Abstract
Nitric oxide, which has been implicated in the development of hyperalgesia in the spinal system, has not been systematically studied in the trigeminal system, especially in the context of inflammatory muscle pain condition. In this study, we investigated the functional role of centrally released nitric oxide in the pathogenesis of orofacial muscle pain. Specifically, we examined the contribution of neuronal, inducible and endothelial nitric oxide synthases, nNOS, iNOS and eNOS, respectively, in mediating masseter hypersensitivity under acute inflammatory condition. Time-dependent changes in nNOS, iNOS and eNOS protein expression in the subnucleus caudalis (Vc) were assessed following capsaicin injection in the masseter muscle of male Sprague Dawley rats. The expression of all three nitric oxide synthases was significantly up-regulated 30–60 min following capsaicin stimulation, which paralleled the time course of the development of capsaicin-induced masseter hypersensitivity. Pretreatment with each NOS inhibitor significantly attenuated the masseter hypersensitivity. These data showed that all three NOS in the Vc are functionally important for the development of craniofacial muscle hyperalgesia and suggest that the three NOS are closely orchestrated to regulate the level of nitric oxide under normal and pathologic conditions.
Keywords: Muscle pain, Nitric oxide synthases, Capsaicin, Trigeminal, Rat
1. Introduction
Nitric oxide (NO) is synthesized by three known isoforms of flavoproteins termed nitric oxide synthase (NOS), namely, neuronal, endothelial and inducible NOS, nNOS, eNOS, and iNOS, respectively, based on the tissue type in which they were first described (Salerno et al., 2002). Among the three isoforms, nNOS is most abundantly expressed in the CNS and exclusively localized in neurons, eNOS in endothelial cells as well as in astrocytes, and iNOS primarily in inflammatory cells, astrocytes and microglia (Dawson et al., 1994; Tao et al., 2004).
The evidence supporting the involvement of nNOS in the central mechanisms of inflammatory hyperalgesia at the spinal cord (SC) level has accumulated over the past two decades (Meller et al., 1992, 1994; Malmberg and Yaksh, 1993), and more recent studies provide compelling evidence that iNOS and eNOS also contribute to various aspects of inflammatory pain (Tao et al., 2003, 2004; Sung et al., 2004, 2005). Peripheral inflammation has been shown to alter the expression of both nNOS and iNOS in the SC (Gühring et al., 2001; Wu et al., 2001), suggesting that the NO level in the SC is closely regulated during the course of inflammation. However, the exact role and relative contribution of NO released from neuronal and non-neuronal sources in inflammatory hyperalgesia at the SC level still remain elusive.
As far as we know, there is no information available on the transcriptional regulation of NO synthases at the level of subnucleus caudalis (Vc), the cranial homolog of the SC dorsal horn, under inflammatory pain conditions. Recently, we have shown that soluble guanylate cyclase (sGC), a physiological receptor for NO, is expressed in Vc neurons and that pharmacological blockade of sGC effectively attenuates masseter hypersensitivity induced by intramuscular injection of capsaicin (Ro et al., 2007), strongly suggesting that NO-sGC pathway in the Vc is involved in mediating orofacial muscle hypersensitivity under acute inflammatory conditions. In the present study, we investigated how acute muscle inflammation alters the pattern and extent of the expression of all three NOS enzymes in the Vc, and studied the functional contribution of each NOS in the development of muscle hypersensitivity under the same condition.
2. Materials
2.1. Animals
Experiments were carried out on male Sprague Dawley rats (250–350 g) housed in a temperature-controlled room under a 12:12 light–dark cycle with access to food and water ad libitum. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996).
