Abstract
Objective
To compare the performance of simulated abbreviated breast MRI (AB-MRI) and full diagnostic (FD)-MRI in distinguishing between benign and malignant lesions detected by MRI and investigate the features of discrepant lesions of the two protocols.
Materials and Methods
An AB-MRI set with single first postcontrast images was retrospectively obtained from an FD-MRI cohort of 111 lesions (34 malignant, 77 benign) detected by contralateral breast MRI in 111 women (mean age, 49.8. ± 9.8; range, 28–75 years) with recently diagnosed breast cancer. Five blinded readers independently classified the likelihood of malignancy using Breast Imaging Reporting and Data System assessments. McNemar tests and area under the receiver operating characteristic curve (AUC) analyses were performed. The imaging and pathologic features of the discrepant lesions of the two protocols were analyzed.
Results
The sensitivity of AB-MRI for lesion characterization tended to be lower than that of FD-MRI for all readers (58.8–82.4% vs. 79.4–100%), although the findings of only two readers were significantly different (p < 0.05). The specificity of AB-MRI for lesion characterization was higher than that of FD-MRI for 80% of readers (39.0–74.0% vs. 19.5–45.5%, p ≤ 0.001). The AUC of AB-MRI was comparable to that of FD-MRI for all readers (p > 0.05). Fifteen percent (5/34) of the cancers were false-negatives on AB-MRI. More suspicious margins or internal enhancement on the delayed phase images were related to the discrepancies.
Conclusion
The overall performance of AB-MRI was similar to that of FD-MRI in distinguishing between benign and malignant lesions. AB-MRI showed lower sensitivity and higher specificity than FD-MRI, as 15% of the cancers were misclassified compared to FD-MRI.
Keywords: Breast neoplasms, Magnetic resonance imaging, Sensitivity, Specificity, Area under the curve
INTRODUCTION
Breast MRI has high sensitivity (75.2–100%) and specificity (83.0–98.4%) for breast cancer screening (1) and superior sensitivity to mammography for identifying aggressive or invasive tumors, which are likely to be biologically important (2). However, breast MRI screening is currently recommended for women with a 20% or higher lifetime risk of breast cancer due to its high cost and limited availability (3). Abbreviated breast MRI (AB-MRI), which obtains the most essential sequences (i.e., precontrast and first postcontrast images) of full diagnostic MRI (FD-MRI) with total scan time of less than 5–10 minutes, has been recently introduced. AB-MRI reduces the MRI examination and interpretation time and allows faster throughput, leading to reduced cost and increased availability of breast MRI screening to intermediate-risk women (1,4,5,6). In several studies involving different populations, AB-MRI screening has consistently shown cancer detection rates and diagnostic accuracy comparable to those of FD-MRI (7,8,9,10,11,12,13,14,15,16,17,18). AB-MRI with first post-contrast images preferentially detects invasive cancers and high-grade ductal carcinoma in situ (DCIS) due to their neoangiogenesis and rapid wash-in of contrast compared with fibrocystic change and low-grade DCIS (7).
In distinguishing between benign and malignant lesions, however, there is limited literature regarding the performance of radiologists by using AB-MRI. It was initially anticipated that restricting the acquisition of sequences by obtaining only first postcontrast T1-weighted images (T1-WIs) could hamper the superior sensitivity and specificity of MRI, as kinetic and morphologic information on delayed contrast-enhanced images is not available on AB-MRI (4). Thus, there might be lesions showing discrepant findings by AB-MRI and FD-MRI, which may lead to discrepant classifications. To use breast AB-MRI more widely in clinical practice, we need to be aware of how often the discrepancy between the findings of the two protocols occurs and the imaging and pathologic features of the discrepant lesions of the two protocols.
Thus, the purpose of our study was to compare the performance of simulated AB-MRI and FD-MRI in distinguishing between benign and malignant lesions detected by MRI and investigate the features of discrepant lesions of the two protocols.
MATERIALS AND METHODS
The Institutional Review Board of our institution approved this retrospective study. The need for written informed consent was waived.
Study Population
Between January 2011 and August 2016, a retrospective search of the Breast Imaging Center database of our institution identified 264 consecutive histologically confirmed lesions on contralateral screening breast MRI during preoperative evaluation in women with recently diagnosed breast cancer. Lesions located contralaterally to recently diagnosed breast cancer, classified as Breast Imaging Reporting and Data System (BI-RADS) final assessment category 4 or 5 on MRI, and subsequently confirmed with surgery were included. Of the 264 lesions, those initially detected by other imaging modalities (mammography, breast ultrasound, CT, or PET-CT) (n = 116) and those in patients treated with neoadjuvant chemotherapy (n = 37) were excluded. Finally, 111 lesions in 111 women (mean age, 49.8. ± 9.8; range, 28-75 years) detected by MRI (34 malignant and 77 benign lesions, median lesion size 1.1 cm; range, 0.3–6.9 cm) were included (Fig. 1). When there were multiple enhancing lesions in the contralateral breast, the most suspicious lesion was chosen for excision. Thus, one lesion per breast was included. The lesions were histologically confirmed using excisional biopsy with ultrasound- (n = 85) or mammography-guided (n = 3) wire localization or without localization (n = 3), surgical excision after percutaneous ultrasound- (n = 15), or MRI-guided (n = 5) biopsy.
Fig. 1. Flow chart showing the study inclusion and exclusion criteria.
