Abstract
Objective
The clinical course of an individual patient with heart failure is unpredictable with left ventricle ejection fraction (LVEF) only. We aimed to evaluate the prognostic value of cardiac magnetic resonance (CMR)-derived myocardial fibrosis extent and to determine the cutoff value for event-free survival in patients with non-ischemic cardiomyopathy (NICM) who had severely reduced LVEF.
Materials and Methods
Our prospective cohort study included 78 NICM patients with significantly reduced LV systolic function (LVEF < 35%). CMR images were analyzed for the presence and extent of late gadolinium enhancement (LGE). The primary outcome was major adverse cardiac events (MACEs), defined as a composite of cardiac death, heart transplantation, implantable cardioverter-defibrillator discharge for major arrhythmia, and hospitalization for congestive heart failure within 5 years after enrollment.
Results
A total of 80.8% (n = 63) of enrolled patients had LGE, with the median LVEF of 25.4% (19.8–32.4%). The extent of myocardial scarring was significantly higher in patients who experienced MACE than in those without any cardiac events (22.0 [5.5–46.1] %LV vs. 6.7 [0–17.1] %LV, respectively, p = 0.008). During follow-up, 51.4% of patients with LGE ≥ 12.0 %LV experienced MACE, along with 20.9% of those with LGE ≤ 12.0 %LV (log-rank p = 0.001). According to multivariate analysis, LGE extent more than 12.0 %LV was independently associated with MACE (adjusted hazard ratio, 6.71; 95% confidence interval, 2.54–17.74; p < 0.001).
Conclusion
In NICM patients with significantly reduced LV systolic function, the extent of LGE is a strong predictor for long-term adverse cardiac outcomes. Event-free survival was well discriminated with an LGE cutoff value of 12.0 %LV in these patients.
Keywords: Non-ischemic cardiomyopathy, Late gadolinium enhancement, Cardiac outcomes
INTRODUCTION
Late gadolinium enhancement-cardiac magnetic resonance (LGE-CMR) has shown that 30–40% of patients with non-ischemic cardiomyopathy (NICM) have a mid-wall pattern of LGE that represents myocardial scarring and provides incremental prognostic information to complement the left ventricule ejection fraction (LVEF) (1,2,3,4,5). However, in NICM patients with severely reduced LV systolic function, the prevalence of LGE can be higher, reaching 71% in certain reports (6,7). Although the presence of LGE itself has predictive value for adverse cardiac outcomes in these patients, LGE quantification and cutoff value determination are also important issues.
Few studies have investigated the possible optimized cutoff value for a myocardial scar size that best stratifies NICM patients with severe LV systolic dysfunction into high- and low-risk subgroups. Two small-scale studies demonstrated the median LGE extent in NICM patients and LVEF < 35%, but they failed to show any improvement of risk stratification using LGE size beyond the presence of LGE (2,3). Although a recent meta-analysis reported that the presence and quantitative burden of LGE provided important prognostic information regarding cardiac mortality and major adverse cardiac events (MACEs) (8), heterogeneous quantitation of LGE and definition of entry criteria and outcome might limit its clinical significance. Therefore, we aimed to evaluate the prognostic value of CMR-derived myocardial LGE extent for predicting overall cardiac outcomes and determining the cutoff value to be used as a discriminator of event-free survival in NICM patients with severely reduced LVEF.
