Abstract
Objective
To investigate the potential value of 18F-fluorodeoxyglucose (FDG) PET/CT in predicting the survival of patients with primary tracheal malignant tumors.
Materials and Methods
An analysis of FDG PET/CT findings in 37 primary tracheal malignant tumor patients with a median follow-up period of 43.2 months (range, 10.8–143.2 months) was performed. Cox proportional hazards regression analyses were used to assess the associations between quantitative 18F-FDG PET/CT parameters, other clinic-pathological factors, and overall survival (OS). A risk prognosis model was established according to the independent prognostic factors identified on multivariate analysis. A survival curve determined by the Kaplan-Meier method was used to assess whether the prognosis prediction model could effectively stratify patients with different risks factors.
Results
The median survival time of the 37 patients with tracheal tumors was 38.0 months, with a 95% confidence interval of 10.8 to 65.2 months. The 3-year, 5-year and 10-year survival rate were 54.1%, 43.2%, and 16.2%, respectively. The metabolic tumor volume (MTV), total lesion glycolysis (TLG), maximum standardized uptake value, age, pathological type, extension categories, and lymph node stage were included in multivariate analyses. Multivariate analysis showed MTV (p = 0.011), TLG (p = 0.020), pathological type (p = 0.037), and extension categories (p = 0.038) were independent prognostic factors for OS. Additionally, assessment of the survival curve using the Kaplan-Meier method showed that our prognosis prediction model can effectively stratify patients with different risks factors (p < 0.001).
Conclusion
This study shows that 18F-FDG PET/CT can predict the survival of patients with primary tracheal malignant tumors. Patients with an MTV > 5.19, a TLG > 16.94 on PET/CT scans, squamous cell carcinoma, and non-E1 were more likely to have a reduced OS.
Keywords: Fluorodeoxyglucose F18, Positron emission tomography computed tomography, Trachea, Prognosis, Survival
INTRODUCTION
Primary tracheal tumors are rare malignancies of the upper airway, accounting for only 1–2% of upper airway tumors (1,2,3,4). Primary tracheal malignant tumors are malignancies located between the cricoid cartilage and carina and are more common at the junction between the cartilage ring and the membrane (3,5). Due to the insidious onset and lack of specific symptoms, this disease is easily misdiagnosed or easily missed (6,7,8). 18F-fluorodeoxyglucose (FDG) PET/CT has high value in staging and prognosticating survival of malignant tumors, which provides a valuable imaging approach for clinical decisions. Since the incidence of primary tracheal malignant tumors is low, few studies have assessed the prognostic efficacy of PET/CT for this disease, and a recognized prognosis prediction model is not currently available. Therefore, this study analyzed the PET/CT findings and clinical data of patients with primary tracheal malignant tumors treated in our institution between June 2007 and December 2017, summarized the prognostic value of PET/CT, and attempted to establish a prognosis prediction model for this disease. This study aimed to investigate the potential value of 18F-FDG PET/CT in predicting the survival of patients with primary tracheal malignant tumors.
MATERIALS AND METHODS
This retrospective, single-institution study was approved by our Institutional Review Board (IRB No. GDREC2019154H). The requirement for written consent was waived by the board.
Patients
This study analyzed the PET/CT findings and clinical data of patients with primary tracheal malignant tumors treated in our institution between June 2007 and December 2017. The inclusion criteria were defined as: 1) patients with pathological diagnosis of primary tracheal malignant tumors; 2) patients who underwent regular treatment and had complete clinical data; and 3) patients who underwent a PET/CT examination within two weeks prior to treatment. The exclusion criteria included: 1) patients with a second primary cancer and 2) patients treated prior to PET/CT examination. Patient-related clinical data were completely documented, including age, sex, tumor location, tumor size, pathological type, degree of tumor invasion, status of lymph node involvement and presence of metastasis, and treatments received.
Between June 2007 and December 2017, 52 patients with tracheal lesions underwent PET/CT scanning at the PET center. We excluded 15 patients, including patients with no pathological results or with incomplete clinical data (n = 7), patients with benign tracheal lesions (n = 6), and patients with a second primary cancer (n = 2). We ultimately included 37 patients with tracheal malignant tumors, also considering patients who were lost to follow-up (n = 2).
According to the literature (9), The definitions of tumor extension classification and the lymph node involvement (N stage) are shown in Table 1.