BI-RADS = Breast Imaging Reporting and Data System, MG = mammography, US = ultrasound
MRI Examinations and Two Imaging Sets
MRI examinations were performed with the patients in the prone position using a 1.5T system (Signa; General Electric Healthcare) with a dedicated 8-channel breast coil or 3T system (Achieva dStream, Philips Healthcare) with a dedicated 16-channel breast coil. Bilateral fat-suppressed T2-weighted image (T2-WI) and T1-WI with one precontrast and five postcontrast series were obtained (Supplementary Material). Subtraction, axial or sagittal reformatted, and maximum intensity projection (MIP) images were generated. From the FD-MRI, precontrast, first postcontrast T1-weighted, and MIP images were selected and saved as anonymized DICOM files for the simulated AB-MRI data set.
Reader Study and Data Collection
Five fellowship-trained radiologists in breast imaging participated as readers and completed the AB-MR Reader Training and Certification Test before participation (19). They had 3, 4, 8, 11, and 11 years of experience in interpreting breast MRI (i.e., FD-MRI) and 0.5, 0.5, 0.5, 1, and 1 year of experience in interpreting AB-MRI. The readers were blinded to the clinical information, images from other modalities, and histopathology results of the breast lesions on MRI data sets by using anonymized DICOM files saved in the research folder on the picture archiving and communication system. As the purpose of this reader study was to evaluate the ability to distinguish between benign and malignant lesions, and not the ability to detect lesions, the locations of the lesions on the images were provided to the readers. They independently reviewed two MRI datasets. A suspicious lesion was defined as a lesion with one of the following features: a mass with a spiculated or irregular margin, an irregular shape, and heterogeneous or rim enhancement; a non-mass enhancement with a linear or segmental distribution, a heterogeneous, clumped, or clustered ring internal enhancement pattern. A probably benign lesion was characterized by the following features: a mass with an oval shape, a circumscribed margin, a homogeneous and persistent enhancement pattern; a focal non-mass enhancement without a suspicious finding.
During the first session, the readers assessed the simulated AB-MRI set composed of MIP, precontrast, and first postcontrast series. During the second session a week later, the readers assessed the FD-MRI set including T2-weighted, precontrast, five postcontrast, subtracted, and delayed postcontrast axial or sagittal images for the same lesions. The final assessment category based on BI-RADS® Atlas for MRI (20) and the likelihood of malignancy of each lesion was classified during each reading session. Both AB- and FD-MRI interpretations were based on the BI-RADS® lexicon for MRI (20), and the standard of reference was the histopathologic result from surgical excision. MRI findings were described at AB-MRI and FD-MRI by two radiologists (with 17 and 1 year of experience) in consensus other than 5 readers.
Discrepant lesions detected by AB-MRI and FD-MRI were defined as lesions with discordant classifications by the two protocols by at least 3 of the 5 readers. The discrepant malignant lesions were malignant lesions that showed discrepancies between the findings of AB-MRI and FD-MRI. The discrepant benign lesions were benign lesions that showed discrepant assessments between AB-MRI and FD-MRI. Two other radiologists analyzed the discrepant lesions by consensus, focusing on the morphologic descriptors, kinetic features based on the BI-RADS lexicon, and signal intensities on T2-WI, to identify presumptive reasons for the discrepant classifications between the two protocols. The histologic type, lesion size, nuclear grade, histologic grade and receptor status, including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression, were reviewed.
Statistical Analysis
The sensitivity and specificity of AB-MRI and FD-MRI determined by the readers were compared using the McNemar test with IBM SPSS Statistics (IBM Corp., version 22.0). The areas under the receiver operating characteristic curve (AUCs) for distinguishing between the benign and malignant lesions classified by the readers were compared using MedCalc Statistical Software version 18.10 (MedCalc Software bv). A p value of < 0.05 was considered statistically significant.
RESULTS
Patient and Lesion Characteristics
Table 1 shows the patient characteristics and MRI findings of 111 histopathologically confirmed benign and malignant lesions detected on contralateral breast MRI screening of 111 women (mean age, 49.8 years; range, 28–75 years) with recently diagnosed breast cancers. The lesion size, defined as the longest dimension on MRI, ranged from 0.3–6.9 cm (median, 1.1 cm). The median size of the benign lesions was 1.0 cm (range, 0.3–6.9 cm) and that of the malignant lesions was 1.2 cm (range, 0.5–6.6 cm) (p = 0.23). The most common benign histopathology was a fibrocystic change (13/77, 16.9%). Of the malignant lesions, 47.1% (16/34) were invasive cancers and 52.9% (18/34) were DCIS (Table 2).
Table 1. Patient Characteristics and MRI Findings of the Histopathologically Confirmed 111 Lesions.
