Abstract
Objective
Our objective was to evaluate the efficacy of the Sitting Together and Reaching to Play (START-Play) intervention in young infants with neuromotor disorders.
Method
This randomized controlled trial compared usual care early intervention (UC-EI) with START-Play plus UC-EI. Analyses included 112 infants with motor delay (55 UC-EI, 57 START-Play) recruited at 7 to 16 months of age across 5 sites. START-Play included twice-weekly home visits with the infant and caregiver for 12 weeks provided by physical therapists trained in the START-Play intervention; UC-EI was not disrupted. Outcome measures were the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley); the Gross Motor Function Measure; reaching frequency; and the Assessment of Problem Solving in Play (APSP). Comparisons for the full group as well as separate comparisons for infants with mild motor delay and infants with significant motor delay were conducted. Piecewise linear mixed modeling estimated short- and long-term effects.
Results
For infants with significant motor delay, positive effects of START-Play were observed at 3 months for Bayley cognition, Bayley fine motor, and APSP and at 12 months for Bayley fine motor and reaching frequency outcomes. For infants with mild motor delay, positive effects of START-Play for the Bayley receptive communication outcome were found. For the UC-EI group, the only difference between groups was a positive effect for the APSP outcome, observed at 3 months.
Conclusion
START-Play may advance reaching, problem solving, cognitive, and fine motor skills for young infants with significant motor delay over UC-EI in the short term. START-Play in addition to UC-EI may not improve motor/cognitive outcomes for infants with milder motor delays over and above usual care.
Impact
Concepts of embodied cognition, applied to early intervention in the START-Play intervention, may serve to advance cognition and motor skills in young infants with significant motor delays over usual care early intervention.
Lay Summary
If you have a young infant with significant delays in motor skills, your physical therapist can work with you to develop play opportunities to enhance your child’s problem solving, such as that used in the START-Play intervention, in addition to usual care to help your child advance cognitive and motor skills.
Keywords: Infant, Motor Development, Early Intervention, Neuromotor Delays, Reaching, Problem-Solving, Embodied Cognition
Introduction
Early intervention for infants with developmental delays is based on the premise of early neuroplasticity.1 Theoretically, building a strong framework for early brain pathways provides the architecture for future complex skills, both cognitive and motor. The Sitting Together and Reaching to Play (START-Play) clinical trial proposed a theory of change in which early motor skills of sitting and reaching interact with and support the development of problem-solving skills to advance cognitive and global development (Fig. 1).2,3 The theory holds that for young infants with motor delays, the critical timing of achieving sitting and reaching within a milieu of environmental learning opportunities could change the trajectory of cognitive advancement.4,5
Figure 1.
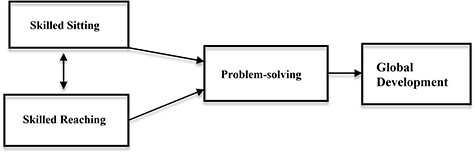
Theory of change: early sitting and reaching interact with problem solving, leading to advances in global development.
Although mandated by law in the United States through the Individuals with Disability Education Improvement Act,6 early intervention (EI) and/or outpatient therapy services (referred to, combined, as usual care-early intervention [UC-EI] in this study) for infants with delays varies widely in both type and amount.7 A recent systematic review supports that EI advances cognitive skills in infants born preterm.8 However, a systematic review of intervention for infants with movement disorders reveals both a lack of evidence and heterogeneity of intervention approaches.9 These reviews show a lack of support for intervention approaches based on either a reflex/maturation model of development or practice of motor skills as presented in motor learning theory. In addition, previous studies measured motor skills in isolation from and without regard to changes in cognition.9 Intervention approaches showing promise include the elements of promoting infant-initiated movement, engaging families in brainstorming for intervention planning, and environmental enrichment/adaptation to facilitate skill development.9,10,11 An evidence gap exists regarding intervention effectiveness when the above key principles are embedded within early therapies.8 For the purposes of this study, although natural environment EI services are theoretically different from clinic-based services in EI, all services being provided to the infants as part of their usual care were included as UC-EI.
The START-Play intervention builds on existing evidence blending movement activity, such as the progression of sitting and reaching, with specific cognitive constructs. Embracing the embodied cognition concept, START-Play espouses that the mind and body are inextricably linked.3,12 Thus, infants learn through performing actions and experiencing their consequences in relation to cognitive constructs.13 In START-Play, the cognitive constructs of focus were means-end relations, object permanence, object affordances, and joint attention.3 The provision of learning opportunities related to these constructs was systematically advanced with incremental changes in motor ability to simultaneously advance action and cognition (Tab. 1).3 The key ingredients of START-Play are: cognitive constructs embedded within motor activities; motor and cognitive skills advanced together at the “just-right” level; parent and therapist brainstorming regarding cognitive-motor interaction; movement flexibility allowed without rigid adherence to “normal patterns”; and all therapy provided within a social, engaging context guided by degrees of joint attention.3
Table 1.
Comparison of START-Play Intervention Content With UC-EI Content, Exemplifying Constructs of START-Play, and Notable Differencesa
| Example Content | UC-EI | START-Play Session | Difference |
|---|---|---|---|
| Sitting and object permanence | 1. Practice sitting balance reactions on ball (isolated motor task) 2. Therapist provides seated support to constrain trunk and suggests presenting toys on tray in front of infant and modeling use of toy | 1. Select activity for motor-based problem solving: finding hidden toy. Infant encouraged to shift weight, re-orient to look behind/under/in containers, thus building sitting balance in service of spatial understanding 2. Dynamic low support in sitting allows infant to re-orient and gain spatial understanding; multiple options for variable sitting support depending on problem-solving task | 1. Cognitive construct selected first and is primary; movements built around cognitive construct 2. Parents taught that chair is passive and not variable. Multiple seated options, with minimal support needed, allow exploration and link motor to problem solving |
| Reaching and means-end | 3. Presents toys in different locations for infant to reach 4. Presents toys of different shapes, colors, weight, and textures for infant to reach | 3. Sets up environment so reaching a proximal object (beads) will cause distal object to move (tied to other toy) 4. Places desirable toy just out of reach but on cloth so infant has to pull cloth to get toy |
3. Cognitive construct of means-end is over-arching theme in motor activities 4. Infant must solve a problem; how to reach “unreachable” toy. Cognitive is end point of motor problem. |
| Reaching and object affordance | 5. Uses toy to have infant reach in a pattern that requires change of trunk posture | 5. Several objects presented that allow combinations that are interesting, eg, small ball that fits in a tube and flies out other end, showing affordance of objects (round affords rolling, tube affords in/out) | 5. Infant discovers properties and uses for objects and what motor change (various sitting and reaching options) allows the action to occur |
a START-Play = Sitting Together and Reaching to Play; UC-EI = usual care-early intervention.
