Abstract
Activation of opioid and cannabinoid receptors expressed in nociceptors induces effective antihyperalgesia. In this study, we examined whether combinations of opioid and cannabinoid receptor agonists directed at the injured site would enhance therapeutic effectiveness. Behavioral pharmacology experiments were performed to compare the effects of DAMGO, a selective agonist for μ-opioid receptor (MOR), ACPA, a specific agonist for CB1, and combinations of DAMGO and ACPA in attenuating complete Freund’s adjuvant (CFA)-induced mechanical hyperalgesia in the rat hindpaw. DAMGO (1 μg–1 mg) or ACPA (1 μg–2 mg) was administered into the inflamed paw when mechanical hyperalgesia was fully developed. When administered individually, DAMGO and ACPA dose-dependently reversed the mechanical hyperalgesia. DAMGO displayed a lower ED50 value (57.4 ± 2.49 μg) than ACPA (111.6 ± 2.18 μg), but ACPA produced longer lasting antihyperalgesic effects. Combinations of DAMGO and ACPA also dose-dependently attenuated mechanical hyperalgesia, but the antihyperalgesic effects were partial and transient even at high doses. Using isobolographic analysis, we determined that combined treatment with DAMGO and ACPA produced antagonistic effects with the observed ED50 of 128.4 ± 2.28 μg. Our findings showed that MOR and CB1 agonists directed at the inflamed site effectively attenuate mechanical hyperalgesia when administered individually, but exert opposing effects when administered together. The antagonistic interactions between the two classes of drugs at the inflamed site suggest distinct mechanisms unique to peripheral nociceptors or inflamed tissue, and therefore require further studies to investigate whether the therapeutic utility of the combined drug treatments in chronic pain conditions can be optimized.
Keywords: DAMGO, ACPA, μ Opioid receptor, CB1, Dorsal root ganglia, Isobologram, Complete freund’s adjuvant
1. Introduction
Classical opioid receptors such as μ, δ and к receptors and cannabinoid receptor type 1 (CB1) and type 2 (CB2) are a family of metabotropic receptors coupled to Gi/o protein. It is well known that activation of both receptor systems invokes intracellular signaling cascades that inhibit adenylyl cyclase (Howlett and Fleming, 1984), decrease Ca2+ channel conductance (Caulfield and Brown, 1992; Seward et al., 1991), and activate inward rectifying and A-type potassium channels (Takeda et al., 2004; Wacnik et al., 2008). Activation of opioid or cannabinoid receptors produces similar pharmacological outcomes, including antinociceptive effects (Bushlin et al., 2010). Potent analgesic effects of both opioids and cannabinoids are, however, offset by serious side effects mediated by their receptors within the CNS.
Preclinical and clinical studies continue to provide strong justification that opioid and cannabinoid receptors localized in primary afferent neurons are viable targets for effective pain management. Recent development of peripherally restricted opioids and cannabinoids (Arendt-Nielsen et al., 2009; Bileviciute-Ljungar et al., 2006; Yu et al., 2010), and novel gene-based therapies to increase peripheral opioid receptor (Raja, 2012) and opioid peptides (Machelska et al., 2009) attest to ongoing efforts to garner maximum therapeutic advantages of peripheral receptors without producing centrally mediated side effects. Interestingly, MOR and CB1 in primary afferent neurons also share remarkable similarities in the transcriptional regulation of their expression. Peripheral inflammation increases μ-opioid receptor (MOR) expression in dorsal root ganglia (DRG) and trigeminal ganglia (TG) (Mousa, 2003; Pol and Puig, 2004; Puehler et al., 2004). Available data show inflammatory cytokines such as interleukin (IL)-1β, IL-4, IL-6, and TNFα induce MOR expression in neuronal as well as in non-neuronal cell lines (Borner et al., 2004; Kraus et al., 2001). We have recently demonstrated that the same inflammatory cytokines induce MOR upregulation in TG (Zhang et al., 2014). Similarly, peripheral inflammation increases CB1 expression in TG, and inflammatory cytokines such as IL-1β and IL-6 induce CB1 expression in TG (Niu et al., 2012). These findings imply that inflammatory cytokines concurrently regulate both CB1 and MOR transcription.
Since the increase in MOR and CB1 densities has been proposed as one of the major mechanisms underlying pronounced antihyperalgesic effects of peripheral opioids and cannabinoids under inflammatory conditions (Niu et al., 2012; Zollner et al., 2003), it is reasonable to assume that targeting both receptor systems in the periphery would lead to greater antihyperalgesic effects in treating inflammatory pain and hyperalgesia. Synergistic or additive interactions between MOR agonist and CB1 agonist have been described for systemic effects mediated primarily by the receptors in the CNS (Cox et al., 2007; Maguire et al., 2013; Tham et al., 2005). However, similar studies evaluating interactions between the peripheral MOR and CB1 under pathological pain conditions have not been conducted.
Mechanical hyperalgesia is a prominent symptom in most chronic pain conditions, especially those associated with deep tissues. Mechanical hyperalgesia is characterized by pain upon touch, palpation, stretching or even movement, all of which could result from sensitization of nociceptors (Mense, 1993). Joseph and Levine showed that most nociceptors paly a role in mechanical hyperalgesia and that MOR on nociceptors attenuate mechanical hyperalgesia (Joseph and Levine, 2010). In trigeminal nociceptors, TRPV1 neurons that mediate inflammatory mechanical hyperalgesia also express MOR (Lee et al., 2016), and that the administration of an MOR agonist at the inflamed tissue effectively attenuate mechanical hyperalgesia (Zhang et al., 2014). Similarly, treatment with a CB1 agonist at the inflamed tissue blocks inflammation-induced mechanical hyperalgesia in a receptor specific manner (Niu et al., 2012). The objective of the present study was to evaluate whether the combination of MOR and CB1 agonists administered directly into the inflamed tissue would lead to additive, synergistic, or antagonistic antihyperalgesic effects on inflammatory mechanical hyperalgesia.
2. Materials and methods
2.1. Subjects
Male Sprague Dawley rats (8 weeks old; 250–300 g, Harlan, Indianapolis) were used in all experiments. Animals were housed in a temperature-controlled room under a 12:12 light–dark cycle with access to food and water ad libitum. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and under a University of Maryland approved Institutional Animal Care and Use Committee protocol.
2.2. Induction of inflammation
Inflammation was induced by the injection of complete Freund’s adjuvant (CFA, 50 μl; 1:1 isotonic saline) into the plantar surface of the right hindpaw with a 27-gauge needle over 5–10 s.