Table 1. The Definition of Tracheal Tumor Extension and Lymph Node Categories.
| Definition | |
|---|---|
| Extension | |
| E1 | Primary tumor was confined to trachea |
| E2 | Primary tumor extending beyond the trachea but not to adjacent organs |
| E3 | Primary tumor involving adjacent organs or other structures including: aortic arch, brachiocephalic vein, azygos vein, carotid sheath, common carotid artery, subclavian artery, jugular arch, phrenic nerves, pretracheal fascia, recurrent laryngeal nerve, vagus nerve, esophagus, main bronchi (originated from trachea), pleura, thymus, thyroid gland, cricoid cartilage, sternum, vertebral column |
| E4 | Further contiguous extension |
| Lymph node | |
| N0 | No regional lymph node involvement |
| N1 | Regional lymph node involved |
| N2 | Distant lymph node involved |
Data Acquisition
All scans were performed using a Sensation Biograph Somatom 16 HR PET/CT scanner (Siemens Healthineers). All patients fasted for at least 6 hours before the PET/CT study. Only patients with blood glucose levels between 72.0 and 144.0 mg/dL (4.0–8.0 mmol/L) were subjected to PET/CT. The patients were instructed to lie still in a quiet room for 60 ± 5 minutes after they received an intravenous injection of 0.1–0.2 mCi/kg (3.7–7.4 MBq/kg) of 18F-FDG.
Whole body PET scans were performed from the upper thigh to the pharynx nasalis immediately after completion of the CT scans using a three-dimensional model with a matrix of 128 × 128 voxels. After a brain CT scan, a five-minute brain PET scan in one bed position was performed from the foramen magnum to the top of the skull.
The lesions are defined as being in the cervical and thoracic segments of the trachea, with the thoracic segment divided into the upper thoracic segment and lower thoracic segments. The mean standardized uptake value (SUVmean), maximum SUV (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) for each patient were measured from each 18F-FDG PET/CT scan using commercially available software (MIM Vista, version 6.9.2, MIM Software Inc.). A spherical volume of interest (VOI) was drawn manually on the primary tracheal lesion on the FDG PET images. The MTV was calculated as the volume of the FDG-avid area with SUV ≥ 2.5. When the VOI defined by the threshold of SUV 2.5 extended beyond the lesion boundaries as seen on CT, we made manual adjustments. TLG was calculated as (MTV) × (SUVmean).
Statistical Analysis
The following variables were retrieved from clinical records: age, sex, tumor location, tumor size, pathologic type, degree of tumor invasion, stage, treatments, and clinical outcomes. Overall survival (OS) served as the outcome measure. OS was defined as the time from the date of diagnosis to the date of death from any cause or the date of the last follow-up.
Differences in MTV, TLG, SUVmean, and SUVmax of various clinical indicators between different groups were assessed using nonparametric tests. All continuous variables in the survival analysis were categorized by specific cutoff values derived from receiver operating characteristic (ROC) analysis. ROC curve analyses were performed for the variables that were predictive of OS. Kaplan-Meier (KM) method was used to assess differences in OS stratified by each variable and to draw a survival curve according to each variable in the different groups. Univariate and multivariate survival analyses were conducted using Cox proportional hazards model. Variables exhibiting prognostic significance (defined as a p value < 0.05) in the univariate survival analysis were then entered into multivariate survival analyses. A multivariate regression model was constructed using Cox proportional hazards regression method with a forward stepwise regression approach. Data were analyzed using SPSS version 21.0 (IBM Corp.). Two-tailed p values < 0.05 were considered statistically significant.
RESULTS
Patient Demographics and Clinical Follow-Up Data
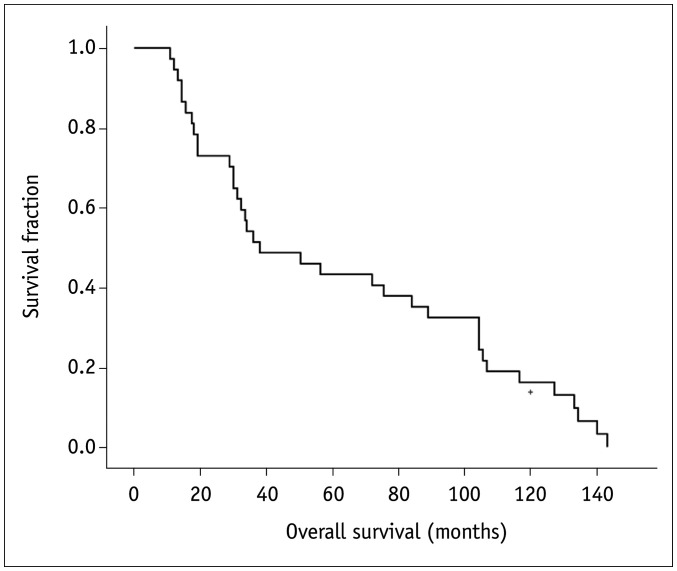
Our sample population included 37 patients (9 females and 28 males) aged 21–80 years (median age, 56.5 years). They were all referred for evaluation of tracheal lesions, which were ultimately diagnosed as primary tracheal malignant tumors. The follow-up period ranged from 10.8 months to 143.2 months (median, 43.2 months). Thirty-seven deaths occurred during this follow-up period. In terms of histology, squamous cell carcinoma was diagnosed in most patients, affecting a total of 16 patients (43.2%). Nine patients (24.3%) were diagnosed with adenoid cystic carcinomas, and only three patients (8.1%) were diagnosed with adenocarcinoma. The remaining nine patients (24.3%) were diagnosed with other histological types, including three patients of mucoepidermoid carcinoma, three patients of carcinoid tumors, one case of large-cell neuroendocrine carcinoma, and two patients of lymphoepithelioma-like carcinoma. The median survival time of the 37 patients with tracheal tumors was 38.0 months, with a 95% confidence interval (CI) of 10.8–65.2. The 3-year, 5-year, and 10-year survival rates were 54.1%, 43.2%, and 16.2%, respectively (Fig. 1). In terms of treatment, most patients (62.2%, 23/37) received surgical treatment. Seven patients underwent radical surgery, fifteen patients underwent electrocautery ablation, one patient underwent local resection, and fourteen patients did not undergo surgery. Eight patients only received surgical treatment, fifteen patients underwent surgery plus radiation or chemotherapy, while fourteen cases received radiation or/and chemotherapy. Details on population characteristics are available in Table 2.