This study compares infants with neuromotor disorders receiving UC-EI with those receiving the START-Play intervention in addition to UC-EI during the emergence of sitting and reaching. Although most of the infants in the UC-EI group received only natural environment Part C services, some of them received only outpatient therapy, and some received both types of services (Tab. 2); thus, all services the children received were considered under the term of “usual care.” Our first primary hypothesis will be addressed in this paper: Compared with the UC-EI group, the START-Play plus UC-EI group will show greater improvements from before the intervention to after the intervention (short term) and in the long term (1-year follow-up) in sitting, reaching, problem solving, and global development outcome measures. In addressing this first primary hypothesis, we opted to expand our investigation to better understand the variability within the sample by comparing intervention efficacy between the intervention groups across the entire sample as well as separately for infants with mild or significant motor delay at baseline to determine whether the short-term rate of change in infant outcomes differs on average (baseline through 3 months after intervention) and whether the long-term rate of change in infant outcomes differs on average (baseline through 12 months after intervention).
Table 2.
Baseline Child and Family Characteristics for the Total Sample and by Intervention Groupa
| Variable | Total (N = 112) | Aggregated Across Severity Levels | Significant Motor Delay at Baseline | Mild Motor Delay at Baseline | |||
|---|---|---|---|---|---|---|---|
| UC-EI Group (n = 55) | START-Play Group (n = 57) | UC-EI Group (n = 25) | START-Play Group (n = 25) | UC-EI Group (n = 30) | START-Play Group (n = 32) | ||
| Sex | |||||||
| Girls | 42.9 | 47.3 | 38.6 | 56.0 | 32.0 | 40.0 | 43.8 |
| Boys | 57.1 | 52.7 | 61.4 | 44.0 | 68.0 | 60.0 | 56.3 |
| Race | |||||||
| White | 70.1 | 66.7 | 73.2 | 60.9 | 80.0 | 71.4 | 67.7 |
| Black | 10.3 | 11.8 | 8.9 | 26.1 | 4.0 | 0.0 | 12.9 |
| Other | 19.6 | 21.5 | 17.9 | 13.0 | 16.0 | 28.6 | 19.4 |
| Ethnicity | |||||||
| Hispanic | 17.6 | 13.5 | 21.4 | 8.3 | 20.0 | 17.9 | 22.6 |
| Not Hispanic | 82.4 | 86.5 | 78.6 | 91.7 | 80.0 | 82.1 | 77.4 |
| Prematurity-adjusted age, mean (SD) mob | 10.80 (2.59) | 10.67 (2.57) | 10.93 (2.63) | 11.96 (2.50) | 12.04 (2.84) | 9.58 (2.11) | 10.07 (2.12) |
| Gestational age at birth, wk | |||||||
| ≥37 | 65.2 | 56.4 | 73.7 | 44.0c | 84.0c | 66.6 | 65.6 |
| 34–36 | 7.1 | 5.5 | 8.8 | 0.0c | 4.0c | 10.0 | 12.5 |
| 32–33 | 6.3 | 10.9 | 1.8 | 16.0c | 0.0c | 6.7 | 3.1 |
| 25–31 | 12.5 | 12.7 | 12.2 | 20.0c | 12.0c | 6.7 | 12.5 |
| <25 | 8.9 | 14.5 | 3.5 | 20.0c | 0.0c | 10.0 | 6.3 |
| Ever had problems seeingb | 27.8 | 28.8 | 26.8 | 50.0 | 56.0 | 10.7 | 3.2 |
| Ever had problems hearing | 18.5 | 25.0 | 12.5 | 29.2 | 24.0 | 21.4c | 3.2c |
| Ever had problems with seizuresb | 19.4 | 15.4 | 23.2 | 20.8 | 44.0 | 10.7 | 6.5 |
| Ever had brain injury or water on the brainb | 26.2 | 21.2 | 30.9 | 33.3 | 52.0 | 10.7 | 13.3 |
| Received early Intervention over past 3 mob | 76.9 | 75.5 | 78.2 | 95.5 | 84.0 | 59.3 | 73.3 |
| Received private practice intervention over past 3 mo | 34.6 | 32.7 | 36.4 | 27.3 | 36.0 | 37.0 | 36.7 |
| Total frequency of therapy sessions/mo over past 3 mo, medianb | 4 | 4.5 | 4 | 6 | 5 | 2.5 | 2.5 |
| Caregiver highest education level | |||||||
| <HS diploma/GED | 2 | 0.0 | 3.6 | 0.0 | 4.2 | 0.0 | 3.2 |
| HS diploma/GED | 13.3 | 16.0 | 10.9 | 13.0 | 20.8 | 18.5 | 3.2 |
| Some college, training certificate, or associate’s degree | 25.7 | 26.0 | 25.5 | 30.4 | 16.6 | 22.2 | 32.2 |
| Bachelor’s degree | 25.7 | 24.0 | 27.3 | 39.2 | 41.7 | 11.2 | 16.2 |
| Postgraduate degree | 33.3 | 34.0 | 32.7 | 17.4 | 16.7 | 48.1 | 45.2 |
| Gross household income, median | 60,000–79,999 | 79,999 | 60,000–79,999 | 35,000–44,999 | 80,000 | 80,000 | 45,000–59,999 |
| Affordances in the home, mean (SD) | 0.72 (0.27) | 0.70 (0.25) | 0.74 (0.29) | 0.68 (0.18) | 0.80 (0.31) | 0.72 (0.29) | 0.67 (0.26) |
a Data are reported as percentages unless otherwise indicated. GED = general equivalency diploma; HS = high school; START-Play = Sitting Together and Reaching to Play; UC-EI = usual care-early intervention.
b Significant differences between mild delay and significant delay groups.
c Significant intervention group differences (P < .05).
