Abstract
Interstitial lung abnormalities (ILAs) are radiologic abnormalities found incidentally on chest CT that are potentially related to interstitial lung diseases. Several articles have reported that ILAs are associated with increased mortality, and they can show radiologic progression. With the increased recognition of ILAs on CT, the role of radiologists in reporting them is critical. This review aims to discuss the clinical significance and radiologic characteristics of ILAs to facilitate and enhance their management.
Keywords: Interstitial lung abnormalities, Interstitial lung disease, Idiopathic pulmonary fibrosis, Smoking
INTRODUCTION
There is a growing awareness of the clinical significance of interstitial lung abnormalities (ILAs) incidentally found on chest CT (1). A possible association between ILAs and idiopathic pulmonary fibrosis (IPF) has been discussed in the literature (2,3,4), and there are reports that ILAs can progress to clinically significant interstitial lung disease (ILD) (3,5). Further, ILA and ILD should be distinguished, as their image features and disease progression may overlap.
With the recent development of anti-fibrotic therapy for patients with IPF, identifying patients with ILAs has become important for their effective management (6). However, the definition of ILA is still unclear, and a standardized definition is imperative to facilitate consistent reporting of ILAs (1). Detecting an ILA is important, not only for thoracic radiologists but also for general radiologists or trainees, because it is a subclinical CT finding that can progress to conspicuous pulmonary fibrosis. The purpose of this review is to discuss the clinical significance of ILAs and their radiologic characteristics.
Background and Definition of ILAs
An ILA is a radiologic abnormality on chest CT thought to be an early or mild form of pulmonary fibrosis. In the 1980s, thoracic radiologists developed the phrase “dirty lung” to describe parenchymal inflammation and remodeling evident on the chest radiographs of smokers (7). Currently, with the increased use of chest CT to screen for lung cancer (8), ILAs are regarded as subtle or mild parenchymal abnormalities identified on CT scans in patients without known ILD (1,9,10,11). ILAs are incidental CT findings in patients with no clinical symptoms or lung function decline, which may include a subclinical stage of progressive fibrotic lung disease such as IPF. Several terms have been used to describe ILA, such as subclinical ILD or asymptomatic ILD. A study showed that ILA was appropriately reported on CT in only 64% of patients who had it; it was reported as incidental findings of no significance, findings of uncertain clinical significance, or age-related changes of no clinical significance (12). Because of the increased awareness of the clinical importance of ILAs on chest CT, it has been emphasized that the definition of ILAs should be standardized. A standardized definition for ILAs is necessary for determining its prevalence and understanding its clinical associations among several cohorts (3).
While progress has been made on the definition of ILAs (1,13), the classification of ILAs as fibrotic or nonfibrotic ILA is not yet clearly categorized. Jin et al. (14) defined fibrotic ILA as a reticular abnormality with or without honeycombing, and 39% of ILA cases in the lung cancer screening population were considered to be fibrotic. Meanwhile, in a study by Araki et al. (15), more than 80% of ILA cases were reticular, but less than 10% were categorized as “definite fibrosis.” Similarly, Putman et al. (16) did not include a reticular abnormality as “definite fibrosis.” Because a fibrotic ILA is more strongly associated with image progression and increased mortality (14,16), its definition, as well as classification, should be standardized.
Prevalence of ILAs
The prevalence of IPF is estimated to range from 1 to 63 per 100000 for the general population (17). Meanwhile, the reported prevalence of ILAs varies, ranging from 0.8–22.8% in smokers and 2–7% in non-smokers (5,10,14,18,19,20,21). ILAs are more prevalent in smokers and patients older than 50 years who exhibit MUC5B promoter polymorphism positivity; the prevalence has been reported to increase with age (10,14,19,20). Possible explanations for the differences in prevalence include the definition of ILAs, which has not yet been established, and the various inclusion criteria used in studies. Putman et al. (19) reported that ILAs were present in 7% of participants in the Age Gene/Environment Susceptibility-Reykjavik study (mean age: 76, general population study). Washko et al. (22) showed that ILAs were prevalent in 10% of participants in a chronic obstructive pulmonary disease (COPD) Gene study (mean age: 61 years; median smoking intensity: 36.7 pack-years). Another study by Jin et al. (14) reported that ILAs were found in about 10% of participants in the National Lung Screening Trial population (age: 55–74 years, ≥ 30 pack-years of smokers). In the Danish Lung Cancer Screening Trial (age: 50–70 years, current or former smokers with > 20 pack-years), ILAs were found in approximately 16.7% of participants on baseline CT scans (21).