2.3. Mechanical sensitivity test
Mechanical sensitivity of the hindpaw was assessed with the Randall–Selitto test, an established rodent model for testing mechanical hypersensitivity of the paw. Experiments were conducted according to the procedure described previously (Auh and Ro, 2012). Briefly, animals were first allowed to habituate to the experimental room for 30 min for three consecutive days. The withdrawal response to noxious paw pressure was assessed using a digital paw pressure Randall–Selitto applicator for rodents (IITC Life Science, Woodland Hills, CA). Each rat was placed in a cloth holder suspended in a sling, and the probe of the pressure applicator was placed under the plantar surface of the hindpaw. A gradually increasing pressure was applied until the rat withdrew its hindpaw. The pressure applicator captures and stores the pressure upon reaction. The lowest pressure necessary to elicit the withdrawal response prior to inflammation was considered as the baseline mechanical threshold.
Antihyperalgesic effects of DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin, Sigma Aldrich, St. Louis, MO, USA), a highly selective MOR agonist, ACPA (Arachidonyl-cyclopropylamide, Tocris, Bristol, United Kingdom), a specific agonist for CB1, or combination of DAMGO and ACPA were measured on day 3 after intraplantar (i.pl) injection of CFA, during which mechanical hyperalgesia was most profound. On day 3, DAMGO (1, 30, 100 μg and 1 mg) or ACPA (1, 30, 100 μg, 1 and 2 mg) or combinations of the two agonists dissolved in phosphate buffer solution (PBS; 20 μl) was administered into the plantar surface of the inflamed hindpaw. The same volume of vehicle control was administered in the identical manner. A pre-drug treatment mechanical threshold for evoking a hindpaw withdrawal response was determined 15 min prior to drug injection. Changes in mechanical sensitivity of the hindpaw were assessed 30, 60, 120 and 180 min after the administration of each drug. The specificity of DAMGO and ACPA for MOR and CB1, respectively, has been well documented in the literature, including our previous studies that confirmed their specificity against selective antagonists in inflammatory muscle pain models (Niu et al., 2012; Nunez et al., 2007). All experimental and control groups consisted of 5 animals per group.
2.4. Statistical analysis
One-way ANOVA was used to compare the differences in baseline mechanical thresholds before and after CFA-induced inflammation for each drug treatment groups. The antihyperalgesic effects of drug treatments were analyzed with a two-way ANOVA with repeated measures. For each treatment, the percent maximum possible effect (%MPE) was calculated using the following formula: [test threshold (g) – baseline (g)/cut off threshold (250 g) – baseline (g)] × 100. We chose the cut off threshold as 250 g since the average mechanical threshold for adult rats under normal condition was around 250 g %MPE was calculated at the time point at which the greatest antihyperalgesic effects were observed. The ED50 (the dose that caused 50% of maximum antihyperalgesia) was generated from standard non-linear regression analysis of the log dose-response curve (Prism 6.0, Graphpad Software, San Diego, CA).
Interactions of agonist combinations were analyzed using fixed ratio design isobolograms whereby combinations of two drugs in known ratio were administered as fractions of their respective ED50 (Tallarida, 2002). The analysis of isobologram was adapted from a published study (Tham et al., 2005). Briefly, the isobologram was constructed by connecting ED50DrugA on the vertical axis to ED50DrugB on the horizontal axis. We then calculated the theoretical dose required for a purely additive interactions using the following formula: Zadd = (f) ED50DrugA + (1 − f) ED50DrugB, where f is the fraction of drug A. Zadd was compared to the actual dose (Zmix determined from the ED50 of the combination dose-response curve) required to achieve the same effect experimentally via the Student’s t-test (Tallarida, 2002). The variance for Zadd was calculated as Var (Zadd) = (f)2 Var (ED50DrugA) + (1 − f)2 Var (ED50DrugB). All data are presented as mean ± standard error of the mean (SE), and differences were considered significant at p < 0.05.
3. Results
3.1. Antihyperalgesic effects of intradermal DAMGO on mechanical hyperalgesia
The mean baseline mechanical thresholds for evoking hindpaw withdrawal responses ranged between 200 and 223 g. There was no significant difference in the baseline mechanical thresholds between control and DAMGO treated groups (F (4,24) = 2.085, p > 0.05). Consistent with our previous study (Auh and Ro, 2012), CFA treatment produced profound mechanical hyperalgesia in all groups. On day 3 following CFA treatment, the mean mechanical thresholds were significantly reduced, ranging from 43 to 60 g. There was no significant group difference in the mean mechanical thresholds at this time point (F (4,24) = 1.235, p > 0.05). Antihyperalgesic effects of DAMGO were tested on day 3 following CFA treatment when CFA-induced mechanical hyperalgesia is most prominent (Auh and Ro, 2012). Intradermal administration of DAMGO dose-dependently reversed CFA-induced mechanical hyperalgesia (Fig. 1A). Two-way ANOVA revealed significant effects of drug (F (4,24) = 23.6, p < 0.001) and time (F (4,24) = 53.8, p < 0.001). PBS or DAMGO at 1 μg or 30 μg did not significantly increase mechanical thresholds during the 3 h of observation. DAMGO at 100 μg and 1 mg significantly attenuated CFA-induced hyperalgesia. The greatest antihyperalgesic responses occurred 30 min after the drug administration. Both 100 μg and 1 mg of DAMGO nearly completely reversed the mechanical thresholds to pre-CFA baseline levels at this time point. DAMGO at 100 μg dose showed the most prominent and longer lasting antihyperalgesic effects whereas the effects of DAMGO at 1 mg were more transient. Based on %MPE calculated for each dose of DAMGO at the 30 min time point, we plotted a log dose-response curve (Fig. 1B). The ED50 of intraplantar DAMGO for this assay was determined to be 57.4 ± 2.49 μg. The mechanical sensitivity of the non-inflamed paw was not altered by the CFA or the drug treatment.
Fig. 1.