Methods
Five clinical sites in different regions of the United States (Seattle, WA; Omaha, NE; Pittsburgh, PA; Newark, DE; and Richmond, VA) participated in this study, allowing for a broad inclusion of UC-EI models and demographic groups. Ethical approval was obtained from the institutional review boards at all sites. Families were recruited through social media, mailings, and websites as well as through medical centers and therapy providers.
Participants
Infants were enrolled based on sitting skill at study entry, so all infants began intervention when they were beginning to sit.14 This stage of motor development was chosen because sitting is critical for multiple motor and cognitive abilities: object exploration,15 visual motor coordination,16 and social interaction with others.17 The inability to sit by 9 months is a common reason for EI referral, making this intervention relevant to the initiation of therapy services.18
Infants were recruited at corrected ages of 7 to 16 months on a rolling basis between 2016 and 2019 and followed-up for 1 year. Initially, 155 potential participants were recruited and assessed for eligibility. Inclusion criteria were a gross motor score of >1.0 SD below the mean on the gross motor subtest of the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley)19; a neuromotor disorder such as cerebral palsy; an increased risk for cerebral palsy due to prematurity or brain damage around birth; a motor delay of an unknown origin; an ability to sit propped up on the arms for support for at least 3 seconds; spontaneous movement of the arms; and an inability to transition in and out of sitting. Exclusion criteria were medical complications limiting participation in assessments and intervention (eg, severe visual disorder); a primary diagnosis of autism, Down syndrome, or spinal cord injury; a diagnosed uncontrolled seizure disorder; or a neurodegenerative disorder.
A total of 134 infants were recruited and participated with written, informed parental consent (see Suppl. Appendix A: CONSORT flow chart). Participants maintained their baseline therapy services because withholding or altering therapy was deemed unethical. Blocked randomization was completed after baseline assessment, with stratification into 3 groups of movement ability designed to achieve equivalent groups. A scale incorporating estimated Gross Motor Function Classification System level,20 Manual Ability Classification System level,21 distribution of motor impairment (eg, quadriplegia, hemiplegia), and active movement (high, medium, low, judged by experienced therapists) defined these groups.3 This stratification ensured that intervention groups were balanced on level of initial movement ability at baseline. Randomized, sealed envelopes with group assignments were created by the data site at the University of Nebraska, Lincoln for each site prior to enrollment and opened after baseline classification.
At the first and last visits, parents completed a health and demographic form. Exclusion criteria were defined a priori. However, in some cases, exclusion could only be determined from post-intervention questionnaires. Based on the outtake information, 14 infants were deemed ineligible due to neurodegenerative diagnoses and/or seizure disorder that became uncontrolled during the study period,22 1 infant was ineligible due to a neurodegenerative diagnosis at baseline, 1 infant was ineligible due to inadequate sitting at baseline, and 6 infants were ineligible due to Bayley gross motor scores ≤1.0 SD below the mean at baseline. The latter 7 infants did not meet our original inclusion criteria and were thus excluded prior to analysis for final outcomes. A total of 112 infants were included in the final analyses. Twenty-three (21%) of those infants dropped out before the end of the study, with nonsignificant differential attrition (9.7%) between groups. Combined, these rates fall under the What Works Clearinghouse23 classification of low attrition (“tolerable threat of bias under optimistic assumptions”)23(p10). We believe optimistic assumptions are justified here, as the reason for dropping out was unrelated to the intervention in many cases (eg, participants moved; catastrophic flooding prevented follow-up). Differences in baseline infant and family characteristics by attrition group are presented in Supplementary Appendix B. Completers were more likely to be White, not Hispanic, have problems seeing, and have higher household income and greater affordances in the home environment and were less likely to have received therapy via private practice. Procedures for reducing bias due to missing data are discussed in the statistical analysis section.
The data from the infants were analyzed together, as well as grouped, by severity of motor delay measured at baseline (minor delay: >1.0 to <2.5 SDs below the mean for the Bayley motor composite score; significant delay: ≥2.5 SDs below the mean). Baseline parent questionnaires did not reveal diagnoses at the early age of recruitment (Tab. 2). Because this study was a home-based community study, medical records were not available and our project closely approximated conditions present for EI services in the United States.
An a priori Monte Carlo simulation–based power analysis indicated a necessary sample size of 152 infants to detect short-term intervention effects with magnitudes of d = 0.66 (reaching), d = 0.48 (sitting), d = 0.56 (problem solving), and d = 0.64 (global development), assuming 10% attrition 3 months after baseline and given α = .05 and power approximately 0.80. Effect sizes were estimated from prior studies.3,4 Given that only 134 infants were randomized and that, of those, only 112 met the criteria to be included in the analyses, the group comparisons presented in this article are underpowered. Implications are discussed in the limitations section.
Procedures
All assessments and interventions were performed consistently in the home, daycare setting, or an assessment site, as dictated by caregiver’s choice at the start of the study. Infants were assessed at baseline and 1.5, 3, 6, and 12 months later by a trained, reliable assessor masked with regard to group assignment. All assessments were video recorded and stored for later scoring by researchers masked to group assignment. Interrater reliability, calculated between coders for 20% of the data, was good to excellent, with ICCs ranging from 0.80 to 0.98 for all measures.24 Datavyu25 was used for behavioral coding. All assessments included in the clinical trial are detailed in the protocol paper and briefly described below.3
A standardized reaching assessment and the Gross Motor Function Measure (GMFM) were administered at every visit. Frequency of contacts with an object presented to infants when seated in a chair was behaviorally coded to demonstrate reaching ability.3 The GMFM sitting dimension from the GMFM-88 was used to quantify sitting ability.
