Abstract
BACKGROUND AND AIMS:
We describe the pathophysiology, treatment, and outcome of Crigler-Najjar type 1 syndrome (CN1) in 28 UGT1A1 c.222C>A homozygotes followed for 520 aggregate patient-years.
APPROACH AND RESULTS:
Unbound (“free”) bilirubin (Bf) was measured in patient sera to characterize the binding of unconjugated bilirubin (BT) to albumin (A) and validate their molar concentration ratio (BT/A) as an index of neurological risk. Two custom phototherapy systems were constructed from affordable materials to provide high irradiance in the outpatient setting; light dose was titrated to keep BT/A at least 30% below intravascular BT binding capacity (i.e., BT/A = 1.0). Categorical clinical outcomes were ascertained by chart review, and a measure (Lf) was used to quantify liver fibrosis. Unbound bilirubin had a nonlinear relationship to BT (R2 = 0.71) and BT/A (R2 = 0.76), and Bf as a percentage of BT correlated inversely to the bilirubin–albumin equilibrium association binding constant (R2 = 0.69), which varied 10-fold among individuals. In newborns with CN1, unconjugated bilirubin increased 4.3 ± 1.1 mg/dL per day. Four (14%) neonates developed kernicterus between days 14 and 45 postnatal days of life; peak BT ≥ 30 mg/dL and BT/A ≥ 1.0 mol:mol were equally predictive of perinatal brain injury (sensitivity 100%, specificity 93.3%, positive predictive value 88.0%), and starting phototherapy after age 13 days increased this risk 3.5-fold. Consistent phototherapy with 33-103 μW/cm2•nm for 9.2 ± 1.1 hours/day kept BT and BT/A within safe limits throughout childhood, but BT increased 0.46 mg/dL per year to reach dangerous concentrations by 18 years of age. Liver transplantation (n = 17) normalized BT and eliminated phototherapy dependence. Liver explants showed fibrosis ranging from mild to severe.
CONCLUSION:
Seven decades after its discovery, CN1 remains a morbid and potentially fatal disorder. (Hepatology 2020;71:1923-1939).
In 1952, John Crigler and Victor Najjar described 7 infants from three families who developed intractable nonhemolytic jaundice within the first week of life(1); 6 died of bilirubin encephalopathy by 15 months of age. Crigler-Najjar syndrome (OMIM 218800) was subsequently linked to deficiency of hepatic uridine 5′-diphosphate glucuronyltransferase (UGT1A1),(2) which mediates the glucuronidation of 4Z,15Z-bilirubin requisite for its biliary excretion (Fig. 1).(3) Residual UGT1A1 activity and its inducibility by phenobarbital allow distinction among type 1 (CN1; absence of UGT1A1), type 2 (CN2; 4%-10% UGT1A1), and type 3 (CN3; 20%-30% UGT1A1; Gilbert syndrome) clinical variants (OMIM PS237450).
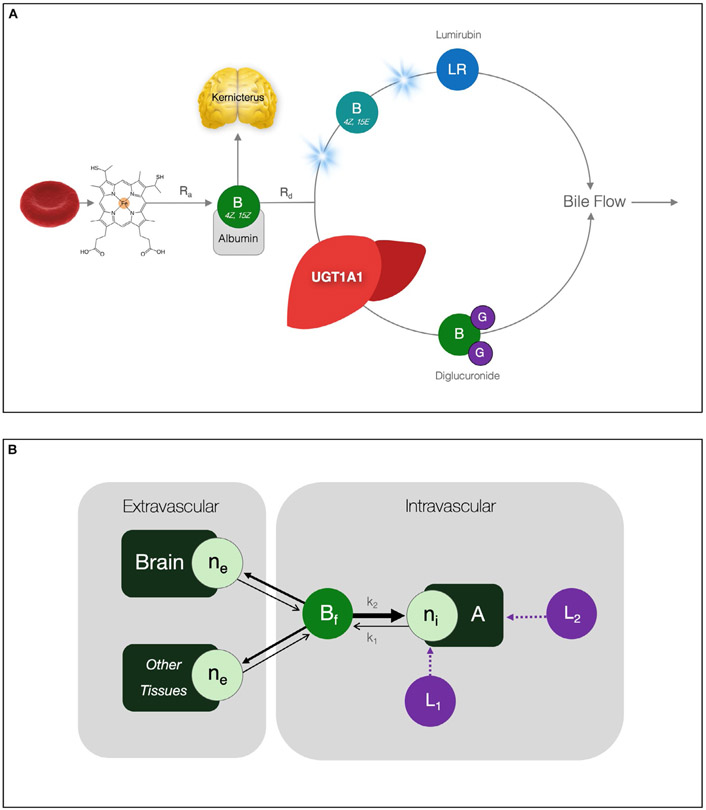
FIG. 1.
Bilirubin metabolism and biodistribution. (A) Native 4Z,15Z-bilirubin is a degradation product of heme derived primarily from senescent erythrocytes. Its rate of appearance (Ra) is governed by the kinetics of red cell destruction. Under normal conditions, hepatocyte UGT1A1 adds glucuronides to native bilirubin and water-soluble diglucuronide enters bile canaliculi for excretion. Bilirubin elimination from the body (Rd) depends on hepatic UGT1A1 activity, diglucuronide transport into canaliculi, and total bile flow. Illumination of bilirubin with 430-490 nm (“blue”) spectrum light reversibly converts 4Z,15Z-bilirubin to its 4Z,15E isomer, a portion of which further isomerizes to lumirubin, the chemically stable phototherapy product that predominates in bile. (B) Unbound (“free”) biliburin (Bf) is the neurotoxic fraction; it has low aqueous solubility (~70 nmol/L; 4 μg/dL) and moves in chemical equilibria between a finite number of intravascular binding sites (ni) on albumin (A) and a larger number of low-affinity extravascular sites (ne) in brain and other tissues. The values of k1 (sec−1) and k2 (μmol/L per second) represent the dissociation and association rate constants, respectively, and k1/k2 is K (L/μmol), the bilirubin–albumin equilibrium association binding constant. Various ligands (e.g., antibiotics) interfere with binding by either competing directly for the bilirubin binding site (L1) or indirectly altering its association constant (L2). Abbreviation: LR, lumirubin.
Native bilirubin is a degradation product of heme, and in the absence of hepatic UGT1A1 activity, accumulates in humans at a relatively constant rate of 3.8 ± 0.6 mg/kg per day.(4) Unconjugated bilirubin has low aqueous solubility at physiological pH (~70 nmol/L; 4 μg/dL),(5) and moves in chemical equilibria between a limited number of high-affinity binding sites on albumin and a much larger reservoir of low-affinity sites on erythrocytes, adipocytes, and endothelial cells (Fig. 1).(6) In vivo, more than 99% of intravascular unconjugated bilirubin (BT) is bound to albumin and interacts minimally with cerebral endothelia.(7) In contrast, unbound (“free”) bilirubin (Bf) readily crosses the blood–brain barrier and binds to neural membranes.(8,9) As intravascular binding capacity saturates, Bf increases as a proportion of the total, driving pigment deposition in brain and other extravascular tissues.
The quantity of bilirubin bound to brain tissue correlates directly with indices of cellular dysfunction and injury.(9-11) In humans, diminished amplitude and velocity of auditory brainstem responses (ABR) are the earliest signs of bilirubin encephalopathy and correlate more closely with Bf than BT.(12,13) A critical threshold of cerebral pigment deposition causes kernicterus (German kern [“nucleus”] and ikterus [“jaundice”]), a devastating static encephalopathy marked by staining of cranial nerve, subthalamic, basal ganglia, and hippocampal neurons that manifests as sudden neurological regression followed by generalized dystonia, choreoathetosis, ataxia, oculomotor palsy, seizures, or death.(14) The advent of effective phototherapy systems between 1958 and 1968(15,16) afforded CN1 patients a mechanism of protection from this catastrophic outcome.(17)
Crigler-Najjar syndrome type 1 is exceedingly rare worldwide (~1 per 1,000,000 live births) but unusually common among the Old Order Amish and Mennonite (Plain) people, who descended from just a few hundred Swiss-German Anabaptists and now comprise about 800,000 individuals inhabiting isolated demes throughout North and South America. A pathogenic missense variant of UGT1A1 (c.222C>A; p.Tyr74*) was introduced separately into Amish and Mennonite populations approximately 300 years ago and likely arose in a common ancestor more than 1,000 years before the formation of the Anabaptist church. The UGT1A1 c.222C>A variant has drifted to allele frequencies of 2.17% (incidence of 1 per 2,124 births) and 0.83% (incidence of 1 per 14,520 births) within extant Mennonite (Weaverland and Groffdal Conferences) and Old Lancaster Amish populations, respectively.