Fig. 1. The overall survival of the tracheal tumor patients.
Table 2. The Characteristics, the Univariate and Multivariate Analysis of the Primary Tracheal Malignant Tumor.
| Covariate | n | Median Survival (Month) (95% CI) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Age (years) | ||||||
| < 56.5 | 19 | 104.400 (83.590–125.210) | 1 | |||
| > 56.5 | 18 | 31.200 (26.081–36.319) | 2.702 (1.320–5.529) | 0.007 | 0.836 (0.263–2.657) | 0.761 |
| Sex | ||||||
| Male | 28 | 34.000 (9.109–58.891) | 1 | |||
| Female | 9 | 72.000 (0.000–171.341) | 1.176 (0.541–2.558) | 0.683 | ||
| MTV (mL) | ||||||
| < 5.19 | 15 | 104.400 (85.310–123.490) | 1 | 1 | ||
| > 5.19 | 22 | 28.800 (14.459–43.141) | 2.701 (1.303–5.598) | 0.008 | 7.428 (1.586–34.789) | 0.011 |
| TLG (g) | ||||||
| < 16.94 | 19 | 104.400 (86.697–122.103) | 1 | 1 | ||
| > 16.94 | 18 | 19.200 (16.721–21.679) | 13.891 (4.399–49.041) | < 0.001 | 11.904 (1.482–95.635) | 0.020 |
| SUVmean | ||||||
| < 2.46 | 15 | 105.600 (92.118–119.082) | 1 | |||
| > 2.46 | 22 | 28.800 (16.665–4.935) | 5.274 (2.412–11.536) | < 0.001 | ||
| SUVmax | ||||||
| < 5.24 | 18 | 104.400 (83.189–125.611) | 1 | 1 | ||
| > 5.24 | 19 | 19.200 (3.422–34.557) | 4.167 (1.999–8.686) | < 0.001 | 1.613 (0.366–7.117) | 0.528 |
| Lesion location | 0.390 | |||||
| Cervical | 1 | 75.600 | 1 | |||
| Upper thoracic | 12 | 34.000 (21.779–46.221) | 1.278 (0.167–9.750) | 0.813 | ||
| Lower thoracic | 24 | 38.000 (0.000–99.933) | 1.672 (0.802–3.485) | 0.170 | ||
| Long-axis diameter (cm) | ||||||
| < 1.95 | 19 | 105.165 (67.795–110.205) | 1 | |||
| > 1.95 | 18 | 13.864 (10.827–65.173) | 2.086 (1.060–4.103) | 0.033 | ||
| Histology | 0.001 | 0.037 | ||||
| Adenoid cystic carcinomas | 9 | 106.800 (15.640–197.960) | 1 | 1 | ||
| Adenocarcinoma | 3 | 32.400 (28.559–36.241) | 1.419 (0.524–3.843) | 0.491 | 2.841 (0.860–9.383) | 0.087 |
| Squamous cell | 16 | 18.000 (15.648–20.352) | 4.992 (1.970–12.654) | 0.001 | 7.213 (1.803–28.853) | 0.007 |
| Others | 9 | 104.400 (90.171–118.629) | 3.545 (0.870–14.442) | 0.077 | 6.199 (0.824–39.202) | 0.060 |
| Extension | < 0.001 | 0.038 | ||||
| E1 | 20 | 104.400 (82.357–126.443) | 1 | 0.001 | 1 | |
| E2 | 12 | 18.000 (0.000–37.350) | 4.964 (1.627–15.142) | 0.005 | 6.613 (1.060–41.260) | 0.043 |
| E3 | 5 | 21.033 (0.000–74.824) | 3.930 (1.780–8.679) | 0.001 | 5.035 (1.457–17.406) | 0.011 |
| E4 | 0 | - | - | - | - | - |
| Lymph node | < 0.001 | |||||
| N0 | 25 | 89.000 (60.799–117.201) | 1 | 1 | ||
| N1 | 12 | 18.000 (0.000–42.443) | 5.844 (2.330–14.655) | < 0.001 | 0.891 (0.226–3.511) | 0.868 |
| N2 | 0 | - | - | - | - | - |
| Treatments | 0.030 | |||||
| Surgery | 8 | 32.400 (0.000–138.839) | 1 | |||
| Surgery + radiation/chemo | 15 | 33.600 (28.551–38.649) | 0.632 (0.119–5.288) | 0.266 | ||
| Others | 14 | 89.000 (62.599–115.401) | 3.866 (1.314–11.374) | 0.014 | ||
chemo = chemotherapy, CI = confidence interval, HR = Hazard ratio, MTV = metabolic tumor volume, SUV = standardized uptake value, SUVmax = maximum SUV, TLG = total lesion glycolysis
18F-FDG PET/CT Findings
Locations
In this study, the primary tracheal malignant tumors were located in the cervical (n = 1), upper thoracic (n = 12), and lower thoracic (n = 24) segments. Significantly more masses were located in the thoracic segment than in the cervical segment (36 vs. 1).