Frequency of self-initiated problem solving was assessed using the Assessment of Problem Solving in Play (APSP), a modification of the Early Problem Solving Indicator for infants with motor impairments.26,27 Infants interacted with 3 toy sets each for 2 minutes while in supported sitting. Behavioral coding documented the frequency of 5 behaviors: look, simple explore, complex explore, function, or solution.25,27 A validated formula weighted the difficulty of the behaviors to calculate a single problem-solving score sensitive to change over time.27
The Bayley cognition, gross and fine motor, and expressive and receptive communication scales were administered at all visits except 1.5 months.19 Raw scores were used to assess change over time and absolute growth, rather than standard scores, which provide a comparison with a typically developing population.28
Intervention
The START-Play intervention was provided in collaboration with at least 1 parent or caregiver 2 times per week for 3 months, up to 24 visits (mean = 21; SD = 3.9). Depending on the infant’s behavioral state, sessions averaged 51.5 minutes (SD = 4.4 minutes) in length, with a range of 40.8 to 60 minutes. Experienced, licensed physical therapists at each site were trained in the provision of START-Play by an investigator. Training for all interventionists included review of pertinent theoretical evidence supporting key ingredients, in-person, hands-on training with infants, ongoing critique and feedback on treatment sessions, and refresher training. Training was provided for the START-Play interventionists during on-site 1-on-1 sessions with the therapists for 3 days, with follow-up intervention videos with children until the therapist reached preset adherence levels of the intervention. UC-EI was not affected or trained for this study; rather, video documentation allowed examination of activities that comprised usual care in the 5 regions of the study. UC-EI was performed by the usual interventionist of the individual child; no training or influence from the START-Play study was imposed on those therapists. Examples of content and differences noted between the START-Play intervention and UC-EI are shown in Table 1.
As part of the methodology, fidelity of intervention measures for adherence and program differentiation were evaluated throughout the 4 years of the study. Adherence to the intervention protocol was evaluated regularly and compared with a priori thresholds for compliance. At least 1 session for each infant in the START-Play group and 1 UC-EI intervention session (for 54% of the UC-EI therapists who provided consent) were video recorded.3 Based on a subsample of 64 videos, interventionists in the START-Play group, on average, utilized at least 1 key ingredient during each minute for >90% of the total session length (mean = 48 minutes; SD = 9.6 minutes). Program differentiation variables were defined and coded to document key differences between START-Play and UC-EI intervention.29,30 Specific differences coded as significantly different (P ≤ .001) between START-Play intervention and UC-EI intervention were greater motor assistance than needed (11% of the sessions for START-Play and 35% of the sessions for UC-EI), rigid adherence to the correct way of moving (9% of the sessions for START-Play and 51% of the sessions for UC-EI), and intervention activities not START-Play related (6% of the sessions for START-Play and 85% of the sessions for UC-EI). Further information on differences in the interventions and fidelity appear in another manuscript and indicates good adherence by the START-Play therapists and strong program differentiation between START-Play and UC-EI.30
Statistical Analysis
Piecewise linear mixed modeling was performed to address the study questions. The specific analytic models and equations are in Supplementary Appendix C. Linear mixed modeling was necessary to account for repeated measures nested within infants. Piecewise modeling, using individually varying time points, allowed separate slopes to be estimated across the intervention (baseline to 3 months after start of intervention) and postintervention (3–12 months) phases and accounted for variation in the time between assessments across infants. All models controlled for intercept-level differences by site as well as intercept- and slope-level differences by baseline-adjusted age and motor severity. Intervention effects were derived via intervention × slope interaction terms. Three-way (intervention × slope × severity) interaction terms were subsequently added to the models to obtain intervention effects stratified by severity. Long-term results are reported as the sum of short-term (0–3 months after start of intervention) and postintervention (3- to 12-month follow-up) effects.
Data were analyzed using Mplus Version 8 software.31 Adhering to an intention-to-treat perspective, all cases were included in the analyses regardless of dropout32 using full information maximum likelihood with robust SE estimation. Full information maximum likelihood assumes data are missing at random. To meet this assumption, baseline characteristics associated with dropout, or that differed between intervention groups within severity levels, were included as auxiliary variables in a saturated correlates model.33 Significance was based on α = .05. Hedges g, corrected for small sample bias, was calculated as a measure of effect size using the model-predicted group differences in rate of change in the numerator and the pooled SD estimated from the 3- or 12-month assessment (for short- and long-term effects, respectively) in the denominator.34 Standardized differences of 0.20, 0.50, and 0.80 were interpreted as small, medium, and large effects, respectively, with those 0.25 or larger being interpreted as substantively important.35,36(p14)
Results
Aggregating across severity levels, there were no differences between the intervention groups at baseline. Among infants with significant motor delay at baseline, the UC-EI group was more likely to be born premature. Infants with significant delays were more likely to have a brain injury (43% vs 12%) than infants with mild delays. Among infants with mild motor delay, the UC-EI group was more likely to have problems hearing. At baseline, those in the mild group were less likely to have a brain injury, problems seeing, or a history of infantile seizures (Tab. 2). Infants in the mild group were younger at baseline with a lower frequency of therapy services. The total numbers of therapy sessions per month were 4.5 for the aggregated group, 5.5 for the children with significant delays, and 2.5 for the children with mild delays; there were no significant differences in the number of therapy sessions between the START-Play and UC-EI groups within the aggregated group, the group with mild delays, or the group with significant delays (Tab. 2).
Table 3 summarizes the statistical significance and magnitude of the baseline, short-term (baseline to 3 months after start of intervention), and long-term (baseline to 12 months) effects by group (aggregated across severity levels vs infants with significant motor delays at baseline vs infants with mild motor delays at baseline), and Figures 2 and 3 provide a graphical representation the results. Figure 2 shows the results in a format mirroring our theory of change, and Figure 3 graphs the predicted outcome trajectories by severity group in both UC-EI and START-Play. Supplementary Table 1 in Supplementary Appendix D provides the raw estimates and SEs as well as exact P values for the target effects. Supplementary Tables 2 to 9 in Supplementary Appendix D provide the estimated coefficients corresponding to the statistical equations used to derive the intervention effects. Below, we organize our summary of the results by effect, and within each effect, by group.
Table 3.