Here, we describe the clinical course, treatment, and outcome of 28 UGT1A1 c.222C>A homozygotes with a severe (type 1) biochemical phenotype over 30 years of follow-up. This study extends the findings of a report published in 2006,(17) which included 17 UGT1A1 c.222C>A homozygotes managed at our center in Pennsylvania (Clinic for Special Children; CSC). We provide a status update on these 17 patients and include new data about 11 additional UGT1A1 c.222C>A homozygotes, 7 of whom were managed at other Midwestern U.S. sites. Our longitudinal observations over 3 decades yield insights into the pathophysiology of bilirubin encephalopathy, underscore principles of effective phototherapy, and provide a framework against which to judge emerging molecular therapies for CN1 and other inborn errors of liver metabolism.
Materials and Methods
PATIENTS
The study was approved by the Penn-Lancaster General Hospital Institutional Review Board, and patients or their parents consented in writing to participate. The CSC database contained 28 UGT1A1 c.222C>A homozygotes (48% female) born between 1984 and 2015 (median age 19.2, range 3.2-34.7 years) and comprised of 520 aggregate patient-years of follow-up. Twenty-one (75%) individuals were followed clinically at CSC(17) and are joined in the present report by 7 additional patients managed at other sites in the Midwestern United States. All patients had a severe (type 1) clinical phenotype, defined here as unconjugated hyperbilirubinemia unresponsive to phenobarbital and necessitating 7 or more hours of high-intensity phototherapy daily to maintain serum bilirubin below a neurotoxic threshold. Based on converging lines of evidence, we define BT ≥ 30 mg/dL (513 μmol/L) and BT/A ≥ 1.0 mol: mol as absolute thresholds for neurotoxicity in vivo. However, to safeguard patients against approaching these limits, we try to keep BT and BT/A approximately 30% lower, at ≤20 mg/dL (340 μmol/L) and ≤0.7 mol:mol, respectively. During severe exacerbations of hyperbilirubinemia, patients were managed according to a standard inpatient protocol that included a provision for intravenous albumin infusions (Table 1).
TABLE 1.
Crigler-Najjar Syndrome Management Guidelines
Principles of Effective Phototherapy
|
Treatment Goals
|
Inpatient Management for Severe Hyperbilirubinemia
|
Different light meters produce variable measurements from the same source. We use the BiliBlanket Meter II (GE Healthcare, Chicago, IL).
Hemolytic conditions should be sought and treated. Consider (1) immunologic/autoimmune, (2) red blood cell enzymopathies, (3) ineffective erythropoiesis, and (4) physical destruction.
Internal hemorrhage, occult tissue hematoma, peripartum blood ingestion by neonates.
10% dextrose solution for infants and toddlers, 5% dextrose for older children and adults.
BILIRUBIN KINETICS, BIODISTRIBUTION, AND RISK ASSESSMENT
Using BT values from newborns naïve to phototherapy, we calculated the postnatal rate of bilirubin formation (mg/kg per day) based on the following assumptions: (1) In the absence of UGT1A1 activity and photoisomerization, unconjugated bilirubin excretion is negligible; (2) the plasma compartment represents about 40 mL/kg body weight; and (3) in jaundiced patients, the total bilirubin load is about equally distributed between intravascular and extravascular binding sites.(13)
The plasma concentration of Bf is more closely linked to neuronal injury than BT, but is a challenging parameter to measure and not widely available for clinical use.(13) We therefore used the serum BT/A molar concentration ratio (mol:mol) as a surrogate for Bf, and thereby an index of neurological risk in which 1 g of human albumin (molecular weight [MW] 66,437 g/mol) binds approximately 9 mg of bilirubin (MW 584.7 g/mol) (https://www.sigmaaldrich.com) with a stoichiometry of about 1:1, and their mass units are converted to concentrations in μmol/L as follows (Fig. 1):
| (1) |
The relationship between BT and Bf is expressed by the following mass action equation:
| (2) |
where P represents the concentration of intravascular protein binding sites (μmol/L) and K is the bilirubin–protein equilibrium association binding constant (L/μmol). In practice, the overwhelming majority of binding sites are represented by albumin (A), and K is effectively the equilibrium association binding constant of the bilirubin–albumin complex. Within clinically relevant values of BT and A, Bf is less than 0.01% of BT and can be ignored, which simplifies the mass action equilibrium to
| (3) |
Various ligands (e.g., drugs, preservatives, contrast agents) alter the relationship between BT and Bf by directly reducing the number of available binding sites or causing conformational changes of albumin that indirectly reduce the binding constant (Fig. 1).
To validate these relationships for clinical practice, we measured Bf from serum of 18 UGT1A1 c.222C>A homozygotes and 7 healthy Amish control individuals on the Arrows UB-analyzer (Arrows Co. Ltd., Osaka, Japan) using the horseradish peroxidase (HRP) method as previously described.(18) The assay was run at two different HRP concentrations to accurately determine equilibrium Bf, and Eq. 3 was used to calculate K for each sample. Results were compared with available data from two high-risk populations: 6 preterm neonates weighing less than 1.0 kg who received an exchange transfusion or had abnormal ABRs at discharge(13) and 8 full-term newborns (weight range 2.6-4.2 kg) with severe idiopathic hyperbilirubinemia (BT 28-34 mg/dL; 479-581 μmol/L).(19)
PHOTOTHERAPY AND LIGHT DOSE
Two different systems were designed and constructed using affordable and commercially available materials to provide high-irradiance light therapy in the outpatient setting. The mainstay of phototherapy was a panel of ten 4-foot, 40W special Billi Blue (BB) fluorescent tubes (Phillips, Amsterdam, Netherlands) housed in a rigid, overhead, aluminum frame (66 × 137 cm).(17) The fully assembled unit weighed 18.2 kg. Newborns received 15-20 hours of phototherapy daily with 60%-70% of body surface exposed and light positioned 30-45 cm from the skin. For older children and adults, the light source was typically placed 45-60 cm from the skin and delivered at night to 35%-50% of skin surface. Mirrors were placed at the sides and head of the bed (Supporting Fig. S1). Patients used white sheets and did not shield their eyes.
More recently, 2 UGT1A1 c.222C>A homozygotes were treated with a custom array of high-intensity light-emitting diodes (LEDs), designed and produced by HJV. The LED system consisted of an assembly of up to five aluminum panels, each with its own power cord, fused power inlet, and switch. A modular design allowed the number of panels to be adjusted to patient size (Fig. S1). Individual panels were mounted and joined along a central 3/4-inch sectional electrical conduit tube to yield a continuous therapeutic array plugged into a standard 110V or 220V AC power outlet. Individual panels were fitted with five 2-foot, 20W blue T8 LED tubes (Grandol Industry, Ltd., Shen Zhen, China), each with nine LEDs (i.e., 45 LEDs per panel) spaced 6 cm apart and emitting a peak wave-length of 468 (spectral range 415-536) nm. Single panels measured 32 × 61 cm and weighed 1.81 kg; the entire five-panel system (25 LED tubes, 225 individual LEDs) measured 168 × 61 cm, weighed 11.5 kg, and could be deconstructed and packed for transport into a 71 × 48 × 30 cm wheeled suitcase.
Daily light dose (W per hour/nm) is the product of irradiance (μW/cm2 per nm), body surface area exposed (cm2), and exposure time (hours). All irradiance measurements were taken with the BiliBlanket Meter II (GE Healthcare, Chicago, IL). The proportion of skin exposed to light is relatively fixed as a function of age. Thus, in practice, the two most relevant variables affecting light dose are skin-level irradiance and exposure time, which were titrated to maintain BT/A < 0.7 mol: mol; this value is 30% below the intravascular binding capacity (i.e., BT/A 1.0 mol:mol), maintains Bf below its aqueous solubility limit,(6) and has proven to be well-tolerated by humans in the absence of ligands that compete for albumin binding.(13,17,19,20)
LIVER FIBROSIS SCORE
Tissue from a liver biopsy or explant was available for 15 UGT1A1 c.222C>A homozygotes. For each tissue sample, a weighted liver fibrosis score (Lf) was calculated using the following equation:
| (4) |
where Fc is the central fibrosis score (range 0-4), Fp is the portal (Ishak) fibrosis score (range 0-6), and the weighted Lf score ranges from 0 (no fibrosis) to 10 (severe fibrosis).