| Characteristics | Benign Lesions (n = 77) | Malignant Lesions (n = 34) | ||
|---|---|---|---|---|
| Mean age (range) (years) | 49.2 (28–75) | 53.3 (41–75) | ||
| Median lesion size on MRI (range) (cm) | 1.0 (0.3–6.9) | 1.2 (0.5–6.6) | ||
| MRI finding | AB-MRI | FD-MRI | AB-MRI | FD-MRI |
| Mass | 56 (72.7) | 55 (71.4) | 26 (76.5) | 24 (70.6) |
| Shape | ||||
| Oval | 24 (42.9) | 23 (41.8) | 4 (15.4) | 5 (20.8) |
| Round | 1 (1.8) | 6 (10.9) | 1 (3.8) | 0 (0) |
| Irregular | 31 (55.4) | 26 (47.3) | 21 (80.8) | 19 (79.2) |
| Margin | ||||
| Circumscribed | 25 (44.6) | 20 (36.4) | 5 (19.2) | 4 (16.7) |
| Not circumscribed | ||||
| Irregular | 27 (48.2) | 31 (56.4) | 13 (50.0) | 14 (58.3) |
| Spiculated | 4 (7.1) | 4 (7.3) | 8 (30.8) | 6 (25.0) |
| Internal enhancement characteristics | ||||
| Homogeneous | 17 (30.4) | 21 (38.2) | 2 (7.7) | 6 (25.0) |
| Heterogeneous | 35 (62.5) | 31 (56.4) | 21 (80.8) | 17 (70.8) |
| Rim enhancement | 3 (5.4) | 2 (3.6) | 3 (11.5) | 1 (4.2) |
| Dark internal septations | 1 (1.8) | 1 (1.8) | 0 (0) | 0 (0) |
| Non-mass enhancement | 21 (27.3) | 22 (28.6) | 8 (23.5) | 10 (29.4) |
| Distribution | ||||
| Focal | 11 (52.4) | 10 (45.5) | 2 (25.0) | 3 (30.0) |
| Linear | 1 (4.8) | 4 (18.2) | 3 (37.5) | 2 (20.0) |
| Segmental | 5 (23.8) | 3 (13.6) | 1 (12.5) | 3 (30.0) |
| Regional | 4 (19.0) | 5 (22.7) | 2 (25.0) | 2 (20.0) |
| Internal enhancement patterns | ||||
| Homogeneous | 1 (4.8) | 3 (13.6) | 2 (25.0) | 0 (0) |
| Heterogeneous | 20 (95.2) | 16 (72.7) | 5 (62.5) | 6 (60.0) |
| Clumped | 0 (0) | 3 (13.6) | 1 (12.5) | 2 (20.0) |
| Clustered ring | 0 (0) | 0 (0) | 0 (0) | 2 (20.0) |
Data are numbers of lesions, and the values in parentheses are percentages. MRI findings were described by two radiologists in consensus who did not participate in the reader study of both protocols. AB = abbreviated breast, FD = full diagnostic
Table 2. Histopathologic Features of 111 Lesions.
| Histopathologic Features | n (%) |
|---|---|
| Benign lesions | 77 (100) |
| Fibrocystic change | 13 (16.9) |
| Ductectasia | 1 (1.3) |
| Fibroadenoma | 10 (13.0) |
| Complex fibroadenoma | 1 (1.3) |
| Adenosis, other than sclerosing adenosis | 4 (5.2) |
| Sclerosing adenosis | 10 (13.0) |
| Atypical apocrine adenosis | 1 (1.3) |
| Usual ductal hyperplasia | 5 (6.5) |
| Florid ductal epithelial hyperplasia | 5 (6.5) |
| Columnar cell change | 3 (3.9) |
| Intraductal papilloma | 11 (14.3) |
| Radial scar | 3 (3.9) |
| Mucocele-like lesion | 1 (1.3) |
| Atypical ductal hyperplasia | 4 (5.2) |
| Atypical lobular hyperplasia | 2 (2.6) |
| Lobular carcinoma in situ | 3 (3.9) |
| Malignant Lesions | 34 (100) |
| Invasive cancer | 16 (47.1) |
| Invasive ductal carcinoma | 12 (35.3) |
| Invasive lobular carcinoma | 4 (11.8) |
| Ductal carcinoma in situ | 18 (52.9) |
Sensitivity, Specificity, and AUC
The sensitivity of AB-MRI tended to be lower than that of FD-MRI for all readers (82.4% [28/34] vs. 85.3% [29/34] for reader 1, p > 0.999; 82.4% [28/34] vs. 100% [34/34] for reader 2, p = 0.031; 67.6% [23/34] vs. 94.1% [32/34] for reader 3, p = 0.004; 58.8% [20/34] vs. 82.4% [28/34] for reader 4, p = 0.077; 76.5% [26/34] vs. 79.4% [27/34] for reader 5, p > 0.999), but a significant difference was only found for two readers (Table 3). The specificity of AB-MRI was significantly higher than that of FD-MRI for four of the five readers (41.6% [32/77] vs. 36.4% [28/77] for reader 1, p = 0.503; 39.0% [30/77] vs. 19.5% [15/77] for reader 2, p = 0.001; 71.4% [55/77] vs. 45.5% [35/77] for reader 3, p < 0.001; 74.0% [57/77] vs. 37.7% [29/77] for reader 4, p < 0.001; 59.7% [46/77] vs. 35.1% [27/77] for reader 5, p = 0.001) (Table 3). The AUCs for AB-MRI and FD-MRI were not significantly different (0.71 vs. 0.71 for reader 1, p = 0.981; 0.70 vs. 0.69 for reader 2, p = 0.764; 0.77 vs. 0.84 for reader 3, p = 0.117; 0.74 vs. 0.70 for reader 4, p = 0.542; 0.74 vs. 0.64 for reader 5, p = 0.078) (Table 3).
