Abstract
Background -
Pulsed field ablation (PFA) is a nonthermal energy with potential safety advantages over radiofrequency ablation (RFA). This study investigated a novel PFA system- a circular multielectrode catheter (“PFA lasso”) and a multichannel generator designed to work with Carto 3® mapping system.
Methods -
A 7.5F bidirectional circular catheter with 10 electrodes and variable expansion was designed for PFA (biphasic, 1800 Volts). This study included a total of 16 swine utilized to investigate the following 3 experimental aims: Aim 1 examined the feasibility to create a right atrial ablation line of block from the superior vena cava (SVC) to the inferior vena cava (IVC). Aim 2 examined the effect of PFA on lesion maturation including durability after a 30-day survival period. Aim 3 examined the effect of high intensity PFA (10 applications) on esophageal and phrenic nerve tissue in comparison to normal intensity RFA (1–2 applications). Histopathological analysis of all cardiac, esophageal and phrenic nerve tissue was performed.
Results -
Acute line of block was achieved in 12/12 swine (100%) and required a total PFA time of 14 sec (IQR:9–24.5) per line. Ablation line durability after 28±3 days was maintained in 11/12 (91.7%) swine. PFA resulted in transmural lesions in 179/183 (97.8%) sections and a median lesion width of 14.2mm. High intensity PFA (9 [IQR:8–14] application) had no effect on the esophagus while standard intensity RFA (1.5 [IQR:1–2] applications) resulted in deep esophageal tissue injury involving the muscularis propria and adventitia layers. High intensity PFA (16 [IQR:10–28] applications) has no effect on phrenic nerve function and structure while standard dose RFA (1.5 [IQR:1–2] applications) resulted in acute phrenic nerve paralysis.
Conclusions -
In this preclinical model, a multielectrode circular catheter and multichannel generator produced durable atrial lesions with lower vulnerability to esophageal or phrenic nerve damage.
Keywords: atrial fibrillation, catheter ablation, ablation, pulmonary vein isolation
Journal Subject Terms: Arrhythmias, Electrophysiology, Catheter Ablation and Implantable Cardioverter-Defibrillator, Translational Studies
Graphical Abstract:

Introduction
Pulsed field ablation (PFA) employs trains of high voltage very-short duration pulses that result in destabilization of the cellular membranes (formation of pores in the cytoplasmic membrane) and death by a mechanism of irreversible electroporation.1 This method has several potential advantages for ablation of cardiac arrhythmias, including higher selectivity to myocardial tissue and smaller thermal effect, reducing the risk for inadvertent injury of blood vessels, nerves, and the esophagus. Pivotal animal studies demonstrated that PFA can produce transmural and durable atrial lesions with minimal effect to the esophagus, phrenic nerve and the coronary arteries.1–4 These potential safety advantages stimulated the interest in PFA as an alternative for radiofrequency ablation (RFA) particularly for atrial fibrillation (AF), where esophageal and phrenic nerve injuries are potential risks.5, 6
Amongst the PFA catheters published to date, the majority are stand-alone ablation catheters without mapping capabilities or integration with electroanatomical mapping (EAM) systems. 7–11
The aim of this study was to investigate the biophysical properties of a novel PFA system comprised on a circular multielectrode catheter and generator designed to work with Carto 3®. We examined its feasibility to create a continuous and transmural line of ablation in the atria, evaluated its durability after 30 days, and compared the vulnerability of the esophagus and phrenic nerve between high-intensity PFA and standard dose RFA.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Animals and Protocols
The study included 16 Yorkshire swine studied under general anesthesia with isoflurane inhalation and mechanical ventilation. The research protocol was approved by the Institutional Animal Care and Use Committee and conformed to the Position of the American Heart Association on Research Animal Use. The study was performed at the Beth Israel Deaconess Medical Center Experimental Electrophysiology Laboratory in Boston, MA, Rambam Hospital in Haifa, Israel and the Absorption Research Laboratory in San Diego, CA.
Experimental Design
The study was designed to answer the following questions: 1) feasibility to create a line of block from the superior vena cava (SVC) to the inferior vena cava (IVC) with PFA delivered from the investigational catheter and generator; 2) examine the effect of PFA on lesion durability after 30-days, and 3) examine the vulnerability of esophageal and phrenic nerve tissue to high-intensity PFA. A total of 16 animals were used to answer these questions and each animal was used for more than one aim in order to minimize animal usage. Supplemental Table 1 details the experiments performed in each animal in regard to each aim of the study.
