Abstract
Purpose
We evaluated the clinical characteristics and severity of diabetic ketoacidosis (DKA) in children before and after the coronavirus disease 2019 (COVID-19) outbreak to identify its indirect effects on DKA incidence.
Patients and Methods
This retrospective study included 19 children with DKA admitted to the emergency room (ER) in two centers in Jeonbuk province, Korea during the first 6 months of the year from 2017 to 2020. Data were collected on age, height, body weight, clinical symptoms, diabetic mellitus (DM) type, and laboratory findings. DKA severity was based on the presence of acute kidney injury, cerebrovascular accident, and altered mental status. The ratio of patients with DKA in all pediatric patients who visited the study ERs and in the Jeonbuk population was also determined.
Results
There were no differences in anthropometric characteristics and complication rates between the pre-COVID-19 and COVID-19 periods; however, the rate of polydipsia was significantly higher in the COVID-19 period. All seven patients admitted during the COVID-19 pandemic (100%) had polydipsia and polyuria and were newly diagnosed with DM. The rate of pediatric patients with DKA admitted to the ER in 2020 (0.459%) was more than twice the mean rate of 0.206% for the four-year period. The incidence of DKA in the Jeonbuk population (0.00141%) also exceeded the mean rate (0.0009%).
Conclusion
The incidence of pediatric DKA might be higher due to the indirect effect of COVID-19 pandemic. Physicians should be aware of nonspecific symptoms related to DKA in children admitted to the ER.
Keywords: coronavirus disease 2019, diabetes, diabetic ketoacidosis, pediatric diabetes
Introduction
The coronavirus disease 2019 (COVID-19) pandemic due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to an unprecedented emergency around the globe. The World Health Organization has declared a public health emergency of international concern on January 30, 2020, and governments in most countries currently recommend limited activity to prevent the spread of infection. In February 2020, the first large COVID-19 outbreak outside China occurred in Daegu, Korea.1 The government of Republic of Korea raised the crisis alert level to the highest level (Level 4) in February 23, 2020, after the first five deaths due to COVID-19. Moreover, it strongly recommended restrictions on movement between cities and personal outings. The Ministry of Education postponed the opening of kindergartens and primary and secondary schools, which disrupted the health and education of millions of children. Since then, the number of patients with COVID-19 has constantly increased. During the publication of this study, policies for decreasing daily activities such as implementing online classes, posing restrictions on eating out, and reducing public transportation time are implemented. This sudden change in lifestyle can also impact individuals with chronic diseases that require lifestyle control. Diabetic mellitus (DM) is one of the most common chronic diseases in patients infected with SARS-CoV-2, and Covid-19 exacerbates the severity and worsens clinical outcomes.2 In particular, recent studies on the bidirectionality of diabetes and COVID-19, which indicates that this infection may be responsible for the expression of diabetes, have been conducted.3,4
The incidence of type 1 DM among the 0–14-year age group is very low in the Republic of Korea (<5% per 100 000 population); however, the incidence of hyperglycemic crisis has increased steadily in the past decade.5 In the Korean city of Jeollabuk-do (below Jeonbuk), the incidence of type 1 DM is below 0.0125%, the average rate of DM in the general Korean population.6 In the Korean province of Jeonbuk, patients with diabetic ketoacidosis (DKA) are most likely to visit one of the two university hospitals, Chonbuk National University Hospital and Wonkwang University Hospital, both of which have pediatric endocrinologists and pediatric emergency specialists on staff. Although the number of pediatric patients admitted to the emergency room (ER) has declined by more than 50% in 2020 compared to 2019 due to the COVID-19 pandemic, the number of pediatric patients with DKA at these two university hospitals in Jeonbuk has increased. Compared with adults who need to maintain economic or social activity, children are expected to be more sensitive to changes imposed by national or parental restrictions due to COVID-19. Therefore, we aimed to determine whether lifestyle changes related to the COVID-19 pandemic might be associated with increased morbidity of latent disease, delayed diagnosis, increased frequency of symptoms, and worsening severity in pediatric patients with DM. The present retrospective study compared the incidence and severity of disease in children with DKA who were admitted to the ER before and after the COVID-19 pandemic to evaluate the indirect effect of COVID-19 on pediatric patients with DM.