Several studies have been conducted on the prevalence of ILAs, but they could not accurately distinguish between an ILA and early-stage IPF. These conditions share similar syndromes; they have overlapping imaging findings, restrictive physiological impairments, increased prevalence of histopathological findings, genetic predictors, and profibrotic biomarkers (23). The important thing is that ILA is reported to be more prevalent than IPF (≒0.5% of the population aged > 65 years) (24,25), and not all ILA cases progress to IPF. One of the essential roles of radiologists is to detect ILAs on chest CT scans and determine which of the ILAs can be considered nonspecific and which are clinically relevant to progressive fibrotic lung disease. A recent paper that reported on Korean Lung Cancer Screening recommended that ILAs should be reported as significant abnormalities on lung cancer screening CT (26); with the increasing number of nation-wide lung cancer screening CT scans, reporting ILAs will become more important, and the role of radiologists will be further emphasized.
Clinical Aspects of ILAs
There has been increased recognition that ILAs may have important clinical associations, even for patients with no previous history of ILD. Clinically, ILAs are associated with older age, smoking, genetic abnormalities, and restrictive lung deficits (19,27). Despite being often undiagnosed and asymptomatic, ILAs among smokers have been associated with reduced lung volumes (10), reduced diffusion capacity of carbon monoxide, and reduced exercise capacity (11). In addition, ILAs are associated with an increased risk of death, specifically death from respiratory failure (19), poor clinical outcomes in advanced lung cancer (28), or postoperative complications of major surgeries (29,30). These results emphasize that it is reasonable to recommend a clinical evaluation to identify any physiologic impairment or an underlying cause of ILD, even if subjects are asymptomatic. For subjects with higher risks of progression, prolonged follow-up and monitoring should also be recommended.
The investigation of ILAs may lead to additional research that may reveal the mechanisms linking pulmonary fibrosis and lung carcinogenesis. Some individuals with ILAs develop clinically significant pulmonary fibrosis, an established risk factor for lung cancer (14). Among the 25041 participants undergoing low-dose CT imaging included in the National Lung Cancer Screening Trial, ILAs were associated with a higher lung cancer incidence in adjusted analyses (incidence rate ratio: 1.33; 95% confidence interval [CI]: 1.07–1.65) (31). According to this research, ILAs, like