Pulsed field Ablation System and Catheter
This ablation system includes a novel circular catheter (VARIPULSE™, Biosense Webster, Irvine, California) and a proprietary PFA generator, both compatible with Carto 3® EAM system (Biosense Webster, Irvine, California). The 7.5Fr circular catheter includes 10 electrodes with individual irrigation pores (Figure 1). The circular “PFA lasso” catheter has an adjustable diameter between 25 and 35mm allowing positioning of the catheter over a wide range of pulmonary vein ostia sizes. The catheter is bidirectional with 1800 deflection to one side and 900 to the other in order to facilitate engagement to all PVs, including the right inferior PV. PFA is applied in a bipolar configuration between skipped electrodes (i.e. electrode 1 to electrode 3) and between each of the adjacent electrodes between them (i.e. electrodes 1–2 and 2–3) with an energy of 1800V. Each PFA application includes trains of microsecond-long biphasic pulses between all three bipolar configurations for a total application duration of ~250 microseconds. The irrigation flow rate during idle as well as ablation is 4mL/min. All procedures were performed under anticoagulation with heparin and activated clotting time range of 300–400sec.
Figure 1:

Description of the Catheter. The investigational catheter is a circular multielectrode catheter with variable expansion ranging from 25 to 35mm. It includes 10 electrodes capable of pulsed field ablation in bipolar configuration. A magnetic sensor allows visualization by the electroanatomical mapping system.
Right atrial line model
In 12 animals, 3 femoral venous sheathes were obtained. A baseline EAM of the right atrium (RA) was created with a Pentaray™ multielectrode mapping catheter (“multielectrode”) and a compatible mapping system (Carto 3®, Biosense Webster, Irvine, California) during atrial pacing from the lateral tricuspid annulus at a cycle length of 500msec as shown in Figure 2. After completion of the baseline map, the investigational ablation catheter was advanced into the RA over a steerable sheath (Vizigo™, Biosense Webster, Irvine CA) and an ablation line was performed along the posterior wall from the superior vena cava (SVC) to the inferior vena cava (IVC) by positioning the investigational catheter on the line and applying pulsed field energy in a point-by-point fashion as shown in Supplementary Figure 1. Tissue contact was evaluated by electrogram shape (i.e. near field electrograms), proximity to the preacquired anatomical shell, and intracardiac echocardiography (ICE; Acuson Acunav™; Siemens, Mountain View, CA). Since pulsed field is created by direct current, there is concern for possible arcing or electrical sparking, a well-known phenomenon of shock-wave related barotrauma that is created by rapid gas formation caused by electrical polarity at the site of ablation. Therefore, visualization with ICE was attempted for each PFA application to evaluate presence or absence of bubbles as a marker for electrical sparking which may occur with PFA.
Figure 2:

Feasibility and Durability of a Right Atrial Ablation Line. The experimental model included a baseline activation map of the right atrium during pacing from the lateral tricuspid annulus (left panel). Pulsed field ablation was then performed along the posterior wall from the superior to the inferior vena cava. The presence or absence of activation line of block was examined by repeat mapping of the chamber during pacing from a similar location, immediately (middle panel) and 30-days after ablation (right panel). Note the presence of a durable block.
Upon completion of the ablation line, the chamber was similarly remapped with a multielectrode mapping catheter and during atrial pacing from the lateral tricuspid annulus. This location was chosen to facilitate evaluation of the conduction across the adjacent posterior ablation line. The effect of PFA was evaluated by the following criteria: 1) bipolar voltage amplitude <0.1mV previously validated as a surrogate for transmural lesions in the swine’s posterior wall 12, 13); 2) activation map demonstrating a line of block; and 3) lack of capture during pacing of the entire length of the line at a strength and duration of 10mA and 5msec, respectively. In the case of incomplete ablation, defined as conduction through the ablation line, ablation was repeated in the gap site(s).
After the ablation procedures, animals were recovered and survived for a period of 4 weeks before a repeat mapping study using the same methodology – i.e., mapping with a multielectrode mapping catheter during atrial pacing from the lateral tricuspid annulus. Similar criteria were used to evaluate the ablation line (voltage amplitude<0.1mV, conduction line of block, and lack of capture with pacing the entire length of the line).
Ablation line dimensions were measured on the EAM system immediately after ablation as well as after the survival period using a bipolar voltage amplitude threshold of 0.1mV. The length of the line was measured from the most superior anatomic point of voltage abnormality to the most inferior continuous voltage abnormality, and the width of the line was measured as the mean of multiple measurements at 5mm intervals along the line.