Baseline and Short- and Long-Term Intervention Effect Sizes (Hedges g) and Statistical Significancea
| Outcome | Aggregated Across Severity Levels | Significant Motor Delay at Baseline | Mild Motor Delay at Baseline | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Short Term | Long Term | Baseline | Short Term | Long Term | Baseline | Short Term | Long Term | |
| Reaching (total toy contacts per minute) | −0.11 | 0.06 | 0.23 | −0.48b | 0.18 | 0.71c | 0.14 | 0.00 | −0.35b |
| GMFM sitting | 0.00 | 0.21c | 0.13 | −0.06 | 0.34b | 0.31b | 0.03 | 0.21 | −0.16 |
| APSP | 0.16 | −0.03 | 0.07 | −0.13 | 0.41c | 0.17 | 0.50c | −0.51d | −0.03 |
| Bayley cognition | 0.03 | 0.18 | 0.08 | −0.09 | 0.43c | 0.17 | 0.18 | −0.06 | 0.01 |
| Bayley gross motor | 0.11 | 0.09 | −0.04 | −0.05 | 0.26b | 0.14 | 0.33b | −0.03 | −0.49b |
| Bayley fine motor | 0.05 | 0.20c | 0.25c | −0.01 | 0.28c | 0.45c | 0.17 | 0.22 | 0.12 |
| Bayley receptive communication | 0.24 | 0.21 | 0.21 | 0.22 | 0.02 | 0.10 | 0.26b | 0.38c | 0.33b |
| Bayley expressive communication | 0.11 | 0.09 | 0.09 | −0.10 | 0.21 | 0.15 | 0.28b | −0.01 | 0.04 |
a Short-term (baseline to 3 mo after start of intervention) and long-term (baseline to 12 mo after start of intervention) effects were adjusted for baseline differences. Effects were not significant and did not have substantively important sizes unless otherwise indicated. APSP = Assessment of Problem Solving in Play; Bayley = Bayley Scales of Infant and Toddler Development, Third Edition; GMFM = Gross Motor Function Measure; START-Play = Sitting Together and Reaching to Play; UC-EI = usual care-early intervention.
b The effect was not statistically significant but had a substantively important size (g ≥ 0.25); a positive value favored the START-Play group, and a negative value favored the UC-EI group.
c The effect was statistically significant (P < .05) in favor of the START-Play group.
d The effect was statistically significant (P < .05) in favor of the UC-EI group.
Figure 2.
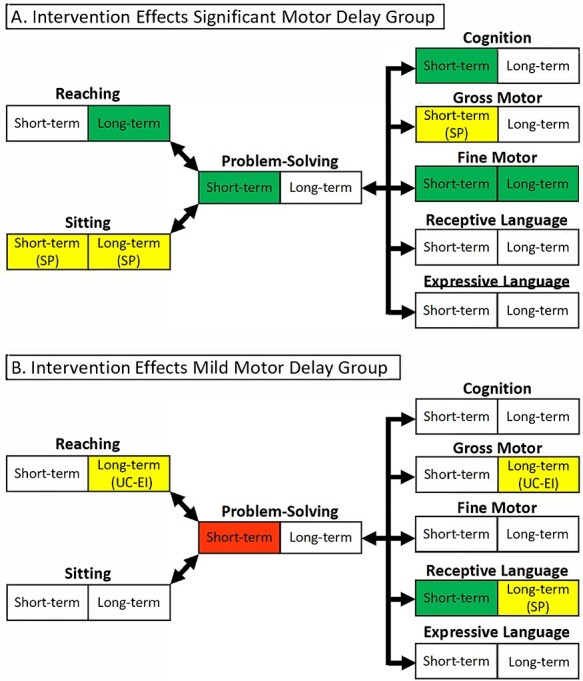
Short-term (0–3 months) and long-term (0–12 months) intervention effects for participants with significant (A) or mild (B) motor delay at the baseline study visit. Green cells represent statistically significant effects (P < .05) in favor of the Sitting Together and Reaching to Play (START-Play) group; red cells represent statistically significant effects (P < .05) in favor of the usual care-early intervention (UC-EI) group; yellow cells represent effects that were not statistically significant but had substantively important sizes (g ≥ 0.25), with the direction of the effects noted as favoring the START-Play (SP) or the UC-EI group; and white cells represent effects that were not significant and did not have substantively important sizes.
Figure 3.
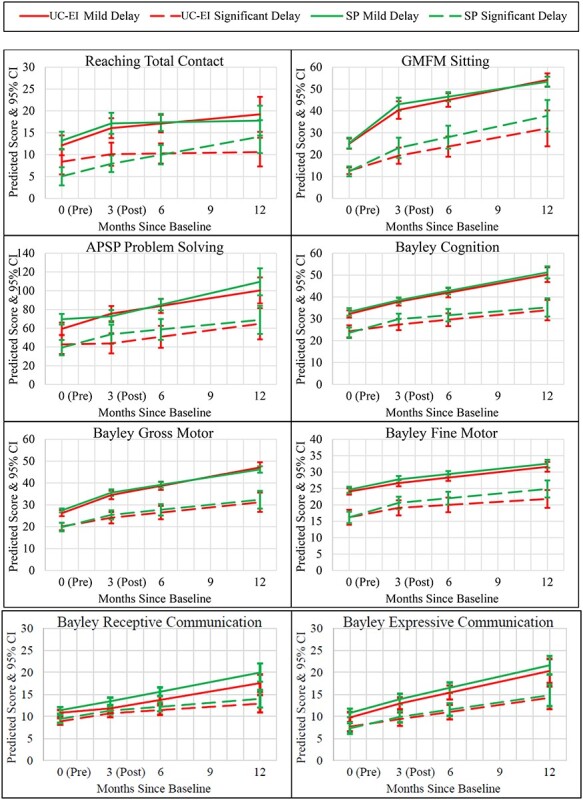
Short-term and long-term predicted outcome trajectories by severity group (usual care-early intervention [UC-EI] and Sitting Together and Reaching to Play [SP]). 0 = preintervention; 3 = postintervention; APSP = Assessment of Problem Solving in Play.
Baseline Comparisons
There were no significant group differences at baseline in infants’ global development when aggregating across severity levels or among infants with significant delays. However, there were substantively important but nonsignificant differences favoring the UC-EI group in reaching frequency (g = −0.48) among infants with significant motor delays. Among infants with mild delays, those in the START-Play group had significantly higher APSP scores (g = 0.50) and substantively important but not significantly higher Bayley gross motor (g = 0.33), receptive communication (g = 0.26), and expressive communication (g = 0.28) skills.