IPF, is an independent risk factor for the development of lung cancer in smokers. With respect to the association with IPF, ILAs are associated with the highest genetic risk locus for IPF, and there are several similarities between the clinical syndromes and the physiologic abnormalities evident in subjects with an ILA and those with IPF (2,27). It is well-known that ILAs are associated with a MUC5B promoter variant, which provides strong evidence for a previously described IPF loci (20). This result can predict common genetically driven biologic pathways between ILAs and IPF (2).
Because of the similarities between ILAs and IPF, nonexpert radiologists and clinicians can be confused about distinguishing early stage of IPF and ILAs. A progressive fibrotic ILA represents a subtype of ILAs; it is subclinical, but it has a high risk of progression to symptomatic fibrotic ILD, of which IPF is one form (6). Therefore, there is not yet clear evidence of whether early treatment for ILAs are is more beneficial to patient survival. However, if a patient develops symptoms or lung function decreases, the case will no longer be an ILA; it should be considered as a disease and efforts should be made for accurate diagnosis and treatment.
Radiologic Findings of ILA
Radiologic Definition of ILAs
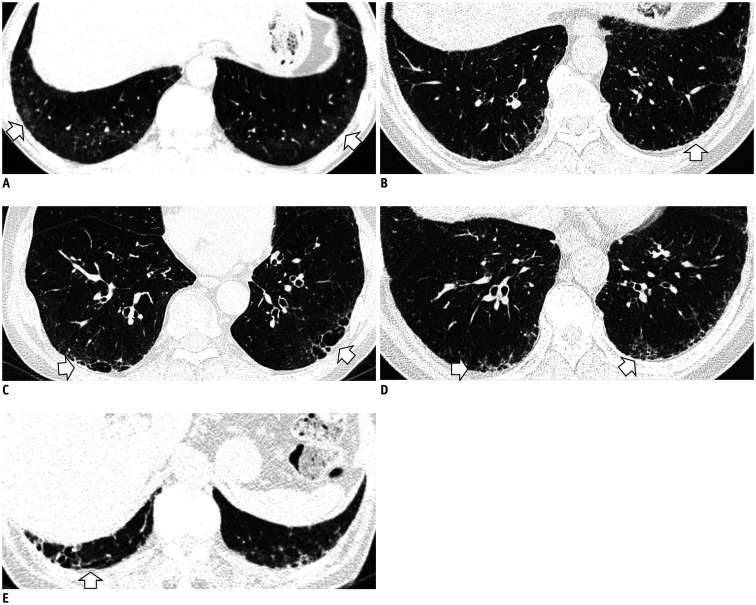
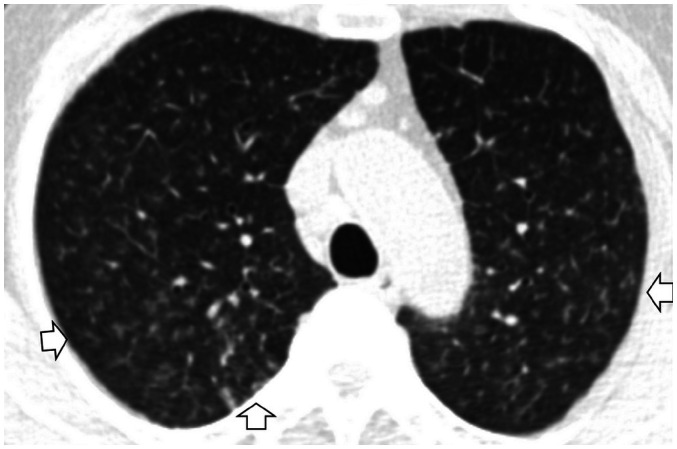
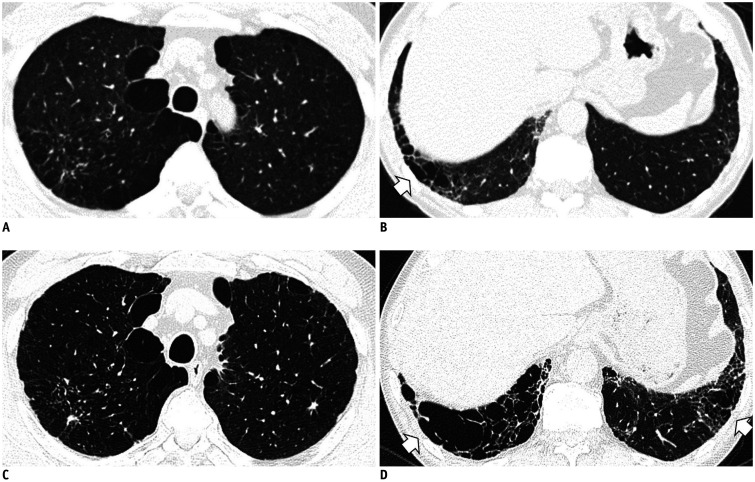
The term ILA was first described by Washko et al. (22) to determine the relationship between a radiographic ILA and lung function impairment in the COPDGene study. They defined ILAs as non-dependent changes affecting more than 5% of any lung zone, which included nondependent ground-glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, and traction bronchiectasis. However, centrilobular nodularity, which is common in smoking-related respiratory bronchiolitis, is usually not associated with pulmonary fibrosis (32). Jin et al. (14) did not consider centrilobular nodularity alone to be sufficient evidence for ILAs, and Putman et al. (16) reported that imaging patterns of centrilobular nodules decreased the likelihood of progression (odds ratio: 0.2; 95% CI: 0.1–0.5), and they were not associated with mortality. Based on these findings, it is likely that ILA should be defined in a format that excludes centrilobular nodularity from the existing description. Figure 1 shows typical imaging features of ILAs, mainly illustrating the following findings: non-dependent ground-glass attenuation (GGA), reticular abnormality, nonemphysematous cyst, honeycombing, and traction bronchiectasis.
Fig. 1. Typical image patterns demonstrating ILA.