The Effect of PFA on the Esophagus
The effect of PFA on the esophagus was examined in 6 swine. In these species, the right inferior pulmonary vein (RIPV) is consistently adjacent to the esophagus, particularly at a location 2–4cm distal to the ostium of the vein. At this location, the distance between the PV and the esophagus is usually <5mm. Therefore, the left atrium (LA) including the RIPV were mapped using the multielectrode mapping catheter. The esophagus was also mapped using a Thermocool™ catheter (Biosense Webster, Irvine, California) and the location of closest proximity between the RIPV and the esophagus was selected for ablation. At this location, high intensity PFA 9 sequential applications without recovery period were performed in order to evaluate the effect of PFA on the esophagus. Animals were then recovered and survived for a period of 4 weeks before euthanasia and histopathological analysis. During the survival period, animals were monitored for fever, pain, appetite, indigestion, and any other unusual signs indicative of poor well-being. To compare the effect of PFA to RFA in this model of esophageal injury, in 2 additional animals, 1–2 RFA application were applied to the RIPV using a 3.5mm irrigated-tip catheter (Thermocool™ STSF, Biosense Webster) at power setting of 40Watts for 30sec. Because the effect of RFA on the esophagus has been previously studied and in order to minimize unnecessary animal suffering, these swine were euthanized immediately after ablation and the esophagus was examined following acute RFA.
The Effect of PFA on the Phrenic Nerve
The effect of PFA on the phrenic nerve was examined in 10 swine. The course of the right phrenic nerve from the SVC to the IVC was determined by pacing with diaphragmatic response, with tags placed on the EAM to mark the course of the phrenic nerve. Multiple PFA applications were delivered over the marked course of the phrenic nerve. Acute phrenic nerve injury was evaluated immediately after ablation by pacing at a similar strength and duration (10mA and 5msec). Chronic phrenic nerve injury was evaluated after a 4-week survival period. The effect of RFA on phrenic nerve function in the same model was examined in 2 swine in whom 1–2 RFA applications (40 watts for 30 sec) were applied over the marked course of the intact phrenic nerve during the terminal study. Acute phrenic nerve injury was similarly evaluated by pacing at a similar strength and duration (10mA and 5msec).
Histopathological Analysis
Triphenyl Tetrazolium Chloride (TTC) was infused 15 minutes prior to euthanasia to facilitate a differentiation between metabolically active from inactive tissue. The redox indicator stains red viable tissue while the non-viable tissue remains white. Following euthanasia, the heart-lung-esophagus complex including the phrenic nerve were dissected and excised en bloc. The RA was opened along the antero-medial wall from the SVC to the IVC. The ablation line was inspected, and width measurements were made at 5mm intervals. The tissue was then fixed in a 10% formalin solution for ≥3 days and embedded in paraffin. The tissue was segmented at 5mm intervals perpendicular to the long axis of the SVC-IVC line, and slides were stained with Hematoxylin-Eosin and Masson’s Trichrome. Lesion continuity, transmurality, and depth were measured for each slide by a pathologist blinded to the intervention. The esophagi and phrenic nerves were also inspected and areas with visualized abnormalities were further sectioned for histological analysis.
Statistical Analysis
Normality of continuous variables was assessed by graphic measures due to the small sample size which precludes robust statistical tests of normality. Normally distributed continuous variables are reported as mean with standard deviations, while non-normally distributed variables are summarized by median (25th–75th percentile). Categorical variables were summarized by frequency and percentage. Statistical analyses were performed with Stata/SE, version 16 (StataCorp, College Station, TX).
Results
Table 1 details the biophysical parameters of ablation, including the width and length of the line at the acute and chronic stages.
Table 1:
Biophysical Experimental of Ablation
| N=12 | Acute Study | Chronic Study (28±3 days) |
|---|---|---|
| Total PFA time (sec) | 14 (9–24.5) | N/A |
| Number of applications | 56 (36–98) | N/A |
| Length of Line (mm)* | 82.6(78.4–120.4) | 86.7(79.1–130.5)‡ |
| Width of Line (mm)* | 14.2 (8.4–24.1) | 13.7 (7.3–24.5)‡ |
| Lesion Depth (mm)† | N/A | 2.1 (1.2–3.6) |
| Activation line of block | 12/12 (100%) | 11/12(91.7%) |
| % Transmurality (% of histology slides) | N/A | 179/182(98.3%) |
measured using voltage amplitude cutoff of 0.1mV
measured using histology
indicates that for each animal, the difference was <10mm between the acute and chronic studies.