MATERIALS AND METHODS
Study Population
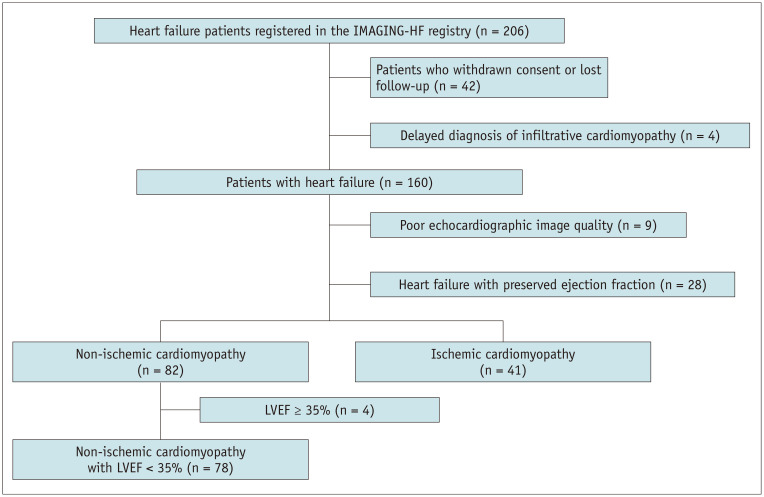
A prospective, international cardiac imaging study of heart failure (IMAGING-HF study) was conducted at two centers in Seoul, Korea, and Rochester, MN, USA, from 2009 to 2013 to evaluate the roles of CMR and echocardiography in patients with heart failure (HF). Patients who met the modified Framingham criteria for the diagnosis of HF (9,10) were included. The exclusion criteria for IMAGING-HF registry were as follows: 1) hemodynamically unstable patients; 2) patients contraindicated to undergo LGE-CMR imaging, such as patients with renal failure (estimated glomerular filtration rate < 30 mL/min), patients who were claustrophobic, and patients with pacemaker, implantable cardiac defibrillator, or metallic implants; and 3) patients who were diagnosed with hypertrophic cardiomyopathy, infiltrative heart disease, arrhythmogenic right ventricular (RV) cardiomyopathy, acute myocarditis, or significant valvular heart disease. After enrollment, each patient with LV systolic dysfunction was diagnosed with ischemic cardiomyopathy (ICM) or NICM based on coronary anatomy using coronary angiography (CAG) or coronary computed tomographic angiography as determined by the patient's primary cardiologist. ICM was defined as follows: 1) patient with a history of myocardial infarction or revascularization with percutaneous coronary intervention or coronary artery bypass graft, 2) patient having ≥ 75% stenosis in the left main or proximal left anterior descending coronary artery, or 3) patient having ≥ 75% stenosis in two or more epicardial coronary arteries. Patients who did not meet the above criteria were diagnosed with NICM. For this study, we consecutively selected 78 NICM patients who showed significant myocardial dysfunction, with LVEF < 35% on echocardiography (Fig. 1). All patients were registered after remaining stable while receiving HF medications for at least 1 month. Our study protocol was approved by the local Institutional Review Board, and all patients provided written informed consent.
Fig. 1. Patient flowchart.
IMAGING-HF = international cardiac imaging study of heart failure, LVEF = left ventricle ejection fraction
Echocardiography and Cardiac Magnetic Resonance Imaging Acquisition and Analysis
Comprehensive transthoracic echocardiography was performed using a commercially available equipment (Vivid 7 or E9, GE Healthcare). Standard M-mode, two-dimensional, color, and tissue Doppler imaging were performed. All echocardiographic measurements were performed independently by observers who were blinded to the patients' clinical characteristics and had more than 2 years of clinical experience. The LV volumes and ejection fraction were assessed using the biplane Simpson's rule via manual tracing. The pulse-wave Doppler transmitral inflow velocity was obtained from the apical four-chamber view to assess diastolic function. For strain analysis, two-, three-, and four-chamber views were obtained at the LV apex. An off-line speckle-tracking analysis using a customized software (EchoPAC PC 7.05, GE Healthcare) was performed by one independent researcher. Global longitudinal strain was automatically provided as the average value of the regional peak systolic longitudinal strain of the three apical views.
All enrolled patients underwent CMR studies using a 1.5T scanner (MAGNETOM Avanto and Syngo MR B15 or B17 version, Siemens Healthineers) with a 32-channel phased-array receiver coil. After localization, cine images for LV mass and volume were acquired using a steady-state free-precession sequence with 8–10 contiguous short-axis slices to cover the entire LV with a slice thickness of 6 mm and gaps of 4 mm. The temporal resolution was 25–30 frames per RR interval. Standard LGE imaging was performed using the phase-sensitive inversion recovery technique after the injection of 0.15 mmol/kg Gadovist (gadobutrol, Bayer AG) with short-axis image acquisition of 10–12 slices of 6-mm thickness with a gap of 4 mm. Inversion delay times were usually 280–360 milliseconds. Each LGE pattern was evaluated at 10–15 minutes after gadolinium administration using a multi-shot turbo field echo breath-hold sequence with a phase-selective inversion recovery. Field of view and image matrix were 35 x 35 cm and 256 x 256, respectively.