Table 3. Performance of Five Readers Assessing 111 Breast Lesions with AB- and FD-MRI.
| Readers | Sensitivity* | Specificity* | AUC† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AB-MRI | FD-MRI | P‡ | AB-MRI | FD-MRI | P‡ | AB-MRI | FD-MRI | P‡ | |
| Reader 1 | 82.4 | 85.3 | > 0.999 | 41.6 | 36.4 | 0.503 | 0.71 [0.61–0.79] |
0.71 [0.61–0.79] |
0.981 |
| (28/34) | (29/34) | (32/77) | (28/77) | ||||||
| [65.5–93.2] | [68.9–95.0] | [30.4–53.4] | [25.7–48.1] | ||||||
| 82.4 | 100.0 | 0.031 | 39.0 | 19.5 | 0.001 | 0.70 [0.61–0.78] |
0.69 [0.59–0.77] |
0.765 | |
| Reader 2 | (28/34) | (34/34) | (30/77) | (15/77) | |||||
| [65.5–93.2] | [89.7–100.0] | [28.0–50.8] | [11.3–30.1] | ||||||
| Reader 3 | 67.6 | 94.1 | 0.004 | 71.4 | 45.5 | < 0.001 | 0.77 [0.68–0.85] |
0.84 [0.76–0.90] |
0.117 |
| (23/34) | (32/34) | (55/77) | (35/77) | ||||||
| [49.5–82.6] | [80.3–99.3] | [60.0–81.2] | [34.1–57.2] | ||||||
| Reader 4 | 58.8 | 82.4 | 0.077 | 74.0 | 37.7 | < 0.001 | 0.74 [0.65–0.82] |
0.70 [0.60–0.78] |
0.542 |
| (20/34) | (28/34) | (57/77) | (29/77) | ||||||
| [40.7–75.4] | [65.5–93.2] | [62.8–83.4] | [26.9–49.4] | ||||||
| Reader 5 | 76.5 | 79.4 | > 0.999 | 59.7 | 35.1 | 0.001 | 0.74 [0.65–0.82] |
0.64 [0.54–0.73] |
0.078 |
| (26/34) | (27/34) | (46/77) | (27/77) | ||||||
| [58.8–89.3] | [62.1–91.3] | [47.9–70.8] | [24.5–46.8] | ||||||
| Mean ± SD | 73.5 ± 10.2 | 88.2 ± 8.6 | 57.1 ± 16.3 | 34.8 ± 9.5 | 0.73 ± 0.03 | 0.72 ± 0.07 | |||
*Numbers are percentages, raw data are in parentheses, and 95% confidence intervals are in brackets, †Numbers in brackets are the 95% confidence intervals of the AUC values, ‡p values refer to the differences in the diagnostic performance between AB-MRI and FD-MRI.
AUC = area under the receiver operating characteristic curve
Discrepant Lesions between AB-MRI and FD-MRI
Of the 34 malignant lesions, 26.5% (9/34) were category 2 or 3 on AB-MRI and 11.8% (4/34) were category 2 or 3 on FD-MRI (Table 4). Of the 77 benign lesions, 42.9% (33/77) were category 4 or 5 on AB-MRI and 64.9% (50/77) were category 4 or 5 on FD-MRI (Table 4).
Table 4. BI-RADS Final Assessments of Five Readers for Malignant and Benign Lesions with AB- and FD-MRI.
| Reader | Benign Lesions (n = 77) | Malignant Lesions (n = 34) | ||||||
|---|---|---|---|---|---|---|---|---|
| AB-MRI | FD-MRI | AB-MRI | FD-MRI | |||||
| BI-RADS | BI-RADS | BI-RADS | BI-RADS | BI-RADS | BI-RADS | BI-RADS | BI-RADS | |
| 2 or 3 | 4 or 5 | 2 or 3 | 4 or 5 | 2 or 3 | 4 or 5 | 2 or 3 | 4 or 5 | |
| 1 | 32 | 45 | 28 | 49 | 6 | 28 | 5 | 29 |
| 2 | 30 | 47 | 15 | 62 | 6 | 28 | 0 | 34 |
| 3 | 55 | 22 | 35 | 42 | 11 | 23 | 2 | 32 |
| 4 | 57 | 20 | 29 | 48 | 14 | 20 | 6 | 28 |
| 5 | 46 | 31 | 27 | 50 | 8 | 26 | 7 | 27 |
| Mean ± SD | 44 ± 12.6 | 33 ± 12.6 | 27 ± 7.3 | 50 ± 7.3 | 9 ± 3.5 | 25 ± 3.5 | 4 ± 2.9 | 30 ± 2.9 |
Data are numbers of lesions. BI-RADS = Breast Imaging Reporting and Data System
Five of the 34 malignant lesions (14.7%) were false negatives on AB-MRI and true positives on FD-MRI according to three or more of the five readers (Table 5). Of the 5 false-negative lesions on AB-MRI, 1 lesion was misclassified by all readers, 3 lesions were misclassified by four readers, and 1 lesion was misclassified by three readers (Table 5). These included three DCISs (low or intermediate nuclear grade; 0.6 cm, 1.5 cm, and 2.5 cm in size) and two invasive ductal carcinomas (low or intermediate histologic grade, ER/PR-positive, HER2-negative, 0.6 cm in size and ER/PR/HER2-negative, 0.1 cm in size). All 5 false-negative lesions (100%) on AB-MRI had more suspicious margins or internal enhancement on the delayed phase of FD-MRI than on AB-MRI (Table 5). Of the five false-negative lesions on AB-MRI, three lesions (60%) showed plateau or washout kinetics on FD-MRI (Table 5). On T2-WI, three lesions showed iso or mixed signal intensities, and two lesions (40%) showed high signal intensities (Table 5, Fig. 2).