Feasibility and Durability of Atrial Line with PFA
PFA resulted in immediate attenuation of local electrograms, loss of tissue capture with pacing, and formation of a continuous line of block in all 12 animals. In 11/12 animals (91.7%), the line remained blocked after a 28±3 days of survival period, while one animal had chronic reconnection at the mid-low aspect of the line at an area where catheter contact during ablation was challenging. Figure 2 shows an example of activation map at baseline, immediately after ablation, and following a 30-day survival period, demonstrating presence of conduction block across the ablation line. Figure 3 shows the corresponding voltage maps. Note that voltage amplitude ≤0.1mV is present throughout the length of the line, corresponding to the location of PFA applications, and remained stable in size between the acute and chronic studies. The median length of the ablation line, measured from the most superior to the most inferior application tags was 82.6 mm (78.4–120.4), consistent with the length of voltage abnormality measured immediately and 30-days after ablation (changes <10mm for each animal). The median width of the line measured 14.2mm (8.4–24.1), and this also remained stable between the acute and chronic studies.
Figure 3:
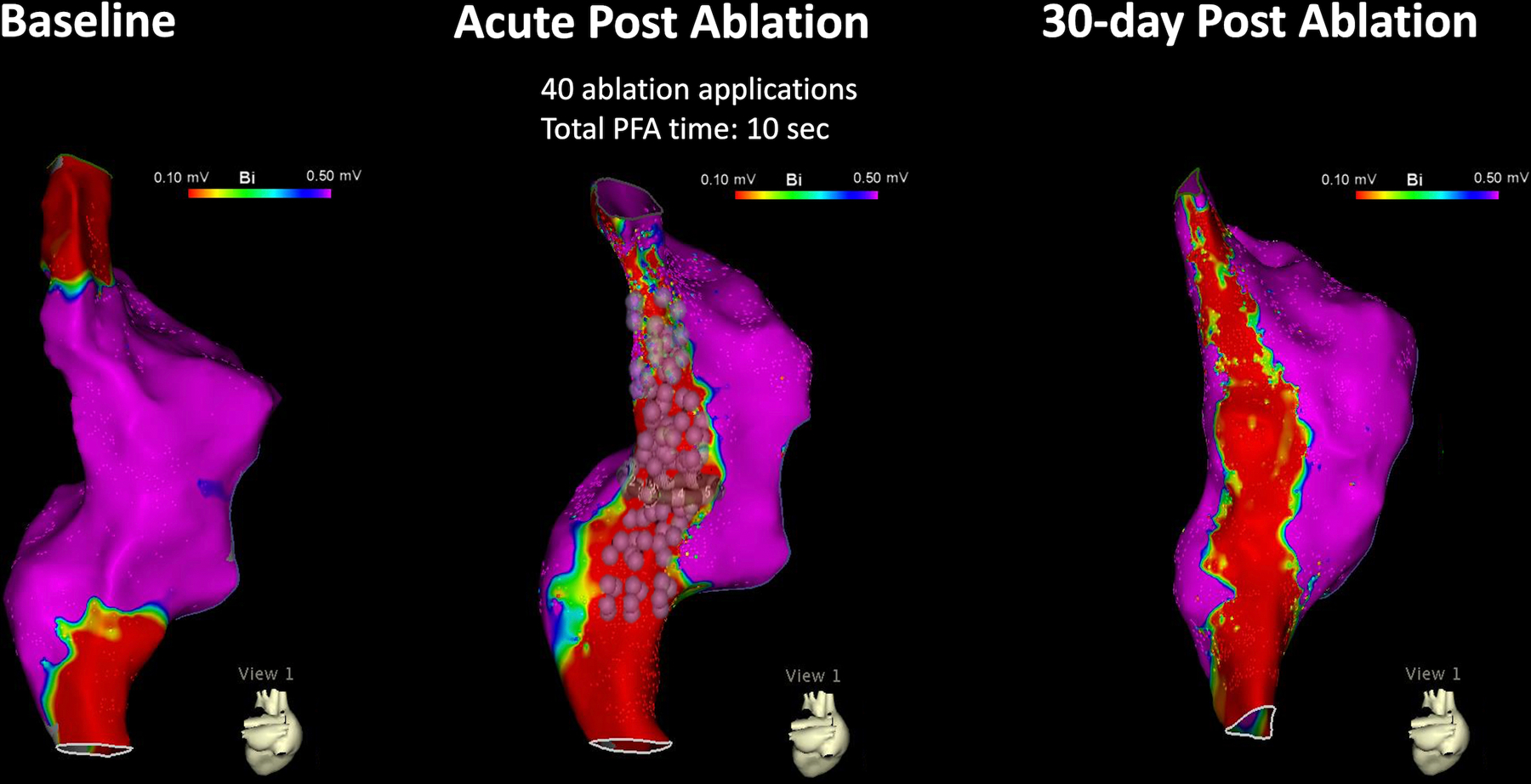
A Time-Dependent Effect of PFA on Voltage Amplitude. This figure shows the bipolar voltage map of the swine depicted in Figure 2. Note a continuous and durable line of voltage amplitude ≤0.1mV corresponding to the location of the ablation tags (each pulsed field ablation application is represented by 3 tags- indicating the participating electrodes).