All measurements were performed at the Samsung Medical Center magnetic resonance imaging core laboratory. Image analysis using a commercial software (Argus version 4.02, Siemens Healthineers) was performed by two experienced CMR imagers (training level III) who were blinded to patient data. When there were discrepancies regarding the presence of LGE between the two readers' interpretation, a third experienced reader confirmed the findings. The extent of LGE by the first observer was analyzed. According to the current guideline, end-diastolic and end-systolic frames to calculate LV volume were selected as the image with the largest (immediately before opening of the aortic valve) and the smallest LV blood volume (after closure of the aortic valve), respectively (11). Endocardial and epicardial borders were traced manually in the selected image frames. Papillary muscles and LV trabeculae were excluded from the endocardium and included in the LV cavity volume. LVEF by CMR was closely associated with echocardiography-derived LVEF (intraclass correlation coefficient with 95% confidence interval, 0.79 [0.66–0.86]; p < 0.001). LGE was considered present when the signal intensity of the index myocardial segment was greater than 5 standard deviation (SD) above the remote normal myocardial signal. The extent of LGE was calculated as the sum of the area of LGE within each segment of the short-axis images multiplied by the slice thickness to cover the entire LV and expressed as the proportion of LGE to LV myocardial volume.
Clinical Outcome and Definitions
The primary endpoint was MACE, defined as a composite of cardiac death, heart transplantation, major arrhythmic event, and hospitalization for congestive HF within 5 years after enrollment. Hospitalization for congestive HF was defined as the first readmission with worsening HF requiring medical or interventional treatment. Major arrhythmic events included sustained ventricular tachycardia, ventricular fibrillation, or appropriate implantable cardioverter-defibrillator (ICD) discharge. We set a month for the window period; therefore, any adverse event that was observed within 1 month after enrollment was excluded from the primary endpoint. Patients were routinely followed up annually for 3 years. After the 3-year follow-up period, we conducted annual telephone follow-up interviews with the subjects. For any reported event, medical reports were retrieved and reviewed by physicians.
Statistical Analyses
Categorical variables are reported as percentages, and continuous variables are presented as mean with SD or median with interquartile range. The difference between two numeric variables was analyzed by independent t test or Wilcoxon rank sum test, and the chi-squared test or Fisher's exact test was used to analyze the difference between non-numeric variables. The Kaplan-Meier analysis log-rank test was used to estimate the event-free survival curve between the two groups. Observer agreement of LGE quantification was validated using the concordance correlation coefficients. To determine independent prognoses, multivariate Cox regression analyses were performed with a model including the potential confounders (hypertension, initial systolic blood pressure [BP], white blood cell [WBC] count, plasma sodium, glucose, N-terminal probrain natriuretic peptide [NT-proBNP], QRS duration, left atrial [LA] volume index, and LGE extent) that showed p < 0.2 in a univariate analysis. The receiver operating characteristic (ROC) curve was determined, and the model included the optimal cutoff values of the independent predictors (p < 0.05) determined by Youden's J statistics and was validated by bootstrap with 10000 iterations (12). All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software version 17.0 (SPSS Inc.). P values were two-tailed, and p values < 0.05 were considered significant.
RESULTS
Among the 78 NICM patients with LVEF < 35% (age, 54.9 ± 13.6 years; 64.1% male), 27 (34.6%) reached the primary endpoint. Thereof, 6 (7.7%) patients died from cardiac cause, 9 (11.5%) underwent heart transplantation, 19 (24.4%) were hospitalized due to worsening of HF, and 2 (0.3%) experienced major arrhythmic events. Cardiac resynchronization therapy with defibrillator was implanted in 15.4% of the patients, 3 and 9 of whom received the therapy within the window period (within 1 month after enrollment) and during the follow-up period, respectively. The median LVEF of all enrolled patients was 25.4% (19.8–32.4%). Table 1 summarizes the baseline characteristics of the patients. Patients with MACE had lower initial BP than those without MACE (systolic BP, 101.9 ± 14.2 mm Hg vs. 110.6 ± 14.4 mm Hg, respectively, p = 0.012; diastolic BP, 65.5 ± 11.5 mm Hg vs. 73.4 ± 13.5 mm Hg, respectively, p = 0.012). Patients with MACE tended to have higher levels of NT-proBNP than those without MACE, but the difference between the two groups was not statistically significant. Discharge medications that were prescribed after the first admission for HF evaluation were similar between the two groups. Data acquired from electrocardiography (ECG) and imaging studies are shown in Table 2. On ECG, QRS duration was significantly longer in patients with MACE than that in patients without MACE (115.0 [99.0–158.5] msec vs. 102.0 [94.0–116.0] msec, respectively, p = 0.019), but it was still within the normal range. LVEF by echocardiography did not differ between the two groups (27.0% [20.0–32.0%] vs. 28.0% [22.0–32.0%], respectively, p = 0.780). LA volume was significantly larger in patients with MACE than that in patients without MACE (57.5 [43.0–80.5] mL/m2 vs. 50.0 [39.0–59.0] mL/m2, respectively, p = 0.026).