Metabolic Indexes
The median (range) MTV, TLG, SUVmean, and SUVmax values were 6.40 (0.85–77.92) mL, 16.29 (1.07–624.34) g, 2.84 (0.96–8.90), and 5.47 (1.10–25.61), respectively. Patients with different pathological types had significantly different MTV (p = 0.007), TLG (p < 0.001), SUVmean (p = 0.001), and SUVmax (p < 0.001) results, with the squamous cell carcinoma patients exhibiting significantly higher values than the patients with other pathological types. More details on the comparison of the PET parameters among subgroups according to the clinical parameters are presented in Table 3.
Table 3. The Relationship between PET Parameters and Clinical Indices.
| Groups | MTV (mL) | TLG (g) | SUVmean | SUVmax | ||||
|---|---|---|---|---|---|---|---|---|
| Median | P | Median | P | Median | P | Median | P | |
| Age (years) | ||||||||
| < 56.5 | 3.57 | 0.078 | 7.02 | 0.21 | 2.12 | 0.001 | 3.30 | 0.003 |
| > 56.5 | 9.00 | 41.21 | 3.54 | 8.20 | ||||
| Sex | ||||||||
| Male | 5.57 | 0.835 | 14.58 | 0.804 | 2.46 | 0.788 | 6.28 | 0.362 |
| Female | 7.35 | 15.29 | 3.11 | 3.86 | ||||
| Lesion location | ||||||||
| Cervical/upper tdoracic | 9.00 | 0.158 | 41.21 | 0.047 | 3.55 | 0.340 | 8.20 | 0.210 |
| Lower tdoracic | 4.47 | 10.13 | 2.51 | 4.37 | ||||
| Long-axis diameter (cm) | ||||||||
| < 1.95 | 2.66 | < 0.001 | 5.77 | < 0.001 | 2.38 | 0.004 | 3.43 | < 0.001 |
| > 1.95 | 11.00 | 65.25 | 3.89 | 9.20 | ||||
| Histology | ||||||||
| Adenoid cystic carcinomas | 3.10 | 0.007 | 4.40 | < 0.001 | 1.42 | 0.001 | 3.80 | < 0.001 |
| Adenocarcinoma | 1.42 | 3.31 | 2.36 | 3.99 | ||||
| Squamous cell | 13.90 | 72.68 | 3.81 | 10.51 | ||||
| Otders | 2.30 | 4.02 | 2.14 | 2.84 | ||||
| Extension | ||||||||
| E1 | 3.04 | < 0.001 | 5.58 | < 0.001 | 2.30 | 0.002 | 5.29 | < 0.001 |
| Non-E1 | 19.77 | 65.26 | 3.90 | 9.21 | ||||
| Lymph node | ||||||||
| N0 | 3.71 | < 0.001 | 8.13 | < 0.001 | 2.40 | 0.007 | 3.75 | 0.006 |
| N1/N2 | 19.40 | 74.29 | 4.17 | 9.28 | ||||
Tumor Extension and Stages
Regarding tumor extension, 20 patients were categorized as E1, 12 patients were categorized as E2, five patients were categorized as E3, and no patient was categorized as E4. In addition, 25 patients were negative for nodal involvement (N0), 12 patients were categorized as N1, and no patient was categorized as N2. None of the patients had distant metastasis.
ROC Curve Analyses and Cutoff Values for Quantitative Measurements of 18F-FDG PET/CT
ROC curve analyses were performed for variables that were predictive of any cause of death. The cutoff values of all continuous variables were calculated from the ROC curve. The results of the ROC curve and area under the curve (AUC) analyses are presented in Table 4. The cutoff values of the parameters in the tumor survival analyses were defined as follows: 56.5 years for age, 5.19 mL for MTV, 16.94 g for TLG, 2.46 for the SUVmean, 5.24 for the SUVmax, and 1.95 cm for the tumor long-axis diameter. However, the AUC for the long-axis diameter was only 0.688 (p = 0.051).