2.2. Western blot analysis
A brainstem block in a region of the Vc (3–6 mm caudal from the obex) was harvested and a dorsolateral portion of the block corresponding to the Vc was excised bilaterally. Total proteins from each tissue were dissolved in RIPA buffer. Protein samples (20–40 μg) were denatured in loading buffer at 90 °C for 5 min, fractionated on NuPAGE gel with running buffer containing SDS (Invitrogen), and blotted onto a PVDF membrane in a semi-dry system. The membranes were blocked with 5% milk once in Tween 20 PBS for 1 h at room temperature. Following primary antibodies were added: nNOS and eNOS (1:1000; mouse monoclonal; BD; San Diego, CA), and iNOS (1:1000; rabbit polyclonal; Stressgen; Victoria, BC). The bound primary antibodies were detected with HRP-conjugated anti-mouse IgG (1:5000, Cell Signaling) and visualized with ECL reagents. The membrane was re-probed with anti-β-actin for normalization. To study time-dependent changes in NOS expression under acute muscle pain condition protein expression of each NOS was assessed at three different time points (30, 60, and 90 min; n = 6 for each time point) following capsaicin injection in the masseter and compared to that of naïve rats (n = 6) with Kruskal–Walis One way ANOVA.
2.3. Behavioral testing of masseter sensitivity
The behavioral model for testing mechanical sensitivity of the masseter muscle has been previously described (Ro et al., 2007). Briefly, rats were initially anesthetized with sodium pentobarbital (40–50 mg/kg, i.p.). A tail vein was cannulated and for continuous infusion of pentobarbital to maintain a relatively light level of anesthesia throughout the duration of experiment (3–5 mg/h). A baseline mechanical threshold for evoking the hindpaw responses was determined 15 min prior to intramuscular capsaicin injection using the electronic von Frey (VF) anesthesiometer (IITC Life Science, Inc, Woodland Hills, CA). A rigid tip attached to the VF meter was applied to the masseter muscle until the animals responded with hindpaw shaking with the threshold defined as the lowest force necessary to evoke the hindpaw response. Changes in masseter sensitivity were assessed at 15, 30 45, 60, 90 min following capsaicin treatments. An experimenter blinded to experimental conditions performed all behavioral testing in order to maintain the consistency in the timing and application of masseter stimulation.
2.4. Drugs and experimental groups
Capsaicin (0.1%, 100 μl dissolved in 7% Tween 80%, 20% ethanol, and 73% isotonic saline) was injected directly into the masseter to induce mechanical hypersensitivity. 7-NI, L-NIO and L-NIL (Tocris Cookson) were used as inhibitors for nNOS, eNOS and iNOS, respectively. 7-NI was dissolved in DMSO and L-NIO and L-NIL in PBS. The final concentration of DMSO did not exceed 0.1%. All inhibitors, freshly prepared on the day of experiment, were given in 10 μl volume. To determine the contribution of each NOS in mediating capsaicin-induced masseter hypersensitivity 7-NI (20 nmol and 1 μmol), L-NIO (100 nmol and 1 μmol), L-NIL (10 nmol and 100 nmol) or the same volume of vehicle was intrathecally administered over the region of Vc 5–10 min prior to capsaicin injection. A group of rats that received intrathecal PBS served as a control for both L-NIO and L-NIL and another group with DMSO as a control for 7-NI. Each group consisted of 5 rats. As a control for the effect of inhibitor alone, the higher dose of each inhibitor was administered intrathecally and masseter sensitivity tested without capsaicin in separate groups of rats (n = 4 each group).
2.5. Behavioral data analysis
Post treatment mechanical thresholds were normalized to the baseline and mean percent changes of mechanical thresholds were for the observed time points were plotted. In order to assess the overall magnitude of drug-induced changes in masseter sensitivity over time, area under the curve (AUC) was calculated for the normalized data for each rat using the Trapezoidal rule. Mean AUC values for experimental and control groups were compared with one-way ANOVA followed by Dunnett’s test. The significance of statistical analyses was set at p < 0.05. All data are presented as mean ± SEM.
3. Results
3.1. Western blot studies
All three NOS were reliably and readily detected in the Vc though iNOS seemed to be expressed only at a low level in naïve rats. There was an immediate and significant increase in all three NOS as early as 30 min that persisted for 60 min before returning to the baseline level at 90 minute following the capsaicin treatment (Fig. 1). The pattern and extent of protein up-regulation were similar for all three NOS (H = 13.1, p < 0.004 for nNOS; H = 11.6, p < 0.009 for iNOS and H = 14.1, p < 0.003 for eNOS). None of the three NOS showed significant changes in expression level in the Vc contralateral to the injected side, suggesting that anesthesia does not have any effect on changes in the NOS expression.
Fig. 1.