STATISTICS
Statistical calculations were done in Prism 8 (GraphPad, La Jolla, CA). Perinatal neurological risk factor data (BT, BT/A, and days to phototherapy) were not normally distributed and were therefore analyzed using the two-tailed Mann-Whitney U test for unpaired observations. Normally distributed continuous variables were compared using the unpaired t test with Welch’s correction (which does not assume equal variances). Associations between paired variables were tested using Pearson coefficients (r) or simple linear correlation. Relationships of Bf to BT and BT/A were fit to an exponential model. Groups of three or more were compared using one-way analysis of variance (ANOVA) followed by the Tukey’s post hoc test for pairwise comparisons. Kaplan-Meier analysis was applied to outcomes of kernicterus, liver transplantation, and survival. Standard contingency tables were constructed to calculate the sensitivity, specificity, positive predictive value (PPV), and likelihood ratio for disease (LRD).
Results
BILIRUBIN BINDING AND TISSUE DISTRIBUTION
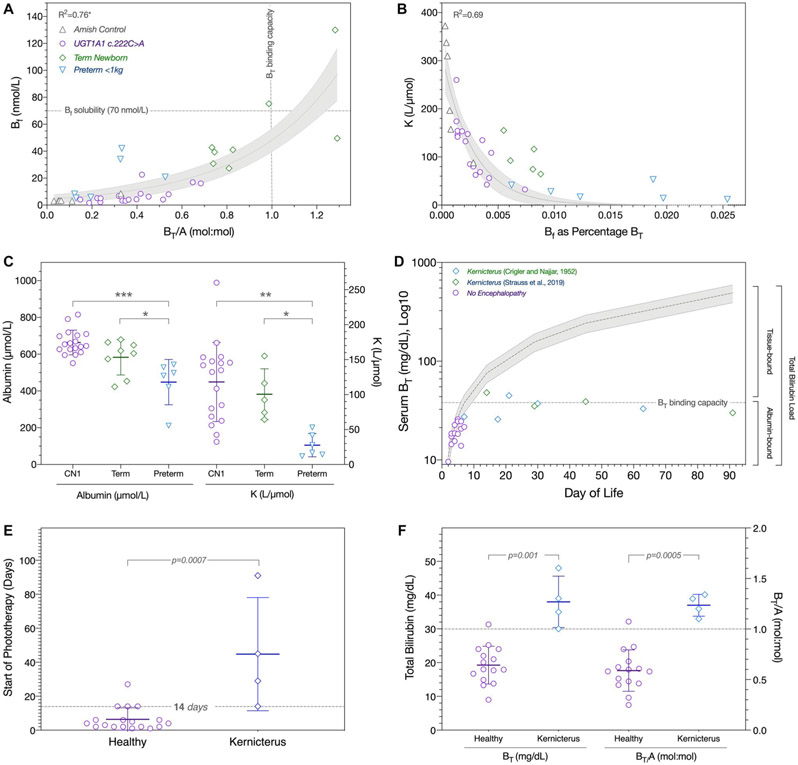
We used sera from 7 healthy Amish controls, 18 UGTA1A c.222C>A homozygotes, 8 term newborns with idiopathic jaundice,(19) and 6 preterm infants weighing less than 1 kg(13) to examine the relationship among BT, A, Bf, and K (Eq. 3) within BT concentrations of 1.5-34.2 mg/dL (25-585 μmol/L) and BT/A values of 0.03-1.29 mol:mol (Table 2). As predicted by the mass action equation, Bf had a strong nonlinear relationship to both BT (R2 = 0.71) and BT/A (R2 = 0.76), and the proportion of BT represented by Bf decreased exponentially with increasing values of K (R2 = 0.69) (Fig. 2).
TABLE 2.
Bilirubin-Albumin Binding in Serum of Control Individuals, Premature Neonates, Jaundiced Term Newborns, and UGT1A1 c.222C>A Homozygotes
| Total Bilirubin |
Albumin |
Unbound Bilirubin |
BT/A |
K |
||||
|---|---|---|---|---|---|---|---|---|
| mg/dL | (μmol/L) | g/dL | (μmol/L) | μg/dL | (nmol/L) | mol:mol | L/mol | |
| Amish control (n = 7) | 3.8 ± 3.4 | (65 ± 59) | 4.8 ± 0.7 | (724 ± 105) | 0.06 ± 0.12 | (1.0 ± 2.0) | 0.10 ± 0.10 | 244 ± 113 |
| UGT1A1 c.222C>A (n = 18) | 14.7 ± 5.7 | (251 ± 98) | 4.4 ± 0.5 | (663 ± 67) | 0.42 ± 0.33 | (7.1 ± 5.7) | 0.38 ± 0.15 | 118 ± 57 |
| Term idiopathic jaundice (n = 8)* | 30.5 ± 2.2 | (521 ± 38) | 3.9 ± 0.6 | (583 ± 96) | 3.2 ± 2.0 | (54.5 ± 33.8) | 0.93 ± 0.24 | 101 ± 36 |
| Preterm < 1 kg (n = 6)† | 6.5 ± 2.9 | (111 ± 49) | 3.0 ± 0.8 | (448 ± 123) | 1.13 ± 0.92 | (19.4 ± 15.7 | 0.27 ± 0.16 | 28 ± 17 |
| ANOVA P value‡ | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |||
| Amish control vs. UGT1A1 c.222C>A | **** | ns | ns | ** | ** | |||
| Amish control vs. term idiopathic jaundice | **** | * | **** | **** | ** | |||
| Amish control vs. preterm < 1 kg | ns | **** | ns | ns | **** | |||
| UGT1A1 c.222C>A vs. term idiopathic jaundice | **** | ns | **** | **** | ns | |||
| UGT1A1 c.222C>A vs. preterm < 1 kg | ** | **** | ns | ns | * | |||
| Term idiopathic jaundice vs. preterm < 1 kg | **** | * | ** | **** | ns | |||
FIG. 2.
Bilirubin–albumin binding, tissue loading, and kernicterus. (A) Gray horizontal and vertical lines indicate Bf aqueous solubility and intravascular BT binding capacity, respectively. There is an exponential relationship between Bf and BT/A (R2 = 0.76; 95% confidence interval shaded gray) (Amish controls, gray triangles; UGTA1A c.222C>A homozygotes, purple circles; term newborns with idiopathic jaundice, green diamonds; preterm infants weighing less than 1 kg, blue triangles). (B) The proportion of BT represented by Bf decreases exponentially with increasing values of K (R2 = 0.69). (C) UGT1A1 c.222C>A homozygotes (CN1) as compared with jaundiced term newborns had similar albumin concentrations (left y-axis) and calculated values of K (right y-axis), whereas preterm babies weighing less than 1 kg had the lowest levels of both albumin and K (ANOVA P < 0.0001; Tukey’s post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001). (D) BT values (log10 scale) are plotted for 5 patients originally described by Crigler and Najjar (green diamonds(1)), 4 neonates from the present cohort who developed kernicterus (blue diamonds), and 14 UGT1A1 c.222C>A homozygotes who escaped neurological injury (purple circles). Gray shading depicts the theoretical increase of BT if produced at a steady rate of 3.7 ± 0.9 mg/kg per day and confined to the circulation. Within a normal range of albumin concentrations (3.5 and 4.5 g/dL), intravascular binding capacity (horizontal dashed line) saturates between 5 and 10 days of age and sets an upper limit on the concentration of intravascular BT. Thereafter, unmeasured pigment binds to brain and other extravascular tissues and comes to comprise 60%-90% of the total bilirubin load at the time of kernicterus. (E) Neonates who developed kernicterus (blue diamonds) started phototherapy at a median age of 37 postnatal days as compared with 4 postnatal days of age for those who remained healthy (purple circles); initiation of phototherapy at 14 or more postnatal days of age (gray line) increased the likelihood of brain injury 3.5 fold. (F) All 4 neonates who suffered brain injury had a BT/A greater than 1.0 mol:mol between 15 and 45 postnatal days of age. The presenting BT/A was about 60% lower among the 24 remaining infants who did not develop kernicterus. As predictors of kernicterus during the neonatal period, BT ≥ 30 mg/dL or BT/A ≥ 1.0 mol:mol had equal specificity (93.3%) and PPV (80.0%).