A. A representative axial CT image showing bilateral subpleural GGA (arrows). To confirm a dependent opacity, this CT was taken in a prone position. B. CT image taken in the prone position shows bilateral subpleural reticulation in both lungs (arrow). C. Subpleural non-emphysematous cysts in both lungs. These present as thin-walled variably sized cysts on CT images (arrows). D. CT images demonstrate honeycombing in both lungs (arrows). These are clustered cystic air spaces, and their sizes are usually uniform (3–10 mm). E. Image shows traction bronchiectasis/bronchiolectasis (arrow). This can be seen as thick-walled bronchial or bronchiolar dilatation in the subpleural area of the lower lobe. GGA = ground-glass attenuation, ILA = interstitial lung abnormality
Findings not Suggesting ILAs
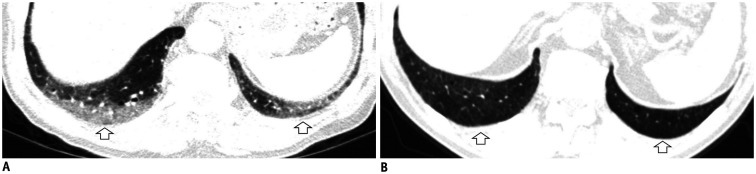
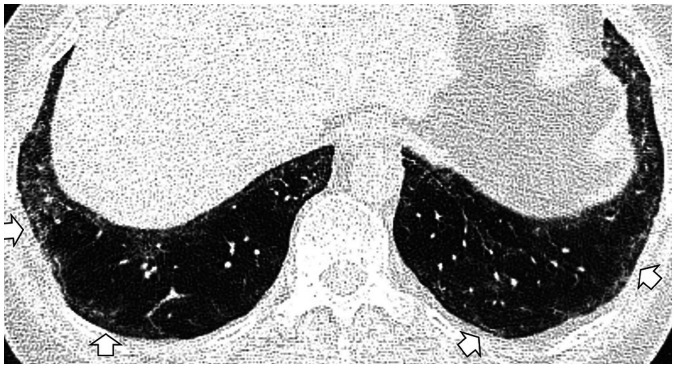
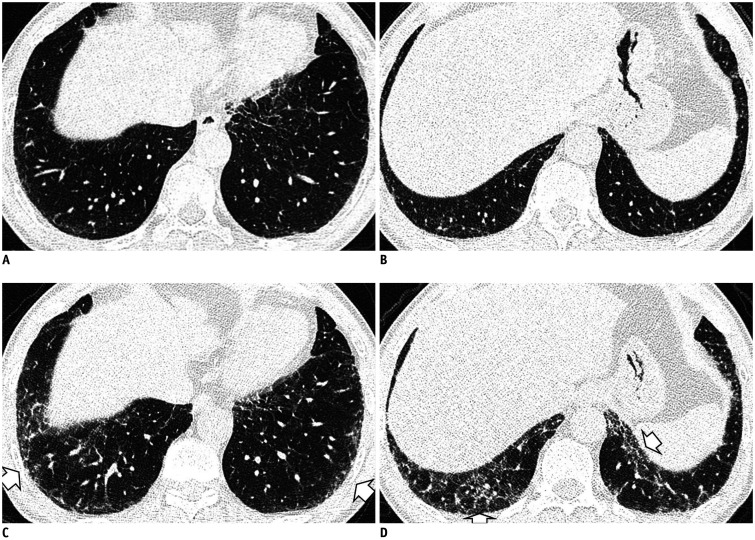
Some CT imaging features should be distinguished from ILAs. Dependent lung atelectasis is a common image finding that is difficult to distinguish from ILA. Dependent opacity is defined by an amorphous increase in attenuation of the dependent lung (in the supine position, the posterobasal segments of the lower lobes) (33), and it is affected by the state of lung inflation (34). It can be confirmed by CT obtained in the prone position to evaluate reversibility (Fig. 2), or it may convincingly mimic a nonfibrotic ILA.
Fig. 2. Dependent opacity of the lung.
A. CT image obtained in the supine position shows diffuse GGA in the subpleural area of the lower lobes and posterior basal segment (arrows). B. The GGA disappeared in the CT obtained in the prone position (arrows).
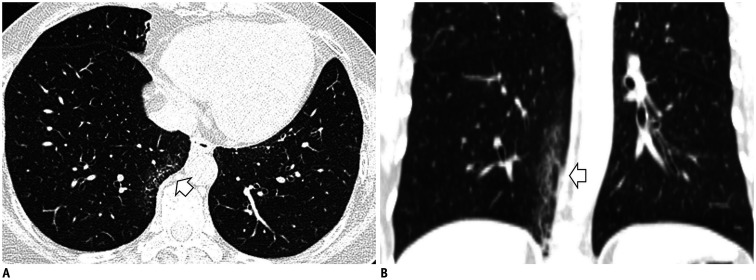
Another finding is fibrosis adjacent to spinal osteophytes. This is observed as a localized GGA or reticular pattern adjacent to osteoarthritic protrusions in the right medial lung, and it does not disappear in the prone position (33,35). Osteophytes cause focal fibrosis in adjacent pulmonary tissue; they can cause the chronic collapse of the subpleural alveolar space and fibrosis. It is easily recognized with a coronal reconstruction (Fig. 3), and it usually presents as focal and unilateral fibrosis (35).
Fig. 3. Fibrosis adjacent to spinal osteophytes.
A. Localized GGA and reticular opacity are seen in the right lower lobe's medial subpleura (arrow). B. Coronal reconstruction shows that the linear fibrotic change is elongated adjacent to the thoracic spine (arrow).