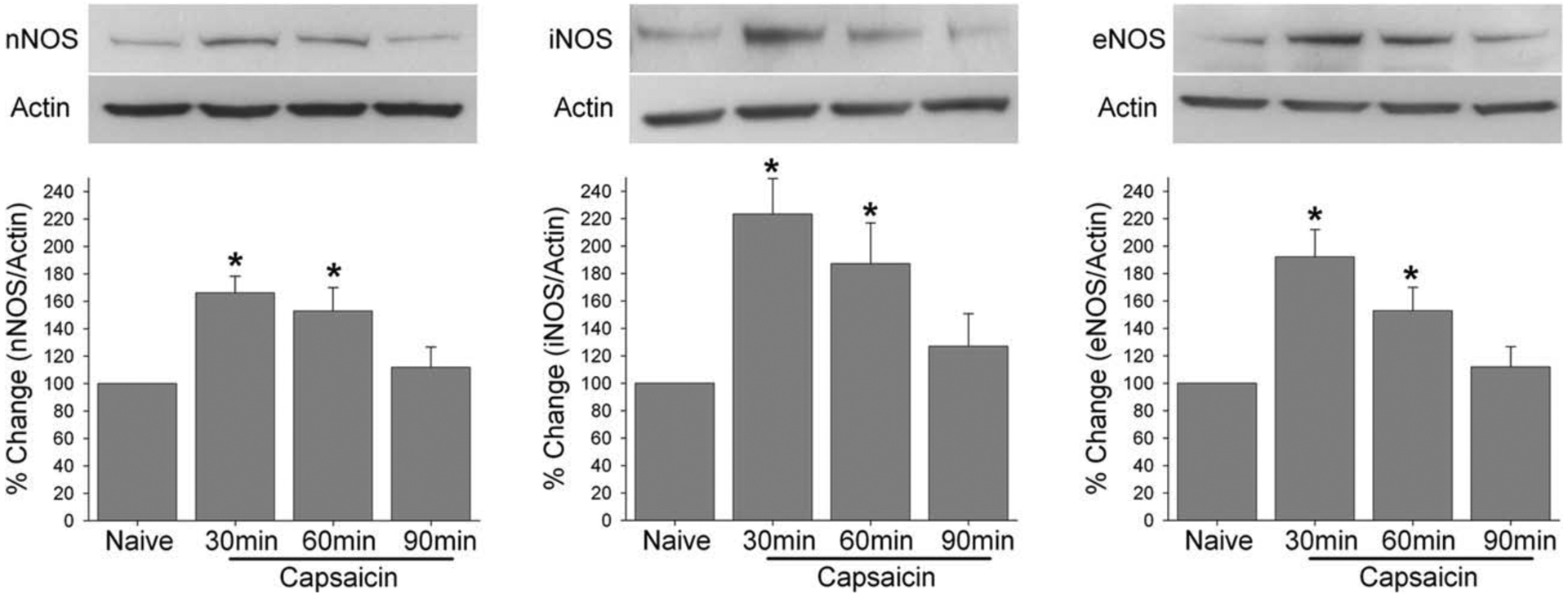
Upper panels show examples of representative blots for each NOS along with actin in lower panels. The group data are from densiometric analysis of the ratio between NOS and actin from each animal. * denotes significant difference at p = 0.05 her and all subsequent figures.
3.2. Behavioral studies
In subsequent behavioral experiments functional contribution of each NOS in capsaicin-induced masseter hypersensitivity was examined. The time course and magnitude of the capsaicin-induced masseter hypersensitivity following intrathecal delivery of vehicle controls were similar to those of capsaicin treatment alone (Ro et al., 2007; Figs. 2–4). Pretreatment with 7-NI in the Vc significantly and dose-dependently attenuated capsaicin-induced masseter hypersensitivity (Fig. 2: F = 11.468, p < 0.001). 7-NI (1 μmol) delivered to the Vc without capsaicin did not significantly altered the baseline masseter sensitivity.
Fig. 2.