At any given value of BT, the serum albumin concentration and bilirubin–albumin equilibrium association binding constant are the main determinants of Bf. UGT1A1 c.222C>A homozygotes as compared with jaundiced term newborns had similar albumin concentrations (4.4 ± 0.5 vs. 3.9 ± 0.6 g/dL) and calculated values of K (118 ± 57 vs. 101 ± 36 L/μmol) (Table 2); in both groups, Bf represented 0.001%-0.009% of BT. Unbound bilirubin was proportionally highest (0.006%-0.025% of BT) in preterm babies less than 1 kg, who had the lowest levels of both albumin (3.0 ± 0.8 g/dL) and K (28 ± 17 L/μmol) (ANOVA P < 0.0001) (Table 2, Fig. 2).
KERNICTERUS: RISK FACTORS AND TIMING
In phototherapy-naïve newborns with CN1, BT increased 4.3 ± 1.1 (range 2.2-6.0) mg/dL per day to reach potentially neurotoxic concentrations by 5-14 days of age. This extrapolates to an endogenous bilirubin formation rate of 3.7 ± 0.9 (range 1.9-5.1) mg/kg per day, remarkably close to the value of 3.8 ± 0.6 mg/kg per day measured in healthy adults.(4) Four (14%) babies developed kernicterus between 14 and 45 days of age with presenting BT and BT/A values of 30-48 mg/dL (513-821 μmol/L) and 1.10-1.34 mol:mol, respectively (Fig. 2). All suffered permanent sequelae, including dystonia-chorea (n = 4), dysphagia (n = 4), mutism (n = 4), hearing impairment (n = 2), electrocortical abnormalities (n = 2), and cerebral atrophy (n = 1); 1 died of pneumonia at the age of 17 years.
Infants who did not develop kernicterus had lower presenting BT (mean 19.3 ± 5.6, range 9.0-31.3 mg/dL; P = 0.001) and BT/A (mean 0.59 ± 0.20, range 0.25-1.07 mol:mol; P = 0.0005) values, and started phototherapy at a median of 4 (range 1-27) as compared to 37 (range 14-91) days of age (P = 0.0007). A presenting BT of at least 30 mg/dL (513 μmol/L) or BT/A of at least 1.0 mol:mol were equally predictive of kernicterus (sensitivity 100%, specificity 93.3%, PPV 80%, LRD 14), and babies who started phototherapy after the age of 13 days were 3.5 times more likely to sustain brain injury (Supporting Table S2, Fig. 2).
One additional patient suffered a fatal neurological crisis during early childhood. He was developing normally, with BT concentrations ranging from 15 to 25 mg/dL on 7-12 hours of phototherapy daily. At 7 years of age, he presented to an urgent care center with Streptococcal pharyngitis and was prescribed amoxicillin for outpatient treatment. The following day, his speech deteriorated and he stopped eating. Four days later, he presented to a regional Midwestern hospital febrile, lethargic, ataxic, and mute. Presenting laboratory studies showed BT = 39 mg/dL (667 μmol/L), albumin = 2.9 g/dL (441 μmol/L), and BT/A = 1.52 mol:mol. An electroencephalogram revealed diffuse slowing and generalized paroxysmal spikes. Magnetic resonance imaging of the brain was normal. He was managed with continuous phototherapy, intravenous crystalloid, albumin, and phenobarbital. His BT decreased to baseline but neurological function did not improve. He died a few weeks later.
HYPERBILIRUBINEMIA AND PHOTOTHERAPY
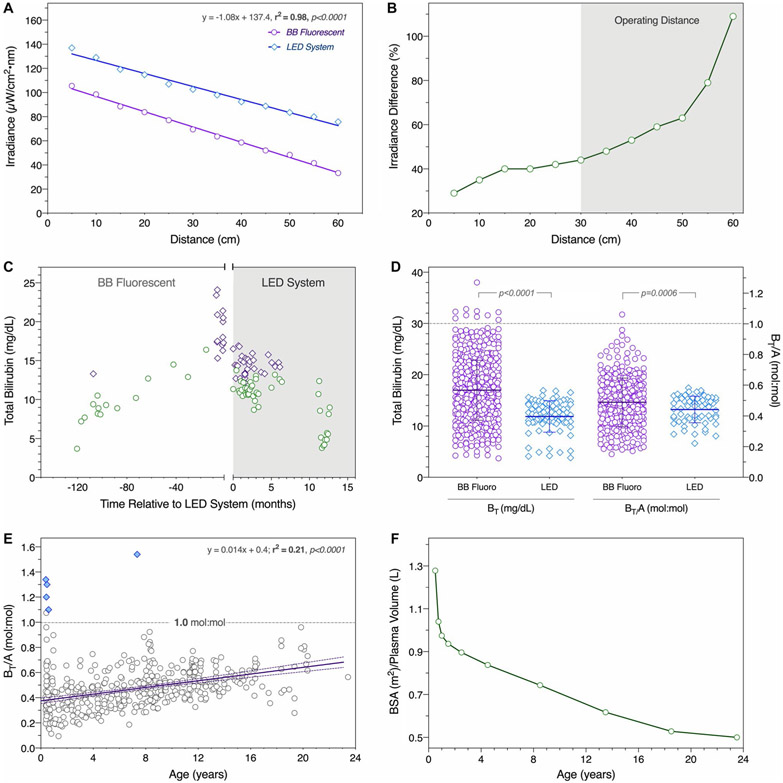
Following the perinatal period, light was delivered 9.2 ± 1.1 (range 7.0-12.5) hours daily to maintain an average BT of 17.0 ± 5.9 (range 3.7-38.7) mg/dL and BT/A of 0.47 ± 0.17 (range 0.10-1.51) mol:mol (Table 3). The serum BT/Alb ratio was lower (0.49 ± 0.15 vs. 0.44 ± 0.09, P = 0.0009) and less variable (F = 3.02, P < 0.0001) in patients treated with LED as compared with BB fluorescent lights (Fig. 3). Irradiance decayed with distance from both light sources (r2 = 0.98, P < 0.0001). LED arrays were 44%-109% more powerful than BB fluorescent tubes (89 ± 10 [13 μW/cm2 per nm vs. 52 ± 13 μW/cm2 per nm; P < 0.0001]) within clinically relevant operating distances of 30-60 cm from the skin. Despite relatively uniform phototherapy conditions, BT and BT/A increased an average of 0.46 mg/dL and 0.013 mol:mol per year (r2 = 0.21; P < 0.0001), respectively, and by 18 years of age reached potentially dangerous values (75%-95% saturation of intravascular binding capacity) (Fig. 3).
TABLE 3.
Biochemical Measurements from UGT1A1 c.222CA Homozygotes Under Phototherapy or Post–Liver Transplantation
| Phototherapy (N = 28)* |
After Liver Transplantation (N = 17) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum Measurement | Reference Range | N† | Mean ± 1 SD | Median | Range | CV | N | Mean ± 1 SD | Median | Range | CV | P | F (P)‡ |
| Total bilirubin, mg/dL | <0.8 | 627 | 17.0 ± 5.9 | 16.0 | 3.7-39.0 | 36% | 113 | 0.9 ± 1.0 | 0.7 | 0.2-9.8 | 108% | <0.0001 | 34.8 (<0.0001) |
| (μmol/L) | (<14) | (278 ± 100) | (274) | (63-667) | (15 ± 17) | (12) | (3-168) | ||||||
| Albumin, g/dL | 3.7-5.6 | 475 | 4.2 ± 0.5 | 4.2 | 2.8-6.7 | 12% | 90 | 4.2 ± 0.4 | 4.3 | 3.3-5.5 | 9% | ns | 1.6 (0.0106) |
| (μmol/L) | (559-846) | (627 ± 77) | (634) | (420-1,012) | (638 ± 57) | (649) | (498-831) | ||||||
| Bilirubin–albumin ratio, mol:mol | — | 475 | 0.47 ± 0.17 | 0.47 | 0.10-1.51 | 35% | 76 | 0.02 ± 0.03 | 0.02 | 0.01-0.26 | 130% | <0.0001 | 28.4 (<0.0001) |
| Alanine aminotransferase, U/L | 10-45 | 401 | 75 ± 46 | 66 | 8-333 | 61% | 134 | 52 ± 126 | 29 | 10-1,427 | 242% | 0.0405 | 7.5 (<0.0001) |
| Alkaline phosphatase, U/L | 120-488 | 384 | 217 ± 60 | 218 | 38-420 | 28% | 94 | 93 ± 62 | 76 | 29-469 | 67% | <0.0001 | 1.1 (ns) |
| Gamma-glutamyltransferase, U/L | 2-39 | 302 | 33 ± 36 | 23 | 3-393 | 111% | 125 | 51 ± 61 | 25 | 7-289 | 120% | 0.0024 | 2.9 (<0.0001) |
The “phototherapy” group is made up of all 28 UGT1A1 c.222C>A homozygotes; the “post–liver transplantation” group is made up of 17 of those same individuals after they received a liver transplant.