Materials and Methods
Subjects
The study included pediatric patients with DKA aged between 0 and 18 years who visited the ER in Chonbuk National University Hospital or Wonkwang University Hospital from January 1, 2017, to June 30, 2020. The data on patients admitted to the study ERs after the start of the COVID-19 pandemic included the 6-month period. The pre-COVID-19 period in the present study encompassed the first 6 months of the year from 2017 to 2019 to control for seasonal variations. Between 2017 and 2020, there were 28 patients with DKA diagnostic codes. Seven patients from the second half of 2017–2019 were excluded, and two patients who did not meet the criteria for the diagnosis of DKA were also excluded; therefore, there were a total of 19 patients with DKA admitted to the study ERs during the study period. The DKA diagnostic criteria were as follows: sugar level ≥ 200 mg/dL (11 mmol/L); bicarbonate level ≤ 15.0 mmol/L; and/or venous pH ≤ 7.3; and significant ketonuria on standard urine dipstick test.7 Thus, the data of 19 children were included in the final analysis.
The study was approved by the Institutional Review Boards of Chonbuk National University Hospital (IRB No. 2020–08-029) and Wonkwang National University Hospital (IRB No. 2020–08-007). This study did not require a written informed consent because data were obtained via a retrospective chart review and personal identifiable information was not revealed.
Methods
We retrospectively analyzed the records of all patients aged 0–18 years who were diagnosed with hyperglycemia or DKA in the ER and/or those admitted to the ER and diagnosed with DKA at the time of discharge from 2017 to June 2020. Patient data were analyzed by the retrospective review of medical records. Anthropometric values were based on the data collected at the time of ER visit. Body mass index was calculated as weight (kg) divided by the square of height (m2). Typical symptoms of DKA, such as altered mental status, polyuria, polydipsia, weight loss, nausea, vomiting, abdominal pain, and dyspnea, were referred to the records of the pediatric emergency specialist at the time of the visit. The patients’ medical history was evaluated to determine if the DM was newly diagnosed and to determine the time from diagnosis to DKA. The hospitalization and discharge records were used to screen for severe DKA complications such as acute kidney injury (AKI), altered mental status, cerebrovascular attack, and death. The data on blood chemistry were based on tests performed immediately after ER admittance and included C-peptide, serum glucose, hemoglobin A1c (HbA1c), creatinine, arterial pH, bicarbonate, anion gap, and urine ketone measurements. To distinguish type 1 from type 2 DM, serum C-peptide, insulin, anti-insulin antibody, and islet cell antibody levels were used in two hospitals. AKI was defined as a glomerular filtration rate (GFR) of ≤90, which was calculated using the original Schwartz equation for patients aged ≤12 years and the Cockcroft–Gault equation for those aged 13–18 years.
Clinical characteristics and laboratory findings were compared among the years of admission, and complication rates were compared between the pre-COVID-19 period between 2017 and 2019 and the COVID-19 period in 2020. To determine the potential increase in DKA incidence during the COVID-19 pandemic, the ratio of patients with DKA to the Jeonbuk population and the ratio of patients with DKA to all patients who visited the two university hospitals for pediatric care were calculated.
Statistical Analysis
The final analyses of 19 patients who fulfilled the study criteria for DKA were performed using the SPSS 25.0 statistical program. The nonparametric x2 and Kruskal–Wallis tests were used to examine differences in clinical characteristics and laboratory findings by the year of admission, and the Mann–Whitney U-test was used to examine differences in the rates of DKA complications between the pre-COVID-19 and COVID-19 periods. A P value of <0.05 was considered to indicate statistical significance.