Table 5. Five Cancers Presenting as False Negative on AB-MRI and as True Positive on FD-MRI.
| Lesion 1 | Lesion 2 | Lesion 3 | Lesion 4 | Lesion 5 | |
|---|---|---|---|---|---|
| Number of radiologists with discrepant categories | 5 | 4 | 4 | 4 | 3 |
| Imaging features on AB-MRI | |||||
| Type | Mass | Mass | Mass | NME | NME |
| Shape/distribution | Oval | Oval | Irregular | Linear | Linear |
| Margin | Circumscribed | Circumscribed | Irregular | NA | NA |
| Internal enhancement characteristics/patterns | Heterogeneous | Heterogeneous | Heterogeneous | Heterogeneous | Heterogeneous |
| Imaging features on FD-MRI | |||||
| Type | Mass | Mass | Mass | NME | NME |
| Shape/Distribution | Oval | Oval | Irregular | Linear | Linear |
| Margin | Irregular | Irregular | Spiculated | NA | NA |
| Internal enhancement characteristics/patterns | Heterogeneous | Heterogeneous | Heterogeneous | Heterogeneous | Heterogeneous |
| Kinetic features initial/delayed phase | Fast/washout | Fast/plateau | Fast/persistent | Fast/plateau | Fast/persistent |
| Signal intensity at T2-WI | Iso | High | Iso | Mixed | High |
| Lesion size at MRI (cm)* | 0.6 | 0.9 | 0.7 | 2.1 | 2.9 |
| Histopathologic features | |||||
| Histologic type | IDC | DCIS | IDC | DCIS | DCIS |
| Tumor size at pathology (cm)† | 0.6 | 1.5 | 0.1 | 0.6 | 2.5 |
| Histologic grade | Low | NA | Low | NA | NA |
| Nuclear grade | Low | Intermediate | Intermediate | Intermediate | Low |
| ER/PR/HER2 | +/+/- | NA | -/-/- | NA | NA |
The analysis was performed by two radiologists in consensus who did not participate in the reader study of both protocols. *Lesion size on MRI was defined as the longest dimension on MRI, †Tumor size at pathology was defined as invasive tumor size in cases of invasive ductal carcinoma. DCIS = ductal carcinoma in situ, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, IDC = invasive ductal carcinoma, NA = not applicable, NME = non-mass enhancement, PR = progesterone receptor, T2-WI = T2-weighted image
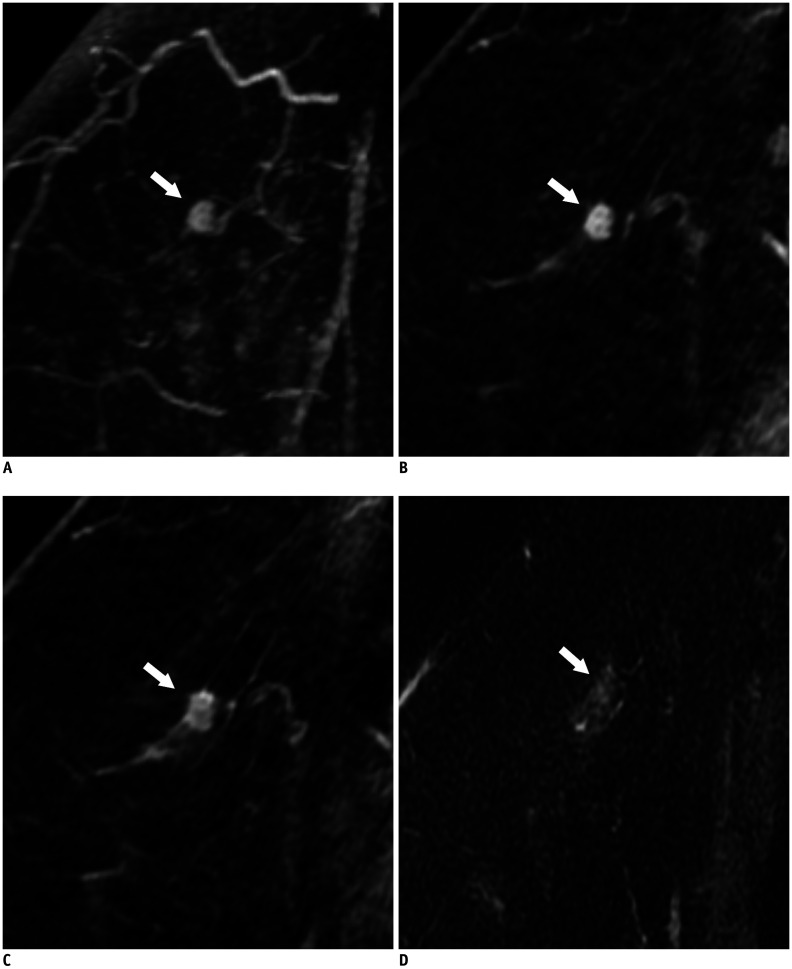
Fig. 2. False-negative lesion on abbreviated MRI in a 55-year-old woman (lesion 1 in Table 5).
MIP reconstruction image (A) and first post-contrast T1-weighted sagittal image (B) show a 0.4 cm oval heterogeneously enhancing mass (arrows) in the right upper outer breast. The fifth post-contrast T1-weighted sagittal image (C) shows the mass (arrow) with a more irregular margin and washout kinetics. T2-weighted sagittal image (D) shows the mass (arrow) with isointense T2 signal intensity compared to breast parenchyma. This lesion was classified as BI-RADS final assessment category 3 by all five radiologists on abbreviated breast MRI and classified as category 4 by all five radiologists on full diagnostic MRI. It was finally confirmed as a 0.6 cm low-grade, ER/PR-positive, HER2-negative invasive ductal carcinoma after breast-conserving surgery. ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, MIP = maximum intensity projection, PR = progesterone receptor
Eleven of 77 benign lesions (14.3%) were false positives on FD-MRI and true negatives on AB-MRI according to three or more of the five readers. Of these 11 lesions, 5 (45.5%) presented more suspicious shapes, margins, or internal enhancement on the delayed phase of FD-MRI than on the first post-contrast images of AB-MRI. Of the 11 false-positive lesions on FD-MRI, 10 (90.9%) showed plateau or washout kinetics. On T2-WI, 8 lesions showed iso- or mixed signal intensities, and three lesions (27.3%) showed high signal intensities (Fig. 3).