Table 1. Baseline Characteristics.
| Without MACE (n = 51) | With MACE (n = 27) | P | |
|---|---|---|---|
| Age, years | 55.9 ± 12.9 | 53.1 ± 14.9 | 0.516 |
| Male, no (%) | 34 (66.7) | 16 (59.3) | 0.621 |
| Obesity, no (%) | 22 (43.1) | 9 (33.3) | 0.400 |
| Diabetes, no (%) | 16 (31.4) | 5 (18.5) | 0.223 |
| Hypertension, no (%) | 14 (27.5) | 3 (11.1) | 0.096 |
| Dyslipidemia, no (%) | 17 (33.3) | 8 (29.6) | 0.739 |
| Smoking, no (%) | 14 (27.5) | 6 (22.2) | 0.615 |
| Previous CVA, no (%) | 1 (2.0) | 2 (7.4) | 0.274 |
| NYHA II–IV, no (%) | 49 (96.1) | 25 (92.6) | 0.606 |
| Initial SBP (mm Hg) | 110.6 ± 14.4 | 101.9 ± 14.2 | 0.012 |
| Initial DBP (mm Hg) | 73.4 ± 13.5 | 65.5 ± 11.5 | 0.012 |
| Initial heart rate (beat/min) | 78.8 ± 16.0 | 76.2 ± 19.0 | 0.528 |
| Initial laboratory findings | |||
| WBC (/μL) | 7470 ± 1807 | 6726 ± 1721 | 0.083 |
| Hemoglobin (g/dL) | 14.3 ± 1.9 | 14.0 ± 1.8 | 0.464 |
| Total cholesterol (mg/dL) | 172.3 ± 37.3 | 175.3 ± 33.4 | 0.729 |
| Sodium (mmol/L) | 140.1 ± 3.0 | 139.0 ± 2.7 | 0.135 |
| Creatinine (mg/dL) | 1.0 ± 0.3 | 1.0 ± 0.2 | 0.635 |
| Glucose (mg/dL) | 119.0 ± 35.9 | 107.6 ± 20.3 | 0.165 |
| NT-proBNP (pg/mL) | 1435.9 ± 1587.1 | 2144.3 ± 2638.9 | 0.166 |
| Initial medications | |||
| Beta blocker, no (%) | 41 (80.4) | 22 (81.5) | 0.908 |
| ACEi/ARB, no (%) | 49 (96.1) | 25 (92.6) | 0.606 |
| Diuretics, no (%) | 43 (84.3) | 22 (81.5) | 0.758 |
| Statin, no (%) | 15 (29.4) | 7 (25.9) | 0.745 |
| Aspirin, no (%) | 30 (58.8) | 13 (48.1) | 0.367 |
| Warfarin, no (%) | 2 (3.9) | 2 (7.4) | 0.606 |
| CRT-D implantation during window period | 2 (0.04) | 1 (0.04) | 1.000 |
Data are presented as n (%), mean ± SD, or median with interquartile ranges. ACEi = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor antagonist, CRT-D = cardiac resynchronization therapy with defibrillator, CVA = cerebrovascular accident, DBP = diastolic blood pressure, ICD = implantable cardioverter-defibrillator, MACE = major adverse cardiac event (cardiac death, heart transplantation, ICD shock, or hospitalization for congestive heart failure), NT-proBNP = N-terminal probrain natriuretic peptide, NYHA = New York Heart Association classification, SBP = systolic blood pressure, SD = standard deviation, WBC = white blood cell
Table 2. Electrocardiography, Echocardiography, and CMR Data.