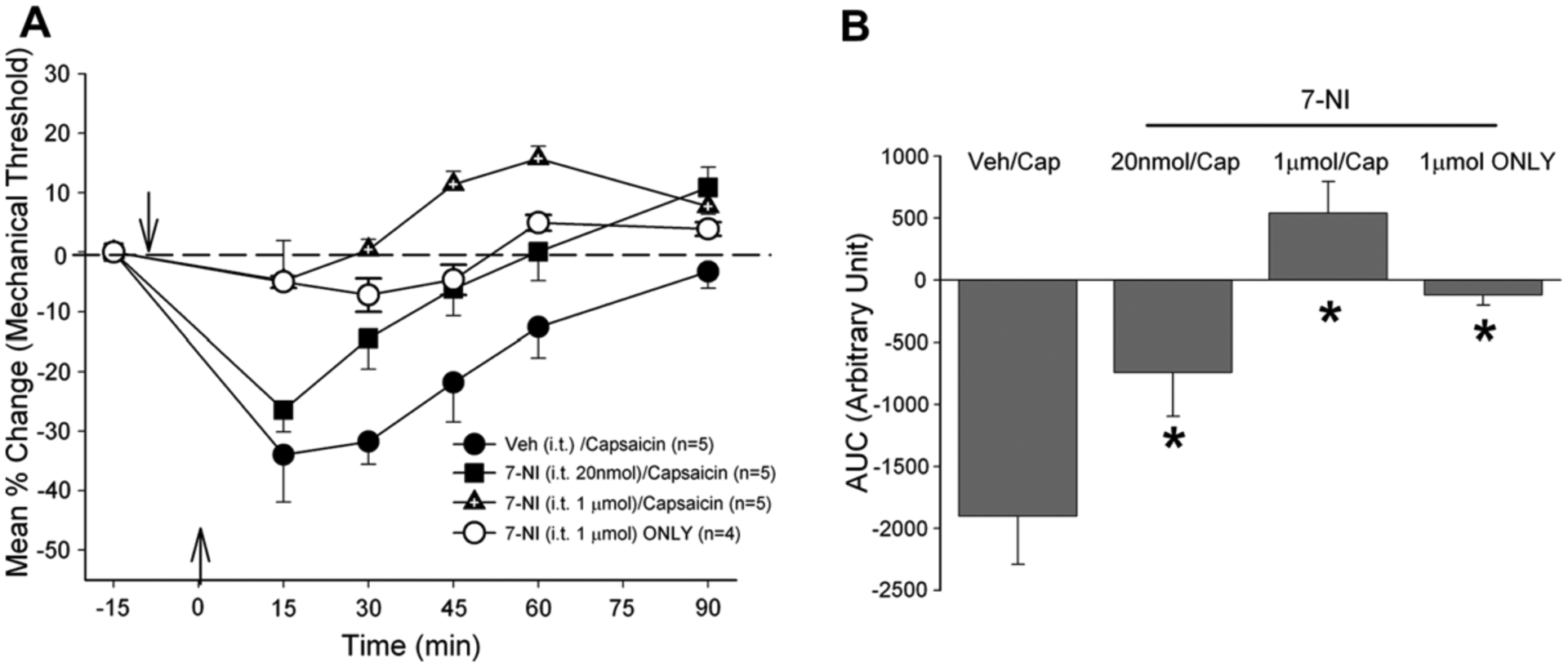
(A) Changes in masseter sensitivity following capsaicin injection with and without it pretreatment of 7-NI (B). Bar graphs compare AUCs from control and 7-NI pretreated rats. Down arrow indicates the time of drug treatment in the Vc and up arrow indicates the time of capsaicin injection in the masseter in this and all subsequent figures.
Fig. 4.