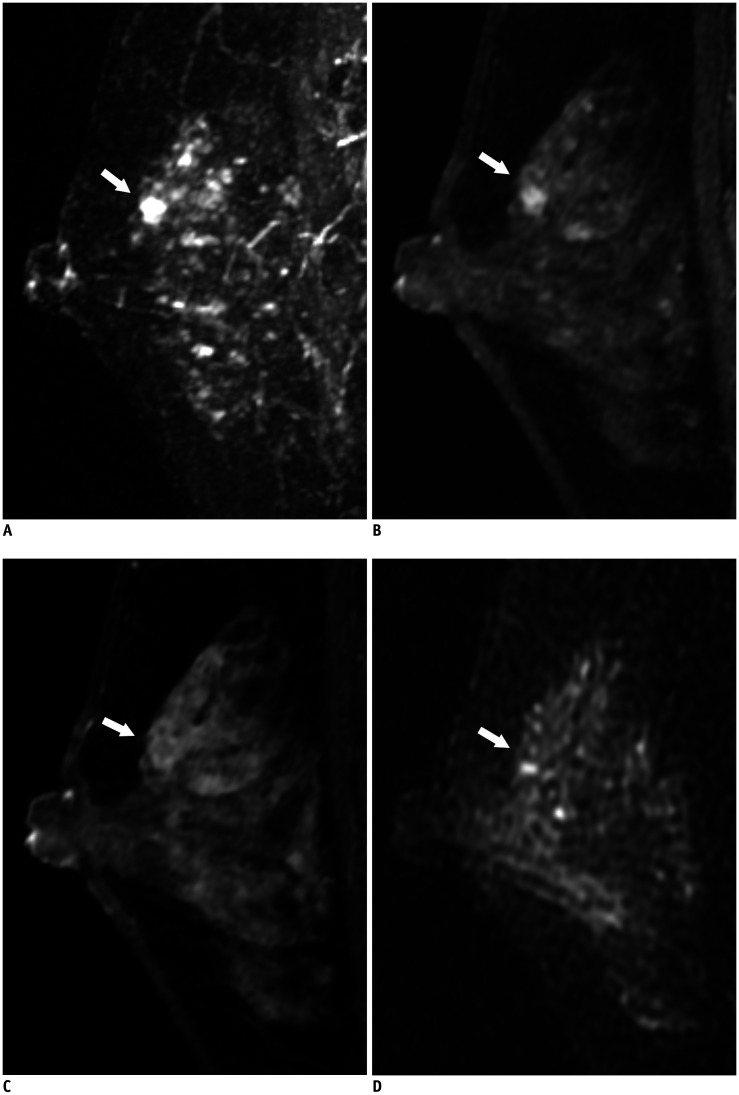
Fig. 3. True-negative lesion on abbreviated MRI in a 49-year-old woman.
MIP reconstruction image (A) and first post-contrast T1-weighted sagittal image (B) show a 0.5 cm irregular, circumscribed mass (arrows) in the left upper breast. The fifth post-contrast T1-weighted sagittal image (C) shows the mass (arrow) with clear washout kinetics. T2-weighted sagittal image (D) shows the mass (arrow) with iso-signal intensity. This lesion was classified as BI-RADS final assessment category 2 or 3 by all five radiologists on abbreviated breast MRI and category 4 by four of five radiologists on full diagnostic MRI. It was finally confirmed as sclerosing adenosis after excision.
There were no discrepant benign or malignant lesions that showed more suspicious findings on AB-MRI (BI-RADS final assessment category 4 or 5) than FD-MRI (BI-RADS category 2 or 3).
DISCUSSION
In our multireader study, simulated AB-MRI with single first post-contrast images tended to show a lower sensitivity and higher specificity than FD-MRI in distinguishing between benign and malignant lesions detected by MRI screening, although AB-MRI and FD-MRI had similar AUCs. Of the 34 malignant lesions, five (15%) were classified as BI-RADS final assessment category 2 or 3 on AB-MRI by three or more of the five readers. All five false-negative cancers on AB-MRI were low- or intermediate-grade DCIS or low- or intermediate-grade invasive cancer of ≤ 1 cm. Not only the absence of kinetic information, but also the more suspicious margins or internal enhancement appearing in the delayed phase of FD-MRI than on the first postcontrast images of AB-MRI, was related to the discrepancies between the two protocols.