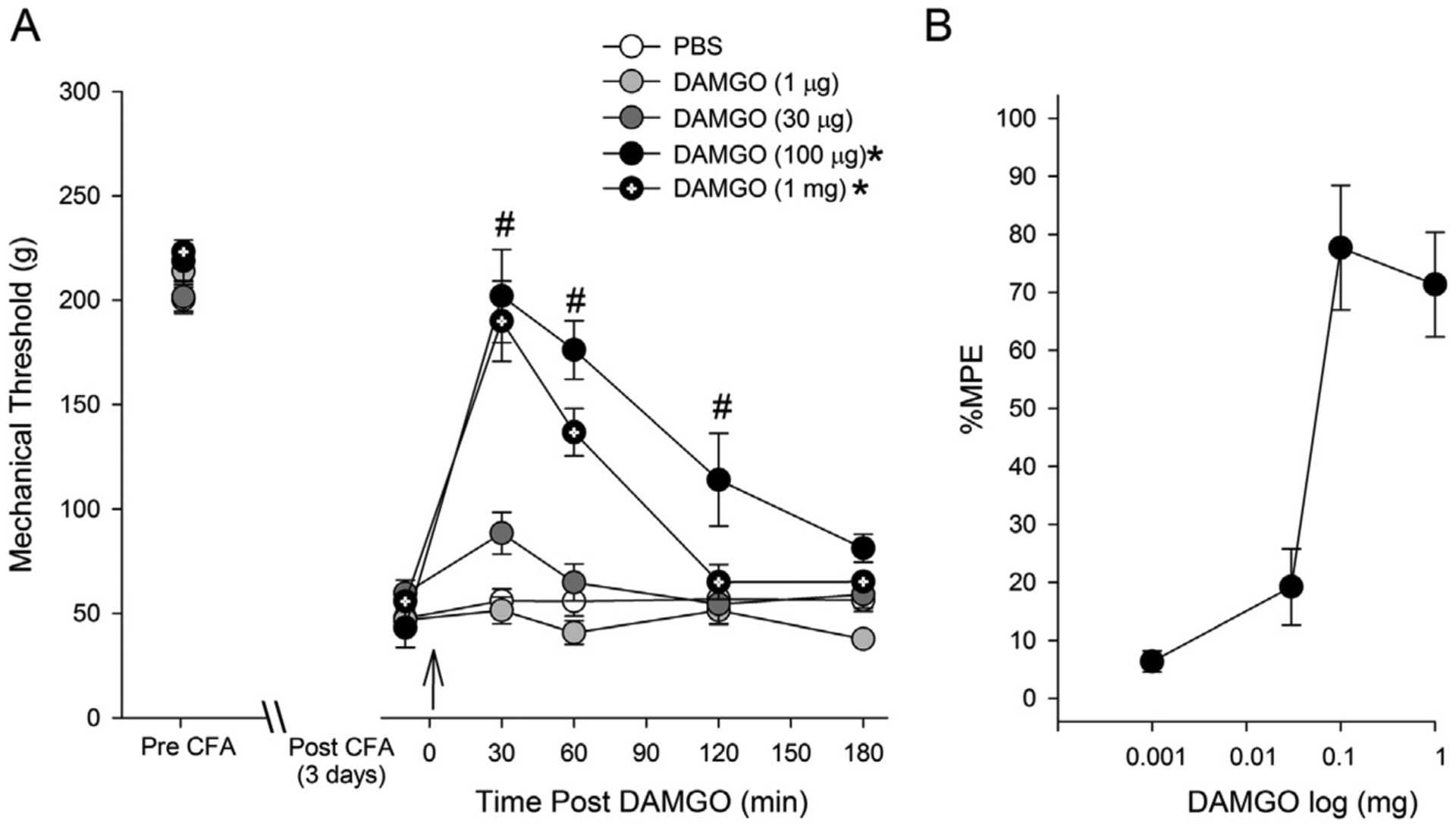
Effects of DAMGO (i.pl) on CFA-induced mechanical hyperalgesia. On day 3 of CFA injection, changes in mechanical thresholds after DAMGO treatment were measured for 180 min. The drug was administered 5 min after pre-treatment measurement of mechanical threshold (indicated by the arrow). * – significant group effect compared to PBS at p < 0.05, # – significant time effect compared to baseline p < 0.05. (n = 5 per group) (B) DAMGO dose-antihyperalgesic response curve. Responses are expressed as %MPE. %MPE values at the time point at which peak antihyperalgesic responses occurred were used to plot the curve.
3.2. Antihyperalgesic effects of intradermal ACPA on mechanical hyperalgesia
As with DAMGO treated groups, the mean mechanical thresholds before and after CFA treatments were not significantly different between vehicle and ACPA treated groups (F (4,24) = 2.65, p > 0.05, F (4,24) = 1.63, p > 0.05, respectively). Antihyperalgesic effects of ACPA were also tested 3 days after CFA treatment. Intradermal administration of ACPA dose-dependently reversed the hyperalgesia (Fig. 2A). Two-way ANOVA revealed significant effects of drug (F (4,24) = 9.82, p < 0.001) and time (F (4, 24) = 9.496, p < 0.001). PBS or ACPA at 1 or 30 μg did not attenuate CFA-induced mechanical hyperalgesia. ACPA at 100 μg significantly attenuated CFA-induced hyperalgesia (Fig. 2A). ACPA at 1 mg completely reversed the antihyperalgesic effects. Interestingly, ACPA at 2 mg did not further increase the mechanical threshold but prolonged the antihyperalgesic effects, suggesting that peripheral ACPA does not lead to analgesic effects even at high doses. The greatest antihyperalgesic responses occurred 30 min after the drug administration. Based on %MPE calculated for each dose of ACPA, we plotted a log dose-response curve (Fig. 2B). From the dose related responses, we determined the ED50 of intraplantar ACPA for this assay to be 111.6 ± 2.18 μg. The mechanical sensitivity of the non-inflamed paw was not altered by the ACPA treatment.
Fig. 2.
