food in all of the treatments and body sizes of females with same Wolbachia infection were similar across treatments (S2 Table), indicating that mosquitoes should have received enough nutrition for larval development. However, it is still unknown whether there has been enough nutrition to support oogenesis, for example, Wolbachia may compete for amino acid and can reduce female fecundity [45]. Multiple blood feeding may rescue this deleterious effect if the reason of fertility loss is nutritional deprivation [47,48], which should be explored further.
The original host of wAlbB, Aedes albopictus, has higher cold tolerance than A. aegypti and prefers cooler temperatures [49–51]. The wAlbB infection may provide fitness benefits to A. aegypti under cool environments, which points to the wAlbB-infected A. aegypti being relatively better adapted to cool environments than uninfected mosquitoes [52]. Under hot environments, wAlbB is relatively more stable than wMel in terms of density and its ability to induce cytoplasmic incompatibility [43]. However, the fertility loss may happen in a short time of egg quiescence, leading to unknown consequences on Wolbachia spread and maintenance. Mosquitoes with wAlbB may not invade readily if releases happen in areas where larval habitats used by A. aegypti are intermittent, requiring long periods of egg quiescence. In such environments, the threshold frequency of wAlbB in a population that needs to be exceeded to get invasion may increase above the currently estimated point (for wMel) of 20–30% [32]. Releases based on wAlbB eggs rather than adults (such as carried out at one site in Malaysia [16]) could also be much less effective than expected if they use eggs that have been stored too long, unless these have been stored under cool conditions. A. aegypti is an opportunistic breeder, taking advantage of locally suitable conditions which are expected to vary spatially and temporally depending on rubbish accumulation, defective building spaces and defective water and sewerage tanks [53–55]. Because of these differences, the contribution of mosquitoes from quiescent eggs to the adult population is expected to vary locally. Releases across different locations show that rates of Wolbachia invasion can vary for both wMel [53] and wAlbB [16]. While various factors may be responsible for site-to-site variation in invasion rates and success including density dependent processes [22], fitness costs associated with quiescence are also likely to be important, particularly for wAlbB.
Once Wolbachia invasions have been completed, there is the potential for Wolbachia infections to decrease the overall size of mosquito populations due to fitness costs [28,56]. Our data suggest that this is particularly likely in locations with strong seasonality, where quiescent eggs persisting during the dry season are more likely to contribute to A. aegypti population continuity. A strategy for crashing A. aegypti populations during the dry season based on the reduced hatch rate of infected quiescent eggs was originally proposed several years ago, based around the wMelPop infection which induces a particularly large fitness costs in quiescent eggs [27,57]. However, this strain cannot easily invade mosquito populations [10,18] whereas the wAlbB strain does establish successfully [16] and may carry fitness costs appropriate for such an intervention.
In summary, we have tested the performance of wMel, wAlbB-infected and uninfected A. aegypti after eggs were stored under cycling temperatures of 11–19°C and 22–30°C. We found that, compared to warm environments that are normally used to culture mosquitoes [34,58], cooler environments may be better for egg storage prior to Wolbachia releases, though various concerns should be taken into account such as selection for better performance under cool conditions which might occur when eggs are repeatedly stored under cold conditions and adversely affect performance under hot field conditions. The local environment may therefore need to be considered. Nevertheless, long-term storage under warm environment greatly reduces the fertility of hatched females, especially for wAlbB-infected, in which a high proportion of females became infertile. Our study helps to guide conditions for appropriate storage of Wolbachia-infected eggs, inform release timing to coincide with periods where quiescent eggs contribute little to population dynamics, and help to explain Wolbachia invasion success or failure in different environments [16].
Supporting information
(A) Diurnal air temperature and (B) air humidity fluctuations in egg storage boxes. Incubators were set to temperature cycles of 11–19°C and 22–30°C. Data were measured by data loggers and shown across a representative 48 hour period to indicate the daily fluctuations during the experiment.
(TIF)
Three spermathecae of an infertile wAlbB-infected female Aedes aegypti were checked for insemination status under a (A) 40X objective lens or (B) 100X objective lens. The two on the left-hand side contain sperm, while the one on the right-hand side lacks sperm.