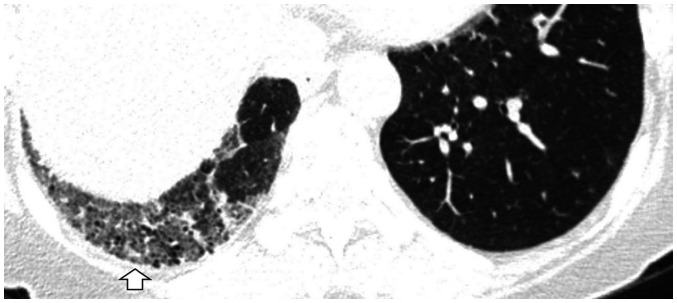
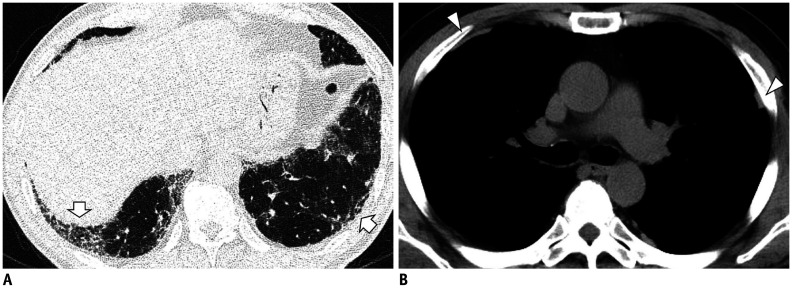
Focal or unilateral abnormalities (Fig. 4) and smoking-related centrilobular nodularities should also be excluded (Fig. 5). Because they are incidental image abnormalities, ILAs associated with connective tissue disease (CTD) (Fig. 6), known ILD with decreased lung function or symptoms, or drug/occupation exposures (Fig. 7) should be excluded. Occasionally, it is difficult to know the patient's occupational information or CTD history. However, if such a pattern is seen in young female non-smokers, it would be better to recommend additional tests to rule out the possibility of a CTD rather than an ILA. Also, efforts to find other ancillary CT findings, such as pleural plaques, will help in ensuring more accurate diagnoses of occupational lung disease (Fig. 7B).
Fig. 4. Unilateral abnormality of the lung.
CT image illustrating unilateral GGA and traction bronchiectasis of the right lower lobe (arrow). In the case of unilateral pulmonary fibrosis, it is better to consider other causes such as chronic inflammatory disease, radiation-induced injury, or circulatory disorders.
Fig. 5. Diffuse centrilobular nodularity.
CT image of a current smoker (30 pack-years) shows ill-defined diffuse centrilobular nodules in the upper lobes (arrows).
Fig. 6. ILA associated with connective tissue disease.
CT image of a 71-year-old female patient shows bilateral subpleural GGA with juxta subpleural sparing (arrows). After the evaluation of connective tissue disease, she was diagnosed as having systemic sclerosis-induced lung disease.
Fig. 7. Occupation-induced lung fibrosis.
A. High-resolution CT image shows bilateral subpleural reticulation, honeycombing, and traction bronchiectasis (arrows). B. The mediastinal window setting image demonstrates bilateral multiple noncalcified pleural plaques (arrowheads). The patient, having a history of long-standing exposure to asbestos, was diagnosed as having asbestosis and asbestos-related pleural plaques.
Subtypes of ILAs
Classifying ILA is important because some specific imaging patterns are associated with progression or risk of mortality. Recently, Putman et al. (16) created an additional subset of “definite fibrosis,” which is characterized by image findings of traction bronchiectasis and honeycombing and associated with the highest risk of progression (odds ratio: 8.4; 95% CI: 2.7–25). Thus, refining the ILA phenotype by stratifying those at a higher risk of adverse clinical outcomes is necessary. ILAs may be subtyped as fibrotic or nonfibrotic.
Fibrotic ILAs
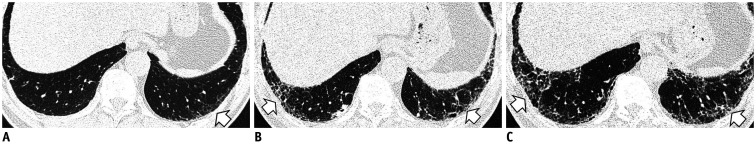
The imaging findings for fibrotic ILAs include lung distortion, traction bronchiectasis, and/or honeycombing. Previous studies demonstrated that image patterns more closely associated with usual interstitial pneumonia (UIP) pattern were more likely to progress on follow-up imaging (11,15,16). Several studies have reported on the rates of imaging progression, from 20% in 2 years (14) to 73% in 5 years (16). Jin et al. (14) reported that none of the fibrotic ILA cases improved, and 37% of them progressed at a 2-year follow-up. Putman et al. (16) showed that all participants with UIP or probable UIP patterns progressed on subsequent imaging, and patients with definite fibrosis patterns had a 70% increase in the risk of death compared with those without definite fibrosis. Figure 8 includes serial CT images showing a progression from a fibrotic ILA to the UIP pattern.