Results
Clinical Characteristics
Table 1 summarizes the clinical characteristics of 19 pediatric patients with DKA included in the study ERs according to the admission year. The study cohort comprised 6 male (31.6%) and 13 female (68.4%) patients, with a mean height of 147.32 ± 20.05 cm and mean weight of 45.35 ± 18.73 kg. During the COVID-19 outbreak, most of the patients had three major symptoms of diabetes, with 100%, 100%, and 85.7% of the patients exhibiting polydipsia, polyuria, and weight loss, respectively. The rate of polyuria was significantly higher in the COVID-19 period than in the pre-COVID-19 period (P < 0.033), whereas the rates of nausea, vomiting, and altered mental status did not differ among the admission years. Within the study cohort, drowsiness and stupor were reported in 5 and 1 patient, respectively, whereas 13 patients were conscious; none of the patients were comatose at the time of ER admission. In the overall cohort, four patients had a previous diagnosis of DM and 15 patients were newly diagnosed with DM. All seven patients with DKA who were admitted to the ER in 2020 were newly diagnosed with DM. Due to the occurrence of DKA, four patients with existing DM visited the ER at an average of 5.75 years after diagnosis.
Table 1.
Clinical Characteristics of Patients with Diabetic Ketoacidosis
| Total (%)(n = 19) | 2017 (%)(n = 4) | 2018 (%)(n = 5) | 2019 (%)(n = 3) | 2020 (%)(n = 7) | p value | ||
|---|---|---|---|---|---|---|---|
| Age (years) Mean ± SD | 11.68 ± 3.65 | 11.50 ± 5.07 | 9.60 ± 4.62 | 13.33 ± 2.08 | 12.57 ± 2.37 | 0.649 | |
| Sex | Male (n) | 6 | 2 (50.0) | 1 (20.0) | 2 (66.7) | 1 (14.3) | 0.306 |
| Female (n) | 13 | 2 (50.0) | 4 (80.0) | 1 (33.3) | 6 (85.7) | ||
| Height (cm) | 147.32 ± 20.05 | 145.75 ± 32.63 | 135.68 ± 21.01 | 158.03 ± 5.17 | 151.93 ± 13.16 | 0.437 | |
| Weight (kg) | 45.35 ± 18.73 | 50.05 ± 28.50 | 34.86 ± 14.07 | 53.13 ± 17.94 | 46.83 ± 16.38 | 0.598 | |
| Polydipsia (n) | 13 (68.4) | 2 (50.0) | 2 (40.0) | 2 (66.7) | 7 (100.0) | 0.125 | |
| Polyuria (n) | 13 (68.4) | 3 (75.0) | 1 (20.0) | 2 (66.7) | 7 (100.0) | 0.033 | |
| Weight loss (n) | 10 (52.6) | 2 (50.0) | 1 (20.0) | 1 (33.3) | 6 (85.7) | 0.129 | |
| Dyspnea (n) | 8 (42.1) | 2 (50.0) | 2 (40.0) | 2 (66.7) | 2 (28.6) | 0.710 | |
| Nausea (n) | 10 (52.6) | 2 (50.0) | 2 (40.0) | 1 (33.3) | 5 (71.4) | 0.621 | |
| Vomiting (n) | 8 (42.1) | 1 (25.0) | 2 (40.0) | 1 (33.3) | 4 (57.1) | 0.745 | |
| Mental status | Alert (n) | 12 (63.2) | 1 (25.0) | 3 (60.0) | 2 (66.7) | 6 (85.7) | 0.254 |
| Drowsy (n) | 6 (31.6) | 3 (75.0) | 2 (40.0) | 1 (33.3) | 0 | 0.076 | |
| Stupor (n) | 1 (5.3) | 0 | 0 | 0 | 1 (14.3) | 0.613 | |
| Coma (n) | 0 | 0 | 0 | 0 | 0 | ||
| New-onset DM | 15 (78.9) | 3 (75.0) | 3 (60.0) | 2 (66.7) | 7 (100.0) | 0.354 | |
| Preexisting DM | 4 (21.1) | 1 (25.0) | 2 (40.0) | 1 (33.3) | 0 | 0.222 | |
| Type of DM | Type 1 (n) | 17 (89.5) | 3 (75.0) | 4 (80.0) | 3 (100.0) | 7 (100.0) | |
| Type 2 (n) | 2 (10.5) | 1 (25.0) | 1 (20.0) | 0 | 0 |
Abbreviations: DM, diabetes mellitus; SD, standard deviation.