“N” represents the number of individual laboratory values analyzed from patients treated with phototherapy (n = 28) as compared with liver transplantation (n = 17).
F is the ratio of SDs squared. P represents the P value for the F ratio; it measures the probability that the SDs (i.e., variance between populations) are the same.
Abbreviations: CV, coefficent of variation; na, not applicable; ns, not significant (ANOVA P > 0.05).
FIG. 3.
Phototherapy. (A) For both BB fluorescent (purpled circles) and LED (blue diamonds) systems, irradiance decayed as a function of distance from the source (r2 = 0.98, P < 0.0001). (B) Decay was less marked for LEDs, which emitted 44%-109% higher irradiance within the standard operating range of 30-60 cm from the skin (gray background). (C) The clinical impact of this difference is shown for 2 representative UGT1A1 c.222C>A homozygotes (indicated by green circles and purple diamonds), whose switch from the BB fluorescent (white background) to LED system (gray background) reduced serum bilirubin concentrations. (D) Total bilirubin (BT, left y-axis) and its molar ratio to albumin (BT/A, right y-axis) were lower in patients treated with the LED system. (E) Despite relative uniform phototherapy conditions over time, total bilirubin (BT) and its ratio to albumin (BT/A) increased with age (n = 28; r2 = 0.21, P < 0.0001; purple line with 95% confidence interval). Patients who developed kernicterus (blue diamonds) all had serum BT concentrations that exceeded intravascular binding capacity (BT/A = 1.0 mol:mol; gray horizontal line). (F) Factors underlying this increase are an age-related decrease of “treatable” body surface area relative to plasma volume (an index of the proportional bilirubin load exposed to blue light), increased skin thickness from infancy to adulthood, and progressive hepatopathy. Abbreviation: BSA, body surface area.
Phototherapy restricted social opportunities and travel but had no serious adverse effects. As previously reported(17) 5 UGT1A1 c.222C>A homozygotes exposed to 100,000 or more hours of phototherapy with unshielded eyes had normal visual acuity and color discrimination as compared with their age-matched siblings. Several fair-complected patients developed chronic dyspigmentation of light-exposed skin, but this required no specific intervention. No member of the cohort developed light-induced erythema or skin cancer.
CHOLELITHIASIS AND CHRONIC HEPATOPATHY
Twelve (43%) of the 28 UGT1A1 c.222C>A homozygotes were diagnosed with cholelithiasis, most often presenting with abdominal pain and exacerbation of hyperbilirubinemia. All underwent cholecystectomy, which revealed numerous small pigmentary stones.
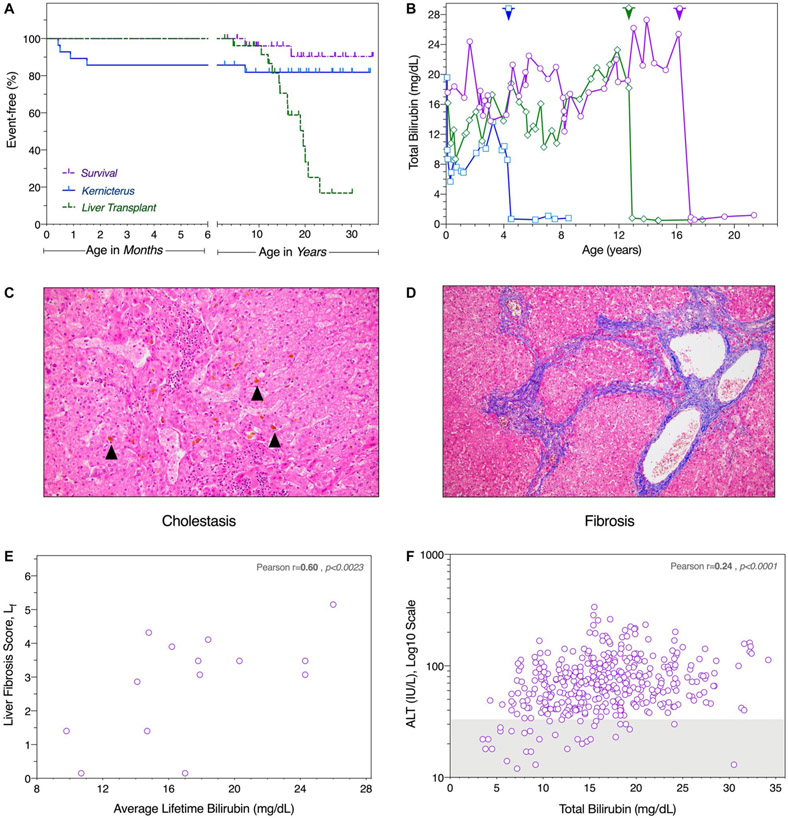
Liver tissue was available from 15 (56%) of 27 UGT1A1 c.222C>A homozygotes (median age 14.6 years, range 4.4-23.6 years). Most tissue sections showed intrahepatic cholestasis and nine (60%) livers had fibrosis ranging from mild to severe (Fig. 4). The degree of fibrosis, as determined by a central–portal weighted fibrosis score (Lf; Eq. 4), correlated with the lifetime average BT (r = 0.60, P < 0.0023; mean 32 values per patient). Using longitudinal data from all 28 UGT1A1 homozygotes (n = 416 paired values), we found a correlation between simultaneously collected BT and alanine aminotransferase concentrations (r = 0.24; P < 0.0001).
FIG. 4.
Clinical outcomes. (A) Four of five cases of kernicterus (blue solid line) occurred within the first 6 weeks of age. Two (7%) patients died (purple dashed line): 1 from respiratory complications of neurological injury and the other from a cause unrelated to CN1 or its treatment. The youngest patient to receive a liver transplant (green dashed line) was 4.7 years old, and median age at transplant was 16.2 years. Perpendicular hatches indicate age at census. Note different scales (months and years) of the divided abscissa. (B) In all 17 transplanted patients, serum bilirubin concentration decreased within a few postoperative days and remained normal thereafter (shown for three representative cases, with timing of liver transplantation indicated by corresponding arrowhead along the upper frame). (C) Most explants showed canalicular bile plugs (black arrows), a sign of intrahepatic cholestasis. (D) Nine (60%) livers had evidence of fibrosis ranging from mild to severe, seen here as central–central bridging on Masson trichrome stain. (E) The weighted liver fibrosis score (Lf) correlated with serum bilirubin averaged over the patient’s lifetime (r = 0.60, P < 0.0023), calculated from a mean of 32 serum bilirubin levels per patient. (F) Based on data from all 28 UGT1A1 c.222C>A homozygotes (416 paired values), there was a modest but statistically significant correlation (r = 0.24; P < 0.0001) between simultaneously measured unconjugated bilirubin and alanine transaminase (ALT; log10 scale). Gray shading represents the normal reference range for ALT.
LIVER TRANSPLANTATION
Seventeen (63%) UGT1A1 c.222C>A homozygotes received an orthotopic liver transplant at a median age of 16.2 (range 4.7-23.2) years (Fig. 4). Fifteen allografts were from deceased unrelated donors; 2 patients were successfully “domino” grafted from living donors homozygous for BCKDHA c.1312T>A, undergoing liver transplantation for treatment of classical maple syrup urine disease(21) Serum BT decreased precipitously within days after transplant and remained stable thereafter. Based on 113 samples from 17 individuals, the mean posttransplant BT was 0.8 ± 0.5 (range 0.1-3.5) mg/dL (Table 3). One transplanted patient died from unrelated circumstances. Sixteen remaining patients are alive and well, with posttransplant graft and patient survival of 100% over 0.1-14.9 (median 7.6) years of follow-up.