Fig. 8. Serial chest CT images show a progression of fibrotic ILA to UIP pattern in a 61-year-old male former smoker with 45 pack-years of cigarette consumption.
A. An initial image at the level of the posterior basal segment shows mild reticular opacity and bronchiolectasis in the subpleural zone (arrow). At this time, he had no respiratory symptoms, and CT was performed for the evaluation of an incidentally detected lung nodule on chest X-ray. B. A follow-up CT image after four years shows the progression of reticular opacity and newly developed honeycombing in the peripheral portion of the lower lung (arrows). C. After 9 years from the baseline CT, a progression of traction bronchiectasis and honeycombing of the lower lobe, which is a UIP pattern, is evident (arrows). UIP = usual interstitial pneumonia
Sometimes, it is difficult to distinguish nonemphysematous cysts from honeycombing observed in the UIP pattern (10,36). Previous studies have shown that nonemphysematous cysts usually present as thin-walled, inhomogeneous-sized cysts, and they are characterized by relatively asymmetric distributions and less involvement of the juxta-subpleural parenchyma (Fig. 9) (37,38,39). It is one of the imaging features that are predictive of ILA progression (odds ratio: 2.5; 95% CI: 1.3–5.1) and increased mortality (hazard ratio: 1.4; 95% CI: 1.1–1.8) (16), albeit not as strongly as the other features, including reticular markings and traction bronchiectasis. Thus, nonemphysematous cysts without architectural distortion may not be regarded as fibrotic ILAs, and continued follow-up studies and multidisciplinary discussion may help with differentiation. Therefore, correctly defining fibrotic ILA and suggesting that it has a considerable risk of developing into advanced pulmonary fibrosis and adverse clinical outcomes is one of the essential roles of radiologists.
Fig. 9. Serial chest CT images show a progression of nonemphysematous cysts in a 59-year-old male former smoker with 50 pack-years of cigarette consumption.
A, B. Initial CT images show emphysema in the upper lobes and nonemphysematous cysts (arrow) in the lower lobes. C, D. After 10 years of follow-up, CTs show an increase in the extent and size of nonemphysematous cysts in the lower lobes (arrows).
Nonfibrotic ILAs
The image findings of nonfibrotic ILAs include GGA and reticular abnormality. A recent study demonstrated that the mortality of ILA without fibrosis (hazard ratio: 1.2 compared with participants without ILA; 95% CI: 1.1–1.3) is not as high as that of an ILA with definite fibrosis (hazard ratio: 1.5; 95% CI: 1.3–1.6) cases; however, it is higher than that of non-ILA cases (16). Figure 10 illustrates CT images showing the progression of nonfibrotic ILAs over time with a decrease in lung function.
Fig. 10. Serial chest CT images show a progression of nonfibrotic ILA in a 57-year-old male former smoker with ten pack-years of cigarette consumption.
A, B. Initial CT images show mild reticular opacity in the subpleural zone. At this time, his lung function was within a normal range. C, D. Follow-up CT images after three years show the progression of reticular opacity and GGA in the peripheral portion of the lower lung (arrows). During this period, the patient experienced a decrease in total lung capacity (79% of predicted) and diffusion capacity (85% of predicted).
Pathologic Findings of ILAs
ILAs are radiologic findings in participants with subclinical stages of ILD, and little is known about their histopathologic features. Recently, a study showed that patients with ILAs had higher rates of subpleural interstitial fibrosis, fibroblastic foci, and honeycombing than those without ILAs (40). The authors suggest that an ILA may be an early stage of IPF based on the common histopathological findings similar to those of UIP. By definition, ILA includes a wide range of interstitial abnormalities, smoking-related interstitial fibrosis (SRIF) is also included in pathologic features of ILA. Therefore, since SRIF characteristics such as upper lobe involvement, subpleural and/or centrilobular fibrosis, and accompanying emphysema and respiratory bronchiolitis can be present in ILA subjects, and it will be appropriate to classify them as SRIF (Fig. 11). Because histopathologic findings of ILAs may include the spectrum of interstitial conditions from benign interstitial abnormalities to the early stage of IPF, a precise definition and detailed subgrouping are necessary for ILA to have clinical significance. Further studies will be required to determine which histopathologic findings are associated with adverse clinical outcomes or disease progression.