Table 4. The Results of the Receiver Operating Characteristic Curve.
| Area | P | Cutoff | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| MTV (mL) | 0.847 | < 0.001 | 5.19 | 0.900 | 0.706 |
| TLG (g) | 0.894 | < 0.001 | 16.94 | 0.850 | 0.941 |
| SUVmean | 0.956 | < 0.001 | 2.46 | 0.950 | 0.824 |
| SUVmax | 0.953 | < 0.001 | 5.24 | 0.850 | 0.882 |
| Long-axis diameter (cm) | 0.688 | 0.051 | 1.95 | 0.750 | 0.765 |
| Age (years) | 0.841 | < 0.001 | 56.50 | 0.750 | 0.765 |
Survival Analysis
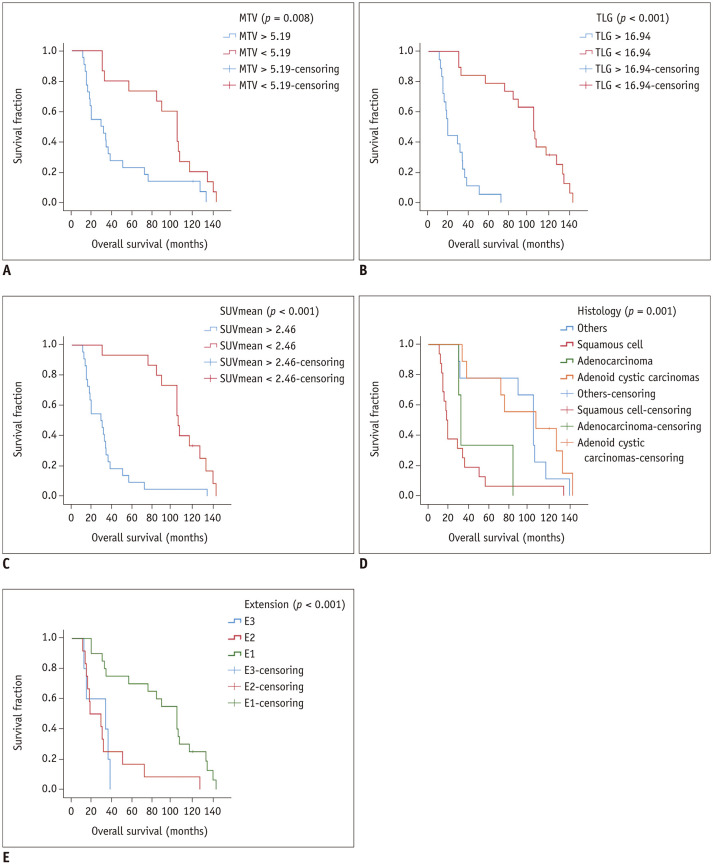
Univariate analysis showed that PET parameters (MTV, TLG, SUVmean, and SUVmax) may help to estimate OS. An MTV > 5.19 (p = 0.008) (Fig. 2A), TLG > 16.94 (p < 0.001) (Fig. 2B), SUVmean > 2.46 (p < 0.001) (Fig. 2C), and SUVmax > 5.24 (p < 0.001) were unfavorable prognostic factors for OS. The univariate analysis of clinical factors considered to be potential predictors of OS are summarized in Table 2. Univariate analysis revealed that an age greater than 56.5 years (p = 0.007) and a long-axis diameter longer than 1.95 (p = 0.033) were significantly correlated with unfavorable OS. Histology (p = 0.001) (Fig. 2D), extension categories (p < 0.001) (Fig. 2E), lymph node stage (p < 0.001), and treatments (p = 0.030) were prognostic factors for OS.
Fig. 2. The overall survival of tracheal tumor patients in different subgroups.
A. Overall survival of patients with an MTV > 5.19 and patients with an MTV < 5.19. B. Overall survival of patients with a TLG > 16.94 and patients with a TLG < 16.94. C. Overall survival of patients with an SUVmean > 2.46 and patients with an SUVmean < 2.46. D. Overall survival of patients with different histological types of tracheal tumors. E. Overall survival of patients in different extension categories. MTV = metabolic tumor volume, SUV = standardized uptake value, TLG = total lesion glycolysis
Since TLG = MTV × SUVmean, to avoid duplication, we included MTV and TLG in multivariate analyses, but not the SUVmean. Univariate analysis showed that not all treatment methods are prognostic factors, and ROC analysis showed that the AUC of the long-axis diameter was only 0.688 (p = 0.051). Therefore, we did not include the treatment methods and long-axis diameter in the multivariate analysis. All the remaining significant variables identified in the univariate analysis (MTV, TLG, SUVmax, age, pathological type, extension categories, and lymph node stage) were included in the multivariate analyses, and Cox proportional hazards model was used for the multivariate analyses to assess the potential independent predictors of OS. Our results showed that the MTV (p = 0.011), TLG (p = 0.020), pathological type (p = 0.037), and extension categories (p = 0.038) were independent prognostic factors for OS, while the prognostic factors shown in the univariate analysis, including the SUVmax (p = 0.528), age (p = 0.761), and lymph node stage (p = 0.868) were not independent prognostic factors (Table 2). Patients with squamous cell carcinoma had the shortest survival duration compared to patients with other pathological types. The survival times in the E2 and E3 groups were shorter than those in the E1 group. Univariate analysis of the E2 and E3 groups showed that the two groups did not differ significantly (p = 0.666).