Each ablation line required a median of 56 applications (36–98) and a total PFA time of 14 sec (9–24.5; Table 1). We suspect that this number of applications was because we utilized a circular catheter to perform a linear ablation line. Furthermore, the number of applications per line decreased as our experience with this ablation model and technique increased.
PFA resulted in immediate attenuation of electrograms as demonstrated in Figure 4. Ablation near the phrenic nerve caused occasional transient diaphragmatic capture that stopped upon completion of the application. There were rare instances of muscle capture during PFA. These were mild, brief and not associated with any particular location. The amount of bubbles visualized with ICE was small and similar before, during and after PFA without evidence for electrical sparking.
Figure 4:

Electrogram Attenuation with Pulsed Field Ablation. Pulsed field ablation was applied between electrodes 4–6 for a duration of ~250 microseconds. During the application period, electrograms (EGMs) are saturated. However, immediately afterward, significant EGM attenuation is observed.
Pathological examination showed a continuous line of transmural fibrosis in 11/12 animals. In these 11 animals, all histological slides, sectioned at 5mm increments, showed transmural fibrosis (171/171; 100%). In one animal with chronic reconnection, an ablation gap measuring 12.6mm in length with non-transmural fibrosis (4/12; 33.3%) was identified at the level of the fossa ovalis (12.6mm out of a total ablation line of 11.3cm). The depth of the line ranged between 1.2–3.6mm (likely an underestimation of the actual ablation effect because collagen replacement with wall thinning). Figure 5 shows a representative example of a gross pathological specimen of the posterior line of ablation with a corresponding histology. Note the clear demarcation of the ablation lesion, its transmurality and lack of PFA effect on nerve fibers embedded in the ablation lesion.
Figure 5:

Histopathological Examination of Pulsed Field Ablation in the Atrium. The left panel shows an example of a pathological specimen of a posterior ablation line demarcated by the dashed lines. The right panel shows histological slides corresponding to the indicated level. The upper panel shows a lower magnification slide in Mason’s Trichrome stain where a blue color indicates collagen. Note replacement of the posterior wall by collagen and fat. The lower panels show higher magnification in mason trichome (left) and Hematoxylins and Eosin (right). Note, the preserved nerve bundles within the ablation lesion. E=endocardium, N=nerve bundle, PW=posterior wall.
The Effect of PFA on the Esophagus
In 6 animals, 9 (8–14) PFA applications were delivered from the RIPV at a median distance of 1.8mm (0.4–2.8) from the esophagus as shown in Figure 6. During the survival period, none of the animals showed signs of acute illness, such as fever, lack of appetite, indigestion or diarrhea. Pathological post-mortem inspection of the entire length of the esophageal adventitia and mucosa layers showed no evidence of injury. In contrast, a single RFA application in one swine and 2 RFA applications in a second swine resulted in acute esophageal injury involving the muscularis propria and adventitia as shown in Figure 6.