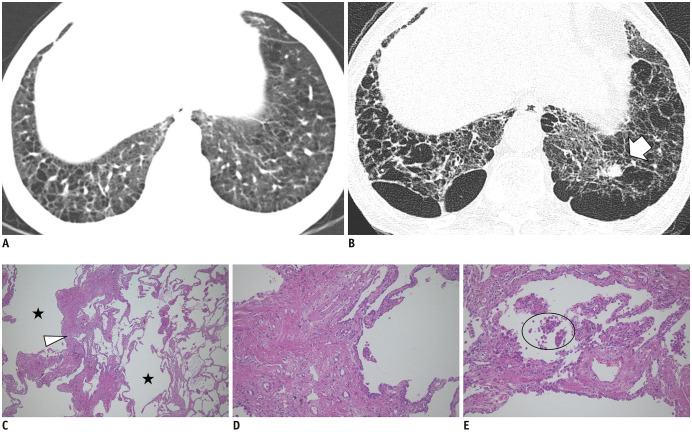
Fig. 11. Chest CT images and histopathologic findings of nonemphysematous cysts in a 60-year-old current smoker.
A. The initial image shows emphysema and nonemphysematous cysts in the lower lobes. B. After 12 years, the CT image shows the progression of emphysema and nonemphysematous cysts, with a newly developed lung nodule in the left lower lobe (arrow), which was proven to be a squamous cell carcinoma by left lower lobectomy. C. Low magnification (× 40) of the histopathologic finding shows marked interstitial thickening (arrowhead) with prominent emphysema (asterisks). D, E. High magnification (× 100) findings show interstitial thickening consists of thick collagen bundles mixed with hyperplastic smooth muscle fibers and pigmented macrophages (circle) are present in air spaces. The histopathologic findings are consistent with smoking-related interstitial fibrosis, which is a pathologic subtype of ILAs (hematoxylin & eosin staining).
CONCLUSION
ILAs are radiologic findings that can be incidentally found in subclinical or asymptomatic subjects. The role of radiologists is not only limited to detecting ILAs, but also to the proper management of ILAs. Radiologists should be aware of relevant and irrelevant radiologic findings of ILAs and review them with consideration of clinical symptoms and pulmonary function. If there is a high probability of an ILD, radiologists should recommend a general approach to ILD. While it is appropriate for ILA, radiologists should mention the possibility of disease progression and recommend continuous CT and pulmonary function follow-up. It is important to remember that ILAs are no longer nonspecific age-related findings. They should be regarded as clinically significant findings that can lead to pulmonary function deterioration and increased mortality.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Hatabu H, Hunninghake GM, Lynch DA. Interstitial lung abnormality: recognition and perspectives. Radiology. 2019;291:1–3. doi: 10.1148/radiol.2018181684. [DOI] [PubMed] [Google Scholar]
- 2.Hobbs BD, Putman RK, Araki T, Nishino M, Gudmundsson G, Gudnason V, et al. Overlap of genetic risk between interstitial lung abnormalities and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200:1402–1413. doi: 10.1164/rccm.201903-0511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells AU, Kokosi MA. Subclinical interstitial lung abnormalities: toward the early detection of idiopathic pulmonary fibrosis? Am J Respir Crit Care Med. 2016;194:1445–1446. doi: 10.1164/rccm.201607-1363ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med. 2012;185:1147–1153. doi: 10.1164/rccm.201108-1420PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh SLF, Richeldi L. Subclinical interstitial lung abnormalities: lumping and splitting revisited. Am J Respir Crit Care Med. 2019;200:121–123. doi: 10.1164/rccm.201901-0180ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser RG, Fraser RS, Renner JW, Bernard C, Fitzgerald PJ. The roentgenologic diagnosis of chronic bronchitis: a reassessment with emphasis on parahilar bronchi seen end-on. Radiology. 1976;120:1–9. doi: 10.1148/120.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369:920–931. doi: 10.1056/NEJMoa1208962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsushima K, Sone S, Yoshikawa S, Yokoyama T, Suzuki T, Kubo K. The radiological patterns of interstitial change at an early phase: over a 4-year follow-up. Respir Med. 2010;104:1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Oldham JM, Adegunsoye A, Khera S, Lafond E, Noth I, Strek ME, et al. Underreporting of interstitial lung abnormalities on lung cancer screening computed tomography. Ann Am Thorac Soc. 2018;15:764–766. doi: 10.1513/AnnalsATS.201801-053RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzilas V, Bouros D. Interstitial lung abnormalities: a word of caution. Chest. 2019;156:1037–1038. doi: 10.1016/j.chest.2019.08.2170. [DOI] [PubMed] [Google Scholar]
- 14.Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putman RK, Gudmundsson G, Axelsson GT, Hida T, Honda O, Araki T, et al. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am J Respir Crit Care Med. 2019;200:175–183. doi: 10.1164/rccm.201809-1652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol. 2013;5:483–492. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sverzellati N, Guerci L, Randi G, Calabrò E, La Vecchia C, Marchianò A, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 19.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyer N, Wille MMW, Thomsen LH, Wilcke T, Dirksen A, Pedersen JH, et al. Interstitial lung abnormalities are associated with increased mortality in smokers. Respir Med. 2018;136:77–82. doi: 10.1016/j.rmed.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunninghake GM. Interstitial lung abnormalities: erecting fences in the path towards advanced pulmonary fibrosis. Thorax. 2019;74:506–511. doi: 10.1136/thoraxjnl-2018-212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur Respir J. 2016;48:179–186. doi: 10.1183/13993003.01653-2015. [DOI] [PubMed] [Google Scholar]