| Without MACE (n = 51) | With MACE (n = 27) | P | |
|---|---|---|---|
| Electrocardiography | |||
| Atrial fibrillation, no (%) | 7 (13.7) | 5 (18.5) | 0.743 |
| QRS duration (msec) | 102.0 (94.0–116.0) | 115.0 (99.0–158.5) | 0.019 |
| Echocardiography | |||
| E (m/sec) | 0.7 (0.5–0.8) | 0.7 (0.5–1.0) | 0.347 |
| A (m/sec) | 0.6 (0.5–0.9) | 0.7 (0.4–0.9) | 0.722 |
| E/E' | 12.5 (10.0–16.7) | 12.9 (8.9–14.9) | 0.530 |
| LA volume index (mL/m2) | 50.0 (39.0–59.0) | 57.5 (43.0–80.5) | 0.026 |
| LVEF (%) | 27.0 (22.0–32.0) | 28.0 (22.0–32.0) | 0.780 |
| Global longitudinal strain (%) | -10.2 (-11.8– -7.7) | -10.6 (-13.6– -6.9) | 0.433 |
| CMR | |||
| LVEDV (mL) | 261.3 (215.3–339.0) | 295.4 (218.8–345.9) | 0.561 |
| LVESV (mL) | 195.6 (151.5–270.8) | 225.2 (154.2–256.6) | 0.491 |
| LVEF (%) | 24.1 (21.2–33.0) | 24.0 (21.1–28.9) | 0.535 |
| LGE, no (%) | 39 (76.5) | 24 (88.9) | 0.186 |
| LGE (%LV) | 6.7 (0–17.1) | 22.0 (5.5–46.1) | 0.008 |
Data are presented as n (%), mean ± SD, or median with interquartile ranges. CMR = cardiac magnetic resonance, LA = left atrium, LGE = late gadolinium enhancement, LV = left ventricle, LVEDV = left ventricle end-diastolic volume, LVEF = left ventricle ejection fraction, LVESV = left ventricle end-systolic volume
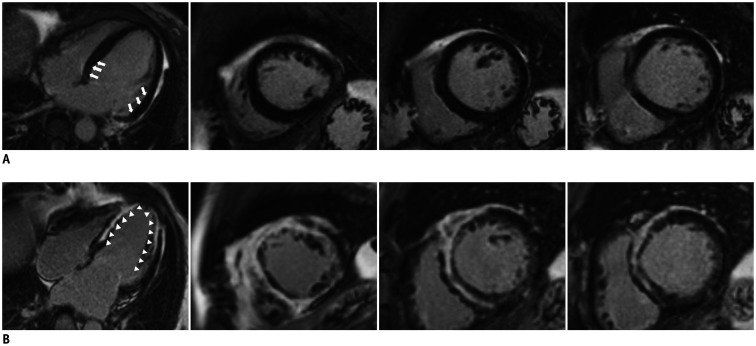
On CMR examination, 63 patients (80.8%) had LGE. Two representative cases of NICM patients with small and extensive LGE are shown in Figure 2. The reliability of LGE by two observers between analyses was excellent (concordance correlation coefficient with 95% confidence interval, 0.92 [0.88–0.95]). The extent of LGE was significantly higher in patients with MACE than that in patients without MACE (median value, 22.0 [5.5–46.1] %LV vs. 6.7 [0–17.1] %LV, respectively, p = 0.008) (Table 2). An extent of LGE more than 12.0 %LV showed a significant predictive value for adverse cardiac events in ROC analysis (area under the curve, 0.68 [0.55–0.81], p = 0.008) with both 66.7% of specificity and sensitivity. According to a multivariate analysis, high WBC count (> 7900/µL ), absence of history of hypertension, high NT-proBNP level, larger LA volume (> 70 mL/m2), and LGE extent larger than 12.0 %LV were independently associated with MACE (Table 3). The incidence of the primary endpoint was significantly different between the patients with LGE ≥ 12.0 %LV and those with LGE < 12.0 %LV (51.4% vs. 20.9%, respectively, p = 0.005). The Kaplan-Meier survival curves revealed a significantly worse cardiac outcome in patients with LGE greater than 12.0 %LV (log-rank p = 0.001) (Fig. 3).
Fig. 2. Representative cardiac magnetic resonance images.
A. A 63-year-old woman with severe LV systolic dysfunction (LVEF, 28.0%) was adverse cardiac event-free for 5 years. LGE analysis by inversion recovery showed small, linear LGE (arrows) in the basal to mid-LV segment (LGE extent, 6.7 %LV). B. A 59-year-old man with severe LV systolic dysfunction (LVEF, 29.0%) experienced hospitalization for congestive heart failure followed by heart transplantation during the follow-up period. Baseline LGE analysis by inversion recovery showed extensive LGE (arrowheads) throughout the LV (LGE extent, 68 %LV). LGE = late gadolinium enhancement
Table 3. Independent Predictors of MACEs.