Figure 6:
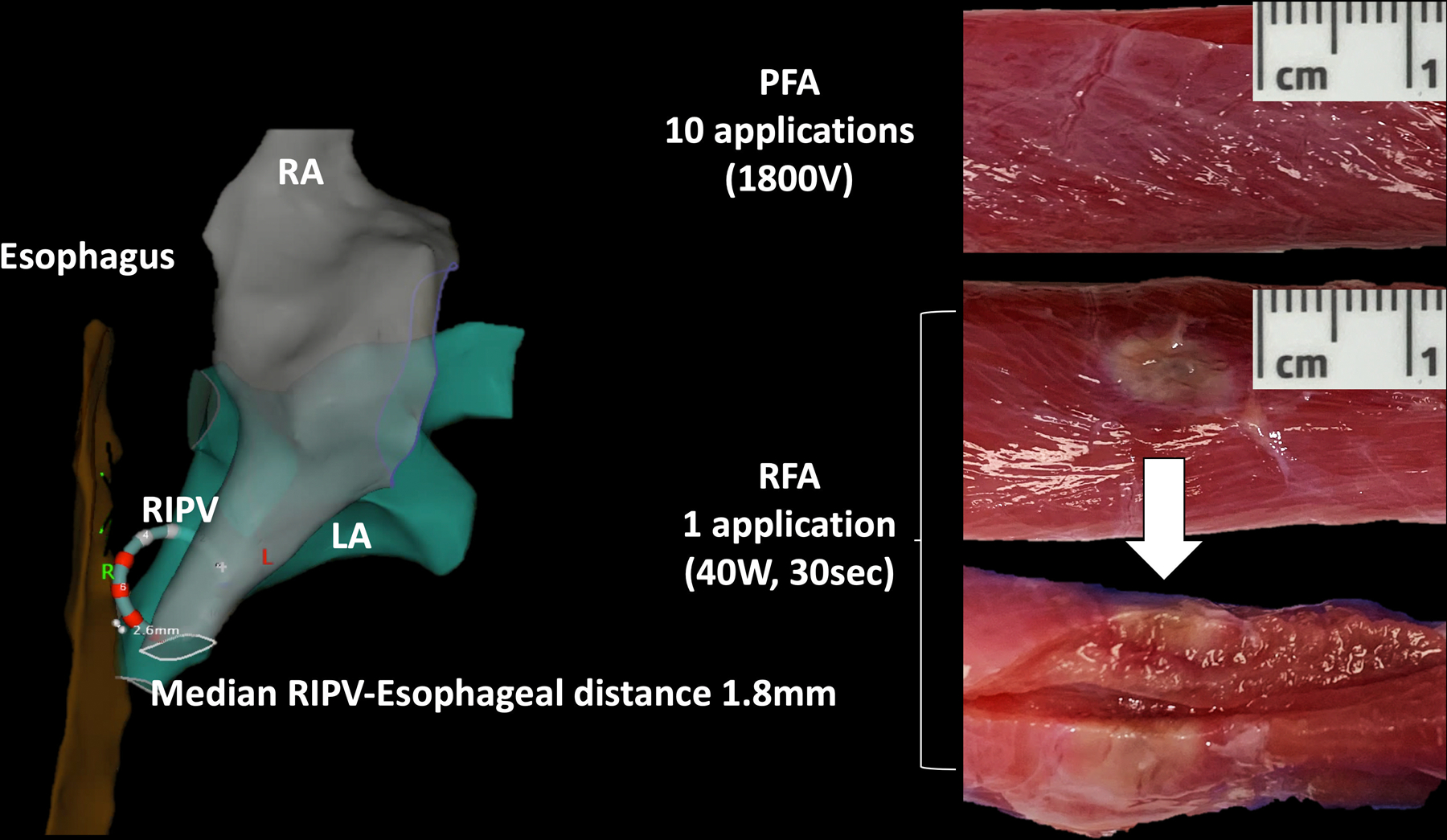
The Effect of Pulsed Field and Radiofrequency Ablation on the Esophagus. The close proximity between the right inferior pulmonary vein (RIPV) and the esophagus in swine served as the basis for comparing the effect of pulsed field ablation (PFA) and radiofrequency ablation (RFA) on the esophagus. The left atrium and RIPV are indicated by the green colored chamber, the esophagus by the brown colored tube, and the right atrium by the gray colored chamber. Insertion of the circular ablation catheter into the RIPV resulted in expansion of the RIPV and juxtaposition between the RIPV and the esophagus (median distance of 1.8mm). PFA had no effect on the esophagus following a 30-day survival period while RFA resulted in acute injury to the muscularis propria.
The Effect of PFA on the Phrenic Nerve
In 10 animals, 16 (10–28) PFA application were delivered over the marked course of the phrenic nerve as shown in Figure 7. Phrenic nerve function was examined immediately after completion of the ablation and again following the survival period. Animals were also clinically monitored for signs of illness or impaired breathing during the survival period. PFA resulted in stimulation of the phrenic nerve during energy delivery, noted by diaphragmatic capture, however, did not impact the nerve’s function after ablation. In all animals, phrenic nerve function was intact immediately after ablation and following the survival period and with no clinical signs of functional injury. In 2 of these swine, 1–2 RFA applications were delivered over the RA course of the phrenic nerve during the chronic remapping study. This resulted in phrenic nerve stimulation during ablation and acute phrenic nerve paralysis following ablation that persisted ≥30 minutes. Figure 7 shows a gross pathological specimen of a phrenic nerve from one of these animals. Note the intact phrenic nerve at sites of PFA in contrast to swelling at a site corresponding to RFA on immediate post-mortem gross pathology.