A Grading System to Predict OS in Primary Tracheal Malignant Tumor Patients
Because no recognized prognostic model specifically designed for primary tracheal tumor patients is available, we proposed a potential predictive system to estimate the prognosis of patients with primary tracheal tumors. Multivariate analysis showed that MTV, TLG, histology, and extension categories were independent predictors of OS. Survival analysis showed that the OS of adenoid cystic carcinoma and adenocarcinoma patients did not differ significantly from that of patients with tumors of other pathological types but differed significantly from the OS of squamous cell carcinoma patients, with the OS of the squamous cell carcinoma patients being the shortest. Therefore, we categorized the pathological types into squamous cell carcinoma and non-squamous cell carcinoma, with squamous cell carcinoma being a risk factor. Among the E stages, survival analysis showed E2 and E3 did not differ significantly from each other; therefore, we categorized the E stages into E1 and non-E1, with non-E1 being a risk factor. Therefore, an MTV > 5.19, TLG > 16.94, squamous cell carcinoma, and non-E1 were the four risk factors. We defined the new evaluation system based on the number of risk factors, and the patients were divided into three groups—group 1: none or only one of the four risk factors, 40.5% (15/37) of the patients; group 2: two or three factors, 29.7% (11/37) of the patients; and group 3: all four risk factors, 29.7% (11/37) of the patients. Figure 3 shows a typical patient in group 3. Survival curves generated by KM analysis were used to display the differences among these three groups, and these showed a favorable capability to further discriminate among the subgroups (Fig. 4).
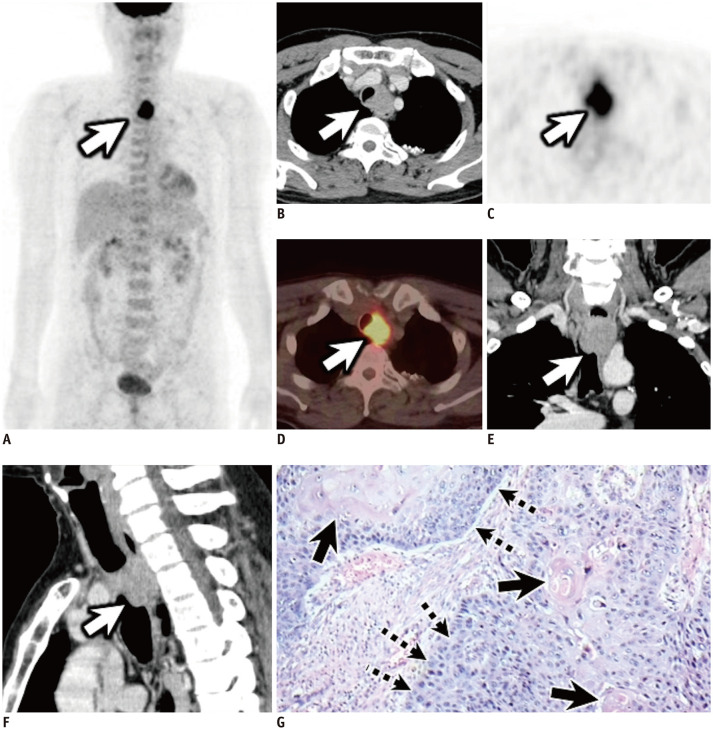
Fig. 3. A 57-year-old man diagnosed with tracheal squamous cell carcinoma.
Anterior MIP (A), transverse contrast-enhanced CT (B), transverse PET (C), transverse PET/CT (D), coronal (E), and sagittal (F) contrast-enhanced CT images show a soft tissue tracheal mass in the esophagus with ill-defined margins and intense uptake within the tumor (white arrows) (maximum SUV, 9.15; SUVmean, 3.72; MTV, 44.24; TLG, 163.75). Pathology photomicrograph (G; hematoxylin and eosin stain) shows squamous cell carcinoma (dotted arrows) with keratin pearl (black arrows). This is a patient in group 3. The patient underwent electrocautery ablation, radiotherapy, and chemotherapy. The patient died after 14.4 months. MIP = maximum intensity projection
Fig. 4. Survival curve obtained by plotting the potential prognostic nomogram for overall survival in primary tracheal malignant tumor patients.