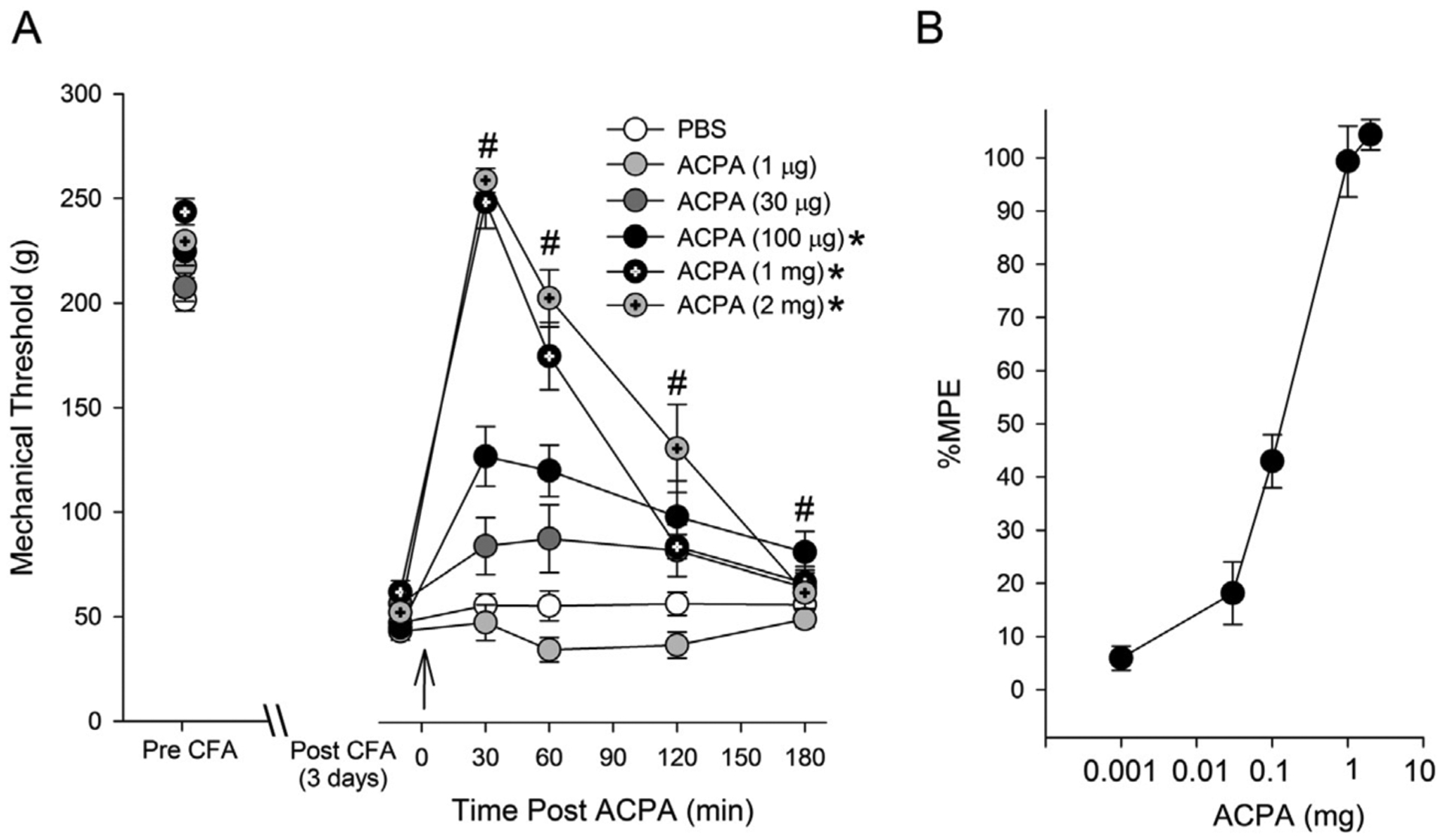
Effects of ACPA (i.pl) on CFA-induced mechanical hyperalgesia. On day 3 of CFA injection, changes in mechanical thresholds after ACPA treatment were measured for 180 min. The drug was administered 5 min after pre-treatment measurement of mechanical threshold (indicated by the arrow). * – significant group effect compared to PBS at p < 0.05, # – significant time effect compared to baseline p < 0.05. (n = 5 per group) (B) ACPA dose-antihyperalgesic response curve. Responses are expressed as %MPE. %MPE values at the time point at which peak antihyperalgesic responses occurred were used to plot the curve.
3.3. Interactions between DAMGO and ACPA
Dose- and time-dependent antihyperalgesic effects of co-administration of DAMGO and ACPA at 1:1.9 fixed ratio of equipotent doses, calculated based on ED50 determined from the above experiments, were examined. Responses to four different combinations of DAMGO and ACPA (i.e., 1 and 1.9, 10 and 19, 30 and 57, and 100 and 190 μg, respectively) co-administered intradermally into the hindpaw 3 days after CFA treatment are shown in Fig. 3. The combination of the lowest doses of DAMGO and ACPA (1 and 1.9 μg) did not significantly alter the mechanical hyperalgesia. Thus this group was used as a control to compare the remaining three combinations of higher doses. The combination of DAMGO and ACPA dose-dependently attenuated CFA-induced mechanical hyperalgesia. Two-way ANOVA revealed significant dose (F (3,19) = 54.1, p < 0.001) and time (F (4,19) = 91.9.2, p < 0.001) effects. Combinations of the three higher doses of agonists significantly reversed CFA-induced mechanical hyperalgesia. However, the significant effect lasted only one hour. We did not use higher doses of DAMGO or ACPA since 1 mg of each agonist completely reversed the mechanical hyperalgesia when administered individually. As with individual DAMGO or ACPA, the greatest antihyperalgesic effects occurred at 30 min. Based on this set of data, we determined the ED50 value for combined drug effects to be 128.4. ± 2.28 μg. The plots of the ED50 value obtained from combinations of drugs in relation to the ED50 values of the individual drugs are shown in Fig. 4. The letter A on the isobologram represents the theoretical additive point for each combination of DAMGO and ACPA, and letter B indicates the experimental point for each drug combination. The theoretical additive ED50 for combined drug treatments was 84.5 ± 9.06 μg while the observed ED50 for ACPA and DAMGO administered together was 128.4 ± 2.28 μg. The isobologram showed that the experimental point lies outside of the confidence intervals of the line of additivity. The theoretical additive mixture ED50 was significantly different from the observed ED50 (p < 0.001) indicating that ACPA and DAMGO act antagonistically when administered together in this inflammatory hyperalgesia assay.
Fig. 3.