Previous reader studies showed the promising performances of AB-MRI with single first post-contrast images; however, these studies were mostly conducted in the breast cancer screening setting (7,8,9,10,11,12,13,15,16,17,18). Regarding the diagnostic setting, Romeo et al. (21) reported that a simplified breast MRI protocol, including the second and fifth postcontrast series, had a comparable performance to FD-MRI in characterization (sensitivity 99% vs. 97%, p = 0.62; specificity 93% vs. 95%, p = 0.72; AUC 0.989 vs. 0.990, p = 0.76). Moschetta et al. (14) also found that AB-MRI consisting of a single third post-contrast T1-WI and morphologic sequences (short TI inversion recovery, T2-WI) had comparable performance to FD-MRI (sensitivity 0.89 vs. 0.92, specificity 0.91 vs. 0.92, AUC 0.91 vs. 0.92, all p > 0.05). However, Gillman et al. (22) recently reported a lower sensitivity and higher specificity of AB-MRI including T2-WI and first postcontrast series than FD-MRI (50% vs. 71%, 96% vs. 77%) in women with a personal history of breast cancer, which is consistent with our results. In addition, in an early pilot study that compared the performances of two simplified breast MRI protocols (1st postcontrast series alone vs. 1st and 2nd postcontrast series) and FD-MRI (1st, 2nd, 3rd, and 4th postcontrast series), Grimm et al. (9) found that the sensitivities and specificities of the protocols were 86% and 52%, 89% and 45%, and 95% and 52%, respectively, although there were no significant differences. Based on these studies, it is notable that while AB-MRI including second or later postcontrast series showed comparable performances to FD-MRI, AB-MRI with single first postcontrast images tended to show inferior sensitivity to FD-MRI in distinguishing between benign and malignant lesions, which was demonstrated in our study.
According to the analysis of the BI-RADS features of the discrepant lesions of the two protocols in our study, the absence of kinetic information and the limited morphologic details in the first postcontrast images of AB-MRI were the main reasons for the discrepancies between the two protocols. Three of the 5 false-negative lesions on AB-MRI and 10 of the 11 false-positive lesions on FD-MRI showed plateau or washout kinetics on FD-MRI. In addition, all 5 false-negative lesions on AB-MRI and 5 of the 11 falsepositive lesions on FD-MRI showed more suspicious shapes, margins, or internal enhancement on the delayed phase of FD-MRI than on the first postcontrast images of AB-MRI. Moreover, readers may have missed suspicious findings of false-negative cancers, such as irregular margins, heterogeneous enhancement, or linear distribution, which appeared to be very subtle in the first postcontrast images, leading to misinterpretation on AB-MRI. In this context, Romeo et al. (21) also emphasized the usefulness of the second and fifth postcontrast series for characterization because these series had better visualization of the margins and internal enhancement patterns than the first postcontrast images alone. Considering that the most important advantage of breast MRI is its outstanding sensitivity, the misclassification of breast cancers, even low to intermediate grade and minimal breast cancers, could be a drawback. Thus, further study is needed to determine whether second or third postcontrast series may help reduce the delayed diagnosis of breast cancers with AB-MRI.
This study has several limitations. First, this was a retrospective study conducted in a single institution, and it included lesions detected by contralateral breast MRI screening in women with newly diagnosed breast cancers. This cohort is not typical for AB-MRI examination, and prospective studies using various populations are needed for further validation of our results. Our study included only histologically confirmed suspicious breast lesions, and 52.9% (18/34) of the cancer cases were DCIS. Previous diagnostic AB-MRI studies, however, included breast lesions detected by mammography or ultrasound examination or palpable lesions (14,21). Second, the 5 breast radiologists who participated in the reader study had less experience with AB-MRI than with FD-MRI. Similar to the ECOG-ACRIN 1141 trial, however, the radiologists in our study had to pass the AB-MR Reader Training and Certification Test before participation (19,23). Third, we did not include T2-WI in the simulated AB-MRI datasets. Nowadays, T2-WI is usually included in the AB-MRI protocol (11,14,24). If T2-WI was included in the AB-MRI protocol in our study, 3 of 5 false-negative cancers on AB-MRI with iso- or mixed signal intensities on T2-WI may have been correctly classified as BI-RADS category 4 or 5 (25,26,27). Ultrafast imaging with fast (< 10 s) temporal resolution retains dynamic information for lesion characterization, and the addition of these protocols in AB-MRI may help improve the performance of AB-MRI (28,29). Lastly, there were no discrepant benign or malignant lesions that showed more suspicious findings on based on the initial FD-MRI interpretations, although this fact may have little effect on the overall results.
In conclusion, our multireader study found that 15% of the cancers were misclassified on AB-MRI compared to FD-MRI because kinetic information is unavailable and suspicious morphology is not well-delineated on AB-MRI. The possibility of delayed diagnosis of breast cancer should be noted in the interpretation of AB-MRI with single first post-contrast images. Further study is needed to determine the diagnostic value of the addition of more than one postcontrast series in AB-MRI.
Footnotes
This study has received funding by grant (no. 03-2019-0330) from the Seoul National University Hospital Research Fund.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.3348/kjr.2020.0311.