Laboratory Findings
The mean C-peptide level was 0.29 in 2018, which was lower than the normal value of 0.5, but there was no statistical significance (Table 2). The mean serum glucose and HbA1c levels of 778.0 ± 455.02 mg/dL and 13.5% ± 0.84%, respectively, were highest in 2017, with no significant difference among the admission years (P > 0.05). The acidosis was more severe in 2018 based on the highest mean arterial pH of 7.09 ± 0.1 and the lowest mean bicarbonate level of 4.72 ± 2.17 mEq/L. However, the anion gap was highest in 2017 (34.5 ± 14.69), followed by 2018 (31.28 ± 10.42). There were no statistically significant differences in any of these three parameters for evaluating the severity of acidosis. The mean urine ketone level was above +3 for all years, and the mean creatinine, GFR, and corrected sodium levels did not exhibit significant differences among the 4 years.
Table 2.
Baseline Laboratory Findings in Patients with Diabetic Ketoacidosis
| Total (n = 19) | 2017 (n = 4) | 2018 (n = 5) | 2019 (n = 3) | 2020 (n = 7) | p value | |
|---|---|---|---|---|---|---|
| C-peptide (nmol/L) | 0.80 ± 0.75 | 1.46 ± 1.20 | 0.29 ± 0.18 | 0.75 ± 0.55 | 0.80 ± 0.59 | 0.080 |
| Serum glucose (mg/dL) | 514.1 ± 273.3 | 778.0 ± 455.0 | 456.2 ± 162.0 | 426.0 ± 235.6 | 442.4 ± 159.3 | 0.433 |
| HbA1c (%) | 12.81 ± 1.67 | 13.50 ± 0.84 | 13.08 ± 1.20 | 12.23 ± 2.83 | 12.46 ± 1.91 | 0.841 |
| Arterial pH | 7.12 ± 0.09 | 7.11 ± 0.09 | 7.09 ± 0.10 | 7.13 ± 0.11 | 7.15 ± 0.10 | 0.737 |
| Bicarbonate (mEq/L) | 6.38 ± 4.61 | 5.50 ± 2.30 | 4.72 ± 2.17 | 6.23 ± 5.27 | 8.14 ± 6.51 | 0.884 |
| Anion gap | 27.14 ± 10.28 | 34.50 ± 14.69 | 31.28 ± 10.42 | 23.77 ± 4.9 | 21.43 ± 5.87 | 0.216 |
| Urine ketone (positive value) | 3.37 ± 0.68 | 3.25 ± 0.50 | 3.40 ± 0.89 | 3.33 ± 0.58 | 3.43 ± 0.79 | 0.914 |
| Corrected Na (mEq/L) | 141.68 ± 6.13 | 144.10 ± 9.90 | 141.90 ± 6.87 | 141.55 ± 6.21 | 140.19 ± 3.57 | 0.947 |
| Creatinine (mg/dL) | 0.82 ± 0.39 | 0.86 ± 0.41 | 0.77 ± 0.60 | 0.80 ± 0.41 | 0.84 ± 0.29 | 0.852 |
| GFR | 120.53 ± 53.56 | 114.59 ± 56.76 | 124.80 ± 65.95 | 139.89 ± 73.78 | 112.57 ± 44.50 | 0.870 |
Note: Values are presented as means ± standard deviation.
Abbreviations: GFR, glomerular filtration rate; HbA1c, hemoglobin A1c.
Rates of Complications
To evaluate changes in the complication rates during the COVID-19 pandemic, we compared the rates of specific complications in 2020 with those in other years of admission (Table 3). The rates of AKI were 33.3% and 57% in the pre-COVID-19 and COVID-19 periods, respectively. In the overall cohort, cerebral edema and cerebral infarction, the most serious complications of DKA, were recorded in only one patient in 2020. The patient was unable to recover from stupor. All six (50%) patients who exhibited altered mental status before the COVID-19 pandemic were drowsy and all achieved consciousness after treatment. There were no deaths during the four-year study period.
Table 3.