| Hazard Ratio (95% CI) | P | |
|---|---|---|
| Hypertension | 0.10 (0.02–0.48) | 0.004 |
| Systolic blood pressure | 1.01 (0.97–1.05) | 0.595 |
| WBC count > 7900/μL | 0.07 (0.01–0.42) | 0.003 |
| Blood glucose | 0.99 (0.98–1.01) | 0.263 |
| Plasma sodium ≤ 140 mmol/L | 2.49 (0.99–6.23) | 0.052 |
| NT-proBNP | 1.00 (1.00–1.00) | 0.002 |
| LA volume index > 70 mL/m2 | 4.17 (1.51–11.53) | 0.006 |
| QRS duration > 106 msec | 0.84 (0.30–2.36) | 0.738 |
| LGE extent ≥ 12.0 %LV | 6.71 (2.54–17.74) | < 0.001 |
Multivariable Cox regression models were adjusted using clinically relevant variables. Adverse cardiac events were cardiac death, heart transplantation, ICD shock, and hospitalization for congestive heart failure. CI = confidence interval
Fig. 3. Kaplan-Meier survival curves.
A. Kaplan-Meier survival curves for major adverse cardiac events (cardiac death, heart transplantation, implantable cardioverter-defibrillator shock, and hospitalization for congestive heart failure). B. Kaplan-Meier survival curves for cardiac death and heart transplantation. C. Kaplan-Meier survival curves for hospitalization for congestive heart failure.
DISCUSSION
In the present study, we prospectively investigated the prognostic value of myocardial LGE on adverse cardiac outcomes in NICM patients with severely reduced LV systolic function. The average prevalence of myocardial LGE was 80.8%, which from a practical standpoint is an important prerequisite for a risk stratification test, in that merely detecting LGE might be inadequate to separate a high-risk group from a low-risk group in these patients. According to our data, total LGE extent, not just the presence of LGE, was independently and strongly associated with cardiac mortality and morbidity, and a cutoff value of 12.0 %LV was the best discriminator of event-free survival in NICM patients with severely reduced LVEF.
In NICM, which is characterized by impairment of cardiac function with the absence of significant coronary artery disease, the annual mortality rate is approximately 7%, with one-third of deaths attributable to sudden cardiac death or critical arrhythmia (13). Although lower LVEF is accepted as the strongest predictor of mortality in NICM patients (14), the identification of particularly high-risk patients within the subgroup with severe LV systolic dysfunction is challenging. Furthermore, despite the fact that ICDs are potentially indicated in patients with LVEF < 35%, most patients do not receive ICD implantation because of various procedural risks and high costs. Therefore, clinicians are currently searching for diagnostic parameters other than LVEF used to improve risk stratification, specifically CMR-LGE.
Compared with the previous studies, our patients showed a higher prevalence of LGE on CMR. Generally, the prevalence of myocardial LGE in NICM patients ranges from 26% to 71% (6,7,15,16). These reports have demonstrated that considering the heterogeneity of the inclusion criteria for patient and scar pattern, such a wide range of reported incidence was not surprising. In our study, we included LGE that show focal, patchy, or diffuse patterns in the RV insertion site (RVIS) of the interventricular septum and the mid-ventricular and epicardial wall, and the presence of myocardial fibrosis may have shown to be more frequent compared to other studies that enrolled limited LGE patterns in the mid-ventricular and epicardial wall only.
Several studies have investigated the optimized cutoff value for myocardial fibrosis that would best stratify NICM patients with severely reduced LVEF into high- and low-risk subgroups (5,6,7). However, these studies not only assessed the prognostic value for arrhythmic event only but also were controversial on whether the LGE extent had prognostic implication. Few studies have evaluated the prognostic value of LGE extent and its cutoff level to discriminate the risk for a composite of adverse cardiac events including HF-related hospitalization and death and arrhythmic events in NICM patients with severe LV systolic dysfunction (2,17,18). Consistent with our data, these studies demonstrated a strong trend toward a significant difference in outcome according to the LGE extent. However, in the previous studies, the follow-up duration was relatively short, and patients with wide range of LVEF were enrolled. Therefore, the fact that the overall therapy for HF and patientspecific therapy were not consistent decreased the power of their results. The homogeneity of our study population and relatively long follow-up duration allow our data to strengthen and clarify the prognostic value of LGE extent with concordant results.