Discussion
BILIRUBIN BIODISTRIBUTION AND TISSUE LOADING
The distribution of unconjugated bilirubin between intravascular and extravascular compartments is a critical determinant of potential toxicity that depends on the interaction between bilirubin and albumin. In CN1 patients, the bilirubin–albumin complex behaves as predicted (Eq. 3), and the correlation between Bf and BT/A is governed by an average equilibrium association binding constant (118 ± 57 L/μmol) remarkably close to that observed in term newborns (101 ± 36 L/μmol). This allows BT/A to serve as a surrogate for Bf. Using aggregate longitudinal data for all CN1 patients, BT/A ≥ 1.0 mol:mol (PPV 83.3%; LRD 473; n = 481) is superior to BT ≥ 30 mg/dL (PPV 35.7%; LRD 70; n = 646) as an index of neurological risk, but provides no predictive advantage over BT alone in newborns (Supporting Table S1).(20)
The coefficient of variation for K between individuals ranges from 36% (term newborns) to 60% (preterm). Such variability can account for 10-fold differences in Bf at any given BT/A (Fig. 3) and ultimately limits the predictive value of both BT and BT/A across clinical populations. Albumin and K values are especially low in preterm babies (Table 2), explaining why BT levels as low as 8.5 mg/dL (145 μmol/L) can cause kernicterus in this vulnerable group. Predicting the relationship among BT, Bf, and kernicterus is further complicated by an unquantified element of tissue exposure. As bilirubin circulates through the brain, some proportion is continually “stripped” from albumin by cerebral membranes.(22) For any particular set of BT, BT/A, and Bf values, more pigment deposits in the brain when cerebral blood flow is high, bilirubin dissociates rapidly from albumin, or blood moves slowly through tissue capillaries.
In experimental animals, cerebral bilirubin uptake is typically 30%-50% lower than predicted by mathematical models.(22) One reason for this discrepancy is extrusion of bilirubin by adenosine triphosphate–dependent drug efflux pumps expressed at the blood–brain barrier (e.g., ABCB1 [i.e., P-glycoprotein, MDR1]),(23) a capacity that is low in newborn brain,(24) matures postnatally,(25) and may adapt to chronic bilirubin exposure.(26) In CN1 patients exposed to high BT concentrations for decades, the ability of cerebral endothelia to extrude bilirubin back to the intravascular space might safeguard the brain against chronic toxicity. This underscores the primacy of tissue bilirubin load, not its intravascular concentration, as the ultimate determinant of neuronal injury (Fig. 2). Unfortunately, the former cannot be measured in vivo.
RISK FACTORS FOR KERNICTERUS
In newborns with CN1, the kinetics of postnatal bilirubin tissue loading is relatively predictable (Fig. 2): BT increases 4.3 ± 1.1 (range 2.2-6.0) mg/dL per day to saturate intravascular binding capacity between 5 and 10 postnatal days of age; thereafter, blood albumin concentration sets an upper limit on measured BT concentration as unmeasured pigment continues to accumulate in the brain and other tissues.(1,13) Knowledge of the UGT1A1 c.222C>A founder allele allows us to anticipate tissue bilirubin loading in high-risk newborns (Table 4), but this advantage is offset by socioeconomic challenges. Most Plain families are uninsured, live in rural areas, and deliver their children at home. Some affected neonates are not seen by a physician for several postnatal weeks, during which jaundice is monitored by the unreliable practice of visual inspection.(27) Thus, within our cohort, each case of perinatal kernicterus befell unsuspecting parents and could have been prevented by systematic bilirubin screening within the first week of life.(28)
TABLE 4.
Crigler-Najjar Syndrome Outcomes: Temporal and Geographical Patterns Over 7 Decades
| Outcomes (% Affected) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cohort | Ref.# | Nationality | Year | N | Brain Injury | Liver Transplant | ET or PP | Death |
| Crigler and Najjar* | 1 | United States | 1952 | 7 | 100% | 0% | 0% | 100% |
| van der Veere et al.† | 42 | International | 1996 | 57 | 26% | 37% | nr | 9% |
| Suresh and Lucey‡ | 43, 44 | International | 1997 | 42 | 23% | 36% | 28% | 0% |
| Shevell et al.§ | 45 | International | 1998 | 63 | 59% | 8% | 10% | nr |
| Nazer et al.∥ | 46 | Saudi Arabia | 1998 | 12 | 50% | 17% | 50% | 8% |
| Aloulou et al.¶ | 47 | Tunisia | 2010 | 30 | 93% | 0% | 0% | nr |
| Present study# | na | United States | 2019 | 28 | 18% | 61% | 7% | 7% |
| Total N/average value** | 239 | 45% | 27% | 19% | 8% | |||
| Range | 7-63 | 18%-100% | 0%-63% | 0%-50% | 0%-100% | |||
Crigler and Najjar’s original report preceded the advent of exchange transfusion, plasmapheresis, phototherapy, and liver transplantation for treatment of Crigler-Najjar syndrome.
Brain injury patients (n = 8) were transplanted later (14.3 ± 5.9 years) than those without brain damage (n = 13, 5.9 ± 5.4 years).
Information is compiled from an international questionnaire involving patients from four continents. There is patient overlap with other studies, including our Amish cohort (12 of the 42 patients reported). In the non-Amish subgroup, neurological disability was present in 9 of 30 patients (30%). The mean ± SD bilirubin values (in mg/dL) varied by subgroup: neonatal peak 26.6 ± 5.8, neonatal typical 19.8 ± 4.5, postnatal typical 20.5 ± 5.
The 59% incidence of neurological injury is based on an extensive review of 37 published articles (1952-1997) by these authors, who further subdivided adverse outcomes into four discrete patterns of disability.
Information from 5 affected siblings, not included in the 12-patient cohort, is included in the paper by Nazer et al. All 5 died with bilirubin-induced brain injury, resulting in a total brain injury rate of 10 of 17 (59%) and a disease-related mortality of 38%. Four patients required more than one exchange transfusion, for a total of 13 exchange procedures among 6 of the 12 patients reported.
Exchange transfusion and liver transplantation were not treatment options, and local resources did not support the use of high-intensity phototherapy at home.
Prior to coming under our care, 2 patients in the cohort required exchange transfusion as neonates and 1 developed kernicterus. However, these outcomes were not observed for patients under our care from 1989 to present.
Average values are calculated from a denominator of 232 (rather than 239), accounting for the limited therapeutic options available in 1952.
Abbreviations: ET, exchange transfusion; na, not applicable; nr, not reported; PP, plasmapheresis.
Intercurrent illness represents a special threat to CN1 patients of all ages, as vividly illustrated by a fatal case of kernicterus in a 7-year-old boy. While infected with Streptococcus, his BT increased 2-fold, albumin decreased 30%, and the BT/A ratio increased from 0.50 to 1.52 mol:mol. Under such conditions, a BT of only 23 mg/dL (390 μmol/L) is sufficient to saturate intravascular binding and increase Bf to its aqueous solubility limit. At the measured BT of 39 mg/dL (438 μmol/L), Bf was likely supersaturated in plasma, driving massive pigment deposition in the brain.(19)
Such biochemical changes associated with the normal acute phase response can be catastrophic in patients with CN1. Inflammatory states are characterized by shortened erythrocyte survival, contraction of plasma volume, and reduced enteral intake (which decreases enterohepatic flow; Fig. 1), all of which increase circulating BT. Albumin is a “negative” acute phase protein, decreasing 10%-40% in response to diverse inflammatory stimuli.(29) Thus, during infectious illnesses, CN1 patients commonly experience a 25%-50% increase of BT accompanied by hypoalbuminemia.
In hospitalized patients with severe hyperbilirubinemia, we treat hypoalbuminemia with intravenous albumin infusions (1-2 g/kg per dose up to every 6 hours) (Table 1). In principle, each gram of albumin per kilogram of body weight should increase the plasma concentration about 1.25 g/dL (assuming a circulating blood volume of 80 mL/kg). The observed increase is consistently lower, likely reflecting some albumin escape into the extravascular space as well as expansion of plasma volume driven by oncotic pressure. In the clinical setting, each albumin infusion reliably decreases BT/A by 10%-20%, but is commonly followed by a transient increase of BT caused by redistribution of tissue-bound pigment to intravascular binding sites.
Certain drugs and exogenous ligands administered during illness also have the potential to precipitate kernicterus. This was first dramatically demonstrated in 1956, when 193 low-birth-weight infants were randomly assigned to receive antibacterial prophylaxis with either oxytetracycline or penicillin-sulfisoxazole.(30) Mortality was more than 2-fold higher in the latter group, attributable not to infections but to a higher incidence of kernicterus observed in postmortem tissue (43.2% vs. 4.5%). Experimental studies subsequently showed that sulfisoxazole competitively displaces bilirubin from intravascular to extravascular sites,(31) and when administered to jaundiced Gunn rats, decreases intravascular BT, exacerbates cerebral staining, and increases mortality.(32) A number of exogenous ligands (most notably antibiotics, drug preservatives, and intravenous contrast agents) interfere with bilirubin–albumin binding and can precipitate neurological injury in any jaundiced patient (Supporting Table S2).