Complications of Patients with Diabetic Ketoacidosis Before and After the COVID-19 Pandemic
| Before COVID-19 (n = 12) | After COVID-19 (n = 7) | Total(n = 19) | p value | |
|---|---|---|---|---|
| AKI | 4 (33.3) | 4 (57.1) | 8 (42.1) | 0.432 |
| CVA | 0 (0.0) | 1 (14.3) | 1 (5.3) | 0.650 |
| Altered mental status | 6 (50.0) | 1 (14.3) | 7 (36.8) | 0.227 |
Note: Patients included in the “Before COVID-19” group were added from 2017 to 2019, “After COVID-19” patients were a record for half a year in 2020.
Abbreviations: AKI, acute kidney injury; COVID, coronavirus disease; CVA, cerebrovascular attack.
Changes in the Rate of DKA in Jeonbuk
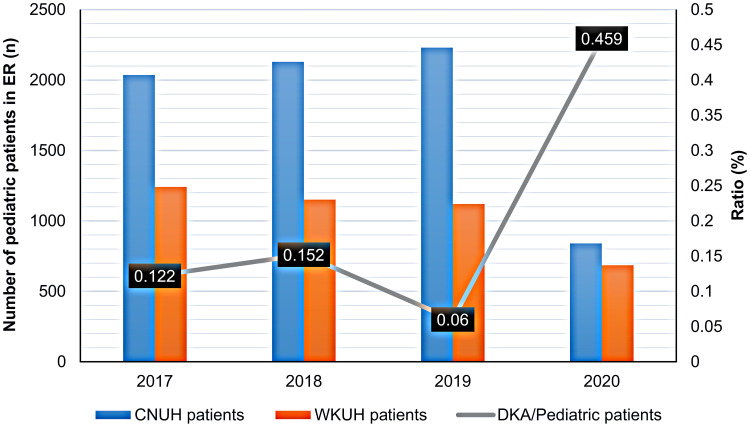
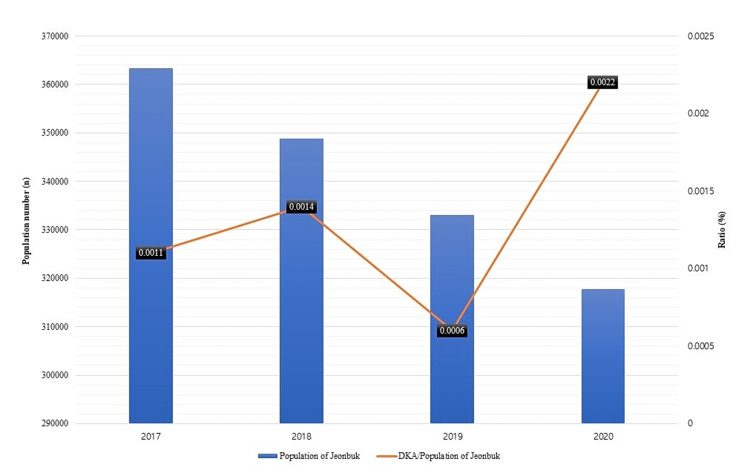
During the first 6 months of the year between 2017 and 2019 before the COVID-19 pandemic, there were about 1000 and 2000 pediatric patients who were admitted to the ERs of Wonkwang University and Chonbuk National University Hospitals, respectively. The number of pediatric patients admitted to the study ERs precipitously declined to less than 1000 after the COVID-19 outbreak. In contrast, the number of patients admitted to the study ERs for DKA was highest in 2020 and the ratio of patients with DKA to all pediatric patients admitted to the study ERs was 0.459%, which was more than twice the average rate of 0.206% (Figure 1). The ratio of pediatric patients with DKA admitted to the study ERs also increased in 2020 although the population in Jeonju, Iksan, and Jeonbuk steadily decreased from 2017 to 2020. Specifically, the ratio of patients with DKA admitted to the study ERs to the population of Jeonbuk was 0.00141% in 2020, which was higher than the average of 0.0009% (Figure 2).
Figure 1.
Trends in the rate of admittance to the ER between patients with DKA and all pediatric patients in two university hospitals.
Abbreviations: CNUH, Chonbuk National University Hospital; DKA, diabetic ketoacidosis; ER, emergency room; WKUH, Wonkwang University Hospital.