Short-term Effects: Baseline to 3 Months After Start of Intervention (START-Play Intervention Period)
Aggregating across severity levels, there were significant positive short-term effects of the START-Play intervention on GMFM sitting (g = 0.21) and Bayley fine motor skills (g = 0.20). Among infants with significant motor delay (Figs. 2A and 3), there were significant positive effects on APSP (g = 0.41), Bayley cognition (g = 0.43), and Bayley fine motor (g = 0.28) scores and substantively important but not significant effects on GMFM sitting (g = 0.34) and Bayley gross motor (g = 0.26) scores. Among infants with mild delays (Figs. 2B and 3), there were significant negative effects on APSP scores (g = −0.51) and positive effects on Bayley receptive communication scores (g = 0.38).
Long-term Effects: Baseline to 12 Months After Start of Intervention
Aggregating across severity levels, there was a significant positive long-term effect of the START-Play intervention on Bayley fine motor scores (g = 0.25). Among infants with significant motor delays, there were significant positive effects on reaching frequency (g = 0.71) and Bayley fine motor scores (g = 0.45) and substantively important but not significant effects on GMFM sitting (g = 0.31) (Figs. 2 and 3). There were no significant long-term effects among infants with mild delays, but there were substantively important negative effects on reaching frequency (g = −0.35) and Bayley gross motor scores (g = −0.49) and positive effects on Bayley receptive communication scores (g = 0.33) (Figs. 2 and 3).
Role of the Funding Source
The funding source had no role in the study’s design, conduct, and reporting.
Discussion
Overall, the infants with significant motor delays who received START-Play intervention showed statistically significant or qualified positive effects (substantively important)36 changes in sitting, reaching, fine motor, problem solving, and global development during the study. However, differences between the START-Play and UC-EI groups were not notable in infants with mild motor delays. The START-Play study results are best understood by examining the baseline characteristics of the participants and the division of infants into significant and mild motor delay groups. This discussion is organized for the short and long-term results, followed by possible clinical implications.
Differences Between Mild and Severe Groupings at Baseline
Infants in the UC-EI and START-Play groups showed a similar distribution of delays, impairments, and supports. There was a range of participants in this study, and to better understand the impact of the intervention across this diverse sample, we examined infants with mild and significant motor delays separately. Infants in the 2 groups had different skills, needs, and potential for change, which allows for better understanding of key intervention ingredients and optimal translation to services.6
Differences in Short-term Outcomes
There were significant, positive differences in problem solving, fine motor, and cognitive ability in the short term (3 months after start of intervention) for the infants with significant delays receiving START-Play. This suggests the key ingredients of START-Play can, in fact, facilitate global development. In addition, substantively important differences in sitting and gross motor in favor of the START-Play group for infants with significant motor delays add to developmental studies showing the important relationship between improving sitting control and potential effects on problem-solving abilities and cognition in the short term.8,10,11
For the group with mild delays, the relationship between reaching and sitting improvement for the START-Play group did not appear related to downstream effects on problem solving or cognition. Possibly the overall higher baseline motor skill level of the infants in the mildly delayed group nullified the effect of START-Play key ingredients. These infants were sitting with greater independence at baseline and quickly became mobile. Thus, they were quite different from the infants with significant motor delays. The other significant finding in receptive communication in favor of the START-Play group may be related to the increased percentage of infants in the mild UC-EI group with hearing difficulties or the greater amount of attention to communicated cognitive constructs during movement in START-Play intervention.
The UC-EI mild delays group showed significantly greater changes in problem solving in the short term. However, the START-Play group was significantly better at problem solving at baseline on average, and by approximately the same magnitude, than the UC-EI group. In effect, the comparison at the 3-month time point may represent a true difference or may simply indicate a regression to the mean, a phenomenon in which individuals who, by chance, perform better than expected at 1 time point are more likely to perform closer to expectation at a subsequent assessment, and vice versa.37 This was the only significant difference between UC-EI and START-Play favoring the UC-EI group.
Differences in Long-term Outcomes
There were significant differences in favor of the START-Play group in infants with significant motor delays noted in reaching and Bayley fine motor scores. For infants with severe movement problems, enhancing reaching, grasping, and manipulation abilities may take longer but is crucial to facilitate cognition, communication, and performance of activities of daily living. In addition, substantively important changes for the infants in the START-Play group existed in sitting.
The long-term substantively important differences in the group with mild motor delays showed advances in the UC-EI group for reaching and gross motor ability. While the gross motor finding may reflect a regression toward the mean similar to short-term problem-solving findings, this also may reflect that START-Play intervention focused less on motor advancement alone but aimed to blend cognitive constructs with motor activities. Substantive positive differences were noted for the mild START-Play group in receptive communication. As stated above, increased communication and interaction with cognitive constructs during the START-Play intervention may be reflected or this may reflect that hearing impairments were more prevalent among the mild UC-EI group.
Implications
Key implications from this study indicate that there may be motor and cognitive benefits of implementing the START-Play intervention for infants with significant but not mild motor delays. UC-EI alone may be sufficient for advancing motor outcomes and problem solving for infants with mild motor delay. A focus on fine motor and cognitive activities within motor intervention may help to maximize outcomes for infants with significant motor delays and may be a neglected area in current EI.
However, the lack of maintenance in gains during the long-term follow-up phase suggests the need for continued focus on areas emphasized in START-Play for infants with significant motor delays. Either a longer period of intervention (>3 months) or a “booster” of START-Play intervention at a later time point may help to extend the effect on global development to the 12-month point or beyond, but this requires further study.
In contrast, minimal differences between groups in the infants with mild motor delays suggests that START-Play has little added value for these infants. These short-term motor gains may not translate to improved cognitive outcomes. Consequently, the key ingredients of START-Play (Tab. 1) may reflect best practice for advancing motor and cognitive outcomes for infants with significant motor delays, but not for infants with mild motor delays. It is possible that infants who become mobile (as in the group of mildly delayed infants) require different key ingredients for improved outcomes.
The START-Play intervention incorporates practices that the EI literature suggest promote developmental outcomes, such as intervention in the natural environment, coaching, a focus on the whole child, and participation within meaningful routines.8–14 It is important to note that the fidelity data in this study suggest that, across the country, EI providers might benefit from improved education regarding providing the right level of support, encouraging flexibility of movement, brainstorming with parents, and developing intervention activities that focus on development across multiple domains.29,30 This type of education could benefit a variety of EI professionals working with infants, including educators, occupational therapists, and physical therapists.