PHOTOTHERAPY
A variety of contemporary phototherapy systems provide high irradiance over a large body surface and, if properly used, afford CN1 patients long-term protection from neurological injury. To optimize phototherapy, any apparatus should be placed as close as tolerable to maximal skin surface (Fig. 3) and surrounded everywhere by white or reflective surfaces (Table 1, Supporting Fig. S1). All available systems that provide sufficient irradiance also generate heat, and patients with Crigler-Najjar often require active nocturnal cooling by electric fans or conditioned air. As previously reported,(17) fiber optic systems produce low irradiance compared with fluorescent BB and LED sources and are not appropriate for treatment of serious hyperbilirubinemia.
Commercial phototherapy systems are expensive and difficult to procure in resource-limited settings. The fluorescent BB unit can be constructed for under US $500 in materials, generates irradiance values equal to or greater than most commercial systems,(17) and has been life-saving for patients with restricted access to medical technology. However, it is large, rigid, and heavy, and uses a light source that decays rapidly with use. Newer systems exploit the higher efficiency and irradiance of LEDs. The full-size (5-panel) LED phototherapy unit costs only about US $1,000 in materials and allows better and tighter control of serum BT levels with lower overall system maintenance (Fig. 3). The LED system is also lighter, cooler, and features a modular design that enables size-matching and portability.
Our data reveal no serious health consequences for individuals exposed to more than 100,000 hours of high-intensity phototherapy over their lifetime. Phototherapy dependence primarily interferes with lifestyle and interpersonal relationships. All high-irradiance light units are cumbersome and require a dedicated power source, limiting opportunities for travel. As CN1 patients mature, nocturnal phototherapy is particularly obstructive to the formation of intimate relationships.
LIVER TRANSPLANTATION
Transplantation of allogeneic liver tissue introduces nearly 100% functional enzyme activity on a whole-body basis, reduces BT into the normal range, and affords decisive protection from kernicterus. Because only 30% or less of hepatic UGT1A1 activity is required to maintain normal bilirubin homeostasis,(33) tissue segments from related (i.e., heterozygous) living donors are sufficient to correct hyperbilirubinemia.(34) Interestingly, 2 patients from our cohort were successfully domino grafted from BCKDHA c.1312T>A homozygotes who underwent liver transplantation for treatment of classical maple syrup urine disease (MSUD). This strategy works because of the large reservoir of branched-chain ketoacid dehydrogenase activity in muscle.(35)
Patients with Crigler-Najjar were in a larger cohort of 115 individuals transplanted between 1997 and the present for various metabolic indications (CN1, n = 17; MSUD, n = 93; progressive familial intrahepatic cholestasis type 1, n = 3; S-adenosylhomocysteine hydrolase deficiency, n = 1; GM3 synthase deficiency, n = 1) as part of a broader collaboration between CSC and UPMC Children’s Hospital of Pittsburgh. Excluding 1 individual who died of unrelated causes, overall patient and graft survival were 100% and 99.1%, respectively, and rates of early postoperative complications (5%-34%) were similar to those reported by others.(36) Among published cases, the overall complication rate is higher (40%-83%) with partial auxiliary grafts, some of which atrophy over time.(37)
Progressive hepatopathy might ultimately necessitate liver transplantation or some other definitive intervention in most CN1 patients (Fig. 3). The causal relationship of hyperbilirubinemia to liver disease is not completely understood. Chronic cholangiopathy has been associated with hepatic fibrosis in other clinical settings,(38) whereas more recent data suggest that BT and/or higher-order heme degradation products might be cytotoxic to certain nonneuronal cells.(39) Two oxidation products (Z-BOX A and Z-BOX B) produced during heme degradation are about 20-fold elevated in serum of both hyperbilirubinemic Gunn rats and humans with cholestasis.(40) In cultured hepatocytes, high concentrations of Z-BOX A and B differentially modulate two nuclear receptor transcription factors (NR1D1 and NR1D2), deplete cells of reduced glutathione, and activate cytoskeletal remodeling, indicating a potential role in the hepatopathy of CN1.
In conclusion, 67 years following its discovery, Crigler-Najjar syndrome remains a morbid and potentially fatal disorder (Table 4).(1,41-46) In developed nations, brain injury occurs in 18%-26% of patients with Crigler-Najjar, a figure that has remained essentially unchanged during the last 2 decades. Kernicterus rates are higher (50%-93%) in countries where medical resources are scarce and home phototherapy is difficult to procure. This same pattern of disparity applies to liver transplantation, which is only available to a small number of patients (0%-17%) who live in developing nations.
Although liver transplantation represents an effective form of “gene therapy” for CN1, it entails well-known surgical, infectious, and malignant risks. Emerging therapies hold promise. In transgenic animal models of CN1, repeated intravenous administration of UGT1A1 mRNA rescues a lethal phenotype and normalizes plasma BT, whereas a single injection of liver-specific adeno-associated virus (AAV) type 9 containing human UGT1A1 controls BT for life.(33,47) However, normal liver growth (e.g., from about 120 g to 1,400 g in humans) dilutes out nonreplicating transgenes delivered by AAV(48); thus, genome editing might prove a more durable solution for very young patients.
Supplementary Material
Acknowledgments:
This paper is dedicated to Drs. John Fielding Crigler, Jr. (1919-2018) and Victor Assad Najjar (1914-2002) and the children and families we serve. The authors thank Dr. D. Holmes Morton for his invaluable contributions to the design and implementation of treatment strategies for CN1.
Abbreviations:
- ANOVA
analysis of variance
- BB
Billi Blue
- CN1
Crigler-Najjar type 1 syndrome
- CSC
Clinic for Special Children
- LEDs
light-emitting diodes
- LRD
likelihood ratio for disease
- MW
molecular weight
- PPV
positive predictive value
- UGT1A1
uridine 5′-diphosphate glucuronyltransferase
Footnotes
Potential conflict of interest: The Clinic for Special Children received grants from Audentes.
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.30959/suppinfo.
REFERENCES
- 1).Crigler JF Jr., Najjar VA. Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics 1952;10:169–180. [PubMed] [Google Scholar]
- 2).Mackenzie PI, Owens IS, Burchell B, Bock KW, Bairoch A, Belanger A, et al. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 1997;7:255–269. [DOI] [PubMed] [Google Scholar]
- 3).Brodersen R, Tygstrup N. Specific determination of serum bilirubin diglucuronide in patients with hepato-biliary diseases. Scand J Clin Lab Invest 1969;23:55–58. [DOI] [PubMed] [Google Scholar]
- 4).Berk PD, Howe RB, Bloomer JR, Berlin NI. Studies of bilirubin kinetics in normal adults. J Clin Invest 1969;48:2176–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Brodersen R, Stern L. Deposition of bilirubin acid in the central nervous system—a hypothesis for the development of kernicterus. Acta Paediatr Scand 1990;79:12–19. [DOI] [PubMed] [Google Scholar]
- 6).Wennberg RP, Ahlfors CE, Rasmussen LF. The pathochemistry of kernicterus. Early Hum Dev 1979;3:353–372. [DOI] [PubMed] [Google Scholar]
- 7).Jacobsen J, Brodersen R. Albumin-bilirubin binding mechanism. J Biol Chem 1983;258:6319–6326. [PubMed] [Google Scholar]
- 8).Wennberg RP. The blood-brain barrier and bilirubin encephalopathy. Cell Mol Neurobiol 2000;20:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Katoh-Semba R, Kashiwamata S. Interaction of bilirubin with brain capillaries and its toxicity. Biochim Biophys Acta 1980;632:290–297. [DOI] [PubMed] [Google Scholar]