Figure 2.
Trends in the rate of patients with DKA patients within the population of Jeollabuk-do before and after the COVID-19 pandemic.
Abbreviation: DKA, diabetic ketoacidosis.
Discussion
This study assessed the trend of DKA among children during the first half of the pandemic. Considering the seasonal variations, patients admitted from January to June of each year were included. In Korea, about 300 pediatric patients with type 1 DM were admitted from 2008 to 2016, and the monthly incidence did not significantly differ even though there were more patients in March, April, and June and less in November and December.8 Based on this information, the study included data obtained in March, April, and June, when the occurrence of pediatric patients with type 1 DM is high. At the same time, the overall survey was not performed from January to December. However, considering that the patients have been evenly distributed every month within the last 10 years, the annual trend can be analyzed via a 6-month survey.
Most pediatric cases of DM are type 1 due to the lack of exogenous insulin; however, the diagnosis of type 2 DM in children has been increasing in parallel with the increase in childhood obesity.9,10 In particular, life-threatening acidosis, ie, DKA, may occur in patients with type 1 DM due to increased levels of counter-regulatory hormones in response to insulin deficiency, alterations in glucose levels, production of ketone bodies following lipolysis, and dehydration resulting from glucosuria.11,12 However, children with DM generally experience polydipsia and polyuria without acidosis for days or weeks and weight loss is observed in about half of the patients. The rates of clinical factors included in the present study did significantly differ from 2017 to 2020, except for polyuria, which was observed significantly more frequently in patients admitted to the study ERs in 2020 compared with those admitted in the previous years. The other patient characteristics and clinical factors that were more frequent in 2020, albeit without statistical significance, were female sex (85.7%), polydipsia (100%), weight loss (85.7%), altered mental status (85.7%), polydipsia (100%), newly onset DM (100%). In addition, all patients developed type 1 DM after acquiring COVID-19. In relation to this, recent studies about the increased incidence and severity of type 1 DM among children after the pandemic support the results of this study.13–15 SARS-CoV-2-infected patients commonly complain of polydipsia and polyuria, which may be attributed to the attachment of hypothalamic cell, thereby affecting its role as a hunger and satiety center.16,17 Considering this theoretical background and the overall rate of symptoms among COVID-19-negative patients in this study, the condition is believed to be associated with an increased incidence of DKA symptoms regardless of the presence of infection. Although exhibiting all three typical symptoms of DM, which could facilitate early diagnosis, these patients visited the ER after developing DKA and the rate of patients with newly diagnosed DM was high, suggesting that patients who were previously detected early may not have been detected for various reasons.
Several recent studies have reported that the rate of DKA has increased as a direct result of SARS-CoV-2 infection. In April 2020, one study from Daegu, Korea reported two cases of acute hyperglycemic crisis after SARS-CoV-2 infection in patients with preexisting DM.18 Subsequent studies reported patients with DKA after confirmed SARS-CoV-2 infection in China and the United States.19,20 Whether COVID-19 is associated with the development of DKA is unclear. However, the virus can bind to angiotensin-converting enzyme 2 (ACE2) receptors in pancreatic beta cells, leading to pleiotropic alterations in glucose metabolism.21,22 One major difference of the present study is the inclusion of pediatric patients with DKA who were not infected with SARS-CoV-2. The observed increase in the number of pediatric patients with DKA admitted to the ER might be explained by the indirect effects of the COVID-19 pandemic such as lifestyle changes due to restriction of outdoor activities, online-oriented classes, and changes in diet. The most important factor that might explain our findings is the failure to properly implement the “urinary glucose test project,” which is performed annually in school-age children. This screening, which has been administered to all children attending school since 1998, has aided in the early diagnosis of DM. The positivity rate of urinary glucose in Seoul was 0.11% during the initial implementation of the screening initiative, and the average positivity rate was 0.77% n Jeonbuk during the 2010–2013 screening period.23,24 According to the Korea Health Insurance Corporation, the general examination acceptance rate was 76.9% in 2018; however, the cumulative examination rate until April 2020 was 8.19% because of the collateral damage of COVID-19 pandemic.25 The importance of screening with the urinary glucose test is highlighted by the finding that all patients with DKA admitted to the study ERs in 2020 were newly diagnosed with DM.