Figure 7:

The Effect of Pulsed Field and Radiofrequency Ablation on the Phrenic Nerve. The vulnerability of the phrenic nerve to pulsed field ablation (PFA) and radiofrequency ablation (RFA) was examined by ablation in the lateral right atrium overlying the right phrenic nerve (marked by the yellow tags). Multiple PFA applications (purple tags) had no effect on phrenic nerve function or structure. In contrast, 2 RFA applications (red tags) resulted in acute phrenic nerve paralysis. The middle and right panels show the right phrenic nerve with localized swelling and whiting corresponding to sites of RFA.
Discussion
Electroporation is a method of cell membrane permeabilization that is widely used today in biotechnology and medicine for delivery of drugs and genes into living cells. The phenomenon of electroporation causes a dramatic increase in membrane permeability caused by externally applied short and intense electric pulses.14 Various theoretical models were developed to describe electroporation, among which the aqueous pore model is the most widely accepted.15 According to this model, hydrophilic pores are formed in the lipid bilayer of a cell membrane when its membrane is exposed to electric pulses. Because electroporation does not produce significant heat and appears to be more sensitive to cardiomyocytes, it provides an opportunity for creating ablation lesions with limited collateral thermal-induced injuries in comparison to RFA. This premise has stimulated the development of PFA catheter technologies particularly for PVI in which the risk for esophageal and phrenic nerve injuries are relatively high.8–10
In this preclinical study, we examined the safety and efficacy of a circular mapping and ablation catheter (VARIPULSE™) designed for PFA with a commercially available EAM system (Carto 3®). The major findings of this investigation include the following:
Pulsed-field energy delivered from this catheter and generator system resulted in irreversible electroporation with creation of a contiguous and transmural line of block in the atria. The location of the applied energy corresponded to the location of the ablation lesions and their size remained stable throughout the lesion maturation period; providing a predictive and irreversible effect. A bipolar voltage of ≤0.1mV recorded immediately after ablation was predictable of irreversible electroporation.
PFA had no effect on esophageal tissue, phrenic nerve function or structure; extending the cardiac selectivity of PFA to this ablation technology. The protocol showed lower vulnerability to esophageal and phrenic nerve injury compared to other previously reported technologies. High intensity ablation (multiple repetitions of PFA applications) did not result in transient or permanent injury to the esophagus or phrenic nerve.3, 4 The improved safety margin of this protocol may potentially be related to the shorter application duration.
PFA requires proximity with tissue. Immediate electrogram attenuation and lesion durability were larger in applications where the catheter was in proximity with the tissue. Although the proximity between the catheter and the tissue was assessed by electrogram morphology, intracardiac echo and proximity of the catheter to the anatomical shell and not by measured contact force, we suspect that tissue proximity plays a role in PFA. The influence of proximity on lesion formation, including the optimal proximity parameters for PFA are unknown and require further investigations.
This multielectrode catheter is designed for mapping as well as for PF ablation allowing to perform PVI using a single transseptal puncture. Although this study focused on its ablation capabilities, a circular mapping catheter is used routinely for left atrial mapping during PVI and may therefore be time and cost effective.
Limitations
The major limitation of this investigation is inherent to its preclinical animal model. Nevertheless, the fundamental differences between RFA and PFA are likely similar between swine and humans, while dosing may need to be optimized. Another limitation of this study is the lack of contact force (CF) indicator in this experimental catheter. We estimated contact using electrogram morphology and frequency, fluoroscopy and ICE. These are valuable for ablation with this technology.
Conclusion
A PFA system that includes a circular catheter and multichannel generator compatible with a commercially available electroanatomical mapping system produced durable atrial lesions and showed lower vulnerability to esophageal or phrenic nerve damage compared to RFA.
Supplementary Material
What Is Known?
Pulsed field ablation (PFA) is an emerging technology with potential safety advantages over radiofrequency ablation (RFA) for atrial fibrillation (AF) ablation.
Emerging PFA technologies include stand-alone ablation catheters without mapping capabilities or integration with commercially available mapping systems.
What the Study Adds?
This preclinical study examined the safety and efficacy of a circular pulsed field ablation (“lasso PFA”) catheter and multichannel generator compatible with a commercially available electroanatomical mapping system.