Over the past decade, a wide range of LGE cutoff (7–17 %LV) to discriminate the risk for adverse cardiac events has been suggested (2,17,18,19). The main reasons for these various cutoff levels might be due to the different LGE analysis methods. First, the optimal method for LGE quantification is not unified. Recently, the 5 SD, 6 SD, and full-width half maximum (FWHM) threshold methods are widely used to quantify LGE (19,20,21,22). The FWHM threshold method measures smaller scars than the two SD techniques, but there are no comparisons to a gold standard of pathological examinations in NICM patients. The accuracy of 5 SD technique for the measurement of the total amount of myocardial fibrosis was well established from the comparison with histopathologic measurement (23). In addition to a recent study that suggested clinically relevant myocardial fibrosis extent using the 5 SD method in NICM patients (21), our data using the 5 SD technique might be informative to physicians. The second issue for the LGE analysis is whether to include myocardial fibrosis of RVIS in the measurement considering that whether this unique distribution of LGE can affect the prognosis in patients with cardiomyopathy is unclear. Some studies reported RVIS as myocardial disarray or interstitial fibrosis rather than replacement fibrosis (24,25). Even in NICM patients, the LGE on RVIS has been reported to have a relatively better prognosis than LGE on the other LV side (20). The LGE cutoff value may vary depending on whether it includes LGE on RVIS, and we included fibrosis of RVIS in the LGE quantification. A future study is required to confirm the clinical impact of LGE on RVIS.
In our data, the LGE extent showed a modest predictive ability for adverse events in NICM patients with severely decreased LV systolic dysfunction. Considering that various clinical factors are affected in the prognosis of HF, it might be acceptable that LGE alone does not show statistically strong predictive value. This result was similar to the results of the previous studies, which had suggested LGE extent as a prognostic parameter (17,21). It should be integrated as a factor providing incremental prognostic information and improvement in risk stratification in addition to the clinical data (26).
Study Limitations
The present study has several limitations. First, the sample size was relatively small to draw definitive conclusions. For the accurate diagnosis of the etiology of HF, we excluded patients whose CAG results could not rule out ischemic heart disease, in contrast to a previous study (5). Second, only patients eligible for CMR examination were included in the present study, and unstable patients with hemodynamic compromise or severe arrhythmia at the time of diagnosis were excluded, which might have caused some selection bias. However, these criteria allowed us to evaluate a clinically homogeneous population. Third, despite the relatively long follow-up duration, we found only a modest number of events in the study. This is possibly attributed to the inclusion criteria, which limited the study to patients who could undergo CMR. This inevitably results in including subjects whose clinical condition was relatively stable. However, this is an unavoidable factor of studies that use CMR evaluation. Fourth, the LVEF < 35% cutoff value was arbitrarily selected to include patients with severe systolic dysfunction, although this cutoff value was used also in the previous CMR study for risk stratification of NICM patients (21). Furthermore, we defined severe LV systolic dysfunction as LVEF < 35% under the consideration of the current guideline for a device therapy in NICM. Finally, contemporary CMR techniques such as T1 mapping, which might facilitate a more accurate and precise assessment of fibrotic burden with less operator dependence, were unavailable in the present data (27,28,29,30).
In summary, the extent of LGE provides prognostic information beyond the identification of the presence of scarring in NICM patients with severely reduced LV systolic function. An LGE cutoff value of 12.0 %LV well discriminated the event-free survival in these patients.