The present study revealed that there was no difference in DKA severity before and after the COVID-19 pandemic. Between 2015 and 2020, only one patient with DKA in Jeonbuk exhibited stupor mental status and cerebral edema with infarction, who was admitted to the ER in 2020. The patient was transferred to the study hospital after hydration with normal saline and administration of regular insulin at other hospitals. The patient’s serum glucose and HbA1c were 428 mg/dL and 8.7% (normal average, 12.8%), respectively. The patient also had severe acidosis with an arterial pH of 7.05 and bicarbonate of 1 mmol/L despite early initial management. The patient’s high HbA1c was positively correlated with urine and plasma ketone levels. Although the HbA1c was not very high, the patient also had cerebral infarction, a serious complication that was not observed in any admitted patients in the last 5 years.26,27 The clinical course in this patient suggests the possibility that the DKA symptoms might have been detected late.
The present study has several limitations, including insufficient sample size. Although the sample mean was calculated, the number of samples in each year was too small, ranging from 3 to 7, leading to higher standard deviations. Second, the data were collected by chart review and there were differences between the actual clinical features and the medical records. Third, the follow-up period was short for patients who were admitted to the study ERs in 2020 as the study end date was July 2020. The DM type was determined by C-peptide levels measured at the time of ER visit, which may not reflect the actual type of DM. The C-peptide and HbA1c levels were measured when the glucose level based on the blood test was higher than 126 mg/dL in all pediatric patients. In addition, in patients who are followed-up in the outpatient department, examination is performed every 6 months. However, in patients who developed DKA in 2020, the C-peptide follow-up test was not performed. Hence, in this study, the type of condition was selected based on the C-peptide level at the time of visit.
Conclusion
The COVID-19 pandemic is associated with an outbreak of DKA among pediatric patients without direct infection. Therefore, pediatric patients visiting the ER with nonspecific symptoms such as nausea, vomiting, and dyspnea should raise the possibility of undiagnosed DM, and early random glucose test and timely initial management of DKA should be considered depending on the case.
Acknowledgments
The authors would like to thank the patients and pediatrician of Chonbuk National University Hospital and Wonkwang National University Hospital.
Funding Statement
The authors received no financial support for the research.
Data Sharing Statement
All data in this study are included in the published manuscript.
Ethics Approval and Informed Consent
The current study was approved by the Institutional Review Boards of Chonbuk National University Hospital (no. 2020-08-029) and Wonkwang National University Hospital (no. 2020-08-007). This study was conducted in accordance with the Declaration of Helsinki. However, it did not require a written informed consent because data were obtained via a retrospective chart review and personal identifiable information was not revealed.
Disclosure
The authors report no conflicts of interest related to this work.
References
- 1.Kim JH, An AR, Min PK, et al. How south korea responded to the covid-19 outbreak in daegu. Vol. 1 No. 4 july–august 2020 NEJM catalyst innovations in care delivery. 2020. [Google Scholar]
- 2.Bornstein SR, Zimmet P, Rubino F, Ludwig B. Management of diabetes in patients with COVID-19–Authors’ reply. Lancet Diabetes Endocrinol. 2020;8:669–670. doi: 10.1016/S2213-8587(20)30223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nina G, Douglas F, James C, Yun-Ni L, Zoe D. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020;166:108291. doi: 10.1016/j.diabres.2020.108291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavan KR, Mohammad SK, Yatin M, Sunil JM. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes Metab Syndr. 2020;14(5):1459–1462. doi: 10.1016/j.dsx.2020.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You JH, Song SO, Park SH, et al. Trends in hyperglycemic crisis hospitalizations and in-and out-of-hospital mortality in the last decade based on Korean national health insurance claims data. Endocrinol Metab. 2019;34:275–281. doi: 10.3803/EnM.2019.34.3.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song SO, Song YD, Nam JY, et al. Epidemiology of type 1 diabetes mellitus in Korea through an investigation of the national registration project of type 1 diabetes for the reimbursement of glucometer strips with additional analyses using claims data. Diabetes Metab J. 2016;40:35–45. doi: 10.4093/dmj.2016.40.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19:155–177. [DOI] [PubMed] [Google Scholar]