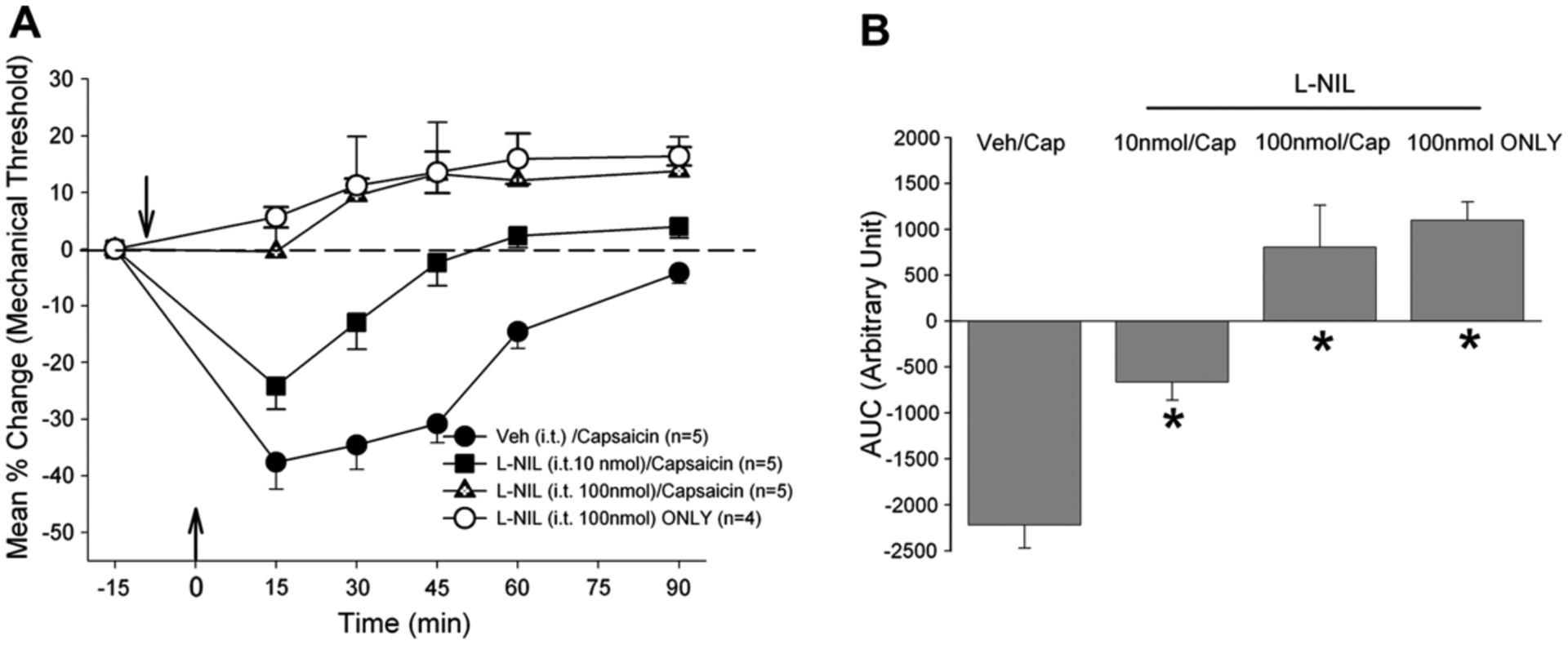
(A) Changes in masseter sensitivity following capsaicin injection with and without it pretreatment of L-NIL (B). Bar graphs compare AUCs from control and L-NIL pretreated rats.
Pretreatment with either L-NIO or N-NIL also reversed the capsaicin-induced masseter hypersensitivity in a dose-dependent manner (Figs. 3 and 4: F = 21.02, p < 0.001; F = 24.8, p < 0.001, respectively). However, in contrast to 7-NI, the higher dose of either L-NIO (1 μmol) or L-NIL (100 nmol) produced significant analgesic responses even in the absence of capsaicin stimulation of the masseter muscle. Pretreatment with the higher dose of L-NIO followed by capsaicin seemed to have produced greater analgesic responses compared to that produced by the same dose of L-NIO alone (Fig. 3B), but the difference was not statistically significant. The lower dose of L-NIO and L-NIL in the absence of capsaicin did not alter the baseline mechanical threshold (data not shown). These behavioral data are consistent with the Western blot data demonstrating that acute muscle inflammation causes an immediate up-regulation of all three NOS enzymes and blockade of any one of the three NOS effectively alleviated capsaicin-induced masseter hypersensitivity.
Fig. 3.