- 10).Roger C, Koziel V, Vert P, Nehlig A. Mapping of the consequences of bilirubin exposure in the immature rat: local cerebral metabolic rates for glucose during moderate and severe hyperbilirubinemia. Early Hum Dev 1995;43:133–144. [DOI] [PubMed] [Google Scholar]
- 11).Waters WJ. The protective action of albumin in bilirubin toxicity in newborn puppies. In: Sass-Kortsak A, ed. Kernicterus. Toronto: University of Toronto Press; 1961:219–221. [Google Scholar]
- 12).Ahlfors CE, Amin SB, Parker AE. Unbound bilirubin predicts abnormal automated auditory brainstem response in a diverse newborn population. J Perinatol 2009;29:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Ahlfors CE, Wennberg RP, Ostrow JD, Tiribelli C. Unbound (free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clin Chem 2009;55:1288–1299. [DOI] [PubMed] [Google Scholar]
- 14).Stern L, Brodersen R. Kernicterus research and the basic sciences: a prospect for future development. Pediatrics 1987;79:154–156. [PubMed] [Google Scholar]
- 15).Lucey J, Ferriero M, Hewitt J. Prevention of hyperbilirubinemia of prematurity by phototherapy. Pediatrics 1968;41:1047–1054. [PubMed] [Google Scholar]
- 16).Cremer RJ, Perryman PW, Richards DH. Influence of light on the hyperbilirubinaemia of infants. Lancet 1958;1:1094–1097. [DOI] [PubMed] [Google Scholar]
- 17).Strauss KA, Robinson DL, Vreman HJ, Puffenberger EG, Hart G, Morton DH. Management of hyperbilirubinemia and prevention of kernicterus in 20 patients with Crigler-Najjar disease. Eur J Pediatr 2006;165:306–319. [DOI] [PubMed] [Google Scholar]
- 18).Ahlfors CE. Effect of serum dilution on apparent unbound bilirubin concentration as measured by the peroxidase method. Clin Chem 1981;27:692–696. [PubMed] [Google Scholar]
- 19).Ahlfors CE, Herbsman O. Unbound bilirubin in a term newborn with kernicterus. Pediatrics 2003;111:1110–1112. [DOI] [PubMed] [Google Scholar]
- 20).Iskander I, Gamaleldin R, El Houchi S, El Shenawy A, Seoud I, El Gharbawi N, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics 2014;134:e1330–e1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Khanna A, Hart M, Nyhan WL, Hassanein T, Panyard-Davis J, Barshop BA. Domino liver transplantation in maple syrup urine disease. Liver Transpl 2006;12:876–882. [DOI] [PubMed] [Google Scholar]
- 22).Robinson PJ, Rapoport SI. Binding effect of albumin on uptake of bilirubin by brain. Pediatrics 1987;79:553–558. [PubMed] [Google Scholar]
- 23).Bockor L, Bortolussi G, Vodret S, Iaconcig A, Jasprova J, Zelenka J, et al. Modulation of bilirubin neurotoxicity by the Abcb1 transporter in the Ugt1−/− lethal mouse model of neonatal hyperbilirubinemia. Hum Mol Genet 2017;26:145–157. [DOI] [PubMed] [Google Scholar]
- 24).Lam J, Baello S, Iqbal M, Kelly LE, Shannon PT, Chitayat D, et al. The ontogeny of P-glycoprotein in the developing human blood-brain barrier: implication for opioid toxicity in neonates. Pediatr Res 2015;78:417–421. [DOI] [PubMed] [Google Scholar]
- 25).Daood M, Tsai C, Ahdab-Barmada M, Watchko JF. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 2008;39:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Gazzin S, Berengeno AL, Strazielle N, Fazzari F, Raseni A, Ostrow JD, et al. Modulation of Mrp1 (ABCc1) and Pgp (ABCb1) by bilirubin at the blood-CSF and blood-brain barriers in the Gunn rat. PLoS One 2011;6:e16165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Moyer VA, Ahn C, Sneed S. Accuracy of clinical judgment in neonatal jaundice. Arch Pediatr Adolesc Med 2000;154: 391–394. [DOI] [PubMed] [Google Scholar]
- 28).Bhutani VK, Johnson LH, Jeffrey Maisels M, Newman TB, Phibbs C, Stark AR, et al. Kernicterus: epidemiological strategies for its prevention through systems-based approaches. J Perinatol 2004;24:650–662. [DOI] [PubMed] [Google Scholar]
- 29).Liao WS, Jefferson LS, Taylor JM. Changes in plasma albumin concentration, synthesis rate, and mRNA level during acute inflammation. Am J Physiol 1986;251:C928–C934. [DOI] [PubMed] [Google Scholar]
- 30).Andersen DH, Blanc WA, Crozier DN, Silverman WA. A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic antibacterial regimens. Pediatrics 1956;18:614–625. [PubMed] [Google Scholar]
- 31).Odell GB. The dissociation of bilirubin from albumin and its clinical implications. J Pediatr 1959;55:268–279. [DOI] [PubMed] [Google Scholar]
- 32).Blanc WA, Johnson L. Studies on kernicterus; relationship with sulfonamide intoxication, report on kernicterus in rats with glucuronyl transferase deficiency and review of pathogenesis. J Neuropathol Exp Neurol 1959;18:165–189; discussion 187–169. [DOI] [PubMed] [Google Scholar]
- 33).Bortolussi G, Zentillin L, Vanikova J, Bockor L, Bellarosa C, Mancarella A, et al. Life-long correction of hyperbilirubinemia with a neonatal liver-specific AAV-mediated gene transfer in a lethal mouse model of Crigler-Najjar Syndrome. Hum Gene Ther 2014;25:844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Ozcay F, Alehan F, Sevmis S, Karakayali H, Moray G, Torgay A, et al. Living related liver transplantation in Crigler-Najjar syndrome type 1. Transplant Proc 2009;41:2875–2877. [DOI] [PubMed] [Google Scholar]
- 35).Mazariegos GV, Morton DH, Sindhi R, Soltys K, Nayyar N, Bond G, et al. Liver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative United Network for Organ Sharing experience. J Pediatr 2012;160:116–121,e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Pett S, Mowat AP. Crigler-Najjar syndrome types I and II. Clinical experience—King’s College Hospital 1972–1978. Phenobarbitone, phototherapy and liver transplantation. Mol Aspects Med 1987;9:473–482. [DOI] [PubMed] [Google Scholar]
- 37).Rela M, Muiesan P, Vilca-Melendez H, Dhawan A, Baker A, Mieli-Vergani G, et al. Auxiliary partial orthotopic liver transplantation for Crigler-Najjar syndrome type I. Ann Surg 1999;229:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Mitchell E, Ranganathan S, McKiernan P, Squires RH, Strauss K, Soltys K, et al. Hepatic parenchymal injury in Crigler-Najjar type I. J Pediatr Gastroenterol Nutr 2018;66:588–594. [DOI] [PubMed] [Google Scholar]
- 39).Elias MM, Comin EJ, Grosman ME, Galeazzi SA, Rodriguez Garay EA. Possible mechanism of unconjugated bilirubin toxicity on renal tissue. Comp Biochem Physiol A Comp Physiol 1987;87:1003–1007. [DOI] [PubMed] [Google Scholar]
- 40).Seidel RA, Claudel T, Schleser FA, Ojha NK, Westerhausen M, Nietzsche S, et al. Impact of higher-order heme degradation products on hepatic function and hemodynamics. J Hepatol 2017;67:272–281. [DOI] [PubMed] [Google Scholar]
- 41).van der Veere CN, Sinaasappel M, McDonagh AF, Rosenthal P, Labrune P, Odievre M, et al. Current therapy for Crigler-Najjar syndrome type 1: report of a world registry. Hepatology 1996;24:311–315. [DOI] [PubMed] [Google Scholar]
- 42).Lucey JF, Suresh GK, Kappas A. Crigler-Najjar syndrome, 1952–2000: learning from parents and patients about a very rare disease and using the internet to recruit patients for studies. Pediatrics 2000;105:1152–1153. [DOI] [PubMed] [Google Scholar]
- 43).Suresh G, Lucey JF. Lack of deafness in Crigler-Najjar syndrome type 1: a patient survey. Pediatrics 1997;100:E9. [DOI] [PubMed] [Google Scholar]
- 44).Shevell MI, Majnemer A, Schiff D. Neurologic perspectives of Crigler-Najjar syndrome type I. J Child Neurol 1998;13:265–269. [DOI] [PubMed] [Google Scholar]
- 45).Nazer H, Al-Mehaidib A, Shabib S, Ali MA. Crigler-Najjar syndrome in Saudi Arabia. Am J Med Genet 1998;79: 12–15. [DOI] [PubMed] [Google Scholar]
- 46).Aloulou H, Ben Thabet A, Khanfir S, Ben Mansour L, Chabchoub I, Labrune P, et al. Type I Crigler Najjar syndrome in Tunisia: a study of 30 cases. Tunis Med 2010;88:707–709. [PubMed] [Google Scholar]
- 47).Apgar JF, Tang JP, Singh P, Balasubramanian N, Burke J, Hodges MR, et al. Quantitative Systems Pharmacology Model of hUG-T1A1-modRNA encoding for the UGT1A1 enzyme to treat Crigler-Najjar Syndrome type 1. CPT Pharmacometrics Syst Pharmacol 2018;7:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Villiger L, Grisch-Chan HM, Lindsay H, Ringnalda F, Pogliano CB, Allegri G, et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat Med 2018;24:1519–1525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.