(TIF)
Uninfected Aedes aegypti females were crossed to wMel-infected and wAlbB-infected Aedes aegypti males derived from eggs stored under a cycling temperature of 11–19°C for 14 weeks, under 22–30°C for 10 (wAlbB-infected) or 11 (wMel-infected) weeks or stored as eggs under 26°C for less than three weeks (control). Each replicate consisted of a cage of five females and five males.
(XLSX)
Uninfected, wAlbB-infected and wMel-infected Aedes aegypti females derived from eggs stored under 26°C for three days (0 week) or under a cycling temperature of 11–19°C for 14 weeks or 22–30°C for 10 (wAlbB-infected) or 11 (wMel-infected) weeks.
(XLSX)
Uninfected, wAlbB-infected and wMel-infected Aedes aegypti females derived from eggs stored under a cycling temperature of 11–19°C for 14 weeks or 22–30°C for 11 (wMel-infected) weeks were mated with uninfected males stored as eggs under 26°C for less than three weeks. For fecundity, means and standard deviations (SDs) are shown. For egg hatch proportion, data are arcsine transformed for computing means and 95% confidence intervals and the back-transformed data are shown.
(XLSX)
wMel-infected females derived from eggs stored under a cycling temperature of 22–30°C for 18 weeks were crossed to wMel-infected males from the same treatment (Control), crossed to wMel-infected males stored as eggs under 26°C for less than three weeks (CI rescue) and crossed to uninfected untreated males (Maternal).
(XLSX)
Acknowledgments
We thank Heng Lin Yeap from the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and Marianne Coquilleau from the University of Melbourne for their advice and assistance.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
AAH was supported by the National Health and Medical Research Council (1132412, 1118640, www.nhmrc.gov.au) and the Wellcome Trust (108508, wellcome.ac.uk). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. elife. 2015;4:e08347. 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiter P. Oviposition, dispersal, and survival in Aedes aegypti: implications for the efficacy of control strategies. Vector Borne Zoonotic Dis. 2007;7(2):261–73. Epub 2007/07/14. 10.1089/vbz.2006.0630 . [DOI] [PubMed] [Google Scholar]
- 3.Meola R. The influence of temperature and humidity on embryonic longevity in Aedes aegypti. Ann Entomol Soc Am. 1964;57(4):468–72. [Google Scholar]
- 4.Faull KJ, Williams CR. Intraspecific variation in desiccation survival time of Aedes aegypti (L.) mosquito eggs of Australian origin. J Vector Ecol. 2015;40(2):292–300. Epub 2015/11/28. 10.1111/jvec.12167 . [DOI] [PubMed] [Google Scholar]
- 5.Zheng M-L, Zhang D-J, Damiens DD, Lees RS, Gilles JRL. Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae)—II—Egg storage and hatching. Parasit Vectors. 2015;8(1):348. 10.1186/s13071-015-0951-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byttebier B, Fischer S. Predation on eggs of Aedes aegypti (Diptera: Culicidae): temporal dynamics and identification of potential predators during the winter season in a temperate region. J Med Entomol. 2019;56(3):737–43. Epub 2019/01/29. 10.1093/jme/tjy242 . [DOI] [PubMed] [Google Scholar]
- 7.Gimenez JO, Fischer S, Zalazar L, Stein M. Cold season mortality under natural conditions and subsequent hatching response of Aedes (Stegomyia) aegypti (Diptera: Culicidae) eggs in a subtropical city of Argentina. J Med Entomol. 2015;52(5):879–85. Epub 2015/09/04. 10.1093/jme/tjv107 . [DOI] [PubMed] [Google Scholar]
- 8.Fischer S, Alem IS, De Majo MS, Campos RE, Schweigmann N. Cold season mortality and hatching behavior of Aedes aegypti L.(Diptera: Culicidae) eggs in Buenos Aires City, Argentina. J Vector Ecol. 2011;36(1):94–9. 10.1111/j.1948-7134.2011.00145.x [DOI] [PubMed] [Google Scholar]
- 9.Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130(3):458–69. 10.1007/s004420100811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476(7361):450–3. 10.1038/nature10355 WOS:000294209400036. [DOI] [PubMed] [Google Scholar]