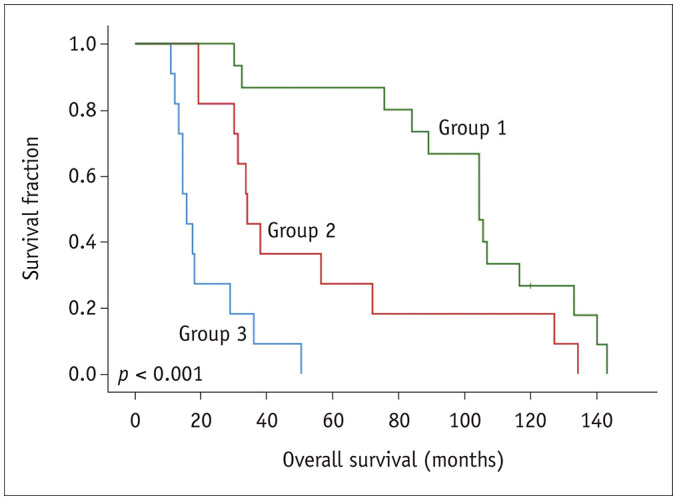
Assessment of the survival curve using the Kaplan-Meier method showed that our prognosis prediction model can effectively stratify patients with different risks factors (p < 0.001). The survival duration in the group 3 was shorter than those in the group 2, patients in group 1 had the longest survival duration compared to patients in group 2 and 3.
DISCUSSION
Primary tracheal tumors are rare and lack specific symptoms, making it easily misdiagnosed (3,10). Surgery is the main treatment for patients with tracheal tumors. Surgical methods include local resection, radical surgery, and electrocautery ablation (9,11). Adenocarcinoma and primary tracheal non-Hodgkin's lymphoma are more sensitive to radiotherapy (12,13,14,15).
Fewer studies are available on the application of PET/CT for primary tracheal malignant tumors; thus, no consensus is available on the prognostic value of PET/CT in primary tracheal malignant tumors. Our study showed that 18F-PET/CT could predict the survival of primary tracheal malignant tumor patients. Univariate analysis showed that PET/CT-relevant parameters, including MTV (p = 0.008), TLG (p < 0.001), SUVmean (p < 0.001), and SUVmax (p < 0.001), could predict the OS of tracheal tumor patients. The prognosis of small tumors with low 18F-FDG uptake is better than that of large tumors with high 18F-FDG uptake. An MTV > 5.19, TLG > 16.94, SUVmean > 2.46, and SUVmax > 5.24 are risk factors for a poor prognosis. Additionally, multivariate analysis showed that MTV (p = 0.004) and TLG (p = 0.020) were independent predictive factors for OS.
18F-FDG uptake differed among tumors of different pathological types, with the metabolism of squamous cell carcinoma being higher than that of other pathological types. Squamous cell carcinoma patients exhibit significantly higher SUVmax values than patients with other pathological types (16). In our study, the median SUVmax values of squamous cell carcinoma, adenocarcinoma, and adenoid cystic carcinoma were 10.51, 3.99, and 3.80, respectively. Additionally, the prognosis differed among tumors of different pathological types. Our study showed that the prognosis of squamous cell carcinoma was also poorer than that of adenocarcinoma and adenoid cystic carcinoma. The results of our study are consistent with those obtained in the study by He et al. (9) and Regnard et al. (17) analyzed differences in the prognosis of adenoid cystic carcinoma and other malignant tracheal tumors and found that the prognosis of adenoid cystic carcinoma was better than that of other malignant tumors.
In a study involving 99 patients with tracheal tumors, Bhattacharyya (8) showed that the median OS and five-year survival rates were 30 months and 40.0%, respectively. The outcomes of patients in the present study were better than those of patients in their study, with median survival, five-year survival, and 10-year survival rates of 38.0 months, 43.2%, and 16.2%, respectively. In a study involving 287 patients with tracheal tumors conducted by He et al. (9), the median survival, five-year survival rate, and 10-year survival rate were 57 months, 48.9%, and 22.2%, respectively, and the survival outcomes in their study were better than those in our study.
Univariate analysis in our study showed that both tumor extension and lymph node stage could predict the survival of tracheal malignant tumor patients, and a late stage was a risk factor for a poor prognosis. Multivariate analysis showed that tumor extension is an independent prognostic factor; therefore, tumor extension has the greatest prognostic value, and the prognosis of patients with E1 was better than that of with non-E1.
Since no comprehensive prognostic evaluation system is currently available, we attempted to establish a new prognostic model. The factors in this prognostic model include the independent factors shown to have prognostic value upon multivariate analysis. Multivariate analysis showed that MTV, TLG, histology, and extension categories were independent factors for predicting OS. We categorized the patients into different risk groups according to the number of risk factors they had. Assessment of the survival curves using the KM method indicated that our prognosis prediction model could effectively stratify patients with different risks factors.
Limitations
Some limitations exist in our study. Our study was retrospective in nature. Because tracheal tumors are rare, the sample size in this study was small, which may have caused some bias in our results. Therefore, further studies are required to validate our prediction model. Additionally, the patients in our study had heterogeneous pathologies. This might have also resulted in some bias in our results.