Limitations
Although we powered for the primary aim, the initial power analysis was based on available data from preterm infants. This may not have been representative of the infants in this study. In addition, our decision to divide the group based on mild or significant motor delay was not part of our initial plan to power the study. Recruitment of infants who met inclusion criteria and our eventual exclusion of infants who developed uncontrolled seizures limited the sample size and resulted in low power. The APSP, although a compelling play assessment for problem solving, may be quite motor biased. Testing for cognition and problem solving in infants with motor deficits is difficult, especially in the natural home setting.38 Finally, the dose of intervention was not equal between groups. The START-Play intervention was provided in addition to UC-EI during the short interval (12 weeks) of study intervention, making it unethical to withdraw from community services. The significant limitations of this study, although large, provide guidance for future studies to address best practice for EI.
Future Directions
The START-Play intervention may include ingredients for best practice during early therapy for infants with significant motor delays. Future directions include analysis of mediators and moderators of the intervention, comparisons for dosage of intervention while infants continue their usual early therapy programs, comparison with dose-matched intervention, and modification of START-Play activities for infants who achieve mobility during the intervention.
Supplementary Material
Acknowledgments
The authors extend their heartfelt thanks to the families, children, and community therapists who participated in this study. The authors are also grateful for the talented team of START-Play interventionists and assessors whose unwavering enthusiasm and hard work made this possible: Maddie Aubuchon, Jamie Barnhill, Amy Beyersdorf, Kelly Bossola, Shaaron Brown, Lynne Capoun, Emily Drew, Brooke Fitterer, Lisa Hennen, Cierra Maloney, Heidi Reelfs, Shawn Rundell, Lisa Schwarcz, Jaclynn Stankus, Tracy Stoner, Tanya Tripathi, Cathy Van Drew, and Allison Yokum.
Contributor Information
Regina T Harbourne, Duquesne University, Pittsburgh, Pennsylvania, USA.
Stacey C Dusing, University of Southern California, Los Angeles, California, USA.
Michele A Lobo, University of Delaware, Newark, Delaware, USA.
Sarah W McCoy, University of Washington, Seattle, Washington, USA.
Natalie A Koziol, University of Nebraska-Lincoln, Lincoln, Nebraska, USA.
Lin-Ya Hsu, University of Washington, Seattle, Washington, USA.
Sandra Willett, Munroe Meyer Institute, University of NE Medical Center, Omaha, Nebraska, USA.
Emily C Marcinowski, Louisiana State University, Baton Rouge, Louisiana, USA.
Iryna Babik, Boise State University, Boise, Idaho, USA.
Andrea B Cunha, University of Delaware, Newark, Delaware, USA.
Mihee An, Kaya University, Gimhae-si, Gyeongsangnam-do, Republic of Korea.
Hui-Ju Chang, Duquesne University, Pittsburgh, Pennsylvania, USA.
James A Bovaird, University of Nebraska-Lincoln, Lincoln, Nebraska, USA.
Susan M Sheridan, University of Nebraska-Lincoln, Lincoln, Nebraska, USA.
Author Contributions
Concept/idea/research design: R. Harbourne, S. Dusing, M. Lobo, S. Westcott-McCoy, N. Koziol, L.-Y. Hsu, E. Marcinowski, I. Babik, J. Bovaird, S. Sheridan
Writing: R. Harbourne, S. Dusing, M. Lobo, S. Westcott-McCoy, N. Koziol, E. Marcinowski, I. Babik, A. Cunha, M. An
Data collection: R. Harbourne, S. Dusing, S. Westcott-McCoy, L-Y. Hsu, E. Marcinowski, I. Babik, A. Cunha, M. An, H.-J. Chang
Data analysis: S. Dusing, M. Lobo, S. Westcott-McCoy, N. Koziol, L.-Y. Hsu, S. Willett, M. An, J. Bovaird
Project management: R. Harbourne, S. Dusing, M. Lobo, S. Westcott-McCoy, N. Koziol, L.-Y. Hsu, S. Willett, E. Marcinowski, H.-J. Chang, J. Bovaird
Fund procurement: R. Harbourne, S. Dusing, M. Lobo, S. Westcott-McCoy, J. Bovaird
Providing participants: R. Harbourne, S. Dusing, S. Westcott-McCoy, S. Willett
Providing facilities/equipment: R. Harbourne, S. Dusing, M. Lobo, S. Westcott-McCoy, S. Willett, J. Bovaird
Providing institutional liaisons: S. Westcott-McCoy, S. Willett
Consultation (including review of manuscript before submitting): N. Koziol, L.-Y. Hsu, S. Willett, J. Bovaird, S. Sheridan
Ethics Approval
Ethical approval was obtained from the institutional review boards at all sites.
Funding
This study was funded by the US Department of Education, Institute of Education Sciences; National Center for Special Education Research, Early Intervention and Early Learning in Special Education, Award no. R324A150103.
Clinical Trial or Systematic Review Registration
This trial is registered at ClinicalTrials.gov (identifier: NCT02593825).
Disclosures
S. Dusing reports receiving continuing education royalties from Medbridge. The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no other conflicts of interest.
References
- 1. Finch-Edmondson M, Morgan C, Hunt RW, Novak I. Emergent prophylactic, reparative and restorative brain interventions for infants born preterm with cerebral palsy. Frontiers Physio. 2019;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adolph KE, Hoch JE. Motor development: embodied, embedded, enculturated, and enabling. Annu Rev Psychol. 2019;70:141–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harbourne RT, Dusing SC, Lobo MA, et al. Sitting Together and Reaching to Play (START-play): protocol for a multisite randomized controlled efficacy trial on intervention for infants with neuromotor disorders. Phys Ther. 2018;98:494–502. [DOI] [PubMed] [Google Scholar]
- 4. Lobo MA, Galloway JC. Enhanced handling and positioning in early infancy advances development throughout the first year. Infant Devel. 2012;83:1290–1302. [DOI] [PubMed] [Google Scholar]
- 5. Needham A, Libertus K. Embodiment in early development. Wiley Interdiscip Rev Cogn Sci. 2011;2:117–123. [DOI] [PubMed] [Google Scholar]
- 6. Individuals With Disabilities Education Act (IDEA), Part C, 34 C.F.R. Part 303 (2004).