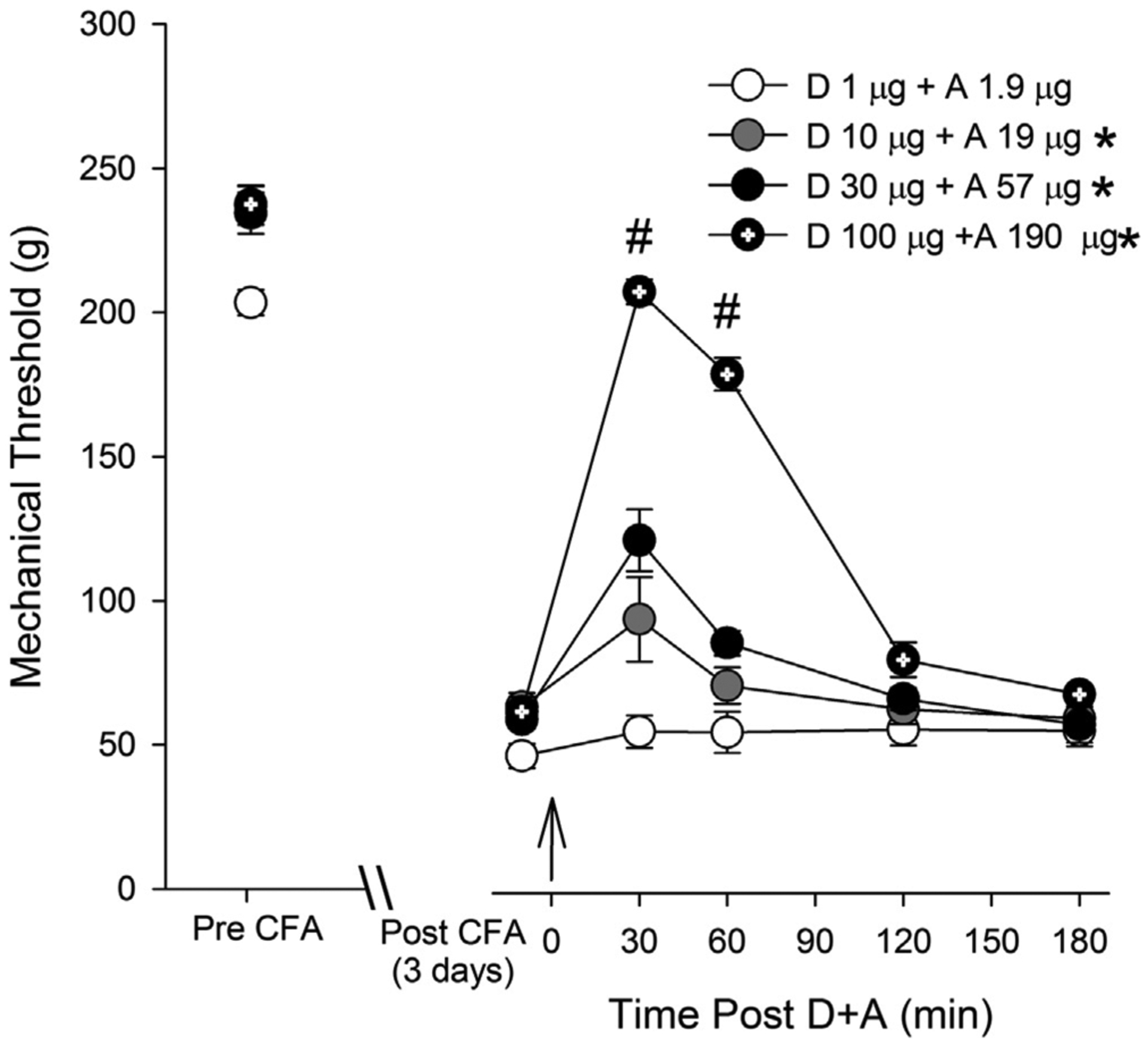
Effects of combinations of ACPA and DAMGO (i.pl) on CFA-induced mechanical hyperalgesia. The four combination doses were 1:1.9 equipotent doses determined from the ED50 values of each drug: 1 and 1.9 μg, 10 and 19 μg, 30 and 57 μg, and 100 and 119 μg of DAMGO and ACPA, respectively. On day 3 of CFA injection, changes in mechanical thresholds after combinations of ACPA and DAMGO treatments were measured for 180 min. The drug combination was administered 5 min after pre-treatment measurement of mechanical threshold (indicated by the arrow). * – significant group effect compared to the combination of lowest doses at p < 0.05, # – significant time effect compared to baseline p < 0.05. (n = 5 per group).
Fig. 4.
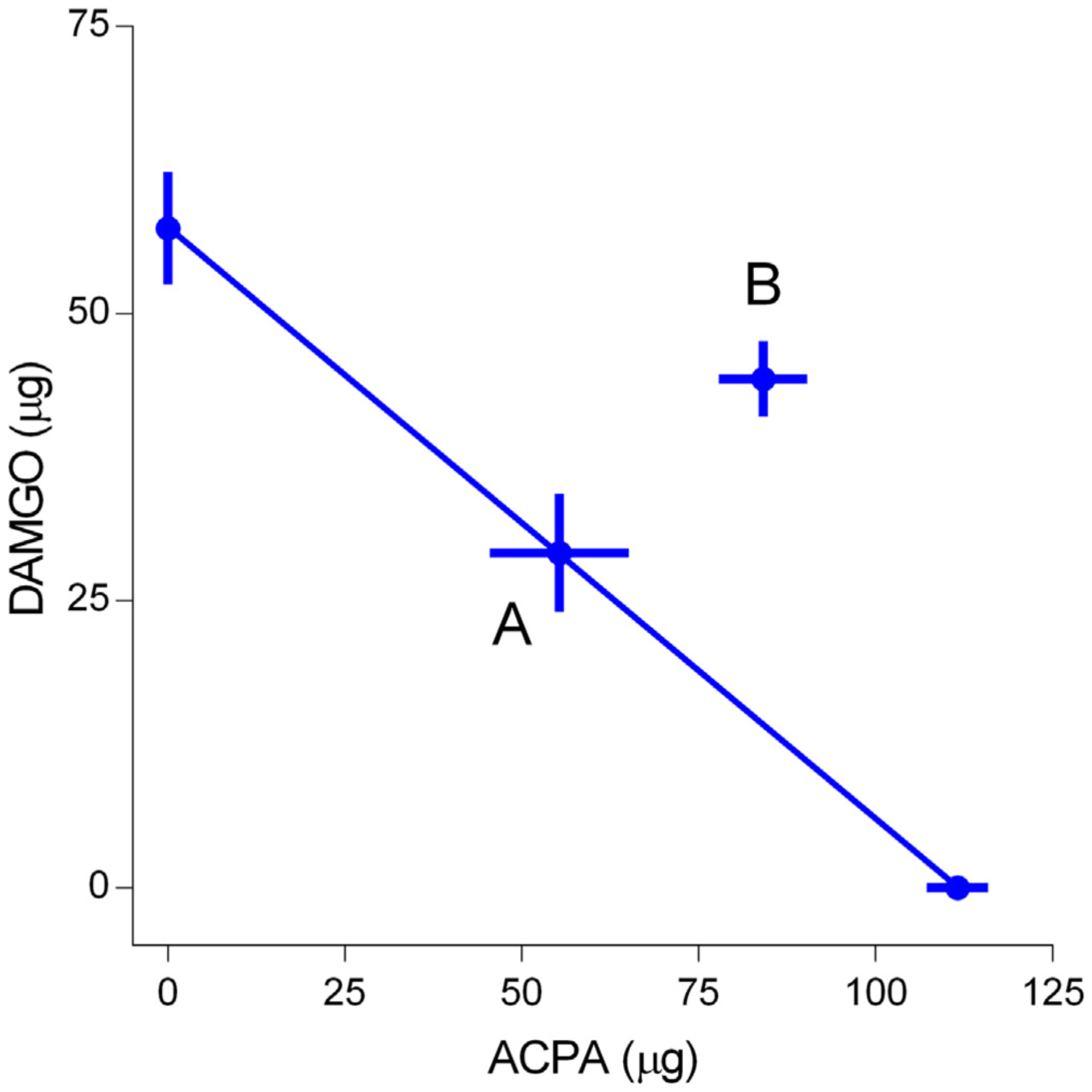
Isobologram of DAMGO and ACPA drug combination in CFA-inflamed rats. The ED50 of ACPA and DAMGO (μg) are plotted along the X and Y axis, respectively, and the line connecting the two values theoretically contains all dose combinations that are additive. Point A represents the theoretical additive value and Point B represents the experimentally determined values for the combination of ED50 values for DAMGO and ACPA. Pont B fell outside of the 95% confidence level of the theoretical additive line.