- 25.Strongman H, Kausar I, Maher TM. Incidence, prevalence, and survival of patients with idiopathic pulmonary fibrosis in the UK. Adv Ther. 2018;35:724–736. doi: 10.1007/s12325-018-0693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon SH, Hong J, Hwang EJ, Kim H, Lim HJ, Suh YJ, et al. Significant abnormalities other than lung cancer in Korean lung cancer CT screening. J Korean Soc Radiol. 2019;80:837–848. [Google Scholar]
- 27.Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189:770–778. doi: 10.1164/rccm.201312-2219PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki T, Dahlberg SE, Hida T, Lydon CA, Rabin MS, Hatabu H, et al. Interstitial lung abnormality in stage IV non-small cell lung cancer: a validation study for the association with poor clinical outcome. Eur J Radiol Open. 2019;6:128–131. doi: 10.1016/j.ejro.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Im Y, Park HY, Shin S, Shin SH, Lee H, Ahn JH, et al. Prevalence of and risk factors for pulmonary complications after curative resection in otherwise healthy elderly patients with early stage lung cancer. Respir Res. 2019;20:136. doi: 10.1186/s12931-019-1087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadoch M, Kitich A, Alqalyoobi S, Lafond E, Foster E, Juarez M, et al. Interstitial lung abnormality is prevalent and associated with worse outcome in patients undergoing transcatheter aortic valve replacement. Respir Med. 2018;137:55–60. doi: 10.1016/j.rmed.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittaker Brown SA, Padilla M, Mhango G, Powell C, Salvatore M, Henschke C, et al. Interstitial lung abnormalities and lung cancer risk in the national lung screening trial. Chest. 2019;156:1195–1203. doi: 10.1016/j.chest.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 32.Remy-Jardin M, Remy J, Gosselin B, Becette V, Edme JL. Lung parenchymal changes secondary to cigarette smoking: pathologic-CT correlations. Radiology. 1993;186:643–651. doi: 10.1148/radiology.186.3.8430168. [DOI] [PubMed] [Google Scholar]
- 33.Hansell DM. Thin-section CT of the lungs: the Hinterland of normal. Radiology. 2010;256:695–711. doi: 10.1148/radiol.10092307. [DOI] [PubMed] [Google Scholar]
- 34.Lee KN, Yoon SK, Sohn CH, Choi PJ, Webb WR. Dependent lung opacity at thin-section CT: evaluation by spirometrically-gated CT of the influence of lung volume. Korean J Radiol. 2002;3:24–29. doi: 10.3348/kjr.2002.3.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otake S, Takahashi M, Ishigaki T. Focal pulmonary interstitial opacities adjacent to thoracic spine osteophytes. AJR Am J Roentgenol. 2002;179:893–896. doi: 10.2214/ajr.179.4.1790893. [DOI] [PubMed] [Google Scholar]
- 36.Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–944. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 37.Chae KJ, Jin GY, Jung HN, Kwon KS, Choi H, Lee YC, et al. Differentiating smoking-related interstitial fibrosis (SRIF) from usual interstitial pneumonia (UIP) with emphysema using CT features based on pathologically proven cases. PLoS One. 2016;11:e0162231. doi: 10.1371/journal.pone.0162231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otani H, Tanaka T, Murata K, Fukuoka J, Nitta N, Nagatani Y, et al. Smoking-related interstitial fibrosis combined with pulmonary emphysema: computed tomography-pathologic correlative study using lobectomy specimens. Int J Chron Obstruct Pulmon Dis. 2016;11:1521–1532. doi: 10.2147/COPD.S107938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe Y, Kawabata Y, Kanauchi T, Hoshi E, Kurashima K, Koyama S, et al. Multiple, thin-walled cysts are one of the HRCT features of airspace enlargement with fibrosis. Eur J Radiol. 2015;84:986–992. doi: 10.1016/j.ejrad.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Miller ER, Putman RK, Vivero M, Hung Y, Araki T, Nishino M, et al. Histopathology of interstitial lung abnormalities in the context of lung nodule resections. Am J Respir Crit Care Med. 2018;197:955–958. doi: 10.1164/rccm.201708-1679LE. [DOI] [PMC free article] [PubMed] [Google Scholar]