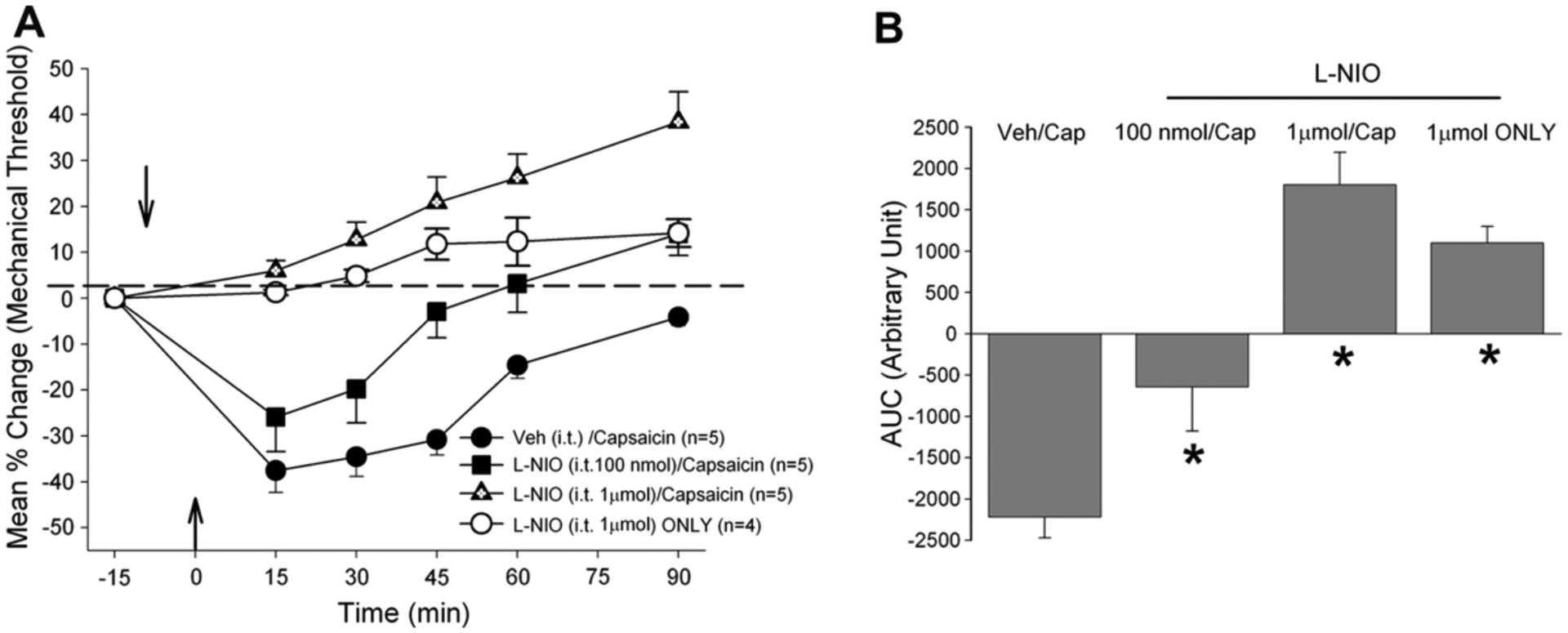
(A) Changes in masseter sensitivity following capsaicin injection with and without it pretreatment of L-NIO (B). Bar graphs compare AUCs from control and L-NIO pretreated rats.
4. Discussion
Functional role of spinally released NO in inflammatory hyperalgesia has been demonstrated by several lines of evidence (Malmberg et al., 1993; Meller et al., 1994; Inoue et al., 1997; Gühring et al., 2001; Okuda et al., 2001; Ikeda et al., 2006). In early behavioral studies that used a non-specific NOS inhibitor, L-NAME, relative activities of constitutive and inducible forms of NOS in the SC in inflammatory pain and their contributions to hyperalgesia could not be clearly delineated. With the renewed interest on the role of spinal glia in pain processing and the development of drugs that target specific NOS enzymes, the data on the role of NO synthesized and released from non-neuronal cells are now accumulating.