4. Discussion
The present study confirmed that DAMGO or ACPA, a specific agonist for MOR or CB1, respectively, significantly reduced inflammatory mechanical hyperalgesia in a dose- and time-dependent manner when administered directly at the inflamed tissue. Our results showed that the two drugs effectively reverse the mechanical hyperalgesia, but have different half maximal effects and pharmacokinetics in the periphery. The analysis of ED50 values showed 1:1.9 DAMGO to ACPA. It seems that DAMGO administered in the inflamed tissue reaches the maximum anti-hyperalgesic effects at about 100 μg and ACPA at around 1 mg. Also there was no marked difference between responses to 1 or 2 mg ACPA administered. It is possible that the DAMGO at 100 μg and ACPA at 1 mg saturate peripherally localized MOR and CB1 receptors. The fact that the high doses of DAMGO or ACPA do not produce analgesic effects would suggest that central action of these drugs is fairly limited. However, it is also possible that these drugs at high doses may have led to unknown off target effects that counter their antihyperalgesic actions. We also demonstrated that a MOR agonist combined with a CB1 agonist displayed antagonistic effects in our mechanical hyperalgesia assay. This study provides interesting novel information to further study mechanistic bases for the antagonistic effects and offer clinically relevant information that combined drug treatments targeting both peripheral MOR and CB1 receptors in inflammatory mechanical hyperalgesia is ineffective.
Due to their similarities in signal transduction mechanisms, distribution in the brain and the spinal cord and behavioral effects, interactions between opioid and cannabinoid receptor systems in the CNS have been extensively explored (Bushlin et al., 2010; Cichewicz, 2004). The synergy between the two classes of drugs is particularly relevant since centrally mediated side effects and tolerance development are dose-limiting factors for both opioid and cannabinoid compounds in the treatment of chronic pain. Early studies have shown that selective antagonists targeting MOR block antinociceptive effects of exogenous cannabinoids, such as delta-9-tetra hydrocannabinol (THC) (Manzanares et al., 1999). These findings were supported by observations that intrathecal administration of THC leads to opioid peptide release and increase the expression of opioid precursor genes (Corchero et al., 1997; Houser et al., 2000; Manzanares et al., 1999; Mason et al., 1999). Similarly, a selective antagonist for CB1 reverses morphine-induced antinociception (Pacheco Dda et al., 2009). Not only the activity of one receptor is mediate by the other, combined treatments with both opioid and cannabinoid receptor agonists produce additive or synergistic antinociceptive effects. Additive or synergistic antinociceptive effects between cannabinoids and opioids in the CNS have been demonstrated in acute pain models (Cichewicz and McCarthy, 2003; Maguire et al., 2013; Smith et al., 1994; Tham et al., 2005; Welch and Stevens, 1992), as well as in inflammatory and neuropathic pain conditions (Cox et al., 2007; Gunduz et al., 2011).
Although there is evidence that antihyperalgesic effects of morphine in the peripheral nervous system involve CB1 (da Fonseca Pacheco et al., 2008), interactions between opioid and cannabinoid agonists preferentially targeting peripheral receptors are less well characterized. Topical cannabinoid enhances topical morphine in acute pain models (Yesilyurt et al., 2003), suggesting an additive or synergistic interactions of the two drugs at the periphery. However, the efficacy of peripheral opioid is not readily detectable in normal tissue, but is greatly augmented under conditions of tissue injury and inflammation (Levine and Taiwo, 1989; Nunez et al., 2007; Schafer et al., 1995). It is not known whether similar interactions can be expected under injury or inflammatory conditions. Mecs and colleagues have shown that endogenous ligands for opioid and cannabinoid receptors lead to greater antinociception when administered together into the inflamed joint compared to individual drug effects (Mecs et al., 2010). These studies suggested positive interactions between the two drug classes at the peripheral level, consistent with the observations made in the CNS. We also hypothesized that the combined treatments would lead to greater antihyperalgesic effects since both CB1 and MOR are upregulated in sensory ganglia with a similar time course following CFA-induced inflammation and transcription of both receptors is regulated by inflammatory cytokines (Niu et al., 2012; Nunez et al., 2007; Zhang et al., 2014). In this study, we utilized the isobolographic analysis, a well-established technique, to clearly delineate additive or synergistic effects of DAMGO and ACPA. To our surprise, we found that combinations of DAMGO and ACPA produced antagonistic effects under inflammatory condition when administered directly to inflamed site. Our unexpected results suggest that cellular mechanisms underlying interactions between opioid and cannabinoid receptors are complex and that drug interactions in primary afferent neurons may be quite different from the observations made in the CNS. In fact, Neelakantan and colleagues have shown that distinct mechanisms of action underlie the interactions between cannabinoid and morphine depending on the underlying pain type and stimulus modality (Neelakantan et al., 2015). In their study, combinations of cannabinoid and morphine produced synergistic effects in reversing acetic acid-stimulated stretching behavior, but sub-additive or antagonistic effects in the hot plate thermal nociceptive assay and the acetic acid-decreased operant responding for palatable food assay.
Cellular and molecular mechanisms underlying the additive, synergistic or antagonistic effects of opioid and cannabinoid receptor agonists in the periphery are currently not known. Additive actions of two pharmacological agents are commonly interpreted as utilizing similar mechanisms of action. Activation of cannabinoid and opioid receptors leads to the binding of GTP to Gi/Go proteins. A combination of opioid and cannabinoid receptor agonists induce an additive stimulation of GTPγS binding in neuroblastoma cells that endogenously express opioid and cannabinoid receptors, suggesting that the receptors do not share the common pool of G proteins (Shapira et al., 2000). Therefore, the lack of additive effects in the peripherally administered DAMGO and ACPA suggest that the two agonists are competing for the common pool of G protein. It is possible that there is only limited amount of G proteins available in sensory neurons at any given time, which could explain the antagonistic effects of the two agonists when given simultaneously. Further studies are warranted to confirm whether MOR and CB1 in primary afferent nociceptors utilize similar intracellular signaling pathways and resources. Alternatively, cannabinoids can bind allosterically to MOR and interfere with DAMGO binding to MOR, which would result in antagonizing effects (Kathmann et al., 2006). It is likely that CB1 and MOR are co-expressed in sub-population of primary afferent nociceptors since both MOR and CB1 are expressed in TRPV1 positive neurons (Amaya et al., 2006; Yamamoto et al., 2008), but a direct receptor-receptor interaction in the same cells is yet to be demonstrated. The synergistic and additive drug effects can also be explained by simultaneous activation of opioid and cannabinoid receptors at multiple levels of brain circuitry involving brain areas such as PAG, RVM and the spinal cord, where these receptors are co-expressed (Herkenham et al., 1991; Hohmann et al., 1999; Mailleux and Vanderhaeghen, 1992; Meng et al., 1998). For example, PAG contributes to bidirectional enhancement of antinociception between morphine and cannabinoids (Wilson-Poe et al., 2013). Thus, dynamic interactions between pain modulating areas can further enhance drug effects originating from a particular brain region, which could also explain the lack of additive effects of the locally administered drugs in our data.