In conclusion, our study showed that PET/CT had an excellent prognosis prediction value for primary tracheal malignant tumors. More importantly, we established a prognosis prediction model for primary tracheal malignant tumors. This prediction model includes four risk factors: MTV, TLG, histology, and extension, and our prognosis prediction model can effectively stratify patients with different risks factors.
Footnotes
This work was supported by grants from the Joint Funds of Basic and Applied Basic Research Foundation of Guangdong Province of China (2019A1515110377), Guangdong Medical Research Foundation (A2019340) and Guangdong Science and Technology Department (2017ZC0250).
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Meyers BF, Mathisen DJ. Management of tracheal neoplasms. Oncologist. 1997;2:245–253. [PubMed] [Google Scholar]
- 2.Azar T, Abdul-Karim FW, Tucker HM. Adenoid cystic carcinoma of the trachea. Laryngoscope. 1998;108:1297–1300. doi: 10.1097/00005537-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol. 2011;34:32–37. doi: 10.1097/COC.0b013e3181cae8ab. [DOI] [PubMed] [Google Scholar]
- 4.Nouraei SM, Middleton SE, Nouraei SA, Virk JS, George PJ, Hayward M, et al. Management and prognosis of primary tracheal cancer: a national analysis. Laryngoscope. 2014;124:145–150. doi: 10.1002/lary.24123. [DOI] [PubMed] [Google Scholar]
- 5.Yang KY, Chen YM, Huang MH, Perng RP. Revisit of primary malignant neoplasms of the trachea: clinical characteristics and survival analysis. Jpn J Clin Oncol. 1997;27:305–309. doi: 10.1093/jjco/27.5.305. [DOI] [PubMed] [Google Scholar]
- 6.Weber AL, Shortsleeve M, Goodman M, Montgomery W, Grillo HC. Cartilaginous tumors of the larynx and trachea. Radiol Clin North Am. 1978;16:261–267. [PubMed] [Google Scholar]
- 7.Wood DE. Management of malignant tracheobronchial obstruction. Surg Clin North Am. 2002;82:621–642. doi: 10.1016/s0039-6109(02)00025-7. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya N. Contemporary staging and prognosis for primary tracheal malignancies: a population-based analysis. Otolaryngol Head Neck Surg. 2004;131:639–642. doi: 10.1016/j.otohns.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 9.He J, Shen J, Huang J, Dai C, Liang W, Ye M, et al. Prognosis of primary tracheal tumor: a population-based analysis. J Surg Oncol. 2017;115:1004–1010. doi: 10.1002/jso.24611. [DOI] [PubMed] [Google Scholar]
- 10.Macchiarini P. Primary tracheal tumours. Lancet Oncol. 2006;7:83–91. doi: 10.1016/S1470-2045(05)70541-6. [DOI] [PubMed] [Google Scholar]
- 11.Rehman S, Lovvorn HN, 3rd, Rickman OB, Wootten CT, Chinnadurai S. Unique application of awake tracheoscopy and endobronchial ultrasound in the management of tracheal mucoepidermoid carcinoma. Head Neck. 2018;40:E58–E61. doi: 10.1002/hed.25147. [DOI] [PubMed] [Google Scholar]
- 12.Chow DC, Komaki R, Libshitz HI, Mountain CF, Ellerbroek N. Treatment of primary neoplasms of the trachea. The role of radiation therapy. Cancer. 1993;71:2946–2952. doi: 10.1002/1097-0142(19930515)71:10<2946::aid-cncr2820711010>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Maziak DE, Todd TR, Keshavjee SH, Winton TL, Van Nostrand P, Pearson FG. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg. 1996;112:1522–1532. doi: 10.1016/S0022-5223(96)70011-9. [DOI] [PubMed] [Google Scholar]
- 14.Luick ML, Hansen EK, Greenberg MS, Kim R, Owens M, Moore CJ, et al. Primary tracheal non-Hodgkin's lymphoma. J Clin Oncol. 2011;29:e193–e195. doi: 10.1200/JCO.2010.32.0309. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal S, Jackson C, Celie KB, Dodhia C, Monie D, Monzon J, et al. Survival trends in patients with tracheal carcinoma from 1973 to 2011. Am J Otolaryngol. 2017;38:673–677. doi: 10.1016/j.amjoto.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Wang SY, Wang SX, Liao JQ, Chen G. 18F-FDG PET/CT and contrast-enhanced CT of primary malignant tracheal tumor. Clin Nucl Med. 2016;41:595–605. doi: 10.1097/RLU.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 17.Regnard JF, Fourquier P, Levasseur P. Results and prognostic factors in resections of primary tracheal tumors: a multicenter retrospective study. The French Society of Cardiovascular Surgery. J Thorac Cardiovasc Surg. 1996;111:808–814. doi: 10.1016/s0022-5223(96)70341-0. [DOI] [PubMed] [Google Scholar]