Multichannel PFA delivered from this catheter resulted in fast and durable tissue effect- transmural and contiguous atrial lesions.
High intensity PFA had no effect on the esophagus or the phrenic nerve. In comparison, moderate intensity RFA resulted in injury to both structures, validating the better safety profile of PFA for AF ablation.
Sources of Funding:
This study was partially supported by a research grant from Biosense Webster Inc.
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- EAM
Electroanatomical mapping
- H&E
hematoxylin and eosin
- MT
Masson's trichrome
- RFA
Radiofrequency ablation
- PFA
Pulsed Field Ablation
- PVI
Pulmonary vein isolation
- RIPV
Right inferior pulmonary vein
Footnotes
Disclosures: Dr. Anter has received research grants and consultation fees from Biosense Webster, Inc. He also receives research grants and consultation fees from Affera Inc., Boston Scientific, Itamar Medical and Phillips Health. Drs, Brem, Zilberman, and Datta are employees at Biosense Webster. All other authors have no conflicts of interest relevant to this study.
References:
- 1.Chang DC, Reese TS. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990;58:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neven K, van Driel V, van Wessel H, van Es R, du Pre B, Doevendans PA, Wittkampf F. Safety and feasibility of closed chest epicardial catheter ablation using electroporation. Circ Arrhythm Electrophysiol. 2014;7:913–9. [DOI] [PubMed] [Google Scholar]
- 3.van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015;12:1838–44. [DOI] [PubMed] [Google Scholar]
- 4.Neven K, van Es R, van Driel V, van Wessel H, Fidder H, Vink A, Doevendans P, Wittkampf F. Acute and Long-Term Effects of Full-Power Electroporation Ablation Directly on the Porcine Esophagus. Circ Arrhythm Electrophysiol. 2017;10:e004672. [DOI] [PubMed] [Google Scholar]
- 5.Bradley CJ, Haines DE. Pulsed field ablation for pulmonary vein isolation in the treatment of atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31:2136–2147. [DOI] [PubMed] [Google Scholar]
- 6.Yavin H, Shapira-Daniels A, Barkagan M, Sroubek J, Shim D, Melidone R, Anter E. Pulsed Field Ablation Using a Lattice Electrode for Focal Energy Delivery: Biophysical Characterization, Lesion Durability, and Safety Evaluation. Circ Arrhythm Electrophysiol. 2020;13:e008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koruth JS, Kuroki K, Kawamura I, Brose R, Viswanathan R, Buck ED, Donskoy E, Neuzil P, Dukkipati SR, Reddy VY. Pulsed Field Ablation vs Radiofrequency Ablation: Esophageal Injury in a Novel Porcine Model. Circ Arrhythm Electrophysiol. 2020;13:e008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, Sediva L, Chovanec M, Dukkipati SR, Jais P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J Am Coll Cardiol. 2019;74:315–326. [DOI] [PubMed] [Google Scholar]
- 9.Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R, Buck ED, Speltz M, Dukkipati SR, Reddy VY. Preclinical Evaluation of Pulsed Field Ablation: Electrophysiological and Histological Assessment of Thoracic Vein Isolation. Circ Arrhythm Ectrophysiol. 2019;12:e007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, Hebeler R, Labrousse L, Barandon L, Kralovec S, et al. Ablation of Atrial Fibrillation With Pulsed Electric Fields: An Ultra-Rapid, Tissue-Selective Modality for Cardiac Ablation. JACC Clin Electrophysiol. 2018;4:987–995. [DOI] [PubMed] [Google Scholar]
- 11.Stewart MT, Haines DE, Verma A, Kirchhof N, Barka N, Grassl E, Howard B. Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model. Heart Rhythm. 2019;16:754–764. [DOI] [PubMed] [Google Scholar]
- 12.Leshem EZI, Tschabrunn CM, Barkagan M, Contreras-Valdes FM, Govari A, Anter E. High-Power and Short-Duration Ablation for Pulmonary Vein Isolation. JACC: Clinical Electrophysiol. 2018;4:467–479. [DOI] [PubMed] [Google Scholar]
- 13.Barkagan M, Leshem E, Rottmann M, Sroubek J, Shapira-Daniels A, Anter E. Expandable Lattice Electrode Ablation Catheter: A Novel Radiofrequency Platform Allowing High Current at Low Density for Rapid, Titratable, and Durable Lesions. Circ Arrhythm Electrophysiol. 2019;12:e007090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsong TY. Electroporation of cell membranes. Biophys J. 1991;60:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