- 7. Chang H, Hsu L, McCoy SW, Harbourne RT. Service delivery of PTs and OTs in early intervention: how are we doing? Ped Phys Ther. 2018;30:E15. [Google Scholar]
- 8. Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. 2015;11:CD005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgan C, Darrah J, Gordon AM, et al. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev Med Child Neurol. 2016;58:900–909. [DOI] [PubMed] [Google Scholar]
- 10. Harbourne RT, Berger SE. Embodied cognition in practice: exploring effects of a motor-based problem-solving intervention. Phys Ther. 2019;99:786–796. [DOI] [PubMed] [Google Scholar]
- 11. Ryalls BO, Harbourne R, Kelly-Vance L, Wickstrom J, Stergiou N, Kyvelidou A. A perceptual motor intervention improves play behavior in infants with moderate to severe cerebral palsy. Front Psych. 2016;7:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lobo MA, Harbourne RT, Dusing SC, McCoy SW. Grounding early intervention: physical therapy cannot just be about motor skills anymore. Phys Ther. 2013;93:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith L, Gasser M. The development of embodied cognition: six lessons from babies. Artif Life. 2005;11:13–29. [DOI] [PubMed] [Google Scholar]
- 14. Harbourne RT, Willett S, Kyvelidou A, Deffeyes J, Stergiou N. A comparison of interventions for infants with cerebral palsy to improve sitting postural control: a clinical trial. Phys Ther. 2010;90:1881–1898. [DOI] [PubMed] [Google Scholar]
- 15. Soska KC, Adolph KE. Postural position constrains multimodal object exploration in infants. Inf Dent. 2014;19:138–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harbourne RT, Ryalls B, Stergiou N. Sitting and looking: a comparison of stability and visual exploration in infants with typical development and infants with motor delay. Phys Occup Ther Ped. 2014;34:197–212. [DOI] [PubMed] [Google Scholar]
- 17. Iverson JM. Developing communication in a developing body: the relationship between motor development and communication development. J Infant Comm. 2010;37:229–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO Multicentre Growth Reference Study Group and de Onis M . WHO motor development study: windows of achievement for six gross motor development milestones. Acta Paediatr. 2006;95:86–95. [DOI] [PubMed] [Google Scholar]
- 19. Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 20. Palisano R, RosenUC-EIm P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in infants with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. [DOI] [PubMed] [Google Scholar]
- 21. Eliasson AC, Krumlinde-Sundholm L, Rösblad B, et al. The manual ability classification system (MACS) for infants with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549–554. [DOI] [PubMed] [Google Scholar]
- 22. Gibbs SN, Choi J, Khilfeh I, Ahmed KH, Yermilov I, Segal E. The humanistic and economic burden of pediatric focal seizures in the United States. J Infant Neuro. 2020;35:543–555. [DOI] [PubMed] [Google Scholar]
- 23. What Works Clearinghouse (WWC) . Procedures Handbook (Version 4.1). Washington, DC: US Department of Education, Institute of Education Sciences, National Center for Education Evaluation and Regional Assistance, What Works Clearinghouse. 2020. Accessed July 13. 2020. https://ies.ed.gov/ncee/wwc/Docs/referenceresources/WWC-Standards-Handbook-v4-1-508.pdf [Google Scholar]
- 24. Nakagawa S, Johnson PCD, Schielzeth H. The coefficient of determination R(2) and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded . J R Soc Interface 2017;14:20170213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Datavyu Team . Datavyu: A Video Coding Tool. Databrary Project. New York: New York University; 2014. Accessed July 13, 2020. http://datavyu.org. [Google Scholar]
- 26. Greenwood CR, Walker D, Carta JJ, Higgins SK. Developing a general outcome measure of growth in the cognitive abilities of infants 1 to 4 years old: the early problem-solving indicator. School Psychology Review. 2006;35:535–551. [Google Scholar]
- 27. Molinini R, Koziol N, Tripathi T, et al. Measuring early problem solving in infants with motor impairments: a validation study. Phys Occup Ther Ped. In press. [DOI] [PubMed] [Google Scholar]
- 28. Shapiro EG, Escolar ML, Delaney KA, Mitchell JJ. Assessments of neurocognitive and behavioral function in the mucopolysaccharidoses. Mol Genet Metab. 2017;122:8–16. [DOI] [PubMed] [Google Scholar]
- 29. An M, Dusing SC, Harbourne RT, Sheridan SM. What really works in intervention? Using fidelity measures to support optimal outcomes. Phys Ther. 2020;100:757–765. [DOI] [PubMed] [Google Scholar]
- 30. An M, Dusing SC, Harbourne RT, Koziol N, Nord J, Kane A. Developing a fidelity measure of early intervention programs for children with neuromotor disorders. Dev Med Child Neurol. 2021;63:97–103. [DOI] [PubMed] [Google Scholar]
- 31. Muthen LK, Muthen BO. Mplus User’s Guide (Version 8). Los Angeles, CA, USA: Muthen & Muthen; 1998–2017. [Google Scholar]
- 32. Fisher LD, Dixon DO, Herson J, Frankowski RK, Hearron MS, Peace KE. Intention to treat in clinical trials. In: Peace KE, ed., Statistical Issues in Drug Research and Development. New York, USA: Marcel Dekker; 1990: 331–350. [Google Scholar]
- 33. Graham JW. Adding missing-data-relevant variables to FIML-based structural equation models. Struct Equ Modeling. 2003;10:80–100. [Google Scholar]
- 34. Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Ed Stat. 1981;6:107–128. [Google Scholar]
- 35. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 36. What Works Clearinghouse (WWC) . Procedures Handbook (Version 4.0). Washington, DC: US Department of Education, Institute of Education Sciences, National Center for Education Evaluation and Regional Assistance, What Works Clearinghouse. 2017. Accessed July 13, 2020. https://ies.ed.gov/ncee/wwc/Docs/referenceresources/wwc_procedures_handbook_v4.pdf. [Google Scholar]
- 37. Chiolero A, Paradis G, Rich B, Hanley JA. Assessing the relationship between the baseline value of a continuous variable and subsequent change over time. Front Public Health. 2013;1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morgan C, Honan I, Allsop A, Novak I, Badawi N. Psychometric properties of assessments of cognition in infants with cerebral palsy or motor impairment: a systematic review. J Pediatr Psychol. 2018;44:238–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