Capsaicin-induced hyperalgesia as well as dorsal horn neuronal responses were partially reversed by the spinal administration of either a selective nNOS or iNOS inhibitor (Wu et al., 2001). Similarly, administration of iNOS inhibitor reduced the hyperalgesic response induce by the spider venom (Zanchet et al., 2004). In this model, iNOS activity was detected as early as 1 h after venom injection. The fast iNOS activity may be explained by a relatively rapid increase in iNOS mRNA expression in the SC that can be detected as early as 30 min following peripheral inflammation (Gühring et al., 2001). Spinal administration of an inflammatory cytokine, IL-1β produces thermal hyperalgesia and the IL-1β induced thermal hyperalgesia can be prevented by pre-treatment with an iNOS inhibitor suggesting that inflammatory cytokines contribute to thermal hyperalgesia by activation of the iNOS-NO cascade (Sung et al., 2004, 2005).
Targeted gene knock out (KO) studies provided additional information on the relative contribution of spinal NOS enzymes in the development and maintenance of inflammatory pain. Gühring and colleagues showed that iNOS KO mice exhibit reduced thermal hyperalgesia that lasts 1–8 h after zymosan injection (Gühring et al., 2000). In wild type mice, the reduced hyperalgesia similar to that seen in iNOS KO was demonstrated following intrathecal treatment with L-NIL, indicating that NO derived from spinal iNOS acts as a fast inductor of thermal hyperalgesia. However, in a carageenan model, thermal hyperalgesia in neither early nor late phase was affected in iNOS KO mice (Tao et al., 2003). In wild type mice, intrathecal administration of L-NIL significantly inhibited carageenan-induced thermal hyperalgesia in the late but not in the early phase suggesting iNOS is not necessary for the early phase. When nNOS inhibitor, 7-NI, was administered in the SC of iNOS KO mice, it significantly reduced carageenan induced thermal hyperalgesia in both the early and late phases suggesting that nNOS might compensate for the function of iNOS in the late phase of carageenan-induced thermal hyperalgesia in the absence of iNOS. In nNOS KO mice, nNOS is also shown to play different roles in the two phases of carageenan-induced inflammatory model and enhanced spinal eNOS appears to compensate for the role of nNOS (Tao et al., 2004). Taken together, these data suggest that the three spinal NOS play different roles during the course of progression of inflammatory pain, and that these enzymes may undergo important compensatory changes dependent on the peripheral pathology.
KO studies also demonstrated that the absence of nNOS up-regulates the basal expression of eNOS, but not iNOS (Tao et al., 2004; Chu et al., 2005). In iNOS KO mice, the absence of iNOS did not affect the baseline expression of either nNOS or eNOS, but inflammation induced expression of nNOS was up-regulated (Tao et al., 2003). These data suggest that the expression of the three NOS proteins is closely orchestrated to ensure the basal level of NO in the SC under normal conditions, and that compensatory changes in the NOS expression following peripheral pathology may provide a continuing source of NO at different phases of inflammatory pain and hyperalgesia. Thus, the efficacy and/or sufficiency of pharmacological blockade of specific NOS may be predicted by the expression pattern of the three NOS enzymes.
In this study, we provided first evidence that the expression level for all three NOS is immediately up-regulated at the level of Vc under acute myositis condition. This observation was paralleled by the behavioral data that showed blockade of any one of the NOS was effective in attenuating the capsaicin-induced muscle hypersensitivity. These data suggest that excess release NO in the Vc, regardless of the source, produces hyperalgesic responses, and that all three NOS enzymes similarly contribute to the final NO level under the acute inflammatory pain model. Thus blockade of any one NOS enzyme would reduce the level of NO and attenuate the masseter hypersensitivity. In our study, higher doses of iNOS and eNOS inhibitors alone produced analgesic responses. Since iNOS is not constitutively expressed under normal condition it is possible that L-NIL at high dose could alter the physiology of brainstem neurons near the Vc region in a non-specific manner. Likewise, the basal level of NO maintained by constitutively expressed eNOS or nNOS may be necessary for normal physiological function of the Vc neurons in that reduction of NO below the basal level may significantly depress Vc neurons. Therefore, our data further suggest that NO level under both normal and inflammatory conditions is regulated by all three NOS enzymes and changes in NO level are associated with the manifestation of hyperalgesic and analgesic responses.
Central mechanisms mediating craniofacial muscle pain is not well understood. In this study, functional role of NO released from both neuronal and non-neuronal cells and non-neuronal cells in acute inflammatory muscle pain condition is proposed. Although the NOS inhibitors used in this study may not be highly selective at high doses behavioral responses at lower doses combined with Western blot data strongly suggest the involvement of all three NOS enzymes. Further studies on NO signaling mechanisms will significantly increase our knowledge on pathophysiological mechanisms underlying chronic orofacial muscle pain conditions, and offer important new insights for the development of treatment alternatives to ameliorate persistent craniofacial muscle pain.