- 11.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018;14(1):e1006815. Epub 2018/01/26. 10.1371/journal.ppat.1006815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268–78. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 13.Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, Brown-Kenyon J, et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2019;3. 10.12688/gatesopenres.13061.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia GdA, Sylvestre G, Aguiar R, da Costa GB, Martins AJ, Lima JBP, et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS neglected tropical diseases. 2019;13(1):e0007023. 10.1371/journal.pntd.0007023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tantowijoyo W, Andari B, Arguni E, Budiwati N, Nurhayati I, Fitriana I, et al. Stable establishment of wMel Wolbachia in Aedes aegypti populations in Yogyakarta, Indonesia. PLoS neglected tropical diseases. 2020;14(4):e0008157. Epub 2020/04/18. 10.1371/journal.pntd.0008157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nazni WA, Hoffmann AA, NoorAfizah A, Cheong YL, Mancini MV, Golding N, et al. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr Biol. 2019;29(24):4241–8. 10.1016/j.cub.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson SL, Fox CW, Jiggins FM. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc R Soc Lond B Biol Sci. 2002;269(1490):437–45. 10.1098/rspb.2001.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TH, Le Nguyen H, Nguyen TY, Vu SN, Tran ND, Le T, et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors. 2015;8(1):563. 10.1186/s13071-015-1174-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross PA, Ritchie SA, Axford JK, Hoffmann AA. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS neglected tropical diseases. 2019;13(4):e0007357. 10.1371/journal.pntd.0007357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt TL, Filipovic I, Hoffmann AA, Rasic G. Fine-scale landscape genomics helps explain the slow spatial spread of Wolbachia through the Aedes aegypti population in Cairns, Australia. Heredity (Edinb). 2018;120(5):386–95. Epub 2018/01/24. 10.1038/s41437-017-0039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng B, Guo W, Hu L, Huang M, Yu J. Complex Wolbachia infection dynamics in mosquitoes with imperfect maternal transmission. Math Biosci Eng. 2018;15(2):523–41. Epub 2017/11/23. 10.3934/mbe.2018024 . [DOI] [PubMed] [Google Scholar]
- 22.Hancock PA, White VL, Callahan AG, Godfray CH, Hoffmann AA, Ritchie SA. Density-dependent population dynamics in Aedes aegypti slow the spread of wMel Wolbachia. J Appl Ecol. 2016;53(3):785–93. [Google Scholar]
- 23.Hu L, Tang M, Wu Z, Xi Z, Yu J. The threshold infection level for Wolbachia invasion in random environments. J Differ Equ. 2019;266(7):4377–93. [Google Scholar]
- 24.Richardson K, Hoffmann AA, Johnson P, Ritchie S, Kearney MR. Thermal sensitivity of Aedes aegypti from Australia: empirical data and prediction of effects on distribution. J Med Entomol. 2011;48(4):914–23. 10.1603/me10204 [DOI] [PubMed] [Google Scholar]
- 25.De Majo MS, Fischer S, Otero M, Schweigmann N. Effects of thermal heterogeneity and egg mortality on differences in the population dynamics of Aedes aegypti (Diptera: Culicidae) over short distances in temperate Argentina. J Med Entomol. 2013;50(3):543–51. 10.1603/me12211 [DOI] [PubMed] [Google Scholar]
- 26.Luz C, Tai MH, Santos AH, Silva HH. Impact of moisture on survival of Aedes aegypti eggs and ovicidal activity of Metarhizium anisopliae under laboratory conditions. Mem Inst Oswaldo Cruz. 2008;103(2):214–5. Epub 2008/04/22. 10.1590/s0074-02762008000200016 . [DOI] [PubMed] [Google Scholar]
- 27.Yeap HL, Mee P, Walker T, Weeks AR, O’Neill SL, Johnson P, et al. Dynamics of the "Popcorn" Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics. 2011;187(2):583–95. 10.1534/genetics.110.122390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritchie SA, Townsend M, Paton CJ, Callahan AG, Hoffmann AA. Application of wMelPop Wolbachia strain to crash local populations of Aedes aegypti. PLoS neglected tropical diseases. 2015;9(7):e0003930. 10.1371/journal.pntd.0003930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farnesi LC, Belinato TA, Gesto JSM, Martins AJ, Bruno RV, Moreira LA. Embryonic development and egg viability of wMel-infected Aedes aegypti. Parasit Vectors. 2019;12(1):211. Epub 2019/05/08. 10.1186/s13071-019-3474-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axford JK, Ross PA, Yeap HL, Callahan AG, Hoffmann AA. Fitness of wAlbB Wolbachia infection in Aedes aegypti: parameter estimates in an outcrossed background and potential for population invasion. Am J Trop Med Hyg. 2016;94(3):507–16. 