Our results add to an overwhelming amount of preclinical and clinical data supporting the role of peripheral opioid and cannabinoid receptors in various pain models. However, despite obvious advantages of avoiding centrally mediated side effects of targeting peripheral opioid and cannabinoid receptors, even peripherally restricted agonists cause side effects involving peripheral organs, such as gastrointestinal tract (Pertwee, 2001; Pinto et al., 2002; Tan-No et al., 2003). The development of novel treatment strategies that may improve the benefit-to-risk ratio of peripheral opioid and cannabinoid agonists for the management of persistent types of pain is therefore important. Our data showed that combined treatments with the agonists for the two receptor systems in the periphery lead to complex interactions and may not be suitable for countering inflammatory mechanical hyperalgesia. Further studies that elucidate mechanical bases for the drug interactions unique to the peripheral nervous system are warranted, and the combined treatment with the two agonists should be tested in different pain conditions with different stimulus modalities. Knowledge gained from those studies can also contribute to the development of novel approaches that enhance peripheral opioid and cannabinoid in ameliorating persistent pain beyond pharmacological means.
Acknowledgement
This study was supported by NIH RO1 DE019448 (JYR) and a grant from the International Scholar Program, Kyung Hee University in 2015.
Footnotes
Conflict of interest
There is no conflict of interest to declare.
References
- Amaya F, Shimosato G, Kawasaki Y, Hashimoto S, Tanaka Y, Ji RR, Tanaka M, 2006. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain 124, 175–183. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Olesen AE, Staahl C, Menzaghi F, Kell S, Wong GY, Drewes AM, 2009. Analgesic efficacy of peripheral kappa-opioid receptor agonist CR665 compared to oxycodone in a multi-modal, multi-tissue experimental human pain model: selective effect on visceral pain. Anesthesiology 111, 616–624. [DOI] [PubMed] [Google Scholar]
- Auh QS, Ro JY, 2012. Effects of peripheral kappa opioid receptor activation on inflammatory mechanical hyperalgesia in male and female rats. Neurosci. Lett 524, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bileviciute-Ljungar I, Spetea M, Guo Y, Schutz J, Windisch P, Schmidhammer H, 2006. Peripherally mediated antinociception of the mu-opioid receptor agonist 2-[(4,5alpha-epoxy-3-hydroxy-14beta-methoxy-17-methylmorphinan-6beta-yl)amino]ace tic acid (HS-731) after subcutaneous and oral administration in rats with carrageenan-induced hindpaw inflammation. J. Pharmacol. Exp. Ther 317, 220–227. [DOI] [PubMed] [Google Scholar]
- Borner C, Kraus J, Schroder H, Ammer H, Hollt V, 2004. Transcriptional regulation of the human mu-opioid receptor gene by interleukin-6. Mol. Pharmacol 66, 1719–1726. [DOI] [PubMed] [Google Scholar]
- Bushlin I, Rozenfeld R, Devi LA, 2010. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr. Opin. Pharmacol 10, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP, Brown DA, 1992. Cannabinoid receptor agonists inhibit Ca current in NG108–15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br. J. Pharmacol 106, 231–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichewicz DL, 2004. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 74, 1317–1324. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, McCarthy EA, 2003. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J. Pharmacol. Exp. Ther 304, 1010–1015. [DOI] [PubMed] [Google Scholar]
- Corchero J, Avila MA, Fuentes JA, Manzanares J, 1997. delta-9-Tetrahydrocannabinol increases prodynorphin and proenkephalin gene expression in the spinal cord of the rat. Life Sci. 61, 39–43. [DOI] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP, 2007. Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur. J. Pharmacol 567, 125–130. [DOI] [PubMed] [Google Scholar]
- da Fonseca Pacheco D, Klein A, de Castro Perez A, da Fonseca Pacheco CM, de Francischi JN, Duarte ID, 2008. The mu-opioid receptor agonist morphine, but not agonists at delta- or kappa-opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Br. J. Pharmacol 154, 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz O, Karadag HC, Ulugol A, 2011. Synergistic anti-allodynic effects of nociceptin/orphanin FQ and cannabinoid systems in neuropathic mice. Pharmacol. Biochem. Behav 99, 540–544. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC, 1991. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci 11, 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Briley EM, Herkenham M, 1999. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 822, 17–25. [DOI] [PubMed] [Google Scholar]
- Houser SJ, Eads M, Embrey JP, Welch SP, 2000. Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55,940 Delta(9)-THC and anandamide. Brain Res. 857, 337–342. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Fleming RM, 1984. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol. Pharmacol 26, 532–538. [PubMed] [Google Scholar]
- Joseph EK, Levine JD, 2010. Mu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in rat. Neuroscience 171, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Trankle C, Schlicker E, 2006. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch. Pharmacol 372, 354–361. [DOI] [PubMed] [Google Scholar]
- Kraus J, Borner C, Giannini E, Hickfang K, Braun H, Mayer P, Hoehe MR, Ambrosch A, Konig W, Hollt V, 2001. Regulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding site. J. Biol. Chem 276, 43901–43908. [DOI] [PubMed] [Google Scholar]
- Lee KS, Zhang Y, Asgar J, Auh QS, Chung MK, Ro JY, 2016. Androgen receptor transcriptionally regulates mu-opioid receptor expression in rat trigeminal ganglia. Neuroscience 331, 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Taiwo YO, 1989. Involvement of the mu-opiate receptor in peripheral analgesia. Neuroscience 32, 571–575. [DOI] [PubMed] [Google Scholar]
- Machelska H, Schroff M, Oswald D, Binder W, Sitte N, Mousa SA, Rittner HL, Brack A, Labuz D, Busch M, Wittig B, Schafer M, Stein C, 2009. Peripheral non-viral MIDGE vector-driven delivery of beta-endorphin in inflammatory pain. Mol. Pain 5, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP, 2013. Interactions between mu-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J. Pharmacol. Exp. Ther 345, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ, 1992. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience 48, 655–668. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA, 1999. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol. Sci 20, 287–294. [DOI] [PubMed] [Google Scholar]