- 8.Chae HW, Seo GH, Song K, et al. Incidence and prevalence of type 1 diabetes mellitus among Korean children and adolescents between 2007 and 2017: an epidemiologic study based on a national database. Diabetes Metab J. 2020;44:866–874. doi: 10.4093/dmj.2020.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsarou A, Gudbjörnsdottir S, Rawshani A, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:1–7. doi: 10.1038/nrdp.2017.16 [DOI] [PubMed] [Google Scholar]
- 10.Tillotson CV, Bowden SA, Boktor SW. Pediatric type 2 diabetes mellitus. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 11.English P, Williams G. Hyperglycemic crises and lactic acidosis in diabetes mellitus. Postgrad Med J. 2004;80:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGarry JD, Woeltje KF, Kuwajima M, Foster DW. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989;5:271–284. doi: 10.1002/dmr.5610050305 [DOI] [PubMed] [Google Scholar]
- 13.Unsworth R, Wallace S, Oliver NS, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43:e170–e171. doi: 10.2337/dc20-1551 [DOI] [PubMed] [Google Scholar]
- 14.Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A. Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care. 2020;43:dc201321. doi: 10.2337/dc20-1321 [DOI] [PubMed] [Google Scholar]
- 15.Tittel SR, Rosenbauer J, Kamrath C, et al. Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care. 2020;43:e172–e173. doi: 10.2337/dc20-1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshiyama H, Hamamoto Y, Honjo S, Wada Y, Lkeda H. Hypothalamic pathogenesis of type 2 diabetes. Med Hypotheses. 2006;67:307–310. doi: 10.1016/j.mehy.2006.02.033 [DOI] [PubMed] [Google Scholar]
- 17.Nampoothiri S, Sauve F, Ternier G, et al. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis. bioRxiv.2020. doi: 10.1101/2020.06.08.139329 [DOI] [Google Scholar]
- 18.Kim NY, Ha E, Moon JS, Lee YH, Choi EY. Acute hyperglycemic crises with coronavirus disease-19. Diabetes Metab J. 2020;44:349. doi: 10.4093/dmj.2020.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chee YJ, Ng SJ, Yeoh E. Reply to comments on letter to the editor–diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;166:108305. doi: 10.1016/j.diabres.2020.108305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palermo NE, Sadhu AR, McDonnell ME. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J Clin Endocrinol Metab. 2020;105:2819–2829. doi: 10.1210/clinem/dgaa360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J-K, Lin -S-S, Ji X-J, Guo L-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubino F, Amiel SA, Zimmet P. New-Onset Diabetes in Covid-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park MJ, Kang YJ, Kim JH, Kim DH. Diagnostic significance of the urine-stick test in middle and high school children in Seoul. J Korean Pediatr Soc. 2000;43:411–416. [Google Scholar]
- 24.Kim MS, Lee DY. Urinary glucose screening for early detection of asymptomatic type 2 diabetes in Jeonbuk province Korean schoolchildren. J Korean Med Sci. 2017;32:985–991. doi: 10.3346/jkms.2017.32.6.985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park BH, Lee BK, Ahn JU, et al. Association of Participation in Health Check-ups with Risk Factors for Cardiovascular Diseases. J Korean Med Sci. 2021;18;36(3):e19. doi: 10.3346/jkms.2021.36.e19. [DOI] [PMC free article] [PubMed]
- 26.Zhu B, Bu L, Zhang M, et al. HbA 1c as a screening tool for ketosis in patients with type 2 diabetes mellitus. Sci Rep. 2016;6:39687. doi: 10.1038/srep39687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamsal R, Azaisi Y, Sharma T, Adeyinka A, Khan M, Pierre L. 471: using the level of glycosylated hemoglobin to predict the severity of diabetic ketoacidosis. Crit Care Med. 2016;44:194. doi: 10.1097/01.ccm.0000509149.57020.8c [DOI] [Google Scholar]