Footnotes
This study was supported by Samsung Medical Center grant #SMO1131501.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 2.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 3.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neilan TG, Coelho-Filho OR, Danik SB, Shah RV, Dodson JA, Verdini DJ, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:944–954. doi: 10.1016/j.jcmg.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perazzolo Marra M, De Lazzari M, Zorzi A, Migliore F, Zilio F, Calore C, et al. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm. 2014;11:856–863. doi: 10.1016/j.hrthm.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Gao P, Yee R, Gula L, Krahn AD, Skanes A, Leong-Sit P, et al. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:448–456. doi: 10.1161/CIRCIMAGING.111.971549. [DOI] [PubMed] [Google Scholar]
- 8.Ganesan AN, Gunton J, Nucifora G, McGavigan AD, Selvanayagam JB. Impact of late gadolinium enhancement on mortality, sudden death and major adverse cardiovascular events in ischemic and nonischemic cardiomyopathy: a systematic review and meta-analysis. Int J Cardiol. 2018;254:230–237. doi: 10.1016/j.ijcard.2017.10.094. [DOI] [PubMed] [Google Scholar]
- 9.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program (NCEP) expert panel on detection evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 11.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): board of trustees task force on standardized post-processing. J Cardiovasc Magn Reson. 2020;22:19. doi: 10.1186/s12968-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 13.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 14.Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564–1575. doi: 10.1056/NEJM199412083312307. [DOI] [PubMed] [Google Scholar]
- 15.Alba AC, Gaztañaga J, Foroutan F, Thavendiranathan P, Merlo M, Alonso-Rodriguez D, et al. Prognostic value of late gadolinium enhancement for the prediction of cardiovascular outcomes in dilated cardiomyopathy: an international, multi-institutional study of the MINICOR group. Circ Cardiovasc Imaging. 2020;13:e010105. doi: 10.1161/CIRCIMAGING.119.010105. [DOI] [PubMed] [Google Scholar]
- 16.Hombach V, Merkle N, Torzewski J, Kraus JM, Kunze M, Zimmermann O, et al. Electrocardiographic and cardiac magnetic resonance imaging parameters as predictors of a worse outcome in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2009;30:2011–2018. doi: 10.1093/eurheartj/ehp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrke S, Lossnitzer D, Schöb M, Steen H, Merten C, Kemmling H, et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011;97:727–732. doi: 10.1136/hrt.2010.205542. [DOI] [PubMed] [Google Scholar]
- 18.Neilan TG, Farhad H, Mayrhofer T, Shah RV, Dodson JA, Abbasi SA, et al. Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging. 2015;8:414–423. doi: 10.1016/j.jcmg.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pöyhönen P, Kivistö S, Holmström M, Hänninen H. Quantifying late gadolinium enhancement on CMR provides additional prognostic information in early risk-stratification of nonischemic cardiomyopathy: a cohort study. BMC Cardiovasc Disord. 2014;14:110. doi: 10.1186/1471-2261-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi JE, Park J, Lee HJ, Shin DG, Kim Y, Kim M, et al. Prognostic implications of late gadolinium enhancement at the right ventricular insertion point in patients with non-ischemic dilated cardiomyopathy: a multicenter retrospective cohort study. PLoS One. 2018;13:e0208100. doi: 10.1371/journal.pone.0208100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barison A, Aimo A, Ortalda A, Todiere G, Grigoratos C, Passino C, et al. Late gadolinium enhancement as a predictor of functional recovery, need for defibrillator implantation and prognosis in non-ischemic dilated cardiomyopathy. Int J Cardiol. 2018;250:195–200. doi: 10.1016/j.ijcard.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Mikami Y, Cornhill A, Heydari B, Joncas SX, Almehmadi F, Zahrani M, et al. Objective criteria for septal fibrosis in non-ischemic dilated cardiomyopathy: validation for the prediction of future cardiovascular events. J Cardiovasc Magn Reson. 2016;18:82. doi: 10.1186/s12968-016-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moravsky G, Ofek E, Rakowski H, Butany J, Williams L, Ralph-Edwards A, et al. Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging. 2013;6:587–596. doi: 10.1016/j.jcmg.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Chan RH, Maron BJ, Olivotto I, Assenza GE, Haas TS, Lesser JR, et al. Significance of late gadolinium enhancement at right ventricular attachment to ventricular septum in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2015;116:436–441. doi: 10.1016/j.amjcard.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 25.Kuribayashi T, Roberts WC. Myocardial dysarray at junction of ventricular septum and left and right ventricular free walls in hypertrophic cardiomyopathy. Am J Cardiol. 1992;70:1333–1340. doi: 10.1016/0002-9149(92)90771-p. [DOI] [PubMed] [Google Scholar]
- 26.Masci PG, Doulaptsis C, Bertella E, Del Torto A, Symons R, Pontone G, et al. Incremental prognostic value of myocardial fibrosis in patiens with non-ischemic cardiomyopathy without congestive heart failure. Circ Heart Fail. 2014;7:448–456. doi: 10.1161/CIRCHEARTFAILURE.113.000996. [DOI] [PubMed] [Google Scholar]
- 27.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 29.Puntmann VO, Carr-White G, Jabbour A, Yu CY, Gebker R, Kelle S, et al. T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. JACC Cardiovasc Imaging. 2016;9:40–50. doi: 10.1016/j.jcmg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Sohal M, Voigt T, Sammut E, Tobon-Gomez C, Child N, et al. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm. 2015;12:792–801. doi: 10.1016/j.hrthm.2014.12.020. [DOI] [PubMed] [Google Scholar]