- Mason DJ Jr., Lowe J, Welch SP, 1999. Cannabinoid modulation of dynorphin A: correlation to cannabinoid-induced antinociception. Eur. J. Pharmacol 378, 237–248. [DOI] [PubMed] [Google Scholar]
- Mecs L, Tuboly G, Toth K, Nagy E, Nyari T, Benedek G, Horvath G, 2010. Peripheral antinociceptive effect of 2-arachidonoyl-glycerol and its interaction with endomorphin-1 in arthritic rat ankle joints. Clin. Exp. Pharmacol. Physiol 37, 544–550. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL, 1998. An analgesia circuit activated by cannabinoids. Nature 395, 381–383. [DOI] [PubMed] [Google Scholar]
- Mense S, 1993. Nociception from skeletal muscle in relation to clinical muscle pain. Pain 54, 241–289. [DOI] [PubMed] [Google Scholar]
- Mousa SA, 2003. Morphological correlates of immune-mediated peripheral opioid analgesia. Adv. Exp. Med. Biol 521, 77–87. [PubMed] [Google Scholar]
- Neelakantan H, Tallarida RJ, Reichenbach ZW, Tuma RF, Ward SJ, Walker EA, 2015. Distinct interactions of cannabidiol and morphine in three nociceptive behavioral models in mice. Behav. Pharmacol 26, 304–314. [DOI] [PubMed] [Google Scholar]
- Niu KY, Zhang Y, Ro JY, 2012. Effects of gonadal hormones on the peripheral cannabinoid receptor 1 (CB1R) system under a myositis condition in rats. Pain 153, 2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez S, Lee JS, Zhang Y, Bai G, Ro JY, 2007. Role of peripheral mu-opioid receptors in inflammatory orofacial muscle pain. Neuroscience 146, 1346–1354. [DOI] [PubMed] [Google Scholar]
- Pacheco Dda F, Klein A, Perez AC, Pacheco CM, de Francischi JN, Reis GM, Duarte ID, 2009. Central antinociception induced by mu-opioid receptor agonist morphine but not delta- or kappa-, is mediated by cannabinoid CB1 receptor. Br. J. Pharmacol 158, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, 2001. Cannabinoids and the gastrointestinal tract. Gut 48, 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Capasso R, Di Carlo G, Izzo AA, 2002. Endocannabinoids and the gut. Prostaglandins Leukot. Essent. Fatty Acids 66, 333–341. [DOI] [PubMed] [Google Scholar]
- Pol O, Puig MM, 2004. Expression of opioid receptors during peripheral inflammation. Curr. Top. Med. Chem 4, 51–61. [DOI] [PubMed] [Google Scholar]
- Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, Stein C, 2004. Rapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience 129, 473–479. [DOI] [PubMed] [Google Scholar]
- Raja SN, 2012. Modulating pain in the periphery: gene-based therapies to enhance peripheral opioid analgesia: bonica lecture, ASRA 2010. Reg. Anesth. Pain Med 37, 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Imai Y, Uhl GR, Stein C, 1995. Inflammation enhances peripheral mu-opioid receptor-mediated analgesia, but not mu-opioid receptor transcription in dorsal root ganglia. Eur. J. Pharmacol 279, 165–169. [DOI] [PubMed] [Google Scholar]
- Seward E, Hammond C, Henderson G, 1991. Mu-opioid-receptor-mediated inhibition of the N-type calcium-channel current: proceedings. Biol. Sci.: Royal Soc 244, 129–135. [DOI] [PubMed] [Google Scholar]
- Shapira M, Vogel Z, Sarne Y, 2000. Opioid and cannabinoid receptors share a common pool of GTP-binding proteins in cotransfected cells, but not in cells which endogenously coexpress the receptors. Cell. Mol. Neurobiol 20, 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PB, Welch SP, Martin BR, 1994. Interactions between delta 9-tetrahydrocannabinol and kappa opioids in mice. J. Pharmacol. Exp. Ther 268, 1381–1387. [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ikeda M, Kadoi J, Nasu M, Matsumoto S, 2004. Opioidergic modulation of excitability of rat trigeminal root ganglion neuron projections to the superficial layer of cervical dorsal horn. Neuroscience 125, 995–1008. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, 2002. The interaction index: a measure of drug synergism. Pain 98, 163–168. [DOI] [PubMed] [Google Scholar]
- Tan-No K, Niijima F, Nakagawasai O, Sato T, Satoh S, Tadano T, 2003. Development of tolerance to the inhibitory effect of loperamide on gastrointestinal transit in mice. Eur. J. Pharm. Sci 20, 357–363. [DOI] [PubMed] [Google Scholar]
- Tham SM, Angus JA, Tudor EM, Wright CE, 2005. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice. Br. J. Pharmacol 144, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacnik PW, Luhr KM, Hill RH, Ljunggren HG, Kristensson K, Svensson M, 2008. Cannabinoids affect dendritic cell (DC) potassium channel function and modulate DC T cell stimulatory capacity. J. Immunol 181, 3057–3066. [DOI] [PubMed] [Google Scholar]
- Welch SP, Stevens DL, 1992. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine in mice. J. Pharmacol. Exp. Ther 262, 10–18. [PubMed] [Google Scholar]
- Wilson-Poe AR, Pocius E, Herschbach M, Morgan MM, 2013. The periaqueductal gray contributes to bidirectional enhancement of antinociception between morphine and cannabinoids. Pharmacol. Biochem. Behav 103, 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Kawamata T, Niiyama Y, Omote K, Namiki A, 2008. Down-regulation of mu opioid receptor expression within distinct subpopulations of dorsal root ganglion neurons in a murine model of bone cancer pain. Neuroscience 151, 843–853. [DOI] [PubMed] [Google Scholar]
- Yesilyurt O, Dogrul A, Gul H, Seyrek M, Kusmez O, Ozkan Y, Yildiz O, 2003. Topical cannabinoid enhances topical morphine antinociception. Pain 105, 303–308. [DOI] [PubMed] [Google Scholar]
- Yu XH, Cao CQ, Martino G, Puma C, Morinville A, St-Onge S, Lessard E, Perkins MN, Laird JM, 2010. A peripherally restricted cannabinoid receptor agonist produces robust anti-nociceptive effects in rodent models of inflammatory and neuropathic pain. Pain 151, 337–344. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang Y, Asgar J, Niu KY, Lee J, Lee KS, Schneider M, Ro JY, 2014. Sex differences in mu-opioid receptor expression in trigeminal ganglia under a myositis condition in rats. Eur. J. Pain 18, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schafer M, 2003. Painful inflammation-induced increase in mu-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol. Pharmacol 64, 202–210. [DOI] [PubMed] [Google Scholar]