10.4269/ajtmh.15-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngugi HN, Mutuku FM, Ndenga BA, Musunzaji PS, Mbakaya JO, Aswani P, et al. Characterization and productivity profiles of Aedes aegypti (L.) breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasit Vectors. 2017;10(1):331. 10.1186/s13071-017-2271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann AA, Montgomery B, Popovici J, Iturbe-Ormaetxe I, Johnson P, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–7. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 33.Xi ZY, Khoo CCH, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310(5746):326–8. 10.1126/science.1117607 WOS:000232670100056. [DOI] [PubMed] [Google Scholar]
- 34.Ross PA, Axford JK, Richardson KM, Endersby-Harshman NM, Hoffmann AA. Maintaining Aedes aegypti mosquitoes infected with Wolbachia. J Vis Exp. 2017;14(126):e56124. 10.3791/56124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winston PW, Bates DH. Saturated solutions for the control of humidity in biological research. Ecology. 1960;41(1):232–7. [Google Scholar]
- 36.McMeniman CJ, O’Neill SL. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS neglected tropical diseases. 2010;4(7):e748. 10.1371/journal.pntd.0000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SF, White VL, Weeks AR, Hoffmann AA, Endersby NM. High-throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl Environ Microbiol. 2012;78(13):4740–3. 10.1128/AEM.00069-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, Axford JK, et al. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS neglected tropical diseases. 2014;8(9):e3115. 10.1371/journal.pntd.0003115 WOS:000342796600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, Rousset F, O’Neill S. Phylogeny and PCR–based classification of Wolbachia strains using wsp gene sequences. Proc R Soc Lond B Biol Sci. 1998;265(1395):509–15. 10.1098/rspb.1998.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika. 1982;69(3):553–66. [Google Scholar]
- 41.Indriani C, Tantowijoyo W, Rancès E, Andari B, Prabowo E, Yusdi D, et al. Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: a quasi-experimental trial using controlled interrupted time series analysis. Gates Open Res. 2020;4:50. Epub 2020/08/18. 10.12688/gatesopenres.13122.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gesto JSM, Ribeiro GS, Rocha MN, Dias FBS, Peixoto J, Carvalho FD, et al. Reduced competence to arboviruses following the sustainable invasion of Wolbachia into native Aedes aegypti from Niterói, Southeastern Brazil. bioRxiv. 2020:2020.09.25.312207. 10.1101/2020.09.25.312207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 2017;13(1):e1006006. Epub 2017/01/06. 10.1371/journal.ppat.1006006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allman MJ, Fraser JE, Ritchie SA, Joubert DA, Simmons CP, Flores HA. Wolbachia’s deleterious impact on Aedes aegypti egg development: The potential role of nutritional parasitism. Insects. 2020;11(11). Epub 2020/10/31. 10.3390/insects11110735 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caragata EP, Rancès E, O’Neill SL, McGraw EA. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb Ecol. 2014;67(1):205–18. 10.1007/s00248-013-0339-4 [DOI] [PubMed] [Google Scholar]
- 46.Telang A, Li Y, Noriega FG, Brown MR. Effects of larval nutrition on the endocrinology of mosquito egg development. J Exp Biol. 2006;209(Pt 4):645–55. Epub 2006/02/02. 10.1242/jeb.02026 . [DOI] [PubMed] [Google Scholar]
- 47.Koella JC, Sørensen FL, Anderson RA. The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc Biol Sci. 1998;265(1398):763–8. Epub 1998/06/17. 10.1098/rspb.1998.0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reyes-Villanueva F. Egg development may require multiple bloodmeals among small Aedes aegypti (Diptera: Culicidae) field collected in northeastern Mexico. Fla Entomol. 2004;87(4):630–2. [Google Scholar]
- 49.Vinogradova EB. Diapause in aquatic insects, with emphasis on mosquitoes. Diapause in aquatic invertebrates: theory and human use. Dordrecht: Springer; 2007. p. 83–113. [Google Scholar]