References
- 1.Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging. 2019;50:377–390. doi: 10.1002/jmri.26654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung JS, Stamler S, Brooks J, Kaplan J, Huang T, Dershaw DD, et al. Breast cancers detected at screening MR imaging and mammography in patients at high risk: method of detection reflects tumor histopathologic results. Radiology. 2016;280:716–722. doi: 10.1148/radiol.2016151419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 4.Kuhl CK. Abbreviated magnetic resonance imaging (MRI) for breast cancer screening: rationale, concept, and transfer to clinical practice. Annu Rev Med. 2019;70:501–519. doi: 10.1146/annurev-med-121417-100403. [DOI] [PubMed] [Google Scholar]
- 5.Deike-Hofmann K, Koenig F, Paech D, Dreher C, Delorme S, Schlemmer HP, et al. Abbreviated MRI protocols in breast cancer diagnostics. J Magn Reson Imaging. 2019;49:647–658. doi: 10.1002/jmri.26525. [DOI] [PubMed] [Google Scholar]
- 6.Leithner D, Moy L, Morris EA, Marino MA, Helbich TH, Pinker K. Abbreviated MRI of the breast: does it provide value? J Magn Reson Imaging. 2019;49:e85–e100. doi: 10.1002/jmri.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32:2304–2310. doi: 10.1200/JCO.2013.52.5386. [DOI] [PubMed] [Google Scholar]
- 8.Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Application of abbreviated protocol of magnetic resonance imaging for breast cancer screening in dense breast tissue. Acad Radiol. 2017;24:316–320. doi: 10.1016/j.acra.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol. 2015;22:1157–1162. doi: 10.1016/j.acra.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol. 2016;13:R74–R80. doi: 10.1016/j.jacr.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Heacock L, Melsaether AN, Heller SL, Gao Y, Pysarenko KM, Babb JS, et al. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol. 2016;85:815–823. doi: 10.1016/j.ejrad.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Jain M, Jain A, Hyzy MD, Werth G. FAST MRI breast screening revisited. J Med Imaging Radiat Oncol. 2017;61:24–28. doi: 10.1111/1754-9485.12502. [DOI] [PubMed] [Google Scholar]
- 13.Machida Y, Shimauchi A, Kanemaki Y, Igarashi T, Harada M, Fukuma E. Feasibility and potential limitations of abbreviated breast MRI: an observer study using an enriched cohort. Breast Cancer. 2017;24:411–419. doi: 10.1007/s12282-016-0718-z. [DOI] [PubMed] [Google Scholar]
- 14.Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated combined MR protocol: a new faster strategy for characterizing breast lesions. Clin Breast Cancer. 2016;16:207–211. doi: 10.1016/j.clbc.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Petrillo A, Fusco R, Sansone M, Cerbone M, Filice S, Porto A, et al. Abbreviated breast dynamic contrast-enhanced MR imaging for lesion detection and characterization: the experience of an Italian oncologic center. Breast Cancer Res Treat. 2017;164:401–410. doi: 10.1007/s10549-017-4264-y. [DOI] [PubMed] [Google Scholar]
- 16.Panigrahi B, Mullen L, Falomo E, Panigrahi B, Harvey S. An abbreviated protocol for high-risk screening breast magnetic resonance imaging: impact on performance metrics and BI-RADS assessment. Acad Radiol. 2017;24:1132–1138. doi: 10.1016/j.acra.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Chhor CM, Mercado CL. Abbreviated MRI protocols: wave of the future for breast cancer screening. AJR Am J Roentgenol. 2017;208:284–289. doi: 10.2214/AJR.16.17205. [DOI] [PubMed] [Google Scholar]
- 18.Sheth D, Abe H. Abbreviated MRI and accelerated MRI for screening and diagnosis of breast cancer. Top Magn Reson Imaging. 2017;26:183–189. doi: 10.1097/RMR.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 19.Society of Breast MRI. Abbreviated breast MRI reader training and certification. Societyofbreastmri.org Web site. [Accessed September 4, 2019]. http://www.societyofbreastmri.org/Training.html.
- 20.American College of Radiology. Breast imaging reporting and data system (BI-RADS) 5th ed. Reston, Va: American College of Radiology; 2013. [Google Scholar]
- 21.Romeo V, Cuocolo R, Liuzzi R, Riccardi A, Accurso A, Acquaviva A, et al. Preliminary results of a simplified breast MRI protocol to characterize breast lesions: comparison with a full diagnostic protocol and a review of the current literature. Acad Radiol. 2017;24:1387–1394. doi: 10.1016/j.acra.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Gillman J, Conant EF, Borthakur A, McDonald ES, Chong A, Weinstein SP. >Assessing the accuracy of an abbreviated breast MRI protocol compared to a full MRI protocol in women with a personal history of breast cancer. Radiological Society of North America 2018 Scientific Assembly and Annual Meeting. Archive.rsna.org Web site. [Accessed March 19, 2020]. http://archive.rsna.org/2018/18016455.html.
- 23.Kuhl CK. Abbreviated breast MRI for screening women with dense breast: the EA1141 trial. Br J Radiol. 2018;91:20170441. doi: 10.1259/bjr.20170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162:283–295. doi: 10.1007/s10549-017-4112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baltzer PA, Dietzel M, Kaiser WA. Nonmass lesions in magnetic resonance imaging of the breast: additional T2-weighted images improve diagnostic accuracy. J Comput Assist Tomogr. 2011;35:361–366. doi: 10.1097/RCT.0b013e31821065c3. [DOI] [PubMed] [Google Scholar]
- 26.Sohns C, Scherrer M, Staab W, Obenauer S. Value of the BI-RADS classification in MR-Mammography for diagnosis of benign and malignant breast tumors. Eur Radiol. 2011;21:2475–2483. doi: 10.1007/s00330-011-2210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhl CK, Klaschik S, Mielcarek P, Gieseke J, Wardelmann E, Schild HH. Do T2-weighted pulse sequences help with the differential diagnosis of enhancing lesions in dynamic breast MRI? J Magn Reson Imaging. 1999;9:187–196. doi: 10.1002/(sici)1522-2586(199902)9:2<187::aid-jmri6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Van Zelst JCM, Vreemann S, Witt HJ, Gubern-Merida A, Dorrius MD, Duvivier K, et al. Multireader study on the diagnostic accuracy of ultrafast breast magnetic resonance imaging for breast cancer screening. Invest Radiol. 2018;53:579–586. doi: 10.1097/RLI.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 29.Mann RM, Cho N, Moy L. Breast MRI: state of the art. Radiology. 2019;292:520–536. doi: 10.1148/radiol.2019182947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.