Acknowledgements
We would like thank Mr. Gregory Haynes for his valuable technical assistance in behavioral experiments.
References
- Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX. Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund’s adjuvant-induced persistent pain. Pain 2005;119:113–23. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci 1994;14:5147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gühring H, Gorig M, Ates M, Coste O, Zeilhofer HU, Pahl A, et al. Suppressed injury-induced rise in spinal prostaglandin E2 production and reduced early thermal hyperalgesia in iNOS-deficient mice. J Neurosci 2000;20:6714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gühring H, Tegeder I, Lotsch J, Pahl A, Werner U, Reeh PW, et al. Role of nitric oxide in zymosan induced paw inflammation and thermal hyperalgesia. Inflamm Res 2001;50:83–8. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 2006;312:1659–62. [DOI] [PubMed] [Google Scholar]
- Inoue T, Mashimo T, Shibuta S, Yoshiya I. Intrathecal administration of a new nitric oxide donor, NOC-18, produces acute thermal hyperalgesia in the rat. J Neurol Sci 1997;153:1–7. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. Pain 1993;54:291–300. [DOI] [PubMed] [Google Scholar]
- Meller ST, Pechman PS, Gebhart GF, Maves TJ. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience 1992;50:7–10. [DOI] [PubMed] [Google Scholar]
- Meller ST, Cummings CP, Traub RJ, Gebhart GF. The role of nitric oxide in the development and maintenance of the hyperalgesia produced by intraplantar injection of carrageenan in the rat. Neuroscience 1994;60: 367–74. [DOI] [PubMed] [Google Scholar]
- Okuda K, Sakurada C, Takahashi M, Yamada T, Sakurada T. Characterization of nociceptive responses and spinal releases of nitric oxide metabolites and glutamate evoked by different concentrations of formalin in rats. Pain 2001;92:107–15. [DOI] [PubMed] [Google Scholar]
- Ro JY, Lee J, Capra NF, Zhang Y. Role of soluble guanylate cyclase in the trigeminal subnucleus caudalis in capsaicin-induced muscle hypersensitivity. Brain Res 2007;1184:141–8. [DOI] [PubMed] [Google Scholar]
- Salerno L, Sorrenti V, Di Giacomo C, Romeo G, Siracusa MA. Progress in the development of selective nitric oxide synthase (NOS) inhibitors. Curr Pharm Des 2002;8:177–200. [DOI] [PubMed] [Google Scholar]
- Sung CS, Wen ZH, Chang WK, Ho ST, Tsai SK, Chang YC, et al. Intrathecal interleukin-1beta administration induces thermal hyperalgesia by activating inducible nitric oxide synthase expression in the rat spinal cord. Brain Res 2004;1015:145–53. [DOI] [PubMed] [Google Scholar]
- Sung CS, Wen ZH, Chang WK, Chan KH, Ho ST, Tsai SK, et al. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem 2005;94:742–52. [DOI] [PubMed] [Google Scholar]
- Tao F, Tao YX, Zhao C, Dore S, Liaw WJ, Raja SN, et al. Differential roles of neuronal and endothelial nitric oxide synthases during carrageenan-induced inflammatory hyperalgesia. Neuroscience 2004;128:421–30. [DOI] [PubMed] [Google Scholar]
- Tao F, Tao YX, Mao P, Zhao C, Li D, Liaw WJ, et al. Intact carrageenan-induced thermal hyperalgesia in mice lacking inducible nitric oxide synthase. Neuroscience 2003;120:847–54. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain 2001;94: 47–58. [DOI] [PubMed] [Google Scholar]
- Zanchet EM, Longo I, Cury Y. Involvement of spinal neurokinins, excitatory amino acids, proinflammatory cytokines, nitric oxide and prostanoids in pain facilitation induced by Phoneutria nigriventer spider venom. Brain Res 2004;1021:101–11. [DOI] [PubMed] [Google Scholar]