- 50.Brady OJ, Golding N, Pigott DM, Kraemer MU, Messina JP, Reiner RC Jr, et al. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit Vectors. 2014;7(1):338. 10.1186/1756-3305-7-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dickens BL, Sun H, Jit M, Cook AR, Carrasco LR. Determining environmental and anthropogenic factors which explain the global distribution of Aedes aegypti and Ae. albopictus. BMJ Glob Health. 2018;3(4):e000801. Epub 2018/09/21. 10.1136/bmjgh-2018-000801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lau MJ, Ross PA, Endersby-Harshman NM, Hoffmann AA. Impacts of low temperatures on Wolbachia (Rickettsiales: Rickettsiaceae)-infected Aedes aegypti (Diptera: Culicidae). J Med Entomol. 2020. Epub 2020/04/21. 10.1093/jme/tjaa074 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt TL, Barton NH, Rašić G, Turley AP, Montgomery BL, Iturbe-Ormaetxe I, et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 2017;15(5):e2001894. 10.1371/journal.pbio.2001894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaCon G, Morrison AC, Astete H, Stoddard ST, Paz-Soldan VA, Elder JP, et al. Shifting patterns of Aedes aegypti fine scale spatial clustering in Iquitos, Peru. PLoS neglected tropical diseases. 2014;8(8):e3038. Epub 2014/08/08. 10.1371/journal.pntd.0003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeffery JA, Thi Yen N, Nam VS, Nghia le T, Hoffmann AA, Kay BH, et al. Characterizing the Aedes aegypti population in a Vietnamese village in preparation for a Wolbachia-based mosquito control strategy to eliminate dengue. PLoS neglected tropical diseases. 2009;3(11):e552. Epub 2009/12/04. 10.1371/journal.pntd.0000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rašić G, Endersby NM, Williams C, Hoffmann AA. Using Wolbachia-based release for suppression of Aedes mosquitoes: insights from genetic data and population simulations. Ecol Appl. 2014;24(5):1226–34. 10.1890/13-1305.1 [DOI] [PubMed] [Google Scholar]
- 57.Ross PA, Axford JK, Callahan AG, Richardson KM, Hoffmann AA. Persistent deleterious effects of a deleterious Wolbachia infection. PLoS neglected tropical diseases. 2020;14(4):e0008204–e. 10.1371/journal.pntd.0008204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morlan HB, Hayes RO, Schoof HF. Methods for mass rearing of Aedes aegypti (L.). Public Health Rep. 1963;78(8):711. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Diurnal air temperature and (B) air humidity fluctuations in egg storage boxes. Incubators were set to temperature cycles of 11–19°C and 22–30°C. Data were measured by data loggers and shown across a representative 48 hour period to indicate the daily fluctuations during the experiment.
(TIF)
Three spermathecae of an infertile wAlbB-infected female Aedes aegypti were checked for insemination status under a (A) 40X objective lens or (B) 100X objective lens. The two on the left-hand side contain sperm, while the one on the right-hand side lacks sperm.
(TIF)
Uninfected Aedes aegypti females were crossed to wMel-infected and wAlbB-infected Aedes aegypti males derived from eggs stored under a cycling temperature of 11–19°C for 14 weeks, under 22–30°C for 10 (wAlbB-infected) or 11 (wMel-infected) weeks or stored as eggs under 26°C for less than three weeks (control). Each replicate consisted of a cage of five females and five males.
(XLSX)
Uninfected, wAlbB-infected and wMel-infected Aedes aegypti females derived from eggs stored under 26°C for three days (0 week) or under a cycling temperature of 11–19°C for 14 weeks or 22–30°C for 10 (wAlbB-infected) or 11 (wMel-infected) weeks.
(XLSX)
Uninfected, wAlbB-infected and wMel-infected Aedes aegypti females derived from eggs stored under a cycling temperature of 11–19°C for 14 weeks or 22–30°C for 11 (wMel-infected) weeks were mated with uninfected males stored as eggs under 26°C for less than three weeks. For fecundity, means and standard deviations (SDs) are shown. For egg hatch proportion, data are arcsine transformed for computing means and 95% confidence intervals and the back-transformed data are shown.
(XLSX)
wMel-infected females derived from eggs stored under a cycling temperature of 22–30°C for 18 weeks were crossed to wMel-infected males from the same treatment (Control), crossed to wMel-infected males stored as eggs under 26°C for less than three weeks (CI rescue) and crossed to uninfected untreated males (